Smooth Muscle Cells from a Rat Model of Obesity and Hyperleptinemia Are Partially Resistant to Leptin-Induced Reactive Oxygen Species Generation
Abstract
1. Introduction
2. Experimental Design
3. Statistical Analysis
4. Results
4.1. General Characteristics of Animals
4.2. Oxidative Stress Markers
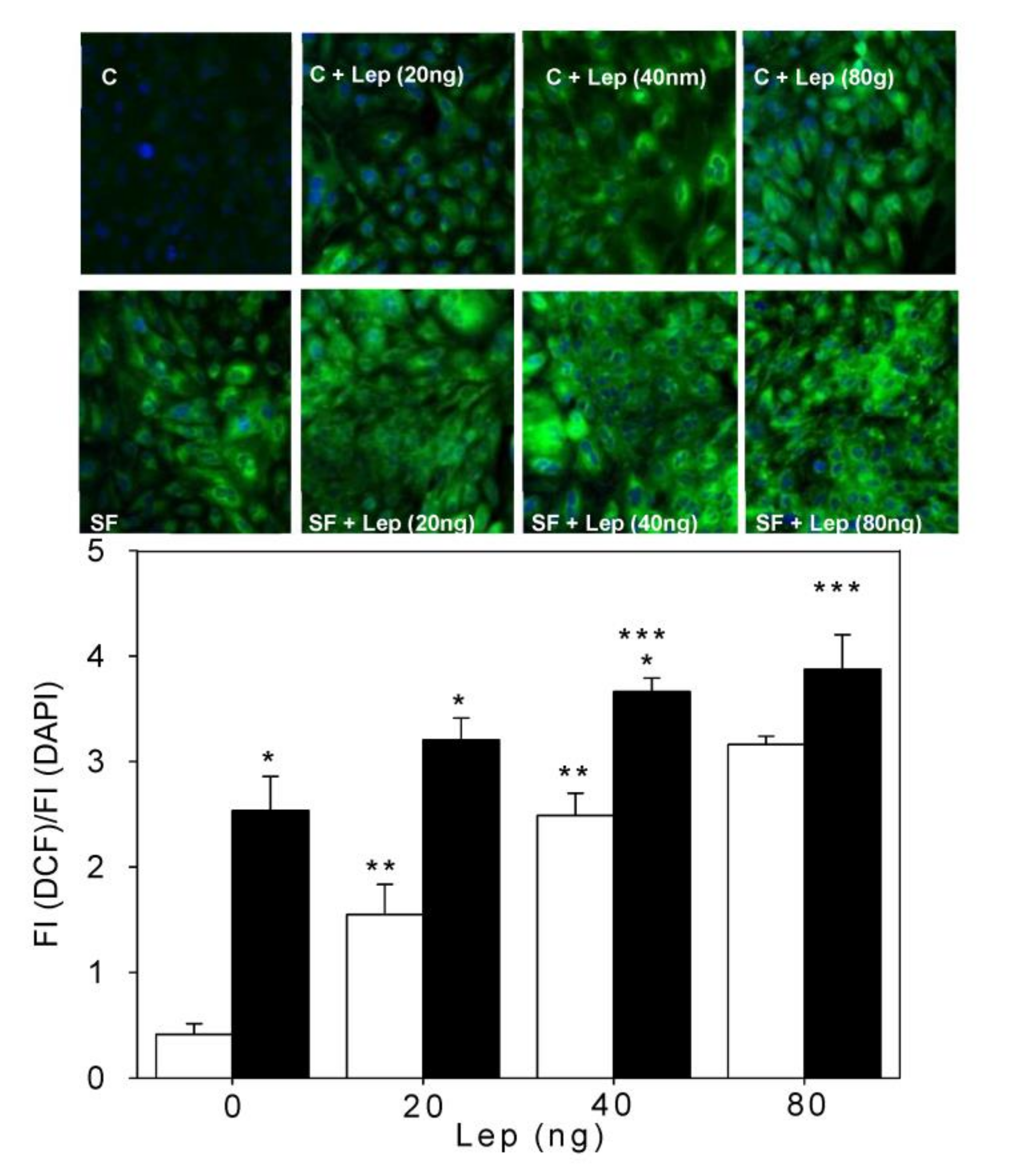
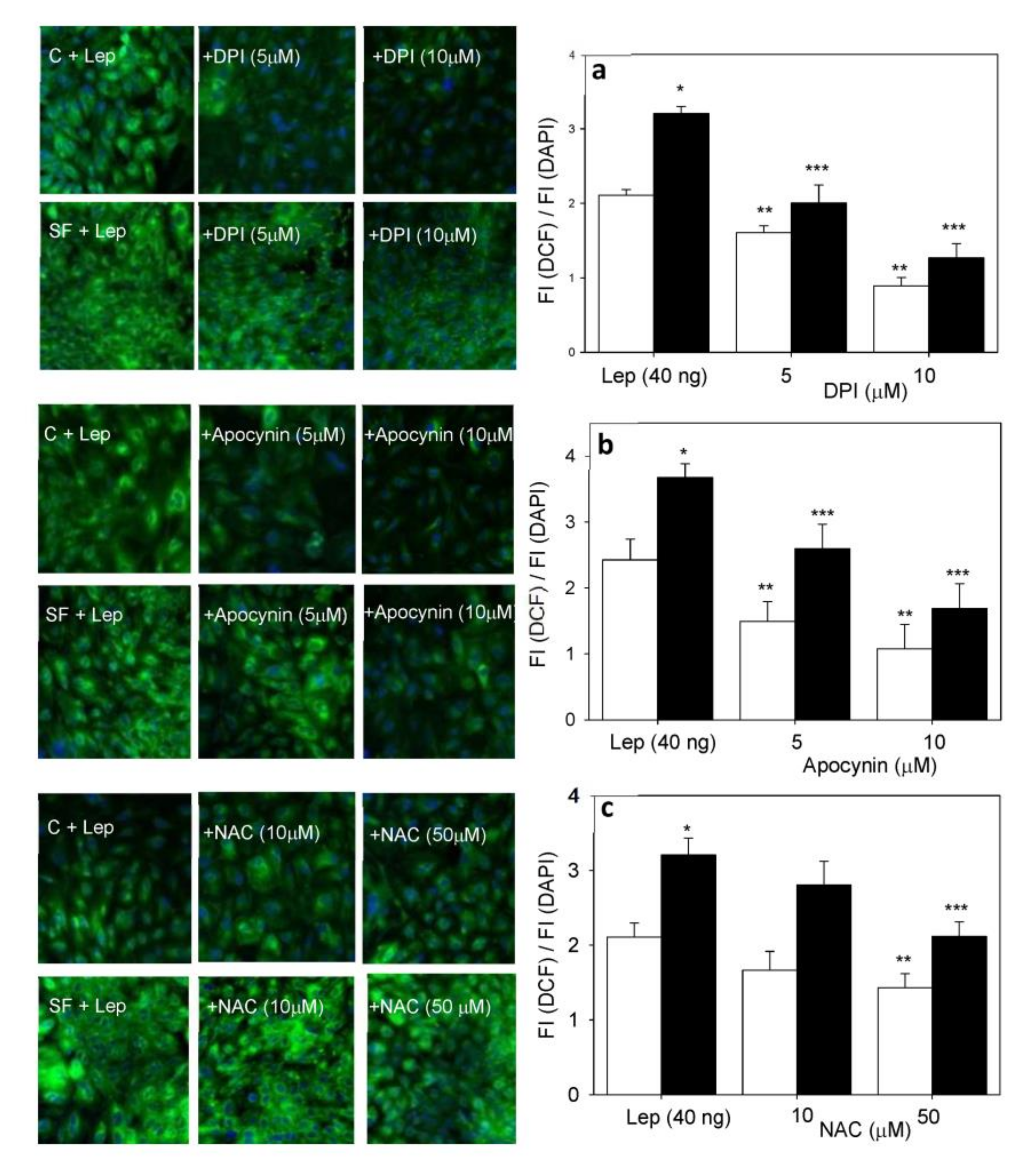
4.3. Smooth Muscle Cell ROS Generation
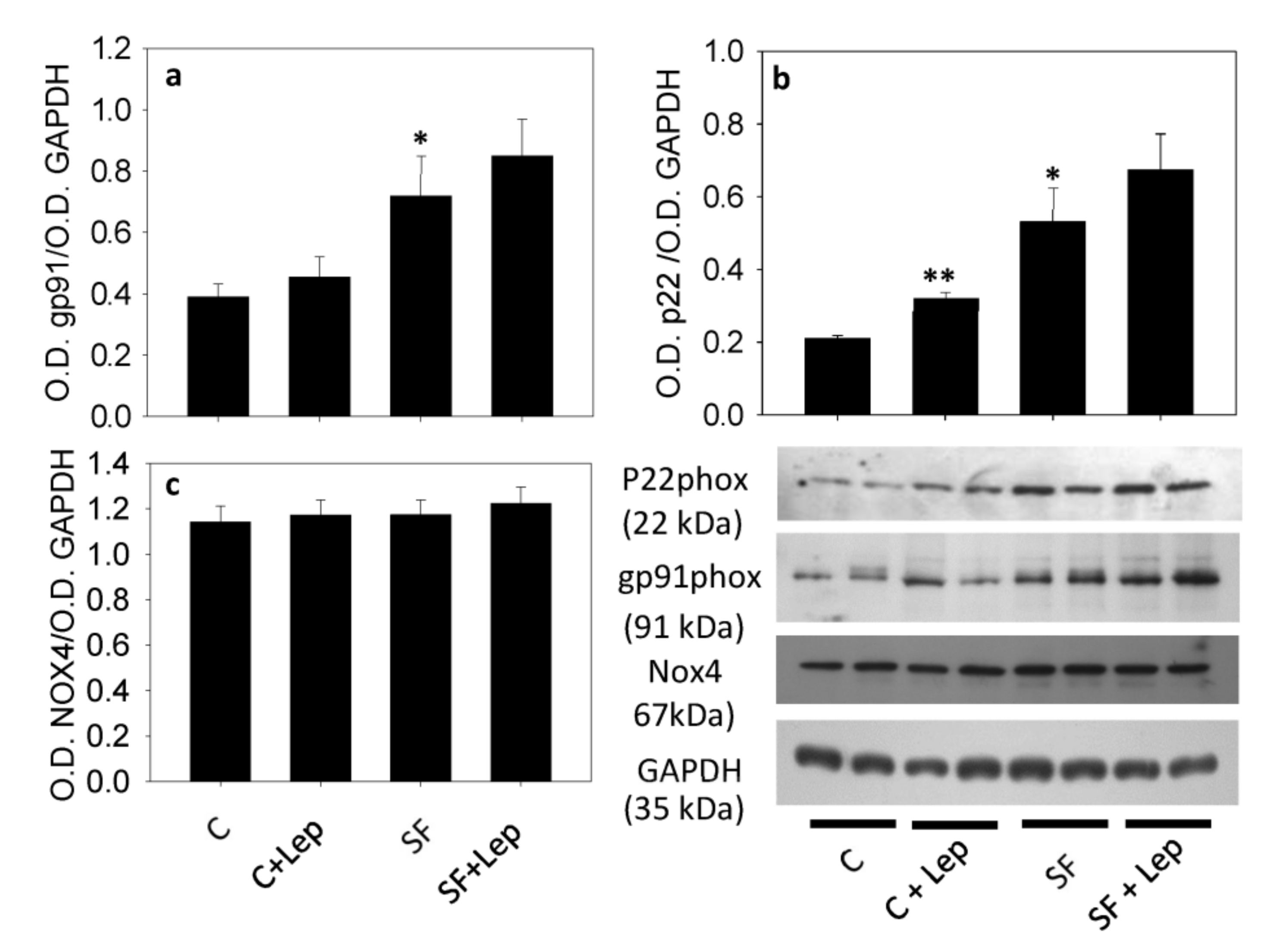
4.4. Western Blot of Nox and Their Subunit Proteins
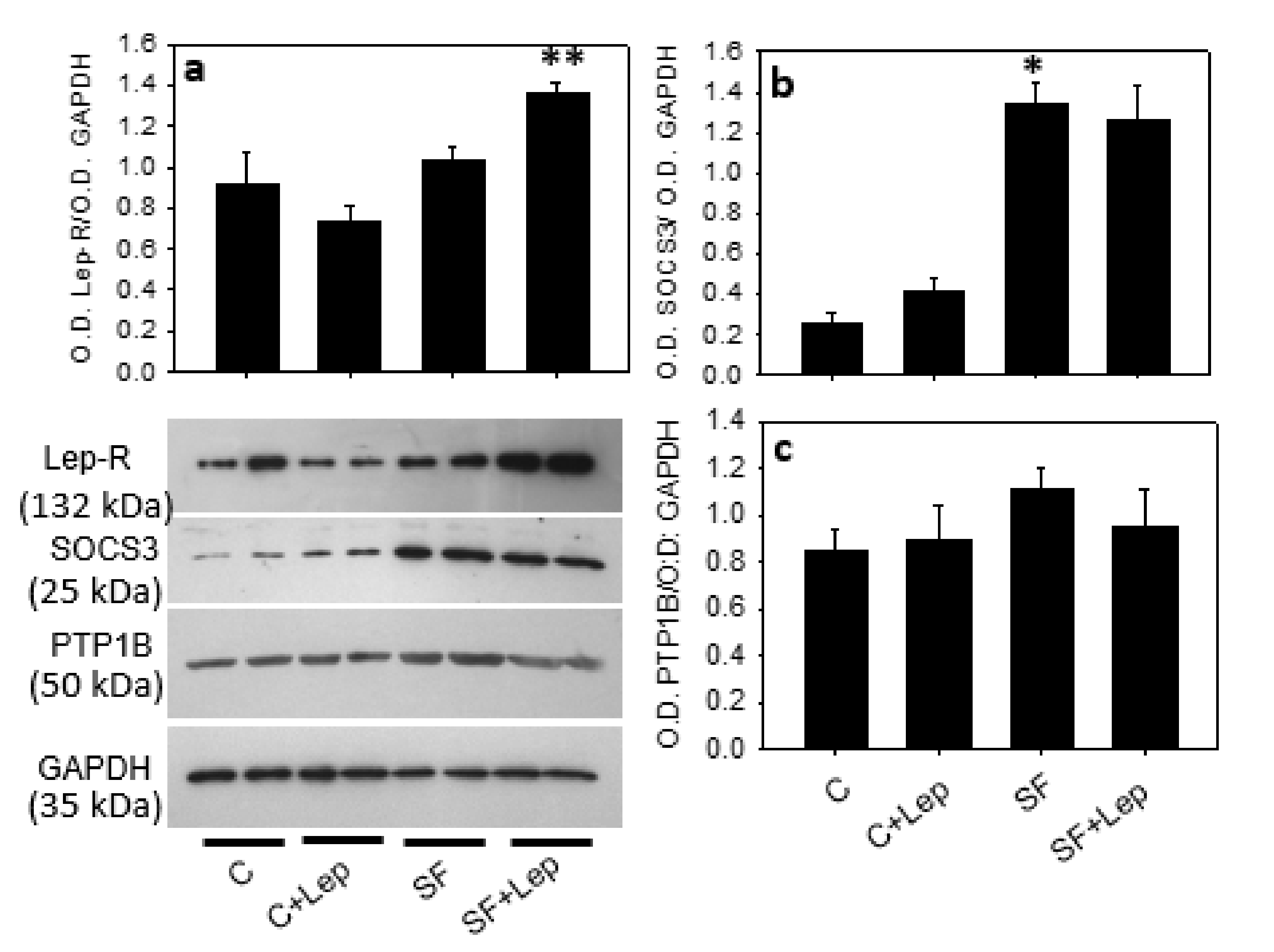
4.5. Leptin Receptor (Lep-R)
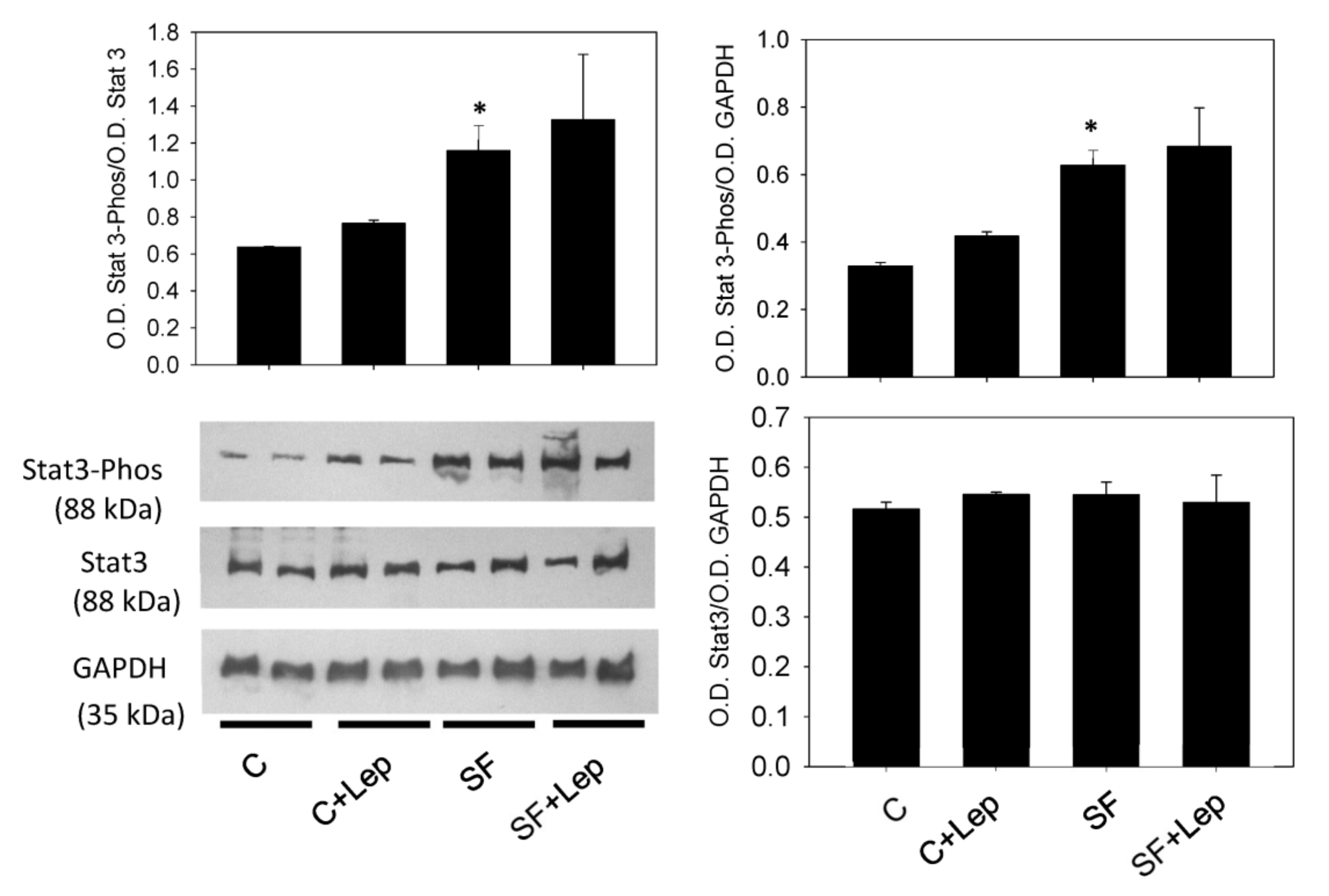
4.6. Effect of Leptin on STAT3, PTP1B and SOCS3
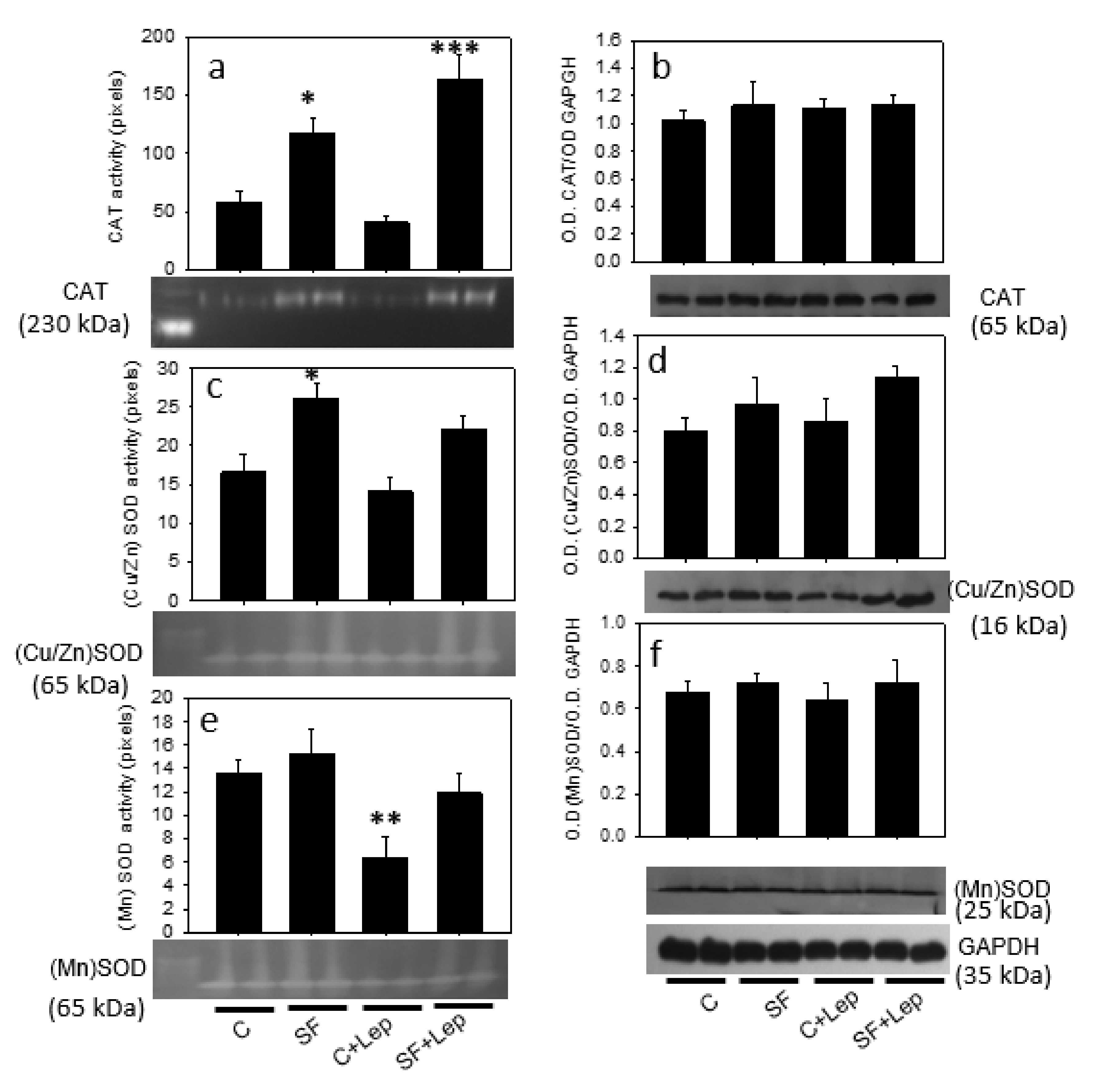
4.7. Effect of Leptin on Catalase and SOD Contents and Activities
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Cowley, A.W., Jr.; Zou, A.P. Increased H(2)O(2) counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R827–R833. [Google Scholar] [CrossRef]
- Durgin, B.G.; Straub, A.C. Redox control of vascular smooth muscle cell function and plasticity. Lab. Investig. 2018, 98, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Xiong, X.; Wang, H.; You, S.; Zeng, H. Leptin-induced vascular smooth muscle cell proliferation via regulating cell cycle, activating ERK1/2 and NF-kappaB. Acta Biochim. Biophys. Sin. 2010, 42, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, H.; Guo, M.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species in vascular formation and development. Oxid. Med. Cell. Longev. 2013, 2013, 374963. [Google Scholar] [CrossRef]
- Gautron, L.; Elmquist, J.K. Sixteen years and counting: An update on leptin in energy balance. J. Clin. Investig. 2011, 121, 2087–2093. [Google Scholar] [CrossRef]
- Scott, M.M.; Lachey, J.L.; Sternson, S.M.; Lee, C.E.; Elias, C.F.; Friedman, J.M.; Elmquist, J.K. Leptin targets in the mouse brain. J. Comp. Neurol. 2009, 514, 518–532. [Google Scholar] [CrossRef]
- Chiu, C.Z.; Wang, B.W.; Shyu, K.G. Molecular regulation of the expression of leptin by hypoxia in human coronary artery smooth muscle cells. J. Biomed. Sci. 2015, 22, 1–14. [Google Scholar] [CrossRef]
- Matsui, H.; Motooka, M.; Koike, H.; Iwasaki, T.; Suzuki, T.; Kurabayashi, M.; Yokoyama, T. Ischemia/reperfusion in rat heart induces leptin and leptin receptor gene expression. Life Sci. 2007, 80, 672–680. [Google Scholar] [CrossRef]
- Zeidan, A.; Purdham, D.M.; Rajapurohitam, V.; Javadov, S.; Chakrabarti, S.; Karmazyn, M. Leptin induces vascular smooth muscle cell hypertrophy through angiotensin II- and endothelin-1-dependent mechanisms and mediates stretch-induced hypertrophy. J. Pharmacol. Exp. Ther. 2005, 315, 1075–1084. [Google Scholar] [CrossRef]
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000Prime Rep. 2014, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chelikani, P.K.; Glimm, D.R.; Kennelly, J.J. Short communication: Tissue distribution of leptin and leptin receptor mRNA in the bovine. J. Dairy Sci. 2003, 86, 2369–2372. [Google Scholar] [CrossRef]
- Gogiraju, R.; Hubert, A.; Fahrer, J.; Straub, B.K.; Brandt, M.; Wenzel, P.; Münzel, T.; Konstantinides, S.; Hasenfuss, G.; Schäfer, K. Endothelial Leptin Receptor Deletion Promotes Cardiac Autophagy and Angiogenesis Following Pressure Overload by Suppressing Akt/mTOR Signaling. Circ. Heart Fail. 2019, 12, e005622. [Google Scholar] [CrossRef]
- Oda, A.; Taniguchi, T.; Yokoyama, M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J. Med. Sci. 2001, 47, 141–150. [Google Scholar] [CrossRef]
- Qin, Z.; Hou, X.; Weisbrod, R.M.; Seta, F.; Cohen, R.A.; Tong, X. Nox2 mediates high fat high sucrose diet-induced nitric oxide dysfunction and inflammation in aortic smooth muscle cells. J. Mol. Cell. Cardiol. 2014, 72, 56–63. [Google Scholar] [CrossRef]
- Ryan, M.J.; Coleman, T.T.; Sasser, J.M.; Pittman, K.M.; Hankins, M.W.; Stec, D.E. Vascular smooth muscle-specific deletion of the leptin receptor attenuates leptin-induced alterations in vascular relaxation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R960–R967. [Google Scholar] [CrossRef]
- Fortuño, A.; Rodríguez, A.; Gómez-Ambrosi, J.; Muñiz, P.; Salvador, J.; Díez, J.; Frühbeck, G. Leptin inhibits angiotensin II-induced intracellular calcium increase and vasoconstriction in the rat aorta. Endocrinology 2002, 143, 3555–3560. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Fortuño, A.; Gómez-Ambrosi, J.; Zalba, G.; Díez, J.; Frühbeck, G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology 2007, 148, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Luo, J.D. Leptin and cardiovascular diseases. Clin. Exp. Pharmacol. Physiol. 2011, 38, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, K.; Halle, M.; Goeschen, C.; Dellas, C.; Pynn, M.; Loskutoff, D.J.; Konstantinides, S. Leptin promotes vascular remodeling and neointimal growth in mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 112–117. [Google Scholar] [CrossRef]
- Schroeter, M.R.; Leifheit-Nestler, M.; Hubert, A.; Schumann, B.; Glückermann, R.; Eschholz, N.; Krüger, N.; Lutz, S.; Hasenfuss, G.; Konstantinides, S.; et al. Leptin promotes neointima formation and smooth muscle cell proliferation via NADPH oxidase activation and signaling in caveolin-rich microdomains. Cardiovasc. Res. 2013, 99, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Salmon, M. NADPH Oxidases in Aortic Aneurysms. Antioxidants 2022, 11, 1830. [Google Scholar] [CrossRef]
- Tong, X.; Khandelwal, A.R.; Wu, X.; Xu, Z.; Yu, W.; Chen, C.; Zhao, W.; Yang, J.; Qin, Z.; Weisbrod, R.M.; et al. Pro-atherogenic role of smooth muscle Nox4-based NADPH oxidase. J. Mol. Cell. Cardiol. 2016, 92, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Duan, Y.; Zhao, N.; Zhu, X.; Yu, X.; Jiao, S.; Song, Y.; Shi, L.; Ma, Y.; Wang, X.; et al. Peroxisome Proliferator-Activated Receptor α Attenuates Hypertensive Vascular Remodeling by Protecting Vascular Smooth Muscle Cells from Angiotensin II-Induced ROS Production. Antioxidants 2022, 11, 2378. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramirez, A.; Ortiz-Balderas, E.; Cardozo-Saldana, G.; Diaz-Diaz, E.; El-Hafidi, M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. 2014, 126, 19–29. [Google Scholar] [CrossRef]
- Castrejón-Téllez, V.; Rubio-Ruiz, M.E.; Cano-Martínez, A.; Pérez-Torres, I.; Del Valle-Mondragón, L.; Carreón-Torres, E.; Guarner-Lans, V. High Sucrose Ingestion during a Critical Period of Vessel Development Promotes the Synthetic Phenotype of Vascular Smooth Muscle Cells and Modifies Vascular Contractility Leading to Hypertension in Adult Rats. Int. J. Hypertens. 2022, 2022, 2298329. [Google Scholar] [CrossRef]
- Ruiz-Ramirez, A.; Chavez-Salgado, M.; Peneda-Flores, J.A.; Zapata, E.; Masso, F.; El-Hafidi, M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1198–E1207. [Google Scholar] [CrossRef]
- Bouloumie, A.; Marumo, T.; Lafontan, M.; Busse, R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999, 13, 1231–1238. [Google Scholar] [CrossRef]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef]
- El-Hafidi, M.; Baños, G. In vivo plasma lipid oxidation in sugar-induced rat hypertriglyceridemia and hypertension. Hypertension 1997, 30, 624–628. [Google Scholar] [CrossRef]
- El-Hafidi, M.; Cuéllar, A.; Ramírez, J.; Baños, G. Effect of sucrose addition to drinking water, that induces hypertension in the rats, on liver microsomal Delta9 and Delta5-desaturase activities. J. Nutr. Biochem. 2001, 12, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Folch, L.; Lee, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipid from animal tissues. J. Biol. Chem. 1957, 226, 497–509. Available online: https://www.ncbi.nlm.nih.gov/pubmed/13428781 (accessed on 6 January 2023). [CrossRef]
- Nagele, U.; Hagele, E.O.; Sauer, G.; Wiedemann, E.; Lehmann, P.; Wahlefeld, A.W.; Gruber, W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J. Clin. Chem. Clin. Biochem. 1984, 22, 165–174. Available online: https://www.ncbi.nlm.nih.gov/pubmed/6716056 (accessed on 6 January 2023). [CrossRef] [PubMed]
- Petzke, K.J.; Proll, J.; Brückner, J.; Metges, C.C. Plasma protein carbonyl concentration is not enhanced by chronic intake of high-protein diets in adult rats. J. Nutr. Biochem. 1999, 10, 268–273. [Google Scholar] [CrossRef] [PubMed]
- El-Hafidi, M.; Franco, M.; Ramírez, A.R.; Sosa, J.S.; Flores, J.A.P.; Acosta, O.L.; Salgado, M.C.; Cardoso-Saldaña, G. Glycine Increases Insulin Sensitivity and Glutathione Biosynthesis and Protects against Oxidative Stress in a Model of Sucrose-Induced Insulin Resistance. Oxid. Med. Cell. Longev. 2018, 2018, 2101562. [Google Scholar] [CrossRef]
- López-Acosta, O.; de Los Angeles Fortis-Barrera, M.; Barrios-Maya, M.A.; Ramírez-Ruiz, A.; Aguilar, F.J.A.; El-Hafidi, M. Reactive Oxygen Species from NADPH Oxidase and Mitochondria Participate in the Proliferation of Aortic Smooth Muscle Cells from a Model of Metabolic Syndrome. Oxid. Med. Cell. Longev. 2018, 2018, 5835072. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. Available online: https://www.ncbi.nlm.nih.gov/pubmed/94205 (accessed on 6 January 2023). [CrossRef]
- Flohe, L.; Otting, F. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. Available online: https://www.ncbi.nlm.nih.gov/pubmed/6328209 (accessed on 6 January 2023).
- El Hafidi, M.; Pérez, I.; Zamora, J.; Soto, V.; Carvajal-Sandoval, G.; Baños, G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1387–R1393. [Google Scholar] [CrossRef]
- Youn, J.Y.; Siu, K.L.; Lob, H.E.; Itani, H.; Harrison, D.G.; Cai, H. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes 2014, 63, 2344–2355. [Google Scholar] [CrossRef]
- Myers, M.G., Jr.; Leibel, R.L.; Seeley, R.J.; Schwartz, M.W. Obesity and Leptin Resistance: Distinguishing Cause from Effect. Trends Endocrinol. Metab. 2010, 21, 643–651. [Google Scholar] [CrossRef]
- Knight, Z.A.; Hannan, K.S.; Greenberg, M.L.; Friedman, J.M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE 2010, 5, e11376. [Google Scholar] [CrossRef]
- Fortuño, A.; Bidegain, J.; Baltanás, A.; Moreno, M.U.; Montero, L.; Landecho, M.F.; Beloqui, O.; Díez, J.; Zalba, G. Is leptin involved in phagocytic NADPH oxidase overactivity in obesity? Potential clinical implications. J. Hypertens. 2010, 28, 1944–1950. [Google Scholar] [CrossRef]
- Bjorbak, C.; Lavery, H.J.; Bates, S.H.; Olson, R.K.; Davis, S.M.; Flier, J.S.; Myers, M.G., Jr. 0SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 2000, 275, 40649–40657. [Google Scholar] [CrossRef]
- Ernst, M.B.; Wunderlich, C.M.; Hess, S.; Paehler, M.; Mesaros, A.; Koralov, S.B.; Kleinridders, A.; Husch, A.; Münzberg, H.; Hampel, B.; et al. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J. Neurosci. 2009, 29, 11582–11593. [Google Scholar] [CrossRef]
- Cheng, L.; Yu, Y.; Szabo, A.; Wu, Y.; Wang, H.; Camer, D.; Huang, X.F. Palmitic acid induces central leptin resistance and impairs hepatic glucose and lipid metabolism in male mice. J. Nutr. Biochem. 2015, 26, 541–548. [Google Scholar] [CrossRef]
- Koch, C.E.; Lowe, C.; Pretz, D.; Steger, J.; Williams, L.M.; Tups, A. High-fat diet induces leptin resistance in leptin-deficient mice. J. Neuroendocrinol. 2014, 26, 58–67. [Google Scholar] [CrossRef]
- Ali, M.I.; Ketsawatsomkron, P.; Belin de Chantemele, E.J.; Chantemele, E.J.; Mintz, J.D.; Muta, K.; Salet, C.; Black, S.M.; Tremblay, M.L.; Fulton, D.J.; et al. Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circ. Res. 2009, 105, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.K.; Park, S.M.; Quon, M.J. Leptin and cardiovascular disease: Response to therapeutic interventions. Circulation 2008, 117, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.A.; Odom, R.S.; Rando, T.A. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Rad. Boil. Med. 1999, 27, 1122–1132. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Edelstein, D.; Du, X.L.; Kaneda, Y.; Guzmán, M.; Brownlee, M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J. Bbiol. Chem. 2001, 276, 25096–25100. [Google Scholar] [CrossRef] [PubMed]
- López-Acosta, O.; Ruiz-Ramírez, A.; Barrios-Maya, M.A.; Alarcon-Aguilar, J.; Alarcon-Enos, J.; Céspedes Acuña, C.L.; El-Hafidi, M. Lipotoxicity, glucotoxicity and some strategies to protect vascular smooth muscle cell against proliferative phenotype in metabolic syndrome. Food Chem. Toxicol. 2023, 172, 113546. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Ishigami, N.; Kujiraoka, T.; Sato, A.; Fujita, M.; Ido, Y.; Adachi, T. Deletion of Superoxide Dismutase 1 Blunted Inflammatory Aortic Remodeling in Hypertensive Mice under Angiotensin II Infusion. Antioxidants 2021, 10, 471. [Google Scholar] [CrossRef]
- Sato, A.; Shiraishi, Y.; Kimura, T.; Osaki, A.; Kagami, K.; Ido, Y.; Adachi, T. Resistance to Obesity in SOD1 Deficient Mice with a High-Fat/High-Sucrose Diet. Antioxidants 2022, 11, 1403. [Google Scholar] [CrossRef]
- Brown, M.R.; Miller, F.J., Jr.; Li, W.G.; Ellingson, A.N.; Mozena, J.D.; Chatterjee, P.; Engelhardt, J.F.; Zwacka, R.M.; Oberley, L.W.; Fang, X.; et al. Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ. Res. 1999, 85, 524–533. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3972612/ (accessed on 6 January 2023). [CrossRef]
- Lee, K.H.; Lim, S.; Kang, S.M.; Kim, D.H.; Cho, H.K.; Chung, J.H.; Kwon, H.M.; Chung, K.H.; Lee, H.; Jang, Y.; et al. Antiproliferative mechanisms of raxofelast (IRFI-016) in H2O2-stimulated rat aortic smooth muscle cells. Eur. J. Pharmacol. 2004, 484, 119–125. [Google Scholar] [CrossRef]
- Vara, D.; Pula, G. Reactive oxygen species: Physiological roles in the regulation of vascular cells. Curr. Mol. Med. 2014, 14, 1103–1125. [Google Scholar] [CrossRef]
| Variables | C | SF |
|---|---|---|
| Blood pressure (mm/Hg) | 121.0 ± 2.2 | 148.2 ± 4.2 * |
| TGs (mM) | 0.8 ± 0.1 | 1.8 ± 0.1 ** |
| FFAs (mM) | 0.6 ± 0.1 | 1.1 ± 0.1 ** |
| Insulin (pM) | 99.6 ± 5.1 | 167.6 ± 7.8 ** |
| Leptin (ng/mL) | 0.6 ± 0.2 | 2.7 ± 0.3 ** |
| Intra-abdominal fat (g) | 7.4 ± 2.2 | 15.3 ± 2.6 * |
| Body weight (g) | 488.0 ± 19.1 | 477.0 ± 31.7 |
| Cholesterol-HDL (mg/dL) | 42.65 ± 3.5 | 28.1 ± 4.1 * |
| Glucose (mM) | 5.9 ± 0.1 | 5.9 ± 0.2 |
| HOMA-IR Total cholesterol (mM) | 3.5 ± 0.3 1.5 ± 0.1 | 6.2 ± 0.8 * 1.4 ± 0.1 |
| Plasma oxidative stress markers: TBARS (mM) | 28.7 ± 6.7 | 43.3 ± 8.2 ** |
| Carbonyl protein (nmol/mg protein) | 3.5 ± 1.2 | 5.25 ± 1.7 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Acosta, O.; Cristóbal-García, M.; Cardoso-Saldaña, G.; Carvajal-Aguilera, K.; El-Hafidi, M. Smooth Muscle Cells from a Rat Model of Obesity and Hyperleptinemia Are Partially Resistant to Leptin-Induced Reactive Oxygen Species Generation. Antioxidants 2023, 12, 728. https://doi.org/10.3390/antiox12030728
López-Acosta O, Cristóbal-García M, Cardoso-Saldaña G, Carvajal-Aguilera K, El-Hafidi M. Smooth Muscle Cells from a Rat Model of Obesity and Hyperleptinemia Are Partially Resistant to Leptin-Induced Reactive Oxygen Species Generation. Antioxidants. 2023; 12(3):728. https://doi.org/10.3390/antiox12030728
Chicago/Turabian StyleLópez-Acosta, Ocarol, Magdalena Cristóbal-García, Guillermo Cardoso-Saldaña, Karla Carvajal-Aguilera, and Mohammed El-Hafidi. 2023. "Smooth Muscle Cells from a Rat Model of Obesity and Hyperleptinemia Are Partially Resistant to Leptin-Induced Reactive Oxygen Species Generation" Antioxidants 12, no. 3: 728. https://doi.org/10.3390/antiox12030728
APA StyleLópez-Acosta, O., Cristóbal-García, M., Cardoso-Saldaña, G., Carvajal-Aguilera, K., & El-Hafidi, M. (2023). Smooth Muscle Cells from a Rat Model of Obesity and Hyperleptinemia Are Partially Resistant to Leptin-Induced Reactive Oxygen Species Generation. Antioxidants, 12(3), 728. https://doi.org/10.3390/antiox12030728






