Oxidative Stress and Antioxidant Therapy in Pulmonary Hypertension
Abstract
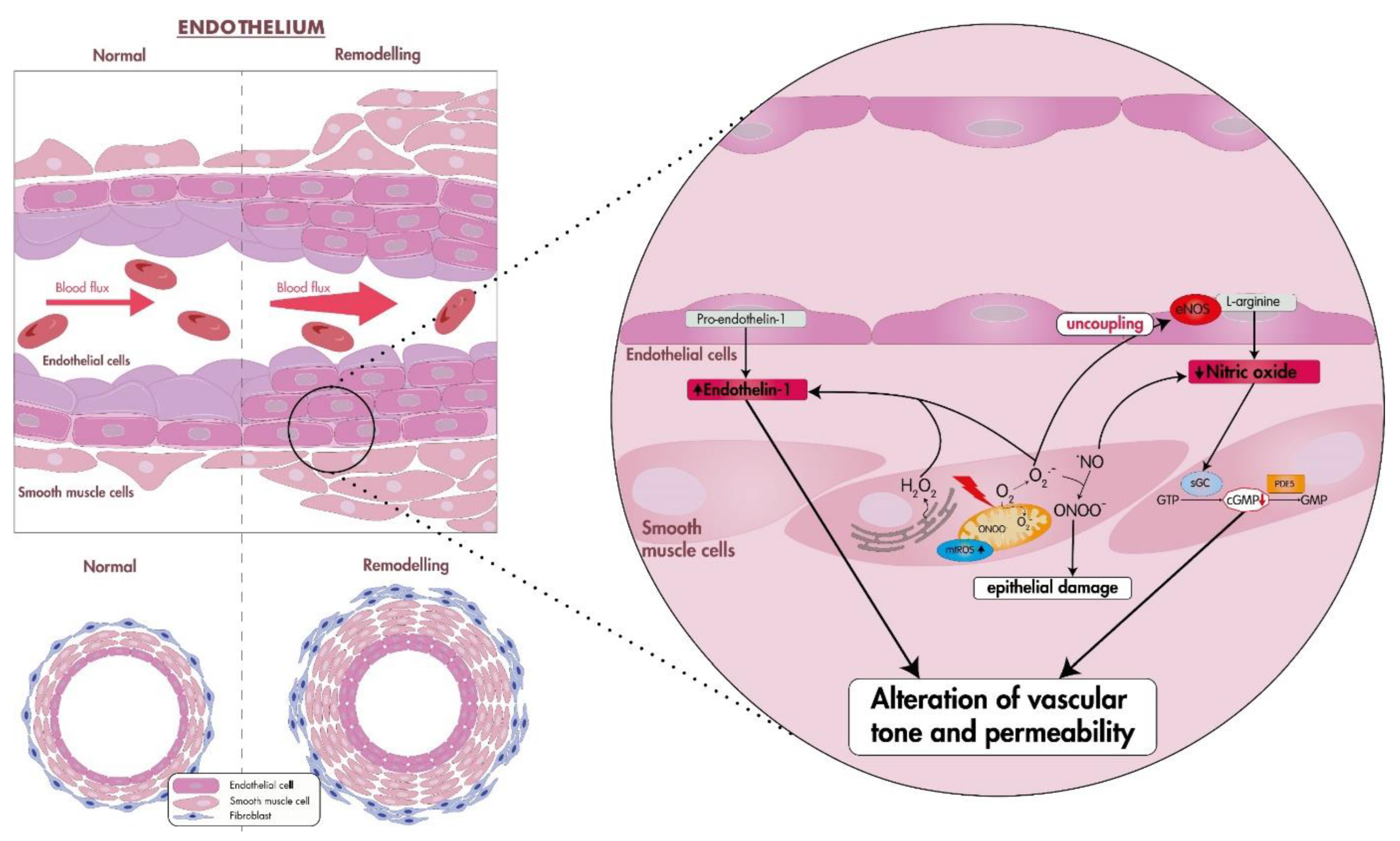
1. Pulmonary Hypertension
2. Oxidative Stress
3. Oxidative Stress in the Different Subtypes of PH
3.1. Oxidative Stress in PAH (Group I PH)
3.2. Pulmonary Hypertension Caused by Left Heart Disease (Group II PH)
3.3. Pulmonary Hypertension Caused by Lung Diseases and/or Hypoxia (Group III PH)
3.4. Oxidative Stress in CTEPH (Group IV PH)
3.5. Pulmonary Hypertension with Unclear and/or Multifactorial Mechanisms (Group V)
| Type of PH | Organism | Oxidative Stress Biomarker | Molecular Changes | Effect on the Pathology | Treatment | Effect of the Treatment | Type of Study | Ref. |
|---|---|---|---|---|---|---|---|---|
| MCT-PAH | Rat | ↑ROS ↓Catalase mRNA ↓GPX1 mRNA | ↑Phosphorylation of PKM2 ↓PKM2 activity | ↑PASMCs proliferation | NAC, apocynin, MnTBAP | ↓ROS ↓Phosphorylation of PKM2 ↑PKM2 activity | In vivo/In vitro | [21] |
| PAH | Human | ↑MAO-A expression | ↑PVR | Clorgyline (MAO-A inhibitor) | ↓MAO-A activity ↓ROS ↓PVR | In vivo/In vitro | [22] | |
| PH-LHD | Rat | ↑Peroxynitrite ↑O2- | ↓PTEN expression | ↑SMC proliferation ↑Vascular remodeling | HO-3867 (synthetic analog of curcumin) | ↓Peroxynitrite ↓O2− ↑PTEN expression ↓Vascular remodeling | In vivo/In vitro | [28] |
| Precapillary PH (Group I, III, IV and V) | Human | ↑iPF2α-III | PGH2 stimulation | Pulmonary vessels constriction | In vivo/In vitro | [23] | ||
| HPH | Rat | ↑PCOOH ↑XO activity | ↑RVH ↑Pulmonary vascular thickening | NAC or Allopurinol | ↓PCOOH ↓RVH ↓Pulmonary vascular thickening | In vivo/In vitro | [34] | |
| HPH | Rat | ↑NOX4 ↑VPO1 ↑HOCl | ↑Expression of cell cycle regulators, apoptosis-related proteins, migration promoters, and NF-κB | Vascular remodeling ↑PASMCs proliferation, apoptosis resistance, and migration | BAY 11-7082 (an inhibitor of NF-κB) | ↓Vascular remodeling ↓PASMCs proliferation, apoptosis resistance, and migration | In vivo/In vitro | [35] |
| CTEPH | Cell | ↑ROS in CTEPH-EC ↑AOPPs ↑PCO ↓GPX4 and GPX1 | Endothelial dysfunction | In vitro | [42] | |||
| CTEPH | Human | ↑MDA ↓TAC activity ↓CAT activity | Adverse clinical outcomes | In vivo/In vitro | [37] | |||
| SCD-PH | Mouse/Human | ↑TSP1 and CD47 expression ↑ROS | Endothelial dysfunction Promotion of PH in SCD | CD47 blockade | ↓ROS ↓RV pressure ↓Mean pulmonary artery pressure | In vivo/In vitro | [51] |
4. Antioxidant Treatment
4.1. Global Antioxidants (Non-Targeted Antioxidant Treatments)
4.1.1. Vitamins
4.1.2. Melatonin
4.1.3. NAC
4.1.4. Polyphenols
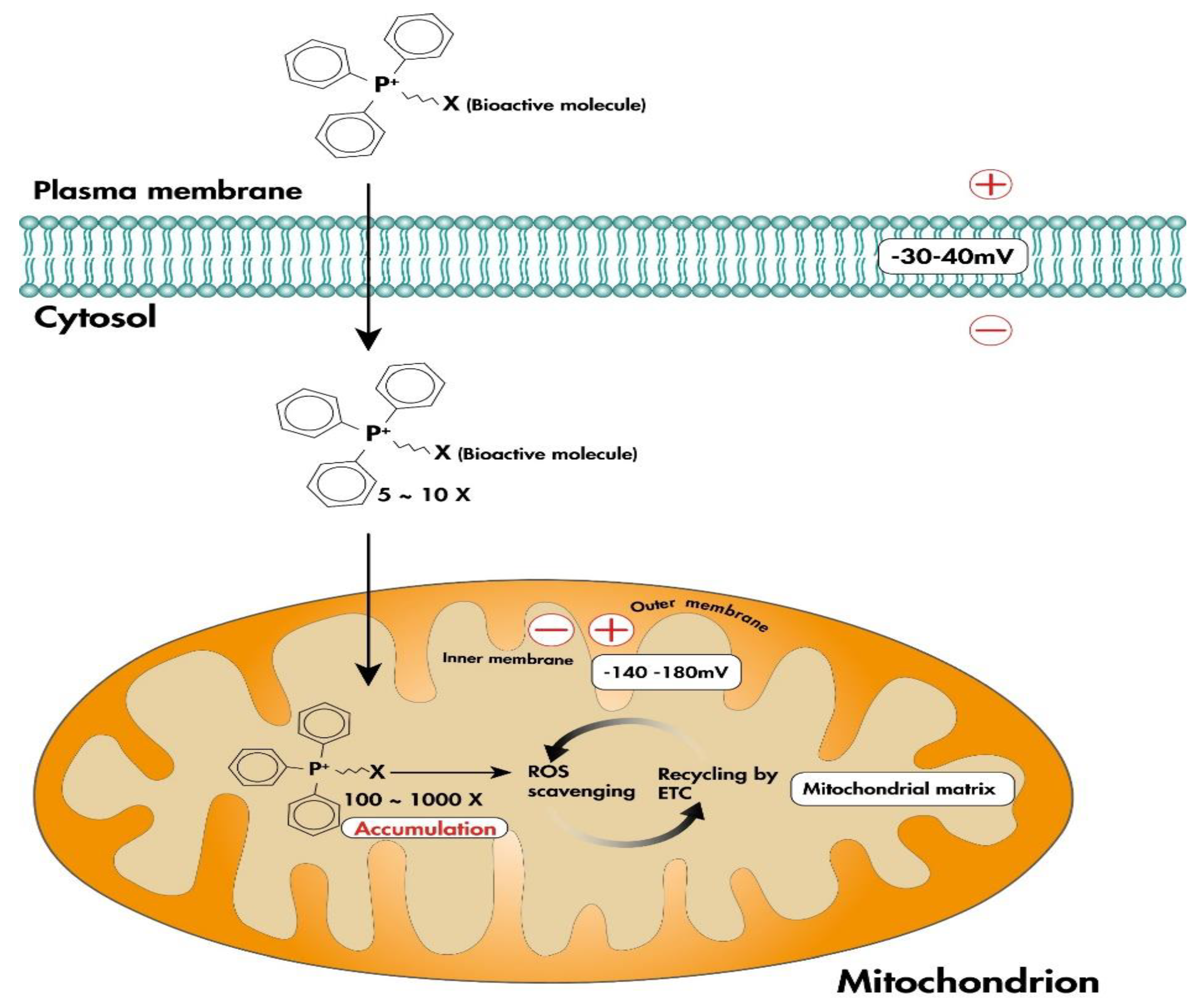
4.2. Mitochondria-Targeted Antioxidants
4.2.1. Lipophilic Cations
MitoQ
SkQ1
4.2.2. Peptide-Based Antioxidants
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Koudstaal, T.; Boomars, K.A.; Kool, M. Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension: An Immunological Perspective. J. Clin. Med. 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, M.; Makar, C.; Wissa, A.; Le, T.; Eghbali, M.; Umar, S. Oxidative stress and its implications in the right ventricular remodeling secondary to pulmonary hypertension. Front. Physiol. 2019, 10, 1233. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 46. [Google Scholar] [CrossRef]
- Morrell, N.W.; Aldred, M.A.; Chung, W.K.; Elliott, C.G.; Nichols, W.C.; Soubrier, F.; Trembath, R.C.; Loyd, J.E. Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801899. [Google Scholar] [CrossRef]
- Anderson, J.J.; Lau, E.M. Pulmonary Hypertension Definition, Classification, and Epidemiology in Asia. JACC Asia 2022, 2, 538–546. [Google Scholar] [CrossRef]
- Mandras, S.A.; Mehta, H.S.; Vaidya, A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin. Proc. 2020, 95, 1978–1988. [Google Scholar] [CrossRef]
- Cuttica, M.J. Pulmonary hypertension associated with lung diseases and hypoxemia. Heart Fail. Rev. 2016, 21, 299–308. [Google Scholar] [CrossRef]
- Lang, I.M.; Campean, I.A.; Sadushi-Kolici, R.; Badr-Eslam, R.; Gerges, C.; Skoro-Sajer, N. Chronic Thromboembolic Disease and Chronic Thromboembolic Pulmonary Hypertension. Clin. Chest Med. 2021, 42, 81–90. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Krylatov, A.V.; Maslov, L.N.; Voronkov, N.S.; Boshchenko, A.A.; Popov, S.V.; Gomez, L.; Wang, H.; Jaggi, A.S.; Downey, J.M. Reactive Oxygen Species as Intracellular Signaling Molecules in the Cardiovascular System. Curr. Cardiol. Rev. 2018, 14, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.S.; Augusto, V.S.; Silveira, A.P.C.; Jordão, A.A.; Baddini-Martinez, J.; Poli Neto, O.; Rodrigues, A.J.; Evora, P.R.B. Oxidative-stress biomarkers in patients with pulmonary hypertension. Pulm. Circ. 2013, 3, 856–861. [Google Scholar] [CrossRef]
- Zimmer, A.; Teixeira, R.B.; Constantin, R.L.; Campos-Carraro, C.; Aparicio Cordero, E.A.; Ortiz, V.D.; Donatti, L.; Gonzalez, E.; Bahr, A.C.; Visioli, F.; et al. The progression of pulmonary arterial hypertension induced by monocrotaline is characterized by lung nitrosative and oxidative stress, and impaired pulmonary artery reactivity. Eur. J. Pharmacol. 2021, 891, 173699. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, H.; Chen, L.; Dhillon, N.K. NADPH oxidase-mediated endothelial injury in HIV- and opioid-induced pulmonary arterial hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L1097–L1108. [Google Scholar] [CrossRef]
- Tabima, D.M.; Frizzell, S.; Gladwin, M.T. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic. Biol. Med. 2012, 52, 1970–1986. [Google Scholar] [CrossRef]
- Crosswhite, P.; Sun, Z. Nitric Oxide, Oxidative Stress and Inflammation in Pulmonary Arterial Hypertension. J. Hypertens. 2010, 28, 201. [Google Scholar] [CrossRef]
- Fulton, D.J.R.; Li, X.; Bordan, Z.; Haigh, S.; Bentley, A.; Chen, F.; Barman, S.A. Reactive oxygen and nitrogen species in the development of pulmonary hypertension. Antioxidants 2017, 6, 54. [Google Scholar] [CrossRef]
- DeMarco, V.G. Contribution of oxidative stress to pulmonary arterial hypertension. World J. Cardiol. 2010, 2, 316. [Google Scholar] [CrossRef]
- Guo, D.; Gu, J.; Jiang, H.; Ahmed, A.; Zhang, Z.; Gu, Y. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to the development of pulmonary arterial hypertension. J. Mol. Cell. Cardiol. 2016, 91, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Q.; Peters, E.L.; Schalij, I.; Axelsen, J.B.; Andersen, S.; Kurakula, K.; Gomez-Puerto, M.C.; Szulcek, R.; Pan, X.; da Silva Goncalves Bos, D.; et al. Increased MAO-A activity promotes progression of pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2021, 64, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.L.; Cracowski, C.; Bessard, G.; Pepin, J.L.; Bessard, J.; Schwebel, C.; Stanke-Labesque, F.; Pison, C. Increased Lipid Peroxidation in Patients with Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2012, 164, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.A.; Steinhorn, R.H.; Wedgwood, S.; Mata-Greenwood, E.; Roark, E.A.; Russell, J.A.; Black, S.M. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: A role for NADPH oxidase. Circ. Res. 2003, 92, 683–691. [Google Scholar] [CrossRef]
- Wedgwood, S.; Steinhorn, R.H.; Bunderson, M.; Wilham, J.; Lakshminrusimha, S.; Brennan, L.A.; Black, S.M. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L660–L666. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Gibbs, J.S.R.; Wachter, R.; De Marco, T.; Vonk-Noordegraaf, A.; Vachiéry, J.-L. Left ventricular heart failure and pulmonary hypertension. Eur. Heart J. 2016, 37, 942–954. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Lang, I.M.; Blindt, R.; Bonderman, D.; Bruch, L.; Diller, G.P.; Felgendreher, R.; Gerges, C.; Hohenforst-Schmidt, W.; Holt, S.; et al. Pulmonary hypertension associated with left heart disease: Updated Recommendations of the Cologne Consensus Conference 2018. Int. J. Cardiol. 2018, 272S, 53–62. [Google Scholar] [CrossRef]
- Ravi, Y.; Selvendiran, K.; Naidu, S.K.; Meduru, S.; Citro, L.A.; Bognár, B.; Khan, M.; Kálai, T.; Hideg, K.; Kuppusamy, P.; et al. Pulmonary hypertension secondary to left-heart failure involves peroxynitrite-induced downregulation of PTEN in the lung. Hypertension 2013, 61, 593–601. [Google Scholar] [CrossRef]
- Sunamura, S.; Satoh, K.; Kurosawa, R.; Ohtsuki, T.; Kikuchi, N.; Elias-Al-Mamun, M.; Shimizu, T.; Ikeda, S.; Suzuki, K.; Satoh, T.; et al. Different roles of myocardial ROCK1 and ROCK2 in cardiac dysfunction and postcapillary pulmonary hypertension in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E7129. [Google Scholar] [CrossRef]
- McGettrick, M.; Peacock, A. Group 3 pulmonary hypertension: Challenges and opportunities. Glob. Cardiol. Sci. Pract. 2020, 2020, e202006. [Google Scholar] [CrossRef]
- Pena, E.; El Alam, S.; Siques, P.; Brito, J. Oxidative Stress and Diseases Associated with High-Altitude Exposure. Antioxidants 2022, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Sham, J.S.K.; Shimoda, L.A.; Kuppusamy, P.; Sylvester, J.T. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am. J. Physiol. Cell. Mol. Physiol. 2003, 285, L322–L333. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, N.; Tadić, A.; Hänze, J.; Rose, F.; Winterhalder, S.; Nollen, M.; Schermuly, R.T.; Ghofrani, H.A.; Seeger, W.; Grimminger, F. Hypoxic vasoconstriction in intact lungs: A role for NADPH oxidase-derived H2O2? Am. J. Physiol. Cell. Mol. Physiol. 2000, 279, L683–L690. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoshikawa, Y.; Ono, S.; Suzuki, S.; Tanita, T.; Chida, M.; Song, C.; Noda, M.; Tabata, T.; Voelkel, N.F.; Fujimura, S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J. Appl. Physiol. 2001, 90, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Liu, Y.; Chen, J.; Huang, X.; Peng, H.; Liu, Z.; Tang, Y.; Zhang, K.; Xu, Q.; Li, X.; et al. Vascular peroxidase 1 mediates hypoxia-induced pulmonary artery smooth muscle cell proliferation, apoptosis resistance and migration. Cardiovasc. Res. 2018, 114, 188–199. [Google Scholar] [CrossRef]
- Pu, X.; Lin, X.; Duan, X.; Wang, J.; Shang, J.; Yun, H.; Chen, Z. Oxidative and Endoplasmic Reticulum Stress Responses to Chronic High-Altitude Exposure during the Development of High-Altitude Pulmonary Hypertension. High Alt. Med. Biol. 2020, 21, 378–387. [Google Scholar] [CrossRef]
- Smukowska-Gorynia, A.; Rzymski, P.; Marcinkowska, J.; Poniedziałek, B.; Komosa, A.; Cieslewicz, A.; Slawek-Szmyt, S.; Janus, M.; Araszkiewicz, A.; Jankiewicz, S.; et al. Prognostic Value of Oxidative Stress Markers in Patients with Pulmonary Arterial or Chronic Thromboembolic Pulmonary Hypertension. Oxid. Med. Cell. Longev. 2019, 2019, 3795320. [Google Scholar] [CrossRef]
- Brandt, M.; Giokoglu, E.; Garlapati, V.; Bochenek, M.L.; Molitor, M.; Hobohm, L.; Schönfelder, T.; Münzel, T.; Kossmann, S.; Karbach, S.H.; et al. Pulmonary Arterial Hypertension and Endothelial Dysfunction Is Linked to NADPH Oxidase-Derived Superoxide Formation in Venous Thrombosis and Pulmonary Embolism in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 1860513. [Google Scholar] [CrossRef]
- Sakao, S.; Hao, H.; Tanabe, N.; Kasahara, Y.; Kurosu, K.; Tatsumi, K. Endothelial-like cells in chronic thromboembolic pulmonary hypertension: Crosstalk with myofibroblast-like cells. Respir. Res. 2011, 12, 109. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Tura-Ceide, O.; Smolders, V.F.E.D.; Aventin, N.; Morén, C.; Guitart-Mampel, M.; Blanco, I.; Piccari, L.; Osorio, J.; Rodríguez, C.; Rigol, M.; et al. Derivation and characterisation of endothelial cells from patients with chronic thromboembolic pulmonary hypertension. Sci. Rep. 2021, 11, 18797. [Google Scholar] [CrossRef] [PubMed]
- Nukala, S.B.; Tura-Ceide, O.; Aldini, G.; Smolders, V.F.E.D.; Blanco, I.; Peinado, V.I.; Castellà, M.; Barberà, J.A.; Altomare, A.; Baron, G.; et al. Protein network analyses of pulmonary endothelial cells in chronic thromboembolic pulmonary hypertension. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Stam, K.; Cai, Z.; van der Velde, N.; van Duin, R.; Lam, E.; van der Velden, J.; Hirsch, A.; Duncker, D.J.; Merkus, D. Cardiac remodelling in a swine model of chronic thromboembolic pulmonary hypertension: Comparison of right vs. left ventricle. J. Physiol. 2019, 597, 4465–4480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, T.; Xu, X.; Wang, M.; Zhong, L.; Yang, Y.; Zhai, Z.; Xiao, F.; Wang, C. Oxidative stress and nitric oxide signaling related biomarkers in patients with pulmonary hypertension: A case control study. BMC Pulm. Med. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.L., Jr.; Oleas, F.; Souza, R. Pulmonary Hypertension: Definition, Classification, and Diagnosis. Semin. Respir. Crit. Care Med. 2017, 38, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Sysol, J.R.; Machado, R.F. Classification and pathophysiology of pulmonary hypertension. Contin. Cardiol. Educ. 2018, 4, 2–12. [Google Scholar] [CrossRef]
- Shilo, N.R.; Morris, C.R. Pathways to pulmonary hypertension in sickle cell disease: The search for prevention and early intervention. Expert Rev. Hematol. 2017, 10, 875–890. [Google Scholar] [CrossRef]
- Novelli, E.M.; Little-Ihrig, L.; Knupp, H.E.; Rogers, N.M.; Yao, M.; Baust, J.J.; Meijles, D.; St. Croix, C.M.; Ross, M.A.; Pagano, P.J.; et al. Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L1150–L1164. [Google Scholar] [CrossRef]
- Morris, C.R.; Vichinsky, E.P. Pulmonary hypertension in thalassemia. Ann. N. Y. Acad. Sci. 2010, 1202, 205–213. [Google Scholar] [CrossRef]
- Willson, C.; Watanabe, M.; Tsuji-Hosokawa, A.; Makino, A. Pulmonary vascular dysfunction in metabolic syndrome. J. Physiol. 2019, 597, 1121–1141. [Google Scholar] [CrossRef]
- Milisav, I.; Ribarič, S.; Poljsak, B. Antioxidant Vitamins and Ageing; Springer: Singapore, 2018; pp. 1–23. [Google Scholar]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and exercise performance: With a focus on vitamin e and c supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef] [PubMed]
- Callejo, M.; Barberá, J.A.; Duarte, J.; Perez-Vizcaino, F. Impact of nutrition on pulmonary arterial hypertension. Nutrients 2020, 12, 169. [Google Scholar] [CrossRef]
- Semen, K.O.; Bast, A. Towards improved pharmacotherapy in pulmonary arterial hypertension. Can diet play a role? Clin. Nutr. ESPEN 2019, 30, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Kupari, M.; Rapola, J. Reversible pulmonary hypertension associated with vitamin C deficiency. Chest 2012, 142, 225–227. [Google Scholar] [CrossRef]
- Callejo, M.; Mondejar-Parreño, G.; Esquivel-Ruiz, S.; Olivencia, M.A.; Moreno, L.; Blanco, I.; Escribano-Subias, P.; Cogolludo, A.; Barbera, J.A.; Perez-Vizcaino, F. Total, bioavailable, and free vitamin D levels and their prognostic value in pulmonary arterial hypertension. J. Clin. Med. 2020, 9, 448. [Google Scholar] [CrossRef]
- Callejo, M.; Morales-Cano, D.; Mondejar-Parreño, G.; Barreira, B.; Esquivel-Ruiz, S.; Olivencia, M.A.; Moreno, L.; Cogolludo, A.; Perez-Vizcaino, F. Restoration of vitamin d levels improves endothelial function and increases task-like k+ currents in pulmonary arterial hypertension associated with vitamin d deficiency. Biomolecules 2021, 11, 795. [Google Scholar] [CrossRef]
- Alamri, A.; Burzangi, A.S.; Coats, P.; Watson, D.G. Untargeted metabolic profiling cell-based approach of pulmonary artery smooth muscle cells in response to high glucose and the effect of the antioxidant vitamins d and e. Metabolites 2018, 8, 87. [Google Scholar] [CrossRef]
- Gonzaléz-Candia, A.; Arias, P.V.; Aguilar, S.A.; Figueroa, E.G.; Reyes, R.V.; Ebensperger, G.; Llanos, A.J.; Herrera, E.A. Melatonin reduces oxidative stress in the right ventricle of newborn sheep gestated under chronic hypoxia. Antioxidants 2021, 10, 1658. [Google Scholar] [CrossRef]
- Figueroa, E.G.; Gonzaléz-Candia, A.; Villanueva, C.A.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Beneficial effects of melatonin on prostanoids pathways in pulmonary hypertensive neonates. Vascul. Pharmacol. 2021, 138, 106853. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, J.; Han, J.; Gong, M.; Liu, L.; Zhang, Y.; Liu, Y.; Wang, D.; Tang, Q.; Wu, N.; et al. Melatonin Attenuates Dasatinib-Aggravated Hypoxic Pulmonary Hypertension via Inhibiting Pulmonary Vascular Remodeling. Front. Cardiovasc. Med. 2022, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef] [PubMed]
- Maarman, G.J. Natural Antioxidants as Potential Therapy, and a Promising Role for Melatonin against Pulmonary Hypertension; Springer: Cham, Switzerland, 2017; pp. 161–178. [Google Scholar]
- Wang, Y.; Li, X.; Niu, W.; Chen, J.; Zhang, B.; Zhang, X.; Wang, Y.; Dang, S.; Li, Z. The alveolar epithelial cells are involved in pulmonary vascular remodeling and constriction of hypoxic pulmonary hypertension. Respir. Res. 2021, 22, 134. [Google Scholar] [CrossRef]
- Yu, W.; Song, X.; Lin, C.; Ji, W. Interventions and mechanisms of N-acetylcysteine on monocrotaline-induced pulmonary arterial hypertension. Exp. Ther. Med. 2018, 15, 5503–5509. [Google Scholar] [CrossRef] [PubMed]
- Elena Soto, M.; Instituto Nacional de Cardiologia Ignacio Chavez. N-Acetyl Cysteine in Post-Reperfusion Pulmonary Injury in Chronic Thromboembolic Pulmonary Hypertension—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04081012?term=NAC&cond=Pulmonary+Hypertension&draw=2&rank=1 (accessed on 6 May 2020).
- Mirhadi, E.; Roufogalis, B.D.; Banach, M.; Barati, M.; Sahebkar, A. Resveratrol: Mechanistic and Therapeutic Perspectives in Pulmonary Arterial Hypertension; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 163, ISBN 9177948564. [Google Scholar]
- Nani, A.; Murtaza, B.; Khan, A.S.; Khan, N.A.; Hichami, A. Antioxidant and anti-inflammatory potential of polyphenols contained in Mediterranean diet in obesity: Molecular mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, H.; Hu, Z. Resveratrol attenuates chronic pulmonary embolism-related endothelial cell injury by modulating oxidative stress, inflammation, and autophagy. Clinics 2022, 77, 100083. [Google Scholar] [CrossRef]
- Shi, W.; Zhai, C.; Feng, W.; Wang, J.; Zhu, Y.; Li, S.; Wang, Q.; Zhang, Q.; Yan, X.; Chai, L.; et al. Resveratrol inhibits monocrotaline-induced pulmonary arterial remodeling by suppression of SphK1-mediated NF-κB activation. Life Sci. 2018, 210, 140–149. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, W.Y.; Wang, C.G.; Huang, J.A.; Jiang, J.H.; Zeng, D.X. xiong Resveratrol prevented experimental pulmonary vascular remodeling via miR-638 regulating NR4A3/cyclin D1 pathway. Microvasc. Res. 2020, 130, 103988. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Xia, T.; Feng, J.; Zhou, R. Pulmonary arterial hypertension and flavonoids: A role in treatment. Chin. J. Physiol. 2021, 64, 115–124. [Google Scholar] [PubMed]
- Zhang, X.; Liu, Q.; Zhang, C.; Sheng, J.; Li, S.; Li, W.; Yang, X.; Wang, X.; He, S.; Bai, J.; et al. Puerarin prevents progression of experimental hypoxia-induced pulmonary hypertension via inhibition of autophagy. J. Pharmacol. Sci. 2019, 141, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhang, H.; Chen, D.; Chen, Y.; Lyu, Y.; Fang, L.; Du, G. Puerarin protects pulmonary arteries from hypoxic injury through the BMPRII and PPARγ signaling pathways in endothelial cells. Pharmacol. Rep. 2019, 71, 855–861. [Google Scholar] [CrossRef]
- Colon Hidalgo, D.; Elajaili, H.; Suliman, H.; George, M.P.; Delaney, C.; Nozik, E. Metabolism, Mitochondrial Dysfunction, and Redox Homeostasis in Pulmonary Hypertension. Antioxidants 2022, 11, 428. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, J.; Chen, J.; Ma, X.; Wu, M.; Liu, G.; Yao, K.; Tan, B.; Yin, Y. Mitochondria-Targeted Antioxidants: A Step towards Disease Treatment. Oxid. Med. Cell. Longev. 2020, 2020, 8837893. [Google Scholar] [CrossRef]
- Fujimoto, C.; Yamasoba, T. Mitochondria-targeted antioxidants for treatment of hearing loss: A systematic review. Antioxidants 2019, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Deus, C.M.; Borges, F.; Oliveira, P.J. Mitochondria: Targeting mitochondrial reactive oxygen species with mitochondriotropic polyphenolic-based antioxidants. Int. J. Biochem. Cell Biol. 2018, 97, 98–103. [Google Scholar] [CrossRef]
- Pak, O.; Scheibe, S.; Esfandiary, A.; Gierhardt, M.; Sydykov, A.; Logan, A.; Fysikopoulos, A.; Veit, F.; Hecker, M.; Kroschel, F.; et al. Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension. Eur. Respir. J. 2018, 51, 1701024. [Google Scholar] [CrossRef]
- Broome, S.C.; Woodhead, J.S.T.; Merry, T.L. Mitochondria-targeted antioxidants and skeletal muscle function. Antioxidants 2018, 7, 107. [Google Scholar] [CrossRef]
- Gioscia-Ryan, R.A.; LaRocca, T.J.; Sindler, A.L.; Zigler, M.C.; Murphy, M.P.; Seals, D.R. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. 2014, 592, 2549–2561. [Google Scholar] [CrossRef]
- Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A. (MitoQ) improves vascular function in healthy older adults. Hypertension 2018, 71, 1056–1063. [Google Scholar]
- Suresh, K.; Servinsky, L.; Jiang, H.; Bigham, Z.; Yun, X.; Kliment, C.; Huetsch, J.; Damarla, M.; Shimoda, L.A. Reactive oxygen species induced Ca2+influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2018, 314, L893–L907. [Google Scholar] [CrossRef]
- Suresh, K.; Servinsky, L.; Jiang, H.; Bigham, Z.; Zaldumbide, J.; Huetsch, J.C.; Kliment, C.; Acoba, M.G.; Kirsch, B.J.; Claypool, S.M.; et al. Regulation of mitochondrial fragmentation in microvascular endothelial cells isolated from the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 317, L639–L652. [Google Scholar] [CrossRef] [PubMed]
- Manskikh, V.N.; Gancharova, O.S.; Nikiforova, A.I.; Krasilshchikova, M.S.; Shabalina, I.G.; Egorov, M.V.; Karger, E.M.; Milanovsky, G.E.; Galkin, I.I.; Skulachev, V.P.; et al. Age-associated murine cardiac lesions are attenuated by the mitochondria-targeted antioxidant SkQ1. Histol. Histopathol. 2015, 30, 353–360. [Google Scholar] [PubMed]
- Zhang, Y.B.; Meng, Y.H.; Chang, S.; Zhang, R.Y.; Shi, C. High fructose causes cardiac hypertrophy via mitochondrial signaling pathway. Am. J. Transl. Res. 2016, 8, 4869–4880. [Google Scholar]
- Zinovkin, R.A.; Romaschenko, V.P.; Galkin, I.I.; Zakharova, V.V.; Pletjushkina, O.Y.; Chernyak, B.V.; Popova, E.N. Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium. Aging 2014, 6, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Victor, V.M. Molecular Strategies for Targeting Antioxidants to Mitochondria: Therapeutic Implications. Antioxid. Redox Signal. 2015, 22, 686. [Google Scholar] [CrossRef]
- Reddy, P.H. Mitochondrial oxidative damage in aging and Alzheimer’s disease: Implications for mitochondrially targeted antioxidant therapeutics. J. Biomed. Biotechnol. 2006, 2006, 31372. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Li, S.; Xie, Y.; Tong, X. The Therapeutic Strategies Targeting Mitochondrial Metabolism in Cardiovascular Disease. Pharmaceutics 2022, 14, 2760. [Google Scholar] [CrossRef]
- Whitson, J.A.; Bitto, A.; Zhang, H.; Sweetwyne, M.T.; Coig, R.; Bhayana, S.; Shankland, E.G.; Wang, L.; Bammler, T.K.; Mills, K.F.; et al. SS-31 and NMN: Two paths to improve metabolism and function in aged hearts. Aging Cell 2020, 19, e13213. [Google Scholar] [CrossRef]
- Lu, H.I.; Huang, T.H.; Sung, P.H.; Chen, Y.L.; Chua, S.; Chai, H.Y.; Chung, S.Y.; Liu, C.F.; Sun, C.K.; Chang, H.W.; et al. Administration of antioxidant peptide SS-31 attenuates transverse aortic constriction-induced pulmonary arterial hypertension in mice. Acta Pharmacol. Sin. 2016, 37, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, A.; Holt, A.; Brown, C.; Cosme, C.; Wipf, P.; Gomez-Marin, A.; Castro, M.R.; Ayala-Peña, S.; McMurray, C.T. Mitochondrial targeting of XJB-5-131 attenuates or improves pathophysiology in HdhQ150 animals with well-developed disease phenotypes. Hum. Mol. Genet. 2016, 25, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
|
|
|
|
|
|
|
|
|
|
| Antioxidant Therapy | Condition | N | Study Design | Findings | Status | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|
| Clinical trials in PH | ||||||
| NAC | CTEPH | 34 | Randomized clinical trial | No finding yet (still recruiting) | Recruiting | NCT04081012 |
| CoQ10 | PAH | 18 | Non-randomized clinical trial | Improved hemoglobin and red cell maturation | Completed | NCT01148836 |
| BQ-123 with or without MitoQ or oral BH4 | PAH | 420 | Non-randomized clinical trial | No finding yet (still recruiting) | Recruiting | NCT02966665 |
| Clinical trials in CVD | ||||||
| Vitamin D | CVD | 80 | Non-randomized clinical trial | VitD did not improve endothelial function, arterial stiffness, or inflammation | Completed | NCT01049048 |
| Vitamin E and C | CVD | 14,641 | Randomized trial | No significant effect on cardiovascular events | Completed | NCT00270647 |
| Melatonin | Smoke-induced Vascular Injury | 68 | Randomized clinical trial | Improved smoke-induced vascular injury | Completed | NCT02591238 |
| NAC | Hypertrophic Cardiomyopathy | 42 | Randomized clinical trial | Small effect on cardiac hypertrophy or fibrosis | Completed | NCT01537926 |
| Resveratrol | Peripheral Arterial Disease | 66 | Randomized clinical trial | No improvement in 6 MWT | Completed | NCT02246660 |
| MitoQ | Peripheral Arterial Disease | 13 | Randomized clinical trial | No finding yet (still recruiting) | Recruiting | NCT03506633 |
| Clinical trials in other diseases | ||||||
| MitoQ | Chronic Obstructive Pulmonary Disease | 24 | Randomized clinical trial | No finding yet | Not yet recruiting | NCT05605548 |
| SkQ1 | Keratoconjunctivitis Sicca | 91 | Randomized clinical trial | Improved dry eye symptoms | Completed | NCT02121301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poyatos, P.; Gratacós, M.; Samuel, K.; Orriols, R.; Tura-Ceide, O. Oxidative Stress and Antioxidant Therapy in Pulmonary Hypertension. Antioxidants 2023, 12, 1006. https://doi.org/10.3390/antiox12051006
Poyatos P, Gratacós M, Samuel K, Orriols R, Tura-Ceide O. Oxidative Stress and Antioxidant Therapy in Pulmonary Hypertension. Antioxidants. 2023; 12(5):1006. https://doi.org/10.3390/antiox12051006
Chicago/Turabian StylePoyatos, Paula, Miquel Gratacós, Kay Samuel, Ramon Orriols, and Olga Tura-Ceide. 2023. "Oxidative Stress and Antioxidant Therapy in Pulmonary Hypertension" Antioxidants 12, no. 5: 1006. https://doi.org/10.3390/antiox12051006
APA StylePoyatos, P., Gratacós, M., Samuel, K., Orriols, R., & Tura-Ceide, O. (2023). Oxidative Stress and Antioxidant Therapy in Pulmonary Hypertension. Antioxidants, 12(5), 1006. https://doi.org/10.3390/antiox12051006







