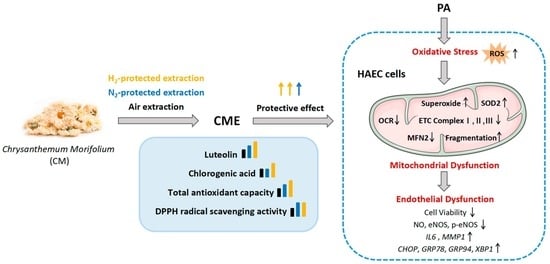
Hydrogen Protection Boosts the Bioactivity of Chrysanthemum morifolium Extract in Preventing Palmitate-Induced Endothelial Dysfunction by Restoring MFN2 and Alleviating Oxidative Stress in HAEC Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CME
2.2. Total Antioxidant Capacity Assay
2.3. DPPH Radical Scavenging Activities Assay
2.4. Liquid Chromatograph Mass Spectrometer (LC-MS) Analysis of Chlorogenic Acid (CA) and Luteolin (LU) Concentration
2.5. Cell Culture and Treatment
2.6. PA Stock Solution Preparation
2.7. Cell Viability Measurement
2.8. NO Production Measurement
2.9. Protein Extraction and Western Blot
2.10. Quantitative Real Time-PCR (qPCR)
2.11. Intracellular ROS Measurement
2.12. Mitochondrial Superoxide Measurement
2.13. Mitochondrial Morphology Measurement
2.14. Mitochondrial DNA (mtDNA) Copy Number Measurement
2.15. Oxygen Consumption Rate (OCR) Measurement
2.16. siRNA Transfection for MFN2 and SOD2 Knockdown
2.17. Statistical Analysis
3. Results
3.1. Hydrogen-Protected Extraction Increased the Content of Polyphenolic Compounds in CME and Enhanced the Antioxidant Capacity of CME
3.2. Hydrogen-Protected Extraction Enhanced the Capacity of CME to Attenuate PA-Induced Endothelial Dysfunction in HAEC Cells
3.3. Hydrogen-Protected Extraction Enhanced the Capacity of CME to Attenuate PA-Induced Oxidative Stress in HAEC Cells
3.4. Hydrogen-Protected Extraction Enhanced the Capacity of CME to Attenuate PA-Induced Mitochondrial Fragmentation and Dysfunction in HAEC Cells
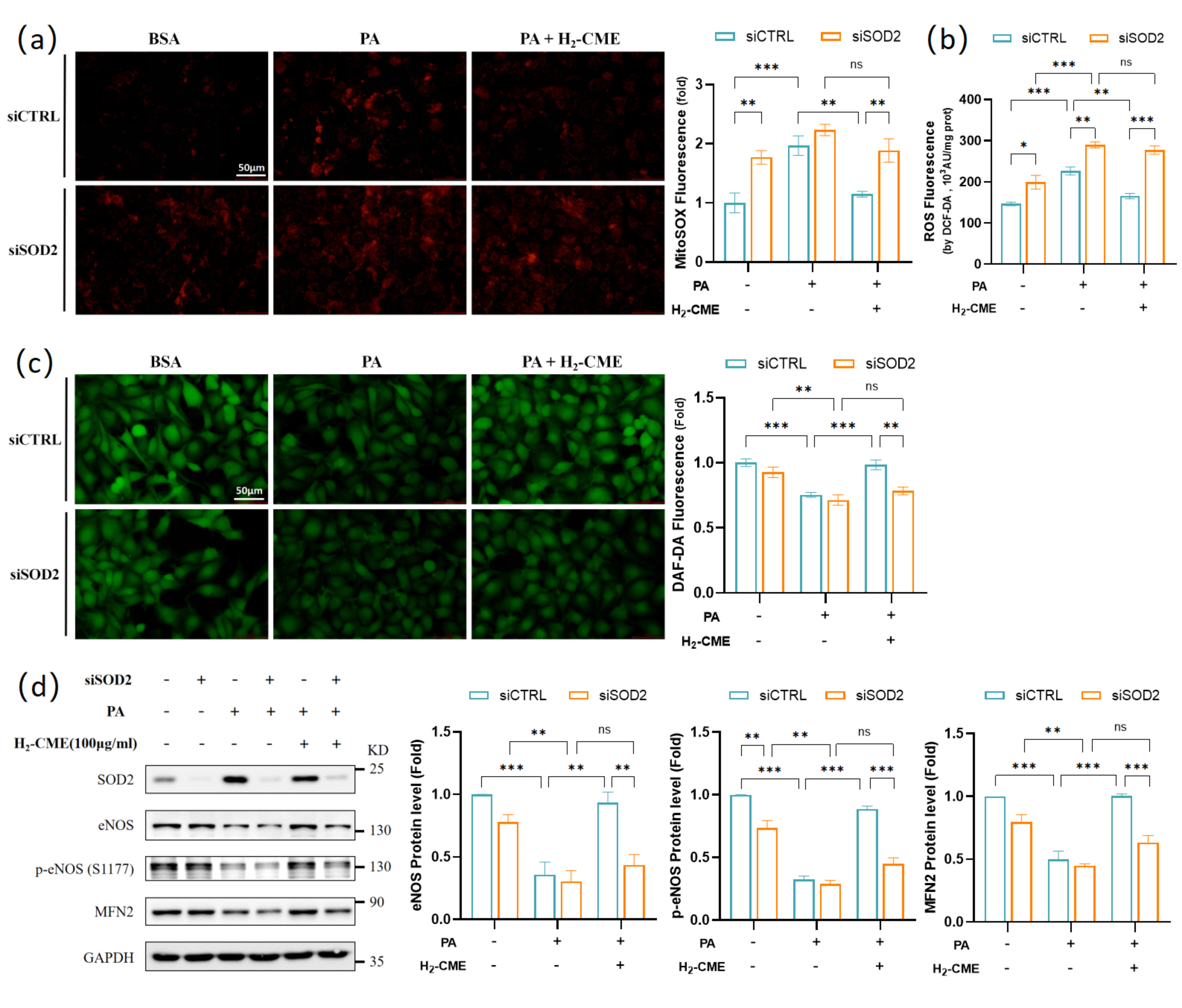
3.5. MFN2 Mediated H2-CME’s Prevention of PA-Induced Decrease of NO Production and Increase of ROS Generation in HAEC Cells
3.6. Oxidative Stress Mediated PA-Induced Endothelial Dysfunction in HAEC Cells
3.7. H2-CME Prevented PA-Induced Decrease in NO Level by Maintaining Redox Balance in HAEC Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, P.; Yang, Y.; Feng, Z.; Jiang, J.; Zhang, P. Six new compounds from the flowers of Chrysanthemum morifolium and their biological activities. Bioorg. Chem. 2019, 82, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; He, Z.; He, S.; Jing, P. Insights into the importance of dietary chrysanthemum flower (Chrysanthemum morifolium cv. Hangju)-wolfberry (Lycium barbarum fruit) combination in antioxidant and anti-inflammatory properties. Food Res. Int. 2019, 116, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Huang, X.; Li, J.; Jiang, G.; Shen, G.; Li, S.; Luo, Q.; Wu, H.; Li, M.; Liu, X.; et al. Chrysanthemum morifoliumExtraction Optimization and Evaluation of the Antioxidant and α-Glucosidase Inhibitory Activity of Polysaccharides from cv. Hangju. Antioxidants 2020, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Youssef, F.; Eid, S.; Alshammari, E.; Ashour, M.; Wink, M.; El-Readi, M. Chrysanthemum indicum and: Chemical Composition of Their Essential Oils and Their Potential Use as Natural Preservatives with Antimicrobial and Antioxidant Activities. Foods 2020, 9, 1460. [Google Scholar] [CrossRef]
- Zhan, G.; Long, M.; Shan, K.; Xie, C.; Yang, R. Chrysanthemum morifoliumAntioxidant Effect of (Chuju) Extract on HO-Treated L-O2 Cells as Revealed by LC/MS-Based Metabolic Profiling. Antioxidants 2022, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Luo, Y.; Gao, B.; Sun, J.; Lu, W.; Liu, J.; Chen, P.; Zhang, Y.; Yu, L. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019, 286, 8–16. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, X.; Li, B.; Yue, R. Chrysanthemum morifoliumFast and efficient identification of hyaluronidase specific inhibitors from Ramat. using UF-LC-MS technique and their anti-inflammation effect in macrophages. Heliyon 2023, 9, e13709. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Xue, J.; Liu, J.; Xie, M. Chrysanthemum morifolium extract attenuates high-fat milk-induced fatty liver through peroxisome proliferator-activated receptor α-mediated mechanism in mice. Nutr. Res. 2014, 34, 268–275. [Google Scholar] [CrossRef]
- Chung, K.; Hong, J.; Lee, J.; Lee, H.; Park, J.; Choi, J.; Park, H.; Hong, J.; Lee, K. Chrysanthemum Borealeβ-Caryophyllene in the Essential Oil from Induces G Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Huang, C.; Li, Z.; Yang, J.; Li, B.; Liang, R.; Dai, Z.; Liu, Z. Chrysanthemum indicum ethanolic extract inhibits invasion of hepatocellular carcinoma via regulation of MMP/TIMP balance as therapeutic target. Oncol. Rep. 2010, 23, 413–421. [Google Scholar] [CrossRef]
- Kim, D.; Won, K.; Yoon, M.; Yu, H.; Park, J.; Kim, B.; Lee, H. Chrysanthemum boreale flower floral water inhibits platelet-derived growth factor-stimulated migration and proliferation in vascular smooth muscle cells. Pharm. Biol. 2015, 53, 725–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, M.; Sunagawa, Y.; Mochizuki, S.; Katagiri, T.; Takai, H.; Iwashimizu, S.; Inai, K.; Funamoto, M.; Shimizu, K.; Shimizu, S.; et al. Chrysanthemum morifolium Extract Ameliorates Doxorubicin-Induced Cardiotoxicity by Decreasing Apoptosis. Cancers 2022, 14, 683. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Jia, H.; Jin, Y.; Wang, M.; Kou, J.; Wang, C.; Rong, X.; Xie, X.; Han, G.; Pang, X. Chrysanthemum extract attenuates hepatotoxicity via inhibiting oxidative stress in vivo and in vitro. Food Nutr. Res. 2019, 63, 1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matacchione, G.; Gurău, F.; Baldoni, S.; Prattichizzo, F.; Silvestrini, A.; Giuliani, A.; Pugnaloni, A.; Espinosa, E.; Amenta, F.; Bonafè, M.; et al. Pleiotropic effects of polyphenols on glucose and lipid metabolism: Focus on clinical trials. Ageing Res. Rev. 2020, 61, 101074. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.; Christou, A.; Kapnissi-Christodoulou, C. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Fraga, C.; Galleano, M.; Verstraeten, S.; Oteiza, P. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.; Liu, J.; Kang, L.; Chen, S.; Ma, B.; Guo, B. Quantitative and Qualitative Analysis of Flavonoids and Phenolic Acids in Snow Chrysanthemum (Coreopsis tinctoria Nutt.) by HPLC-DAD and UPLC-ESI-QTOF-MS. Molecules 2016, 21, 1307. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Xue, X.; Zhang, Z. Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 2016, 91, 132–142. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Yang, J.; Hu, Y.; Peng, L.; Zheng, H.; Cao, J. Vesicle based ultrasonic-assisted extraction of saponins in Panax notoginseng. Food Chem. 2020, 303, 125394. [Google Scholar] [CrossRef]
- Ding, Q.; Jiang, H.; Chen, Y.; Luo, L.; He, R.; Ma, H.; Wu-Chen, R.A.; Zhang, T. Influence of nitrogen protection on the extraction yield and antioxidant activities of polyphenols by ultrasonic-assisted extraction from rapeseed meal. J. Food Process Eng. 2019, 42, e13104.13101–e13104.13111. [Google Scholar] [CrossRef]
- Mark, G.; Tauber, A.; Laupert, R.; Schuchmann, H.; Schulz, D.; Mues, A.; von Sonntag, C. OH-radical formation by ultrasound in aqueous solution—Part II: Terephthalate and Fricke dosimetry and the influence of various conditions on the sonolytic yield. Ultrason. Sonochem. 1998, 5, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shen, Y.; Fan, X.; Martín, J.; Wang, X.; Song, Y. Free radical generation induced by ultrasound in red wine and model wine: An EPR spin-trapping study. Ultrason. Sonochem. 2015, 27, 96–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwazeer, D.; Liu, F.; Wu, X.; LeBaron, T. Combating Oxidative Stress and Inflammation in COVID-19 by Molecular Hydrogen Therapy: Mechanisms and Perspectives. Oxidative Med. Cell. Longev. 2021, 2021, 5513868. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxidative Med. Cell. Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef]
- Qian, L.; Wu, Z.; Cen, J.; Pasca, S.; Tomuleasa, C. Medical Application of Hydrogen in Hematological Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3917393. [Google Scholar] [CrossRef]
- Fujita, K.; Seike, T.; Yutsudo, N.; Ohno, M.; Yamada, H.; Yamaguchi, H.; Sakumi, K.; Yamakawa, Y.; Kido, M.; Takaki, A.; et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS ONE 2009, 4, e7247. [Google Scholar] [CrossRef] [Green Version]
- Kiyomi, N.; Takashi, A.; Ikuroh, O.; Etsuko, N.; Chiaki, I.; Takashi, Y.; Naomi, K.; Shigeo, O. Effects of Molecular Hydrogen Assessed by an Animal Model and a Randomized Clinical Study on Mild Cognitive Impairment. Curr. Alzheimer Res. 2017, 15, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Iketani, M.; Sekimoto, K.; Igarashi, T.; Takahashi, M.; Komatsu, M.; Sakane, I.; Takahashi, H.; Kawaguchi, H.; Ohtani-Kaneko, R.; Ohsawa, I. Administration of hydrogen-rich water prevents vascular aging of the aorta in LDL receptor-deficient mice. Sci. Rep. 2018, 8, 16822. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, T.; Liu, L.; Li, S.; Zhang, Z.; Zhang, R.; Zhou, Y.; Liu, F. Effect of hydrogen-rich water on the Nrf2/ARE signaling pathway in rats with myocardial ischemia-reperfusion injury. J. Bioenerg. Biomembr. 2019, 51, 393–402. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C.; Ping, N.; Sun, Y.; Wang, Z.; Zhao, G.; Yuan, S.; Zibrila, A.; Soong, L.; Liu, J. Hydrogen-rich water alleviates cyclosporine A-induced nephrotoxicity via the Keap1/Nrf2 signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22467. [Google Scholar] [CrossRef]
- Du, H.; Sheng, M.; Wu, L.; Zhang, Y.; Shi, D.; Weng, Y.; Xu, R.; Yu, W. Hydrogen-Rich Saline Attenuates Acute Kidney Injury After Liver Transplantation via Activating p53-Mediated Autophagy. Transplantation 2016, 100, 563–570. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, W.; Sun, H.; Fan, D.; Nakao, A.; Cai, J.; Yan, G.; Zhou, W.; Shen, R.; Yang, J.; et al. Hydrogen-rich saline protects against liver injury in rats with obstructive jaundice. Liver Int. Off. J. Int. Assoc. Study Liver 2010, 30, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Pi, X.; Xie, L.; Patterson, C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef]
- Vanhoutte, P.; Zhao, Y.; Xu, A.; Leung, S. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef] [Green Version]
- Qu, K.; Yan, F.; Qin, X.; Zhang, K.; He, W.; Dong, M.; Wu, G. Mitochondrial dysfunction in vascular endothelial cells and its role in atherosclerosis. Front. Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef]
- Ma, H.; Liu, S.; Qu, W.; Huang, Q.; Li, L.; Chu, F.; Zhu, Y.; Lv, X.; Wang, Z.; Zhu, J. Chrysanthemum morifoliumComparison of the antioxidant activities of nonfumigated and sulphur-fumigated cv. Hang-ju induced by oxidative stress. Pharm. Biol. 2021, 59, 40–46. [Google Scholar] [CrossRef]
- Kim, H. Extracts of Chrysanthemum zawadskii attenuate oxidative damage to vascular endothelial cells caused by a highly reducing sugar. Cytotechnology 2017, 69, 915–924. [Google Scholar] [CrossRef]
- Lii, C.; Lei, Y.; Yao, H.; Hsieh, Y.; Tsai, C.; Liu, K.; Chen, H. Chrysanthemum morifolium Ramat. reduces the oxidized LDL-induced expression of intercellular adhesion molecule-1 and E-selectin in human umbilical vein endothelial cells. J. Ethnopharmacol. 2010, 128, 213–220. [Google Scholar] [CrossRef]
- Jiang, H.; Cai, J.; Xu, J.; Zhou, X.; Xia, Q. Endothelium-dependent and direct relaxation induced by ethyl acetate extract from Flos Chrysanthemi in rat thoracic aorta. J. Ethnopharmacol. 2005, 101, 221–226. [Google Scholar] [CrossRef]
- He, D.; Ru, X.; Wen, L.; Wen, Y.; Jiang, H.; Bruce, I.; Jin, J.; Ma, X.; Xia, Q. Total flavonoids of Flos Chrysanthemi protect arterial endothelial cells against oxidative stress. J. Ethnopharmacol. 2012, 139, 68–73. [Google Scholar] [CrossRef]
- Quiles, J.; Gustafsson, Å. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022, 19, 723–736. [Google Scholar] [CrossRef]
- Gao, S.; Hu, J. Mitochondrial Fusion: The Machineries In and Out. Trends Cell Biol. 2021, 31, 62–74. [Google Scholar] [CrossRef]
- Tang, X.; Luo, Y.; Chen, H.; Liu, D. Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Filadi, R.; Pendin, D.; Pizzo, P. Mitofusin 2: From functions to disease. Cell Death Dis. 2018, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Krantz, S.; Jambusaria, A.; Toth, P.; Moon, H.; Gunarathna, I.; Park, G.; Rehman, J. Mitofusin-2 stabilizes adherens junctions and suppresses endothelial inflammation via modulation of β-catenin signaling. Nat. Commun. 2021, 12, 2736. [Google Scholar] [CrossRef]
- Forrester, S.; Preston, K.; Cooper, H.; Boyer, M.; Escoto, K.; Poltronetti, A.; Elliott, K.; Kuroda, R.; Miyao, M.; Sesaki, H.; et al. Mitochondrial Fission Mediates Endothelial Inflammation. Hypertension 2020, 76, 267–276. [Google Scholar] [CrossRef]
- Shenouda, S.; Widlansky, M.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.; Hamburg, N.; Frame, A.; Caiano, T.; Kluge, M.; et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011, 124, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Diebold, I.; Hennigs, J.; Miyagawa, K.; Li, C.; Nickel, N.; Kaschwich, M.; Cao, A.; Wang, L.; Reddy, S.; Chen, P.; et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015, 21, 596–608. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Youn, S.; Sudhahar, V.; Das, A.; Chandhri, R.; Cuervo Grajal, H.; Kweon, J.; Leanhart, S.; He, L.; Toth, P.; et al. Redox Regulation of Mitochondrial Fission Protein Drp1 by Protein Disulfide Isomerase Limits Endothelial Senescence. Cell Rep. 2018, 23, 3565–3578. [Google Scholar] [CrossRef]
- Liu, X.; Cao, K.; Lv, W.; Feng, Z.; Liu, J.; Gao, J.; Li, H.; Zang, W.; Liu, J. Punicalagin attenuates endothelial dysfunction by activating FoxO1, a pivotal regulating switch of mitochondrial biogenesis. Free. Radic. Biol. Med. 2019, 135, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Bi, Y.; Sowers, J.; Hetz, C.; Zhang, Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat. Rev. Cardiol. 2021, 18, 499–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [Green Version]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Wang, Y.; Ying, M.; Jin, C.; Li, J.; Hu, X. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct. Target. Ther. 2021, 6, 242. [Google Scholar] [CrossRef]
- Soriano, F.; Virág, L.; Szabó, C. Diabetic endothelial dysfunction: Role of reactive oxygen and nitrogen species production and poly(ADP-ribose) polymerase activation. J. Mol. Med. 2001, 79, 437–448. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Song, O.; Wang, M.; Guo, X.; Zhang, G.; Liu, X.; Liu, J.; Zhao, L. Hydrogen Protection Boosts the Bioactivity of Chrysanthemum morifolium Extract in Preventing Palmitate-Induced Endothelial Dysfunction by Restoring MFN2 and Alleviating Oxidative Stress in HAEC Cells. Antioxidants 2023, 12, 1019. https://doi.org/10.3390/antiox12051019
Gao Y, Song O, Wang M, Guo X, Zhang G, Liu X, Liu J, Zhao L. Hydrogen Protection Boosts the Bioactivity of Chrysanthemum morifolium Extract in Preventing Palmitate-Induced Endothelial Dysfunction by Restoring MFN2 and Alleviating Oxidative Stress in HAEC Cells. Antioxidants. 2023; 12(5):1019. https://doi.org/10.3390/antiox12051019
Chicago/Turabian StyleGao, Yilin, Oumeng Song, Min Wang, Xin Guo, Guanfei Zhang, Xuyun Liu, Jiankang Liu, and Lin Zhao. 2023. "Hydrogen Protection Boosts the Bioactivity of Chrysanthemum morifolium Extract in Preventing Palmitate-Induced Endothelial Dysfunction by Restoring MFN2 and Alleviating Oxidative Stress in HAEC Cells" Antioxidants 12, no. 5: 1019. https://doi.org/10.3390/antiox12051019
APA StyleGao, Y., Song, O., Wang, M., Guo, X., Zhang, G., Liu, X., Liu, J., & Zhao, L. (2023). Hydrogen Protection Boosts the Bioactivity of Chrysanthemum morifolium Extract in Preventing Palmitate-Induced Endothelial Dysfunction by Restoring MFN2 and Alleviating Oxidative Stress in HAEC Cells. Antioxidants, 12(5), 1019. https://doi.org/10.3390/antiox12051019