Maternal and Neonatal Factors Modulating Breast Milk Cytokines in the First Month of Lactation
Abstract
:1. Introduction
2. Materials and Methods
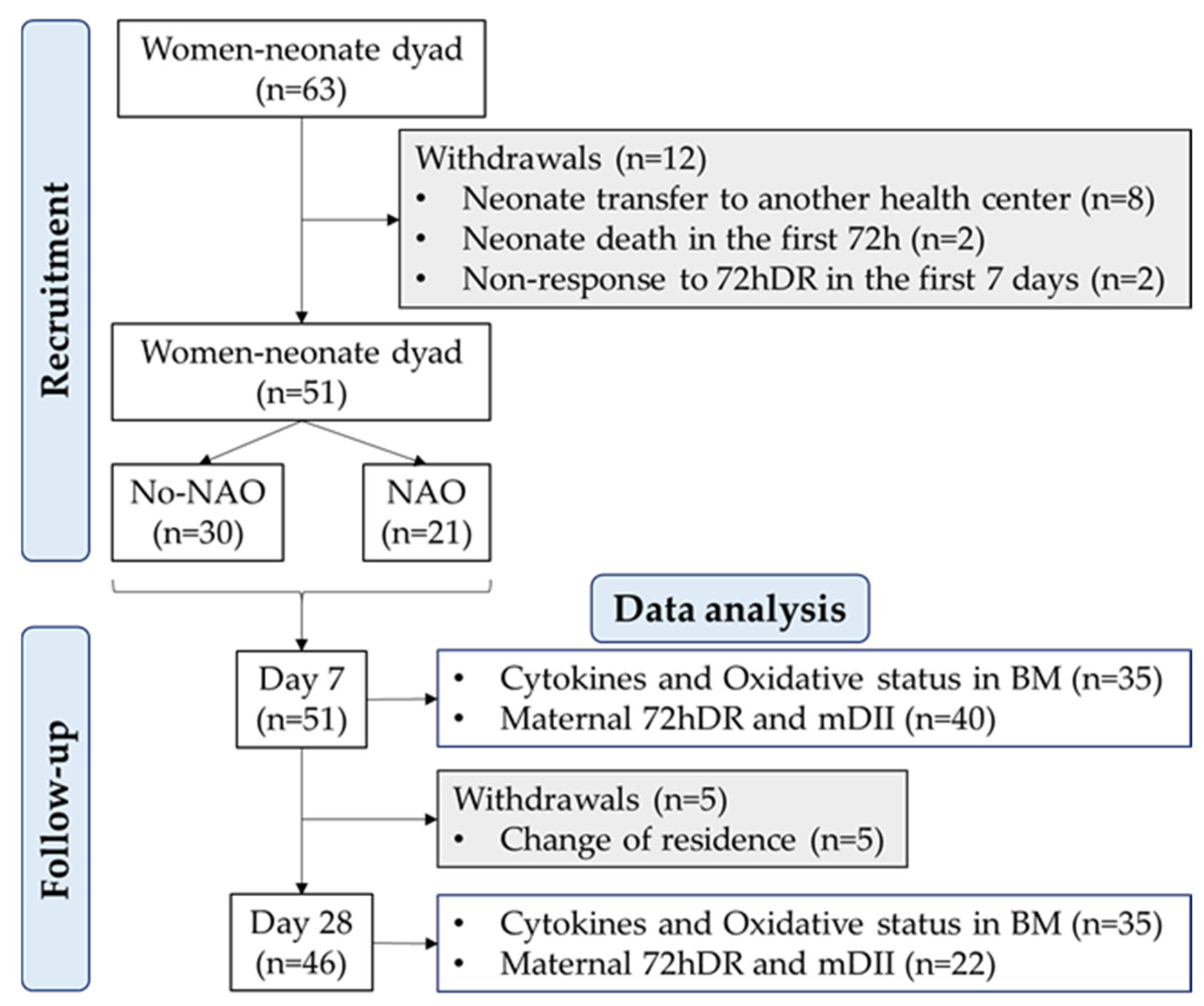
2.1. Study Design and Cohort
2.2. Social and Clinical Data
2.3. Maternal 72-h Dietary Intake
2.4. Calculation of the Maternal Dietary Inflammatory Index
2.5. Breast Milk Collection and Processing to Obtain the Defatted Phase
2.6. Breast Milk Cytokine Detection
2.7. Breast Milk Oxidative Status Analysis
2.8. Statistical Analysis
3. Results
3.1. Sociodemographic Context and Obstetric and Neonatal Characteristics at Birth
3.2. Physiological Levels of BM Cytokines and Antioxidant Status during Lactation
3.3. Influence of Sex, Gestational Age, and NAO on BM Cytokines and Oxidative Status
3.4. Influence of mDII, C-Section, and Obstetric Complications on BM Cytokines and Oxidative Status
3.5. Association Factors Influencing BM Cytokines
4. Discussion
Strengths, Limitations, and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiełbasa, A.; Gadzała-Kopciuch, R.; Buszewski, B. Cytokines-Biogenesis and Their Role in Human Breast Milk and Determination. Int. J. Mol. Sci. 2021, 22, 6238. [Google Scholar] [CrossRef]
- Dawod, B.; Marshall, J.S. Cytokines and Soluble Receptors in Breast Milk as Enhancers of Oral Tolerance Development. Front. Immunol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Lokossou, G.A.G.; Kouakanou, L.; Schumacher, A.; Zenclussen, A.C. Human Breast Milk: From Food to Active Immune Response With Disease Protection in Infants and Mothers. Front. Immunol. 2022, 13, 849012. [Google Scholar] [CrossRef]
- Duale, A.; Singh, P.; Al Khodor, S. Breast Milk: A Meal Worth Having. Front. Nutr. 2021, 8, 800927. [Google Scholar] [CrossRef]
- Meki, A.-R.M.; Saleem, T.H.; Al-Ghazali, M.H.; Sayed, A.A. Interleukins -6, -8 and -10 and Tumor Necrosis Factor-Alpha and Its Soluble Receptor I in Human Milk at Different Periods of Lactation. Nutr. Res. 2003, 23, 845–855. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Peroni, D.G.; Fanos, V. Human Breast Milk: Bioactive Components, from Stem Cells to Health Outcomes. Curr. Nutr. Rep. 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune Markers in Breast Milk and Fetal and Maternal Body Fluids: A Systematic Review of Perinatal Concentrations. J. Hum. Lact. 2011, 27, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Filteau, S.M. Milk Components with Immunomodulatory Potential. Adv. Nutr. Res. 2001, 10, 327–350. [Google Scholar] [CrossRef]
- Gila-Diaz, A.; Arribas, S.M.; Algara, A.; Martín-Cabrejas, M.A.; López de Pablo, Á.L.; Sáenz de Pipaón, M.; Ramiro-Cortijo, D. A Review of Bioactive Factors in Human Breastmilk: A Focus on Prematurity. Nutrients 2019, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Hrdý, J.; Novotná, O.; Kocourková, I.; Prokešová, L. Cytokine Expression in the Colostral Cells of Healthy and Allergic Mothers. Folia Microbiol. 2012, 57, 215–219. [Google Scholar] [CrossRef]
- Olivares, M.; Albrecht, S.; De Palma, G.; Ferrer, M.D.; Castillejo, G.; Schols, H.A.; Sanz, Y. Human Milk Composition Differs in Healthy Mothers and Mothers with Celiac Disease. Eur. J. Nutr. 2015, 54, 119–128. [Google Scholar] [CrossRef]
- Rentea, R.M.; Wagner, A.J.; Gourlay, D.M.; Christensen, M.; Liedel, J.L. Effects of Anticipated Neonatal Surgical Intervention on Maternal Milk Cytokine Production. J. Pediatr. Surg. 2017, 52, 45–49. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Sinkiewicz-Darol, E.; Gadzała-Kopciuch, R. The Impact of Environmental Pollution on the Quality of Mother’s Milk. Environ. Sci. Pollut. Res. Int. 2019, 26, 7405–7427. [Google Scholar] [CrossRef] [PubMed]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Göpel, W.; Härtel, C.; German Neonatal Network, German Center for Lung Research and Priming Immunity at the Beginning of Life (PRIMAL) Consortium. Preterm Birth and Sustained Inflammation: Consequences for the Neonate. Semin. Immunopathol. 2020, 42, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Richard, C.; Larsen, B.M.; Field, C.J. The Importance of Human Milk for Immunity in Preterm Infants. Clin. Perinatol. 2017, 44, 23–47. [Google Scholar] [CrossRef]
- Nolan, L.S.; Parks, O.B.; Good, M. A Review of the Immunomodulating Components of Maternal Breast Milk and Protection Against Necrotizing Enterocolitis. Nutrients 2019, 12, 14. [Google Scholar] [CrossRef]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, A. Premature Delivery Influences the Immunological Composition of Colostrum and Transitional and Mature Human Milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef]
- Berdi, M.; de Lauzon-Guillain, B.; Forhan, A.; Castelli, F.A.; Fenaille, F.; Charles, M.-A.; Heude, B.; Junot, C.; Adel-Patient, K.; EDEN Mother-Child Cohort Study Group. Immune Components of Early Breastmilk: Association with Maternal Factors and with Reported Food Allergy in Childhood. Pediatr. Allergy Immunol. 2019, 30, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zambruni, M.; Villalobos, A.; Somasunderam, A.; Westergaard, S.; Nigalye, M.; Turin, C.G.; Zegarra, J.; Bellomo, S.; Mercado, E.; Ochoa, T.J.; et al. Maternal and Pregnancy-Related Factors Affecting Human Milk Cytokines among Peruvian Mothers Bearing Low-Birth-Weight Neonates. J. Reprod. Immunol. 2017, 120, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Santana, Y.; Peña-Quintana, L. Cytokines and Maternal Omega-3 LCPUFAs Supplementation. In Maternal and Child Health Matters Around the World; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Bopp, M.; Lovelady, C.; Hunter, C.; Kinsella, T. Maternal Diet and Exercise: Effects on Long-Chain Polyunsaturated Fatty Acid Concentrations in Breast Milk. J. Am. Diet. Assoc. 2005, 105, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Afeiche, M.C.; Iroz, A.; Thielecke, F.; De Castro, A.C.; Lefebvre, G.; Draper, C.F.; Martínez-Costa, C.; Haaland, K.; Marchini, G.; Agosti, M.; et al. The Dietary Inflammatory Index Is Associated with Subclinical Mastitis in Lactating European Women. Nutrients 2022, 14, 4719. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; de Oliveira, A.C.M.; Goulart, M.O.F. Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia. Oxid Med. Cell. Longev. 2019, 2019, 8238727. [Google Scholar] [CrossRef]
- De Mendonça, E.L.S.S.; Fragoso, M.B.T.; de Oliveira, J.M.; Xavier, J.A.; Goulart, M.O.F.; de Oliveira, A.C.M. Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies. Antioxidants 2022, 11, 129. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística (INE). Estadística de Nacimientos. Movimiento Natural de La Población. Available online: www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736177007&menu=ultiDatos&idp=1254735573002 (accessed on 16 November 2021).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bass, W.T.; Jones, M.A.; White, L.E.; Montgomery, T.R.; Aiello, F.; Karlowicz, M.G. Ultrasonographic Differential Diagnosis and Neurodevelopmental Outcome of Cerebral White Matter Lesions in Premature Infants. J. Perinatol. 1999, 19, 330–336. [Google Scholar] [CrossRef]
- Mantoo, M.R.; Deorari, A.K.; Jana, M.; Agarwal, R.; Sankar, M.J.; Thukral, A. Preterm White Matter Injury: A Prospective Cohort Study. Indian Pediatr. 2021, 58, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Luna, M.; Moreno Hernando, J.; Botet Mussons, F.; Fernández Lorenzo, J.R.; Herranz Carrillo, G.; Rite Gracia, S.; Salguero García, E.; Echaniz Urcelay, I. Comisión de Estándares de la Sociedad Española de Neonatología [Bronchopulmonary Dysplasia: Definitions and Classifications]. An. Pediatr. 2013, 79, 262.e1–262.e6. [Google Scholar] [CrossRef]
- Patel, R.M.; Ferguson, J.; McElroy, S.J.; Khashu, M.; Caplan, M.S. Defining Necrotizing Enterocolitis: Current Difficulties and Future Opportunities. Pediatr. Res. 2020, 88, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.; Lemieux, S.; Lamarche, B.; Laramée, C.; Corneau, L.; Lapointe, A.; Tessier-Grenier, M.; Robitaille, J. Development of a Web-Based 24-h Dietary Recall for a French-Canadian Population. Nutrients 2016, 8, 724. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Lemieux, S.; Lafrenière, J.; Laramée, C.; Robitaille, J.; Morisset, A.-S. Validation of a Self-Administered Web-Based 24-Hour Dietary Recall among Pregnant Women. BMC Pregnancy Childbirth 2018, 18, 112. [Google Scholar] [CrossRef]
- Gómez Cándela, C.; Loria Kohen, V.; Lourenço Nogueira, T. Guía Visual de Alimentos y Raciones, 1st ed.; Editores Médicos, S.A., Ed.; EDIMSA: Madrid, Spain, 2008. [Google Scholar]
- Dehghan, P.; Nejati, M.; Vahid, F.; Almasi-Hashiani, A.; Saleh-Ghadimi, S.; Parsi, R.; Jafari-Vayghan, H.; Shivappa, N.; R Hébert, J. The Association between Dietary Inflammatory Index, Dietary Antioxidant Index, and Mental Health in Adolescent Girls: An Analytical Study. BMC Public Health 2022, 22, 1513. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; Gila-Diaz, A.; Herranz Carrillo, G.; Cañas, S.; Gil-Ramírez, A.; Ruvira, S.; Martin-Cabrejas, M.A.; Arribas, S.M. Influence of Neonatal Sex on Breast Milk Protein and Antioxidant Content in Spanish Women in the First Month of Lactation. Antioxidants 2022, 11, 1472. [Google Scholar] [CrossRef]
- Ustundag, B.; Yilmaz, E.; Dogan, Y.; Akarsu, S.; Canatan, H.; Halifeoglu, I.; Cikim, G.; Aygun, A.D. Levels of Cytokines (IL-1beta, IL-2, IL-6, IL-8, TNF-Alpha) and Trace Elements (Zn, Cu) in Breast Milk from Mothers of Preterm and Term Infants. Mediat. Inflamm. 2005, 2005, 137261. [Google Scholar] [CrossRef]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The Good, the Bad, and the Gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef]
- Buescher, E.S.; Malinowska, I. Soluble Receptors and Cytokine Antagonists in Human Milk. Pediatr. Res. 1996, 40, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Sferruzzi-Perri, A.N.; Lopez-Tello, J.; Salazar-Petres, E. Placental Adaptations Supporting Fetal Growth during Normal and Adverse Gestational Environments. Exp. Physiol. 2023, 108, 371–397. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Smink, C.J.; Robinson, R.C.; Tian, T.; Guerrero, A.; Parker, E.A.; Smilowitz, J.T.; Hettinga, K.A.; Underwood, M.A.; Lebrilla, C.B.; et al. Endogenous Human Milk Peptide Release Is Greater after Preterm Birth than Term Birth. J. Nutr. 2015, 145, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef]
- Bry, A.; Wigert, H. Psychosocial Support for Parents of Extremely Preterm Infants in Neonatal Intensive Care: A Qualitative Interview Study. BMC Psychol. 2019, 7, 76. [Google Scholar] [CrossRef]
- Gila-Díaz, A.; Herranz Carrillo, G.; Arribas, S.M.; Ramiro-Cortijo, D. Healthy Habits and Emotional Balance in Women during the Postpartum Period: Differences between Term and Preterm Delivery. Children 2021, 8, 937. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Bhandari, V. Pulmonary Biomarkers of Bronchopulmonary Dysplasia. Biomark Insights 2008, 3, BMI.S834. [Google Scholar] [CrossRef] [PubMed]
- Baier, J.R.; Loggins, J.; Kruger, T.E. Monocyte Chemoattractant Protein-1 and Interleukin-8 Are Increased in Bronchopulmonary Dysplasia: Relation to Isolation of Ureaplasma Urealyticum. J. Investig. Med. 2001, 49, 362–369. [Google Scholar] [CrossRef]
- Miclat, N.N.; Hodgkinson, R.; Marx, G.F. Neonatal Gastric PH. Anesth. Analg. 1978, 57, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, K.G.; Badovinac, V.P.; Griffith, T.S.; Knoop, K.A. Sepsis, Cytokine Storms, and Immunopathology: The Divide between Neonates and Adults. Immunohorizons 2021, 5, 512–522. [Google Scholar] [CrossRef]
- Platt, M.J. Outcomes in Preterm Infants. Public Health 2014, 128, 399–403. [Google Scholar] [CrossRef]
- Zheng, Y.; Corrêa-Silva, S.; de Souza, E.C.; Maria Rodrigues, R.; da Fonseca, F.A.M.; Gilio, A.E.; Carneiro-Sampaio, M.; Palmeira, P. Macrophage Profile and Homing into Breast Milk in Response to Ongoing Respiratory Infections in the Nursing Infant. Cytokine 2020, 129, 155045. [Google Scholar] [CrossRef]
- Peelen, M.J.C.S.; Kazemier, B.M.; Ravelli, A.C.J.; De Groot, C.J.M.; Van Der Post, J.A.M.; Mol, B.W.J.; Hajenius, P.J.; Kok, M. Impact of Fetal Gender on the Risk of Preterm Birth, a National Cohort Study. Acta Obstet. Gynecol. Scand. 2016, 95, 1034–1041. [Google Scholar] [CrossRef]
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.M.; Cladis, F.; Davis, P.J. Outcomes for Extremely Premature Infants. Anesth. Analg. 2015, 120, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Zozaya, C.; Avila-Alvarez, A.; Arruza, L.; García-Muñoz Rodrigo, F.; Fernandez-Perez, C.; Castro, A.; Cuesta, M.T.; Vacas, B.; Couce, M.L.; Vento Torres, M.; et al. The Effect of Morbidity and Sex on Postnatal Growth of Very Preterm Infants: A Multicenter Cohort Study. Neonatology 2019, 115, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, M.F.; Jenmalm, M.C.; Björkstén, B.; Garofalo, R.P. Chemoattractant Factors in Breast Milk from Allergic and Nonallergic Mothers. Pediatr. Res. 2000, 47, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Impact of Maternal Diet on Human Milk Composition and Neurological Development of Infants. Am. J. Clin. Nutr. 2014, 99, 734S–741S. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, Y.-M. Dietary Patterns and Their Association with Breast Milk Macronutrient Composition among Lactating Women. Int. Breastfeed. J. 2020, 15, 52. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of Maternal Nutrition on Breast-Milk Composition: A Systematic Review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Selma-Royo, M.; Hervas, D.; Yang, B.; Intonen, L.; González, S.; Martínez-Costa, C.; Linderborg, K.M.; Collado, M.C. Breast Milk Lipidome Is Associated With Maternal Diet and Infants’ Growth. Front. Nutr. 2022, 9, 854786. [Google Scholar] [CrossRef]
- Aumeistere, L.; Ciproviča, I.; Zavadska, D.; Andersons, J.; Volkovs, V.; Ceļmalniece, K. Impact of Maternal Diet on Human Milk Composition Among Lactating Women in Latvia. Medicina 2019, 55, 173. [Google Scholar] [CrossRef]
- Codini, M.; Tringaniello, C.; Cossignani, L.; Boccuto, A.; Mirarchi, A.; Cerquiglini, L.; Troiani, S.; Verducci, G.; Patria, F.F.; Conte, C.; et al. Relationship between Fatty Acids Composition/Antioxidant Potential of Breast Milk and Maternal Diet: Comparison with Infant Formulas. Molecules 2020, 25, 2910. [Google Scholar] [CrossRef]
- Karbasi, S.; Bahrami, A.; Asadi, Z.; Shahbeiki, F.; Naseri, M.; Zarban, A.; Ferns, G.A. The Association of Maternal Dietary Quality and the Antioxidant-Proxidant Balance of Human Milk. Int. Breastfeed. J. 2022, 17, 56. [Google Scholar] [CrossRef]
- Kugananthan, S.; Gridneva, Z.; Lai, C.T.; Hepworth, A.R.; Mark, P.J.; Kakulas, F.; Geddes, D.T. Associations between Maternal Body Composition and Appetite Hormones and Macronutrients in Human Milk. Nutrients 2017, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.S.; Bryan, D.-L.; Makrides, M.; Neumann, M.A.; Gibson, R.A. A Randomized Trial of Supplementation with Docosahexaenoic Acid-Rich Tuna Oil and Its Effects on the Human Milk Cytokines Interleukin 1 Beta, Interleukin 6, and Tumor Necrosis Factor Alpha. Am. J. Clin. Nutr. 2002, 75, 754–760. [Google Scholar] [CrossRef]
- Mozurkewich, E.L.; Berman, D.R.; Vahratian, A.; Clinton, C.M.; Romero, V.C.; Chilimigras, J.L.; Vazquez, D.; Qualls, C.; Djuric, Z. Effect of Prenatal EPA and DHA on Maternal and Umbilical Cord Blood Cytokines. BMC Pregnancy Childbirth 2018, 18, 261. [Google Scholar] [CrossRef]
- Rodriguez-Santana, Y.; Ochoa, J.J.; Lara-Villoslada, F.; Kajarabille, N.; Saavedra-Santana, P.; Hurtado, J.A.; Peña, M.; Diaz-Castro, J.; Sebastian-Garcia, I.; Machin-Martin, E.; et al. Cytokine Distribution in Mothers and Breastfed Children after Omega-3 LCPUFAs Supplementation during the Last Trimester of Pregnancy and the Lactation Period: A Randomized, Controlled Trial. Prostaglandins Leukot. Essent. Fat. Acids 2017, 126, 32–38. [Google Scholar] [CrossRef]
- Vedin, I.; Cederholm, T.; Freund Levi, Y.; Basun, H.; Garlind, A.; Faxén Irving, G.; Jönhagen, M.E.; Vessby, B.; Wahlund, L.-O.; Palmblad, J. Effects of Docosahexaenoic Acid-Rich n-3 Fatty Acid Supplementation on Cytokine Release from Blood Mononuclear Leukocytes: The OmegAD Study. Am. J. Clin. Nutr. 2008, 87, 1616–1622. [Google Scholar] [CrossRef]
- Noakes, P.S.; Vlachava, M.; Kremmyda, L.-S.; Diaper, N.D.; Miles, E.A.; Erlewyn-Lajeunesse, M.; Williams, A.P.; Godfrey, K.M.; Calder, P.C. Increased Intake of Oily Fish in Pregnancy: Effects on Neonatal Immune Responses and on Clinical Outcomes in Infants at 6 Mo. Am. J. Clin. Nutr. 2012, 95, 395–404. [Google Scholar] [CrossRef]
- Zou, H.; Sun, M.; Liu, Y.; Xi, Y.; Xiang, C.; Yong, C.; Liang, J.; Huo, J.; Lin, Q.; Deng, J. Relationship between Dietary Inflammatory Index and Postpartum Depression in Exclusively Breastfeeding Women. Nutrients 2022, 14, 5006. [Google Scholar] [CrossRef] [PubMed]
- Parra-Llorca, A.; Pinilla-Gonzlez, A.; Torrejón-Rodríguez, L.; Lara-Cantón, I.; Kuligowski, J.; Collado, M.C.; Gormaz, M.; Aguar, M.; Vento, M.; Serna, E.; et al. Effects of Sepsis on Immune Response, Microbiome and Oxidative Metabolism in Preterm Infants. Children 2023, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Tirone, C.; Pezza, L.; Paladini, A.; Tana, M.; Aurilia, C.; Lio, A.; D’Ippolito, S.; Tersigni, C.; Posteraro, B.; Sanguinetti, M.; et al. Gut and Lung Microbiota in Preterm Infants: Immunological Modulation and Implication in Neonatal Outcomes. Front. Immunol. 2019, 10, 2910. [Google Scholar] [CrossRef]



| Cytokine and Oxidative Status | Day 7 (n = 20) | Day 28 (n = 18) | p |
|---|---|---|---|
| IL-10 Ln (pg/mL) | −0.20 [−0.21; −0.16] | −0.17 [−0.22; −0.15] | 0.79 |
| IL-13 Ln (pg/mL) | −0.49 [−0.52; −0.33] | 0.51 [−0.15; 0.79] | <0.001 |
| IL-8 Ln (pg/mL) | −0.32 [−0.54; 0.20] | −0.47 [−0.63; −0.37] | 0.024 |
| MCP-1 Ln (pg/mL) | 0.63 [−0.25; 1.32] | −0.92 [−0.96; −0.41] | <0.001 |
| TNFα Ln (pg/mL) | −0.26 [−0.32; −0.08] | −0.31 [−0.40; −0.26] | 0.10 |
| Inflammatory index | 0.00 [−0.93; 0.81] | −0.13 [−0.49; 0.32] | 0.87 |
| ABTS Ln (mg TE/mL) | 0.32 [−0.02; 1.14] | −0.88 [−1.14; −0.36] | <0.001 |
| MDA+HNE Ln (μM) | 0.05 [−0.26; 0.60] | −0.49 [−1.02; −0.17] | 0.001 |
| IL-10 | IL-13 | IL-8 | MCP-1 | TNFα | BM-II | |
|---|---|---|---|---|---|---|
| mDII (Ref. “High”) | −0.03 ± 0.03 (p = 0.39) | −0.03 ± 0.12 (p = 0.12) | −0.06 ± 0.27 (p = 0.82) | −0.24 ± 0.25 (p = 0.34) | 0.10 ± 0.21 (p = 0.63) | −3.89 ± 2.53 (p = 0.12) |
| Gestational age (Ref. Full-term infants) | - | - | 0.55 ± 0.27 (p = 0.041) | 0.15 ± 0.31 (p = 0.63) | 0.60 ± 0.41 (p = 0.14) | - |
| Lactation period (Ref. day 7) | - | 0.85 ± 0.12 (p < 0.001) | −0.64 ± 0.27 (p = 0.019) | −0.98 ± 0.22 (p < 0.001) | - | - |
| NAO (Ref. “no”) | - | - | - | 0.17 ± 0.31 (p = 0.59) | - | - |
| Women as random effects | 17.3% | 8.8% | 7.4% | 35.4% | 89.2% | 4.2% |
| AIC/BIC | −57.5/−49.5 | 54.9/64.2 | 158.0/169.9 | 136.2/149.6 | 163.1/173.1 | 327.9/335.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramiro-Cortijo, D.; Herranz Carrillo, G.; Singh, P.; Rebollo-Hernanz, M.; Rodríguez-Rodríguez, P.; Ruvira, S.; Martín-Trueba, M.; Martin, C.R.; Arribas, S.M. Maternal and Neonatal Factors Modulating Breast Milk Cytokines in the First Month of Lactation. Antioxidants 2023, 12, 996. https://doi.org/10.3390/antiox12050996
Ramiro-Cortijo D, Herranz Carrillo G, Singh P, Rebollo-Hernanz M, Rodríguez-Rodríguez P, Ruvira S, Martín-Trueba M, Martin CR, Arribas SM. Maternal and Neonatal Factors Modulating Breast Milk Cytokines in the First Month of Lactation. Antioxidants. 2023; 12(5):996. https://doi.org/10.3390/antiox12050996
Chicago/Turabian StyleRamiro-Cortijo, David, Gloria Herranz Carrillo, Pratibha Singh, Miguel Rebollo-Hernanz, Pilar Rodríguez-Rodríguez, Santiago Ruvira, María Martín-Trueba, Camilia R. Martin, and Silvia M. Arribas. 2023. "Maternal and Neonatal Factors Modulating Breast Milk Cytokines in the First Month of Lactation" Antioxidants 12, no. 5: 996. https://doi.org/10.3390/antiox12050996









