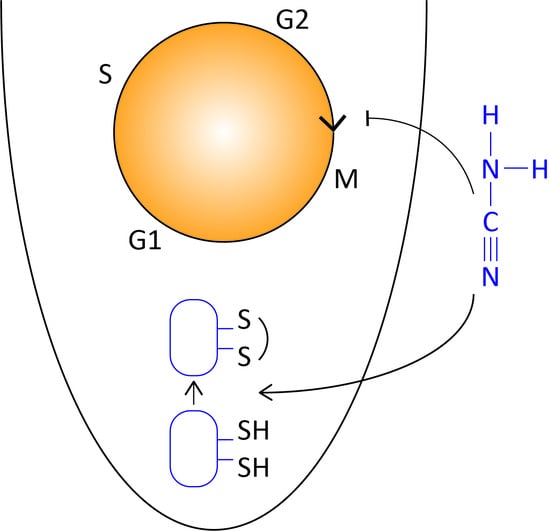
Hydrogen Cyanamide Causes Reversible G2/M Cell Cycle Arrest Accompanied by Oxidation of the Nucleus and Cytosol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Growth Conditions
2.3. Root Growth Rate
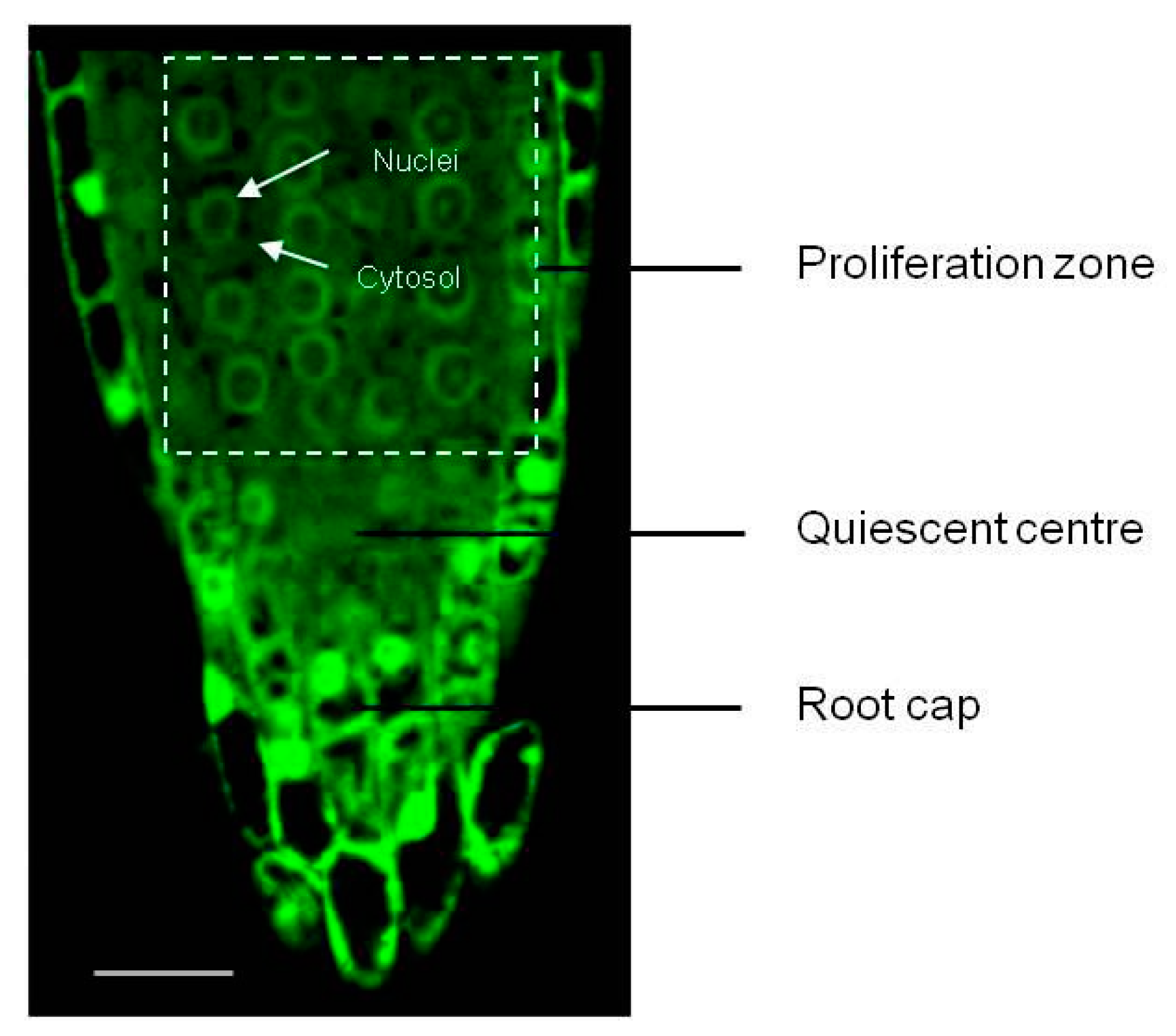
2.4. Visualisation of Redox Status
2.5. Calibration of roGFP2 Probe
2.6. Calculation of Redox Potential
2.7. Cell Cycle Status
2.7.1. Flow Cytometry
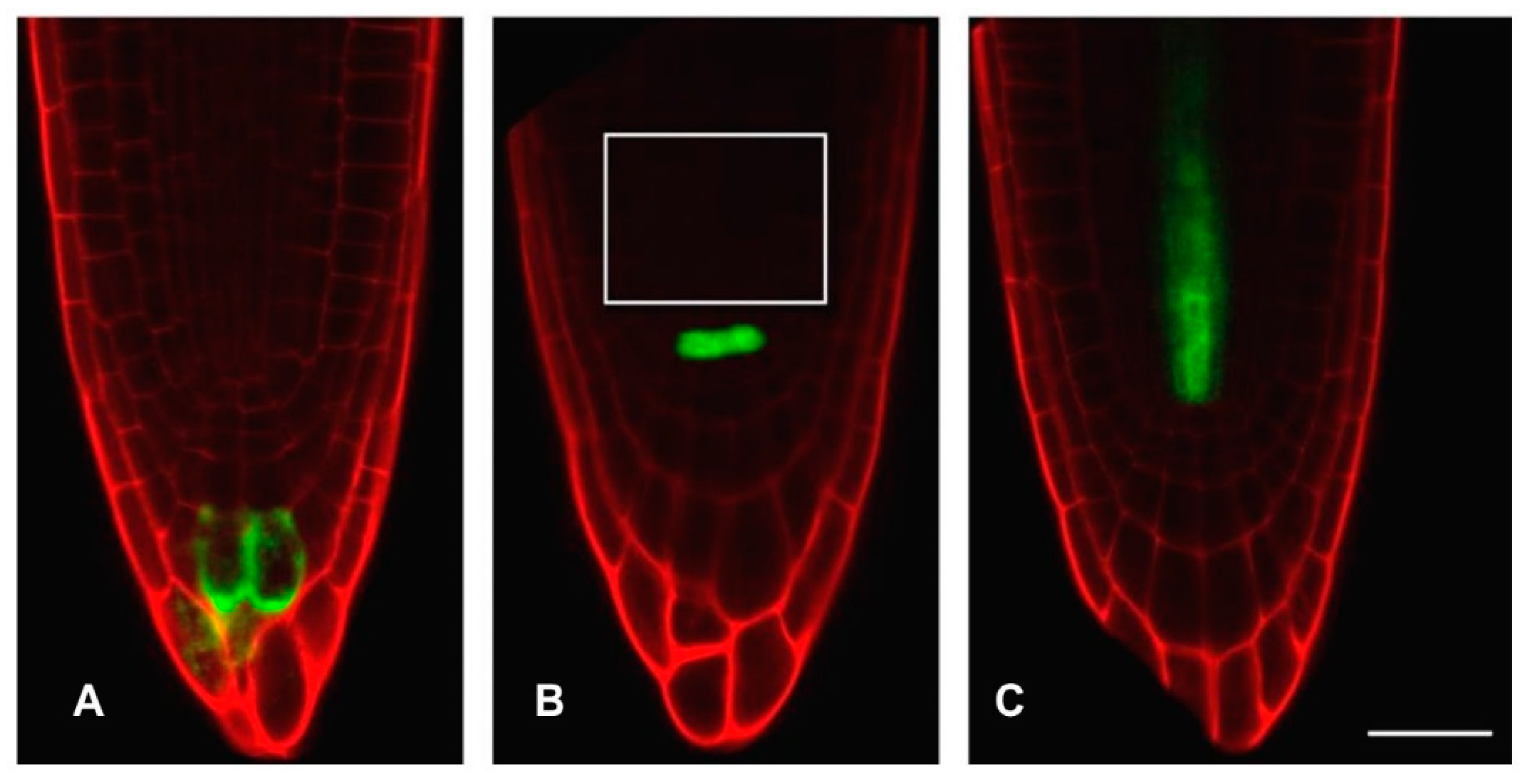
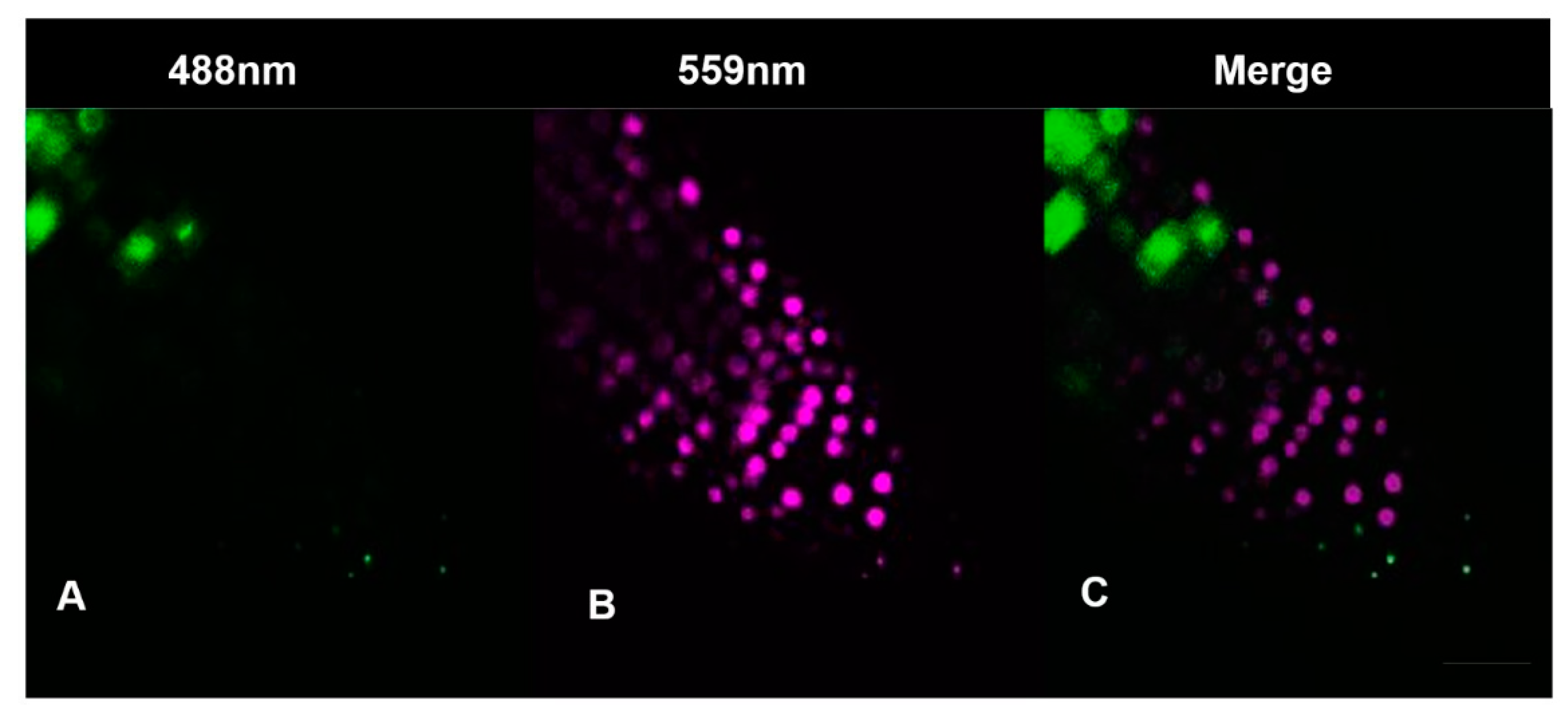
2.7.2. Cytrap
2.8. Data Analysis and Statistical Analysis
3. Results
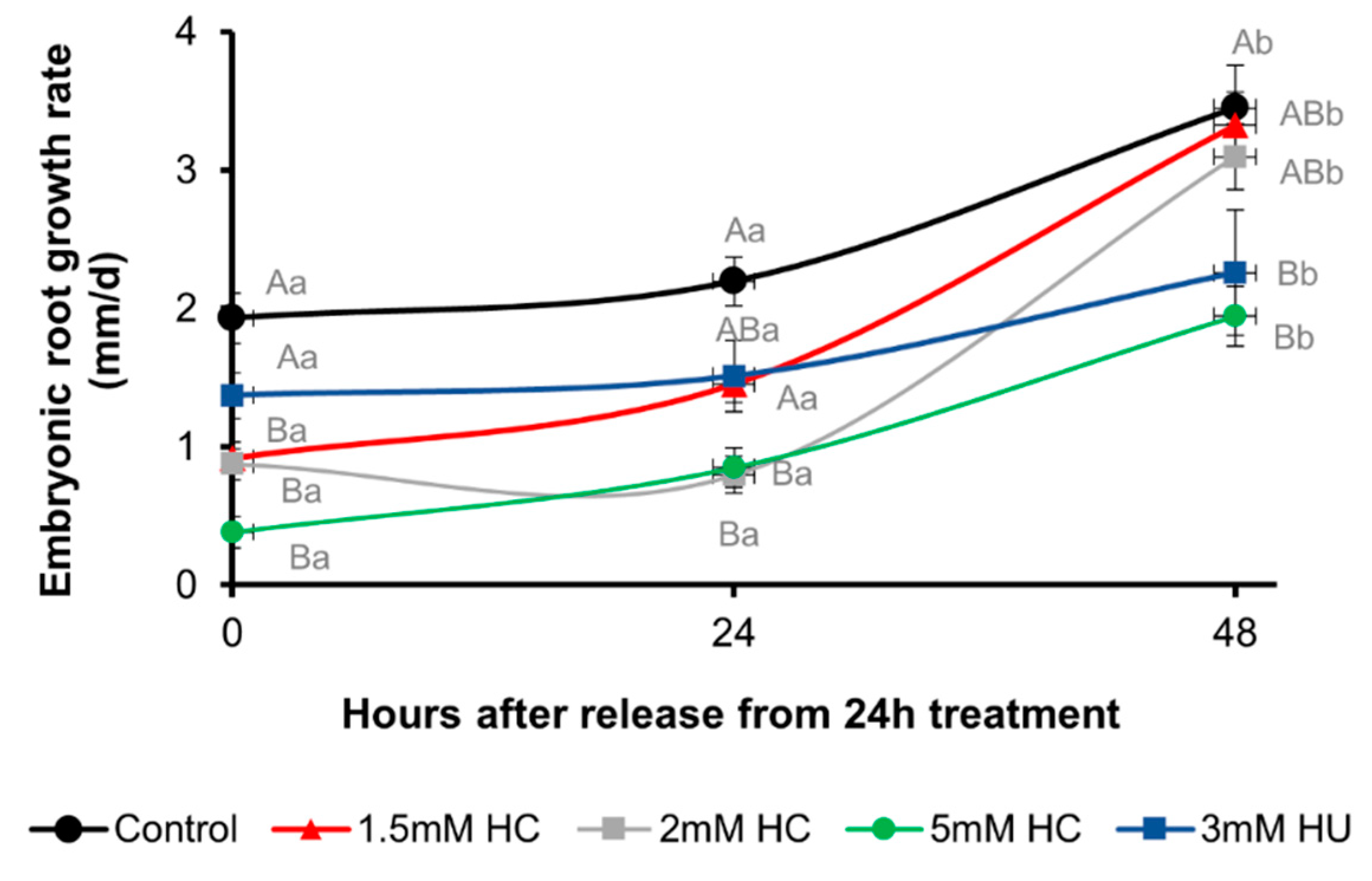
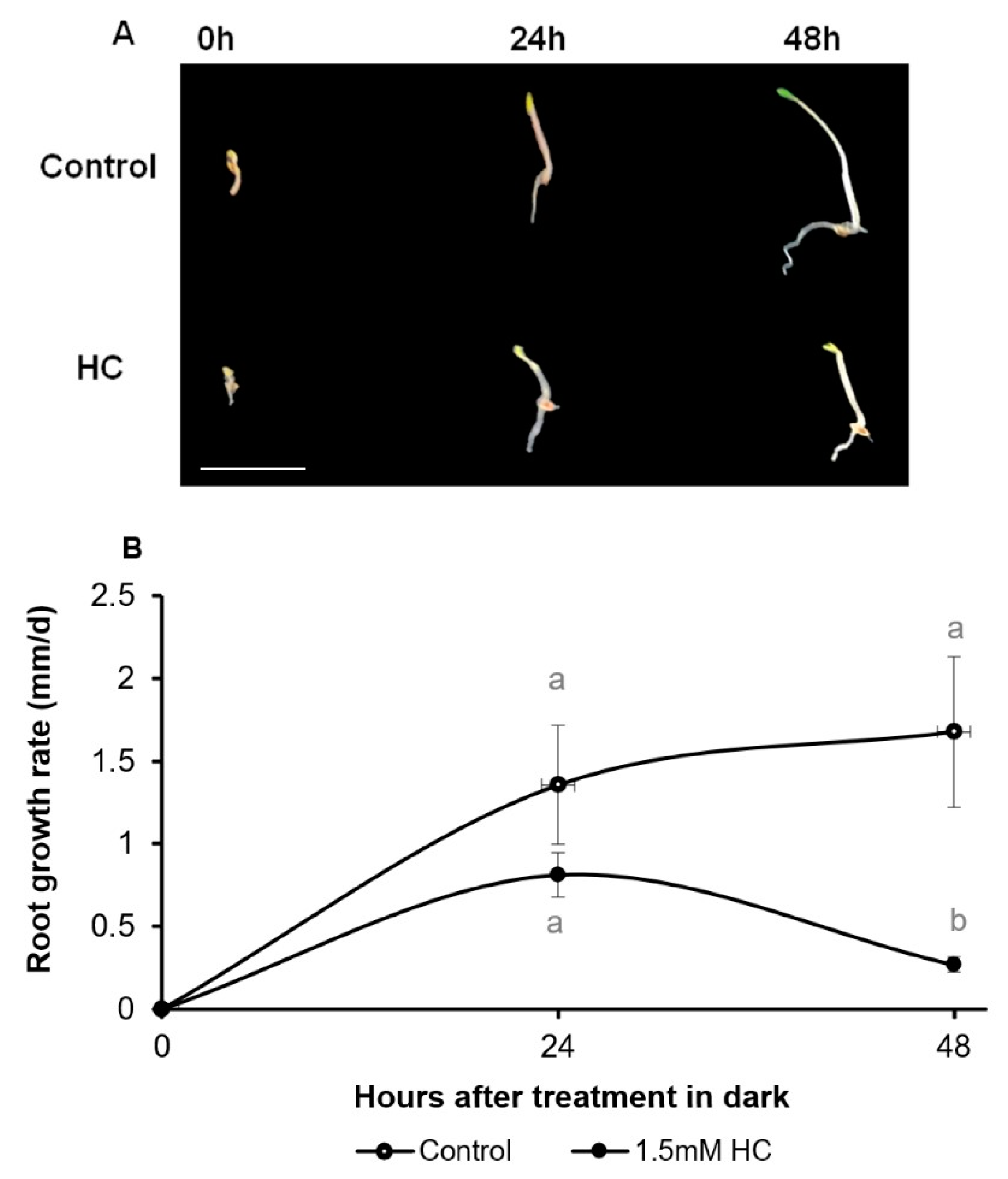
3.1. Hydrogen Cyanamide Causes a Reversible Effect on Embryonic Root Growth of A. thaliana
3.2. Hydrogen Cyanamide Decreases Root Growth of A. thaliana Seedlings in a Time-Dependent Manner
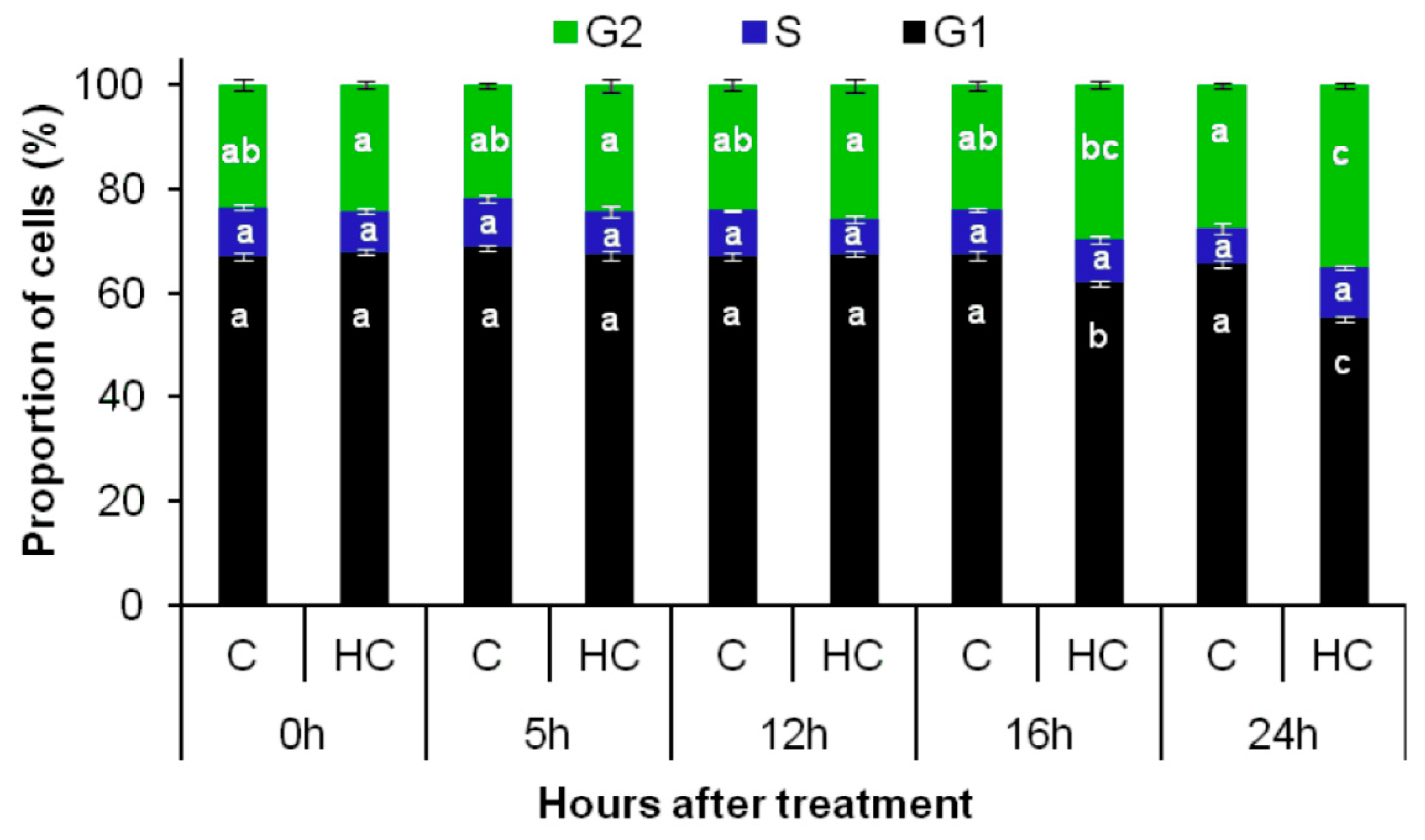
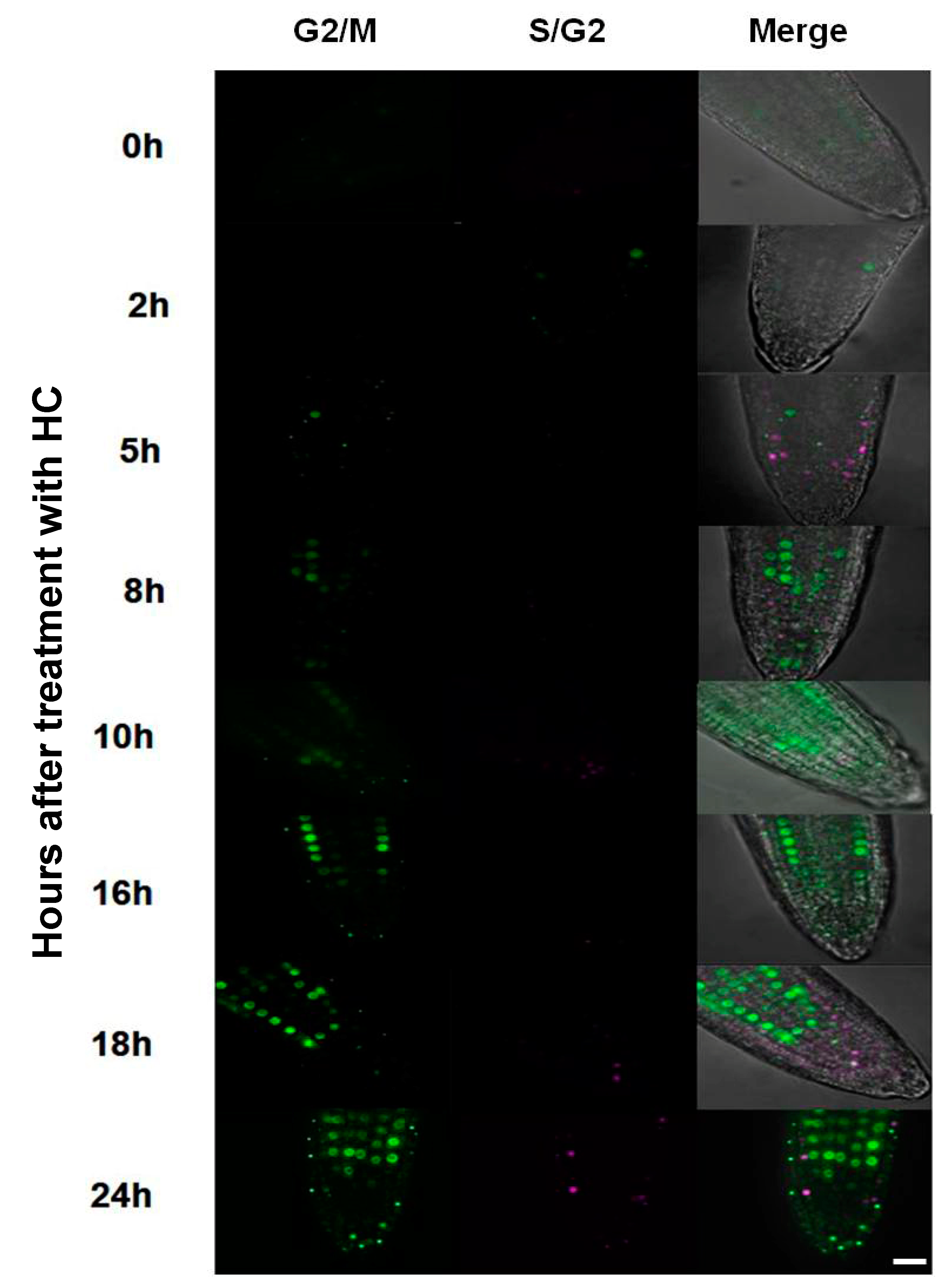
3.3. Hydrogen Cyanamide Treatment of Embryonic Root Tips Causes Gradual Accumulation of G2/M Phase Cells
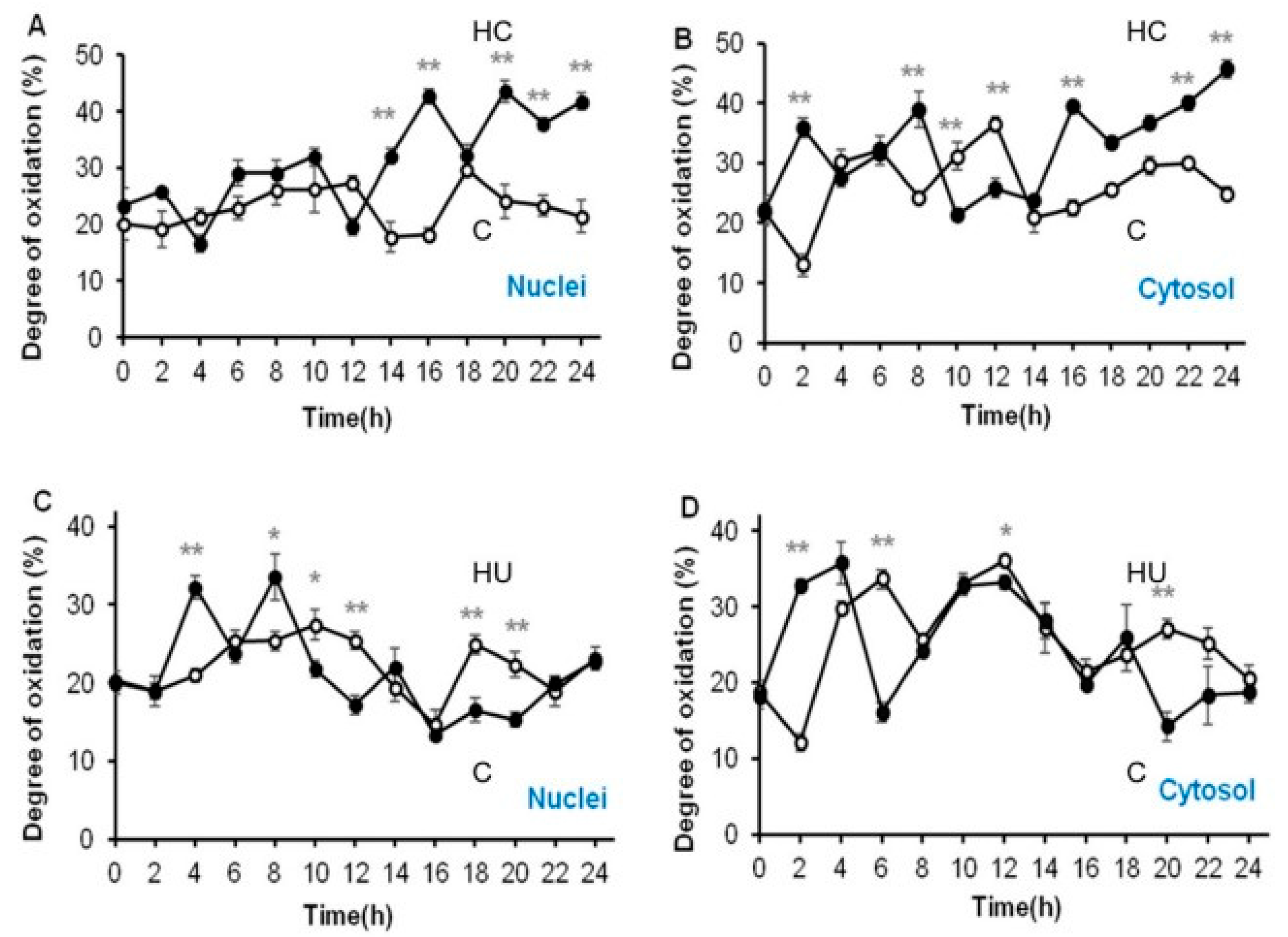
3.4. Hydrogen Cyanamide Triggers a Higher Cellular Oxidation Compared to Hydroxyurea
3.5. Accumulation of G2/M Cells Is Accompanied by Increased Oxidation in Hydrogen Cyanamide Treated Root Tips
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vergara, R.; Noriega, X.; Parada, F.; Dantas, D.; Pérez, F.J. Relationship between Endodormancy, FLOWERING LOCUS T and Cell Cycle Genes in Vitis Vinifera. Planta 2016, 243, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Soltys, D.; Rudzińska-Langwald, A.; Gniazdowska, A.; Wiśniewska, A.; Bogatek, R. Inhibition of Tomato (Solanum lycopersicum L.) Root Growth by Cyanamide Is Due to Altered Cell Division, Phytohormone Balance and Expansin Gene Expression. Planta 2012, 236, 1629–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltys, D.; Rudzińska-Langwald, A.; Kurek, W.; Gniazdowska, A.; Sliwinska, E.; Bogatek, R. Cyanamide Mode of Action during Inhibition of Onion (Allium Cepa L.) Root Growth Involves Disturbances in Cell Division and Cytoskeleton Formation. Planta 2011, 234, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Chhajed, S.; Vashisth, T.; Olmstead, M.A.; Olmstead, J.W.; Colquhoun, T.A. Transcriptomic Study of Early Responses to the Bud Dormancy-Breaking Agent Hydrogen Cyanamide in ‘TropicBeauty’ Peach. J. Am. Soc. Hortic. Sci. 2019, 144, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Lyublinskaya, O.G.; Borisov, Y.G.; Pugovkina, N.A.; Smirnova, I.S.; Obidina, J.V.; Ivanova, J.S.; Zenin, V.V.; Shatrova, A.N.; Borodkina, A.V.; Aksenov, N.D.; et al. Reactive Oxygen Species Are Required for Human Mesenchymal Stem Cells to Initiate Proliferation after the Quiescence Exit. Oxid. Med. Cell Longev. 2015, 2015, 502105. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, J.S.; Pugovkina, N.A.; Neganova, I.E.; Kozhukharova, I.V.; Nikolsky, N.N.; Lyublinskaya, O.G. Cell Cycle-Coupled Changes in the Level of Reactive Oxygen Species Support the Proliferation of Human Pluripotent Stem Cells. Stem Cells 2021, 39, 1671–1687. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Oxygen and Reactive Oxygen Species-Dependent Regulation of Plant Growth and Development. Plant Physiol. 2021, 186, kiaa077. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Redox Regulation of Plant Development. Antioxid. Redox Signal 2014, 21, 1305–1326. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Vivancos, P.; Dong, Y.; Ziegler, K.; Markovic, J.; Pallardó, F.V.; Pellny, T.K.; Verrier, P.J.; Foyer, C.H.; Diaz Vivancos, P.; Dong, Y.; et al. Recruitment of Glutathione into the Nucleus during Cell Proliferation Adjusts Whole-Cell Redox Homeostasis in Arabidopsis Thaliana and Lowers the Oxidative Defence Shield. Plant J. 2010, 64, 825–838. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Wolff, T.; Markovic, J.; Pallardó, F.V.; Foyer, C.H. A Nuclear Glutathione Cycle within the Cell Cycle. Biochem. J. 2010, 431, 169–178. [Google Scholar] [CrossRef] [Green Version]
- De Simone, A.; Hubbard, R.; De La Torre, N.V.; Velappan, Y.; Wilson, M.; Considine, M.J.; Soppe, W.J.J.; Foyer, C.H. Redox Changes during the Cell Cycle in Embryonic Root Meristem of Arabidopsis Thaliana. Antioxid. Redox. Signal 2017, 27, 1505–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velappan, Y.; Signorelli, S.; Considine, M.J. Cell Cycle Arrest in Plants: What Distinguishes Quiescence, Dormancy and Differentiated G1? Ann. Bot. 2017, 120, 495–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Gu, M.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Effects of Phenylcarboxylic Acids on Mitosis, Endoreduplication and Expression of Cell Cycle-Related Genes in Roots of Cucumber (Cucumis sativus L.). J. Chem. Ecol. 2009, 35, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Cools, T.; Iantcheva, A.; Maes, S.; Van Den Daele, H.; De Veylder, L. A Replication Stress-Induced Synchronization Method for Arabidopsis Thaliana Root Meristems. Plant J. 2010, 64, 705–714. [Google Scholar] [CrossRef]
- Ding, L.; Jing, H.; Qin, B.; Qi, L.; Li, J.; Wang, T.; Liu, G. Regulation of Cell Division and Growth in Roots of Lactuca Sativa LSeedlings by the Ent-Kaurene Diterpenoid Rabdosin B. J. Chem. Ecol. 2010, 36, 553–563. [Google Scholar] [CrossRef]
- Meyer, A.J.; Brach, T.; Marty, L.; Kreye, S.; Rouhier, N.; Jacquot, J.-P.P.; Hell, R. Redox-Sensitive GFP in Arabidopsis Thaliana Is a Quantitative Biosensor for the Redox Potential of the Cellular Glutathione Redox Buffer. Plant J. 2007, 52, 973–986. [Google Scholar] [CrossRef]
- Yin, K.; Ueda, M.; Takagi, H.; Kajihara, T.; Sugamata Aki, S.; Nobusawa, T.; Umeda-Hara, C.; Umeda, M.; Aki, S.S.; Nobusawa, T.; et al. A Dual-Color Marker System for in Vivo Visualization of Cell Cycle Progression in Arabidopsis. Plant J. 2014, 80, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis Root Development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [Green Version]
- De Simone, A. Redox Regulation of the Cell Cycle in Arabidopsis Thaliana; University of Leeds: Leeds, UK, 2016. [Google Scholar]
- Arumuganathan, K.; Earle, E.D. Nuclear DNA Content of Some Important Plant Species. Plant Mol. Biol. Rep. 1991, 9, 208–218. [Google Scholar] [CrossRef]
- Shulman, Y.; Nir, G.; Lavee, S. Oxidative Processes in Bud Dormancy and the Use of Hydrogen Cyanamide in Breaking Dormancy. Acta Hortic. 1986, 179, 141–148. [Google Scholar] [CrossRef]
- Or, E.; Nir, G.; Vilozny, I. Timing of Hydrogen Cyanamide Application to Grapevine Buds. Vitis 1999, 38, 1–6. [Google Scholar]
- Shulman, Y.; Nir, G.; Fanberstein, L.; Lavee, S.; Nir, J.; Lavee, S.; Nir, G.; Fanberstein, L.; Lavee, S. The Effect of Cyanamide on the Release from Dormancy of Grapevine Buds. Sci. Hort. 1983, 19, 97–104. [Google Scholar] [CrossRef]
- Fuchigami, L.; Nee, C.C. Degree Growth Stage Model and Rest-Breaking Mechanisms in Temperate Woody Perennials. Hort. Sci. 1987, 22, 836–845. [Google Scholar] [CrossRef]
- Siller-Cepeda, J.H.; Fuchigami, L.H.; Chen, T.H.H. Hydrogen Cyanamide-Induced Budbreak and Phytotoxicity in `Redhaven’ Peach Buds. Hort. Sci. 1992, 27, 874–876. [Google Scholar] [CrossRef] [Green Version]
- Soltys, D.; Rudzińska-Langwald, A.; Kurek, W.; Szajko, K.; Sliwinska, E.; Bogatek, R.; Gniazdowska, A. Phytotoxic Cyanamide Affects Maize (Zea Mays) Root Growth and Root Tip Function: From Structure to Gene Expression. J. Plant Physiol. 2014, 171, 565–575. [Google Scholar] [CrossRef]
- Kamo, T.; Hiradate, S.; Fujii, Y. First Isolation of Natural Cyanamide as a Possible Allelochemical from Hairy Vetch Vicia Villosa. J. Chem. Ecol. 2003, 29, 275–283. [Google Scholar] [CrossRef]
- Gutierrez, C. The Arabidopsis Cell Division Cycle. Arab. Book 2009, 7, e0120. [Google Scholar] [CrossRef] [Green Version]
- Inzé, D.; De Veylder, L. Cell Cycle Regulation in Plant Development. Annu. Rev. Genet. 2006, 40, 77–105. [Google Scholar] [CrossRef]


| Redox Potential (mV) | Degree of Oxidation (%) | |||||
|---|---|---|---|---|---|---|
| Control | HC | HU | Control | HC | HU | |
| Nuclei | −296.8 ± 0.9 | −291.4 ± 1.9 | −297.4 ± 1.1 | 22.90 ± 2.3 | 31.22 ± 1.6 | 21.35 ± 1.3 |
| Cytosol | −294.1 ± 1.4 | −289.6 ± 1.4 | −295.2 ± 1.6 | 26.31 ± 1.6 | 32.48± 1.4 | 24.44 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velappan, Y.; de Simone, A.; Signorelli, S.; Considine, J.A.; Foyer, C.H.; Considine, M.J. Hydrogen Cyanamide Causes Reversible G2/M Cell Cycle Arrest Accompanied by Oxidation of the Nucleus and Cytosol. Antioxidants 2023, 12, 1330. https://doi.org/10.3390/antiox12071330
Velappan Y, de Simone A, Signorelli S, Considine JA, Foyer CH, Considine MJ. Hydrogen Cyanamide Causes Reversible G2/M Cell Cycle Arrest Accompanied by Oxidation of the Nucleus and Cytosol. Antioxidants. 2023; 12(7):1330. https://doi.org/10.3390/antiox12071330
Chicago/Turabian StyleVelappan, Yazhini, Ambra de Simone, Santiago Signorelli, John A. Considine, Christine H. Foyer, and Michael J. Considine. 2023. "Hydrogen Cyanamide Causes Reversible G2/M Cell Cycle Arrest Accompanied by Oxidation of the Nucleus and Cytosol" Antioxidants 12, no. 7: 1330. https://doi.org/10.3390/antiox12071330
APA StyleVelappan, Y., de Simone, A., Signorelli, S., Considine, J. A., Foyer, C. H., & Considine, M. J. (2023). Hydrogen Cyanamide Causes Reversible G2/M Cell Cycle Arrest Accompanied by Oxidation of the Nucleus and Cytosol. Antioxidants, 12(7), 1330. https://doi.org/10.3390/antiox12071330