Seed Priming with Salicylic Acid Alleviates Salt Stress Toxicity in Barley by Suppressing ROS Accumulation and Improving Antioxidant Defense Systems, Compared to Halo- and Gibberellin Priming
Abstract
:1. Introduction
2. Materials and Methods
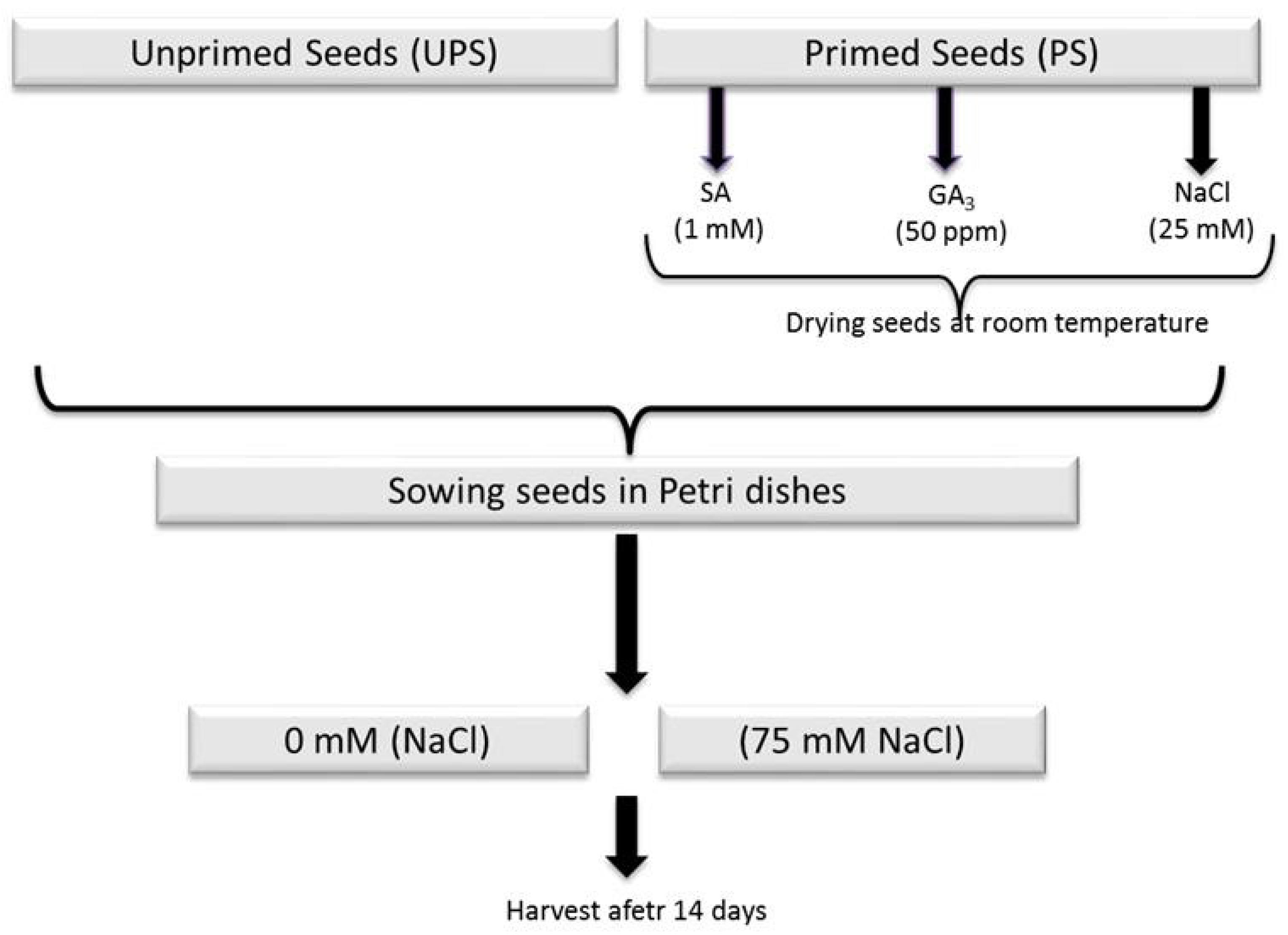
2.1. Priming Treatment and Growth Conditions
2.2. Germination Traits
2.3. Relative Water Content (RWC) Measurement
2.4. Total Chlorophyll Content
2.5. Total Sugar Content
2.6. Sodium (Na+) and Potassium (K+) Analysis
2.7. Hydrogen Peroxide (H2O2) Content
2.8. Lipid Peroxidation (MDA) Content
2.9. Proline Accumulation
2.10. Ascorbic Acid (AsA) Content
2.11. Polyphenol Quantification
2.12. Antioxidant Enzyme Extraction and Assay
2.13. Statistical Analysis
3. Results
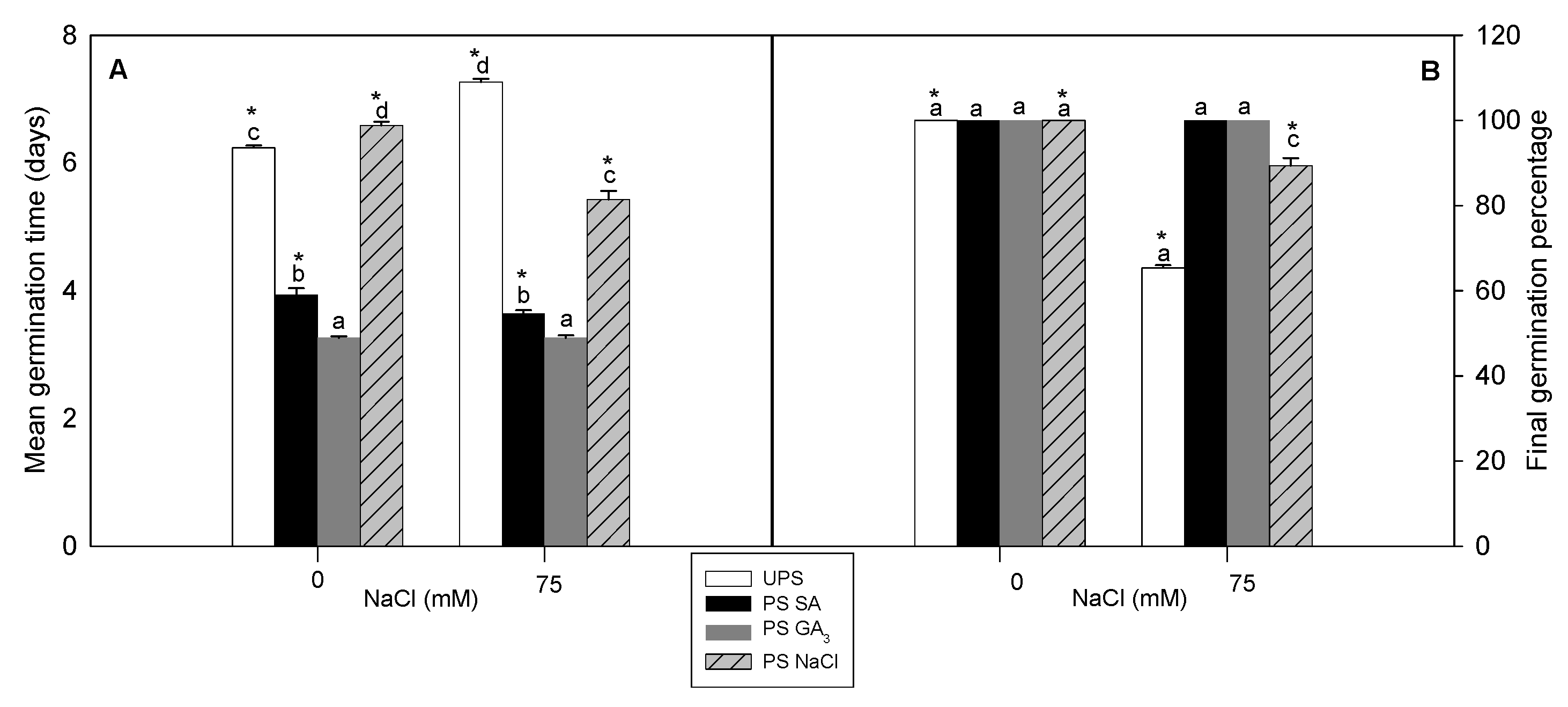
3.1. Effect of Seed Priming on Germination Traits
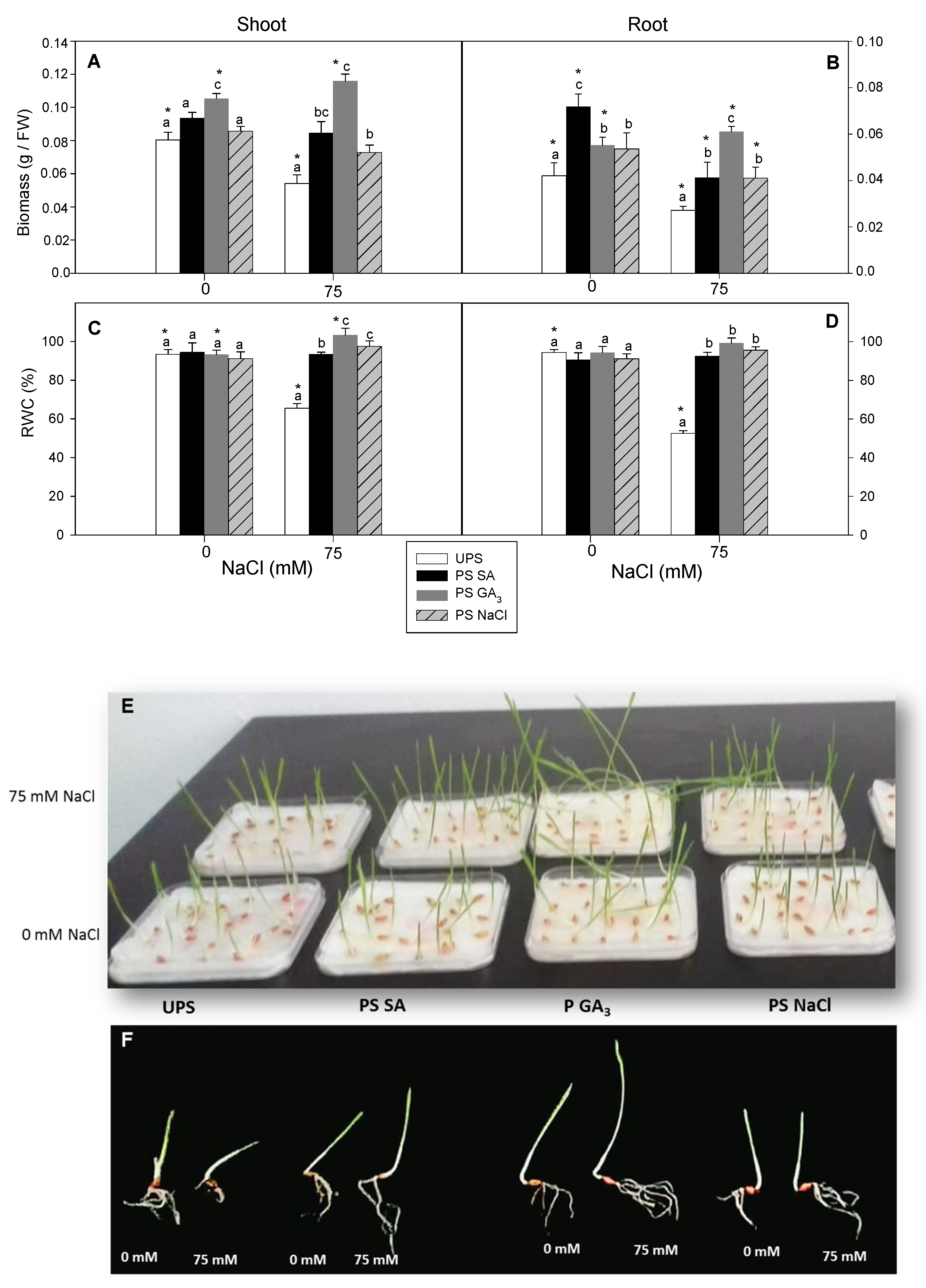
3.2. Effect of Seed Priming on Plant Growth and Water Status
3.3. Effect of Seed Priming on Chlorophyll Content
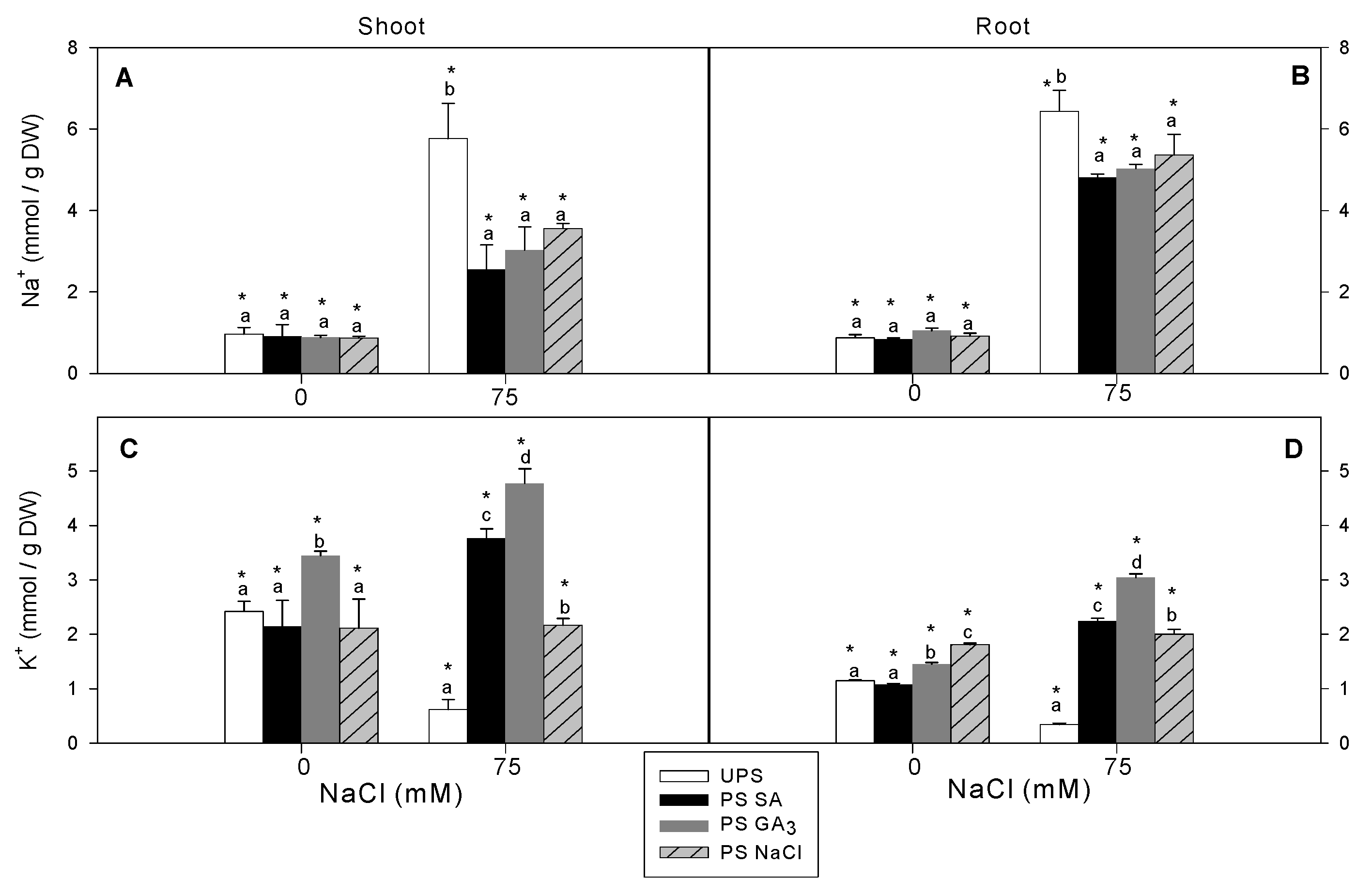
3.4. Effect of Seed Priming on Na+ and K+ Accumulation
3.5. Effect of Seed Priming on H2O2 and MDA Contents
3.6. Effect of Seed Priming on Total Sugar and Proline Contents
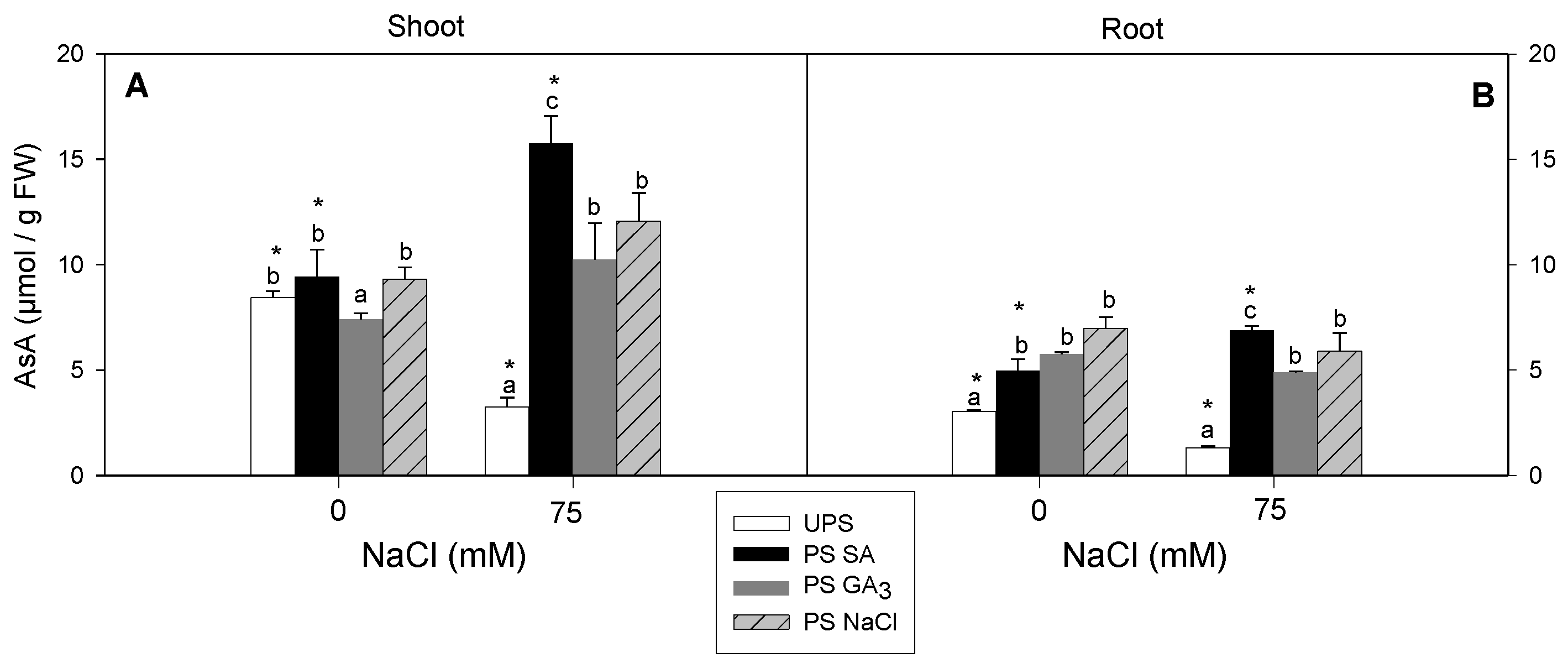
3.7. Effect of Seed Pre-Treatment on AsA Accumulation
3.8. Effect of Seed Priming on Antioxidant Enzyme Activities (SOD, CAT, and GPX)
3.9. Effect of Seed Priming on Shoot PAL and TAL Activities and Polyphenol Content
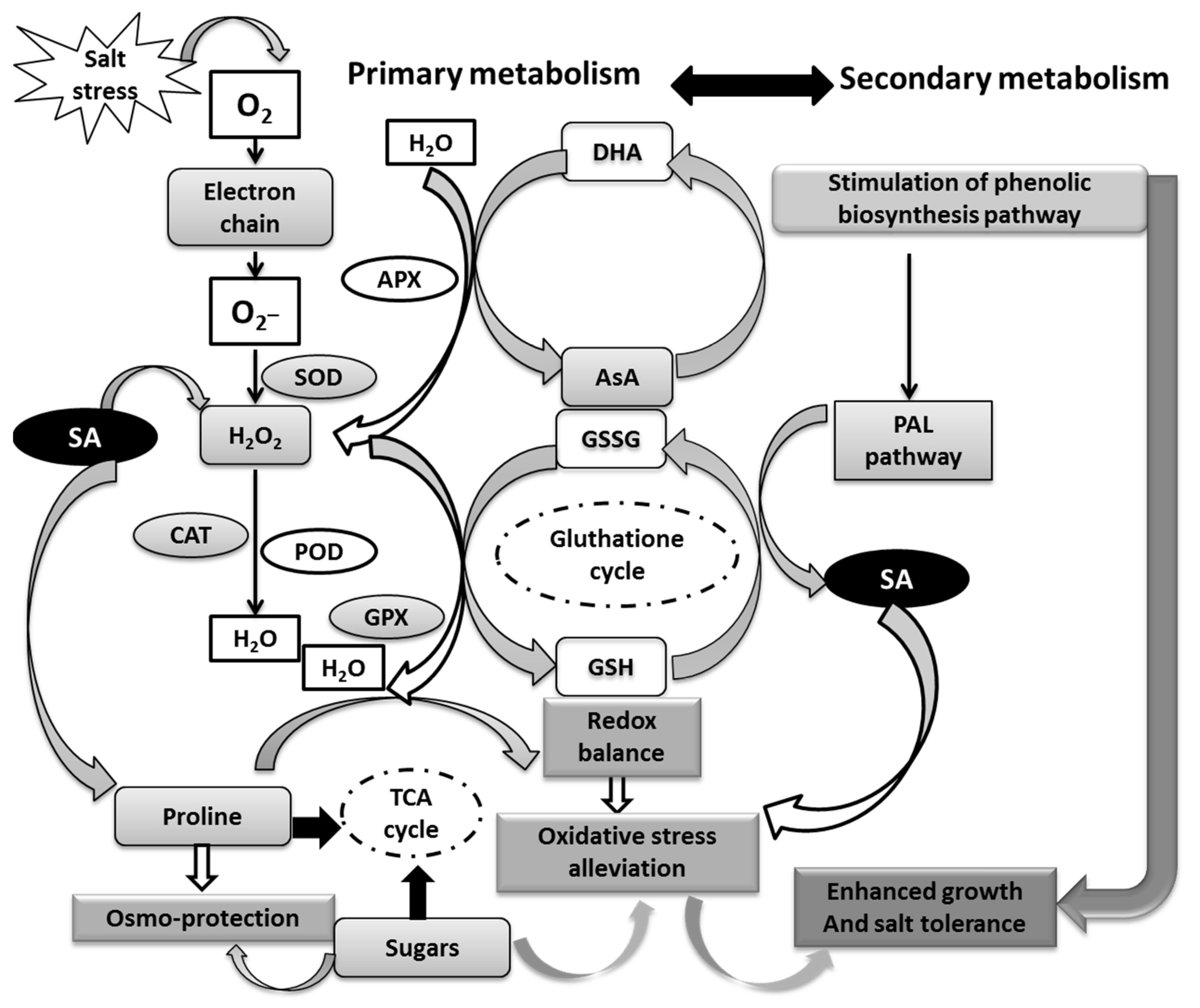
4. Discussion
4.1. Growth and Physiological Alterations with GA3 and NaCl Seed Priming
4.2. SA Seed Priming Enhances Antioxidant Defense
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hongna, C.; Leyuan, T.; Junmei, S.; Xiaori, H.; Cheng Xianguo, C. Exogenous salicylic acid signal reveals an osmotic regulatory role in priming the seed germination of Leymus chinensis under salt-alkali stress. Environ. Exp. Bot. 2021, 188, 104–498. [Google Scholar] [CrossRef]
- Khan, A.; Anwar, Y.; Hasan, M.M.; Iqbal, A.; Ali, M.; Alharby, H.F.; Hakeem, K.R.; Hasanuzzaman, M. Attenuation of drought stress in brassica seedlings with exogenous application of Ca2+ and H2O2. Planta 2019, 6, 20. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Benidire, L.; Daoui, K.; Fatemi, Z.A.; Achouak, W.; Bouarab, L.; Oufdou, K. Effect of salt stress on germination and seedling of Vicia faba L. J. Mater. Environ. Sci. 2015, 6, 840–851. [Google Scholar]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2020, 135, 385–394. [Google Scholar] [CrossRef]
- Wojtyla, L.; Paluch-Lubawa, E.; Nowicka, E.S.; Garnczarska, M. Drought stress memory and subsequent drought stress tolerance in plants. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 115–131. [Google Scholar]
- Biswas, S.; Seal, P.; Majumder, B.; Biswas, A.K. Efficacy of seed priming strategies for enhancing salinity tolerance in plants: An overview of the progress and achievements. Plant Stress 2023, 9, 100186. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W.; Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: An overview. Planta 2022, 255, 45. [Google Scholar] [CrossRef] [PubMed]
- Paparella, S.; Araujo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef]
- Karimi, S.; Torki, Z.; Nazok-kar Maher, M.; Yegane, N. Pre-transplant silicon priming improved drought tolerance and biomass partitioning in young tomato plants. Int. J. Veg. Sci. 2022, 28, 349–365. [Google Scholar] [CrossRef]
- Ellouzi, H.; Rabhi, M.; Khedher, S.; Debez, A.; Abdelly, C.; Zorrig, W. Silicon seed priming enhances salt tolerance of barley seedlings through early ROS detoxification and stimulation of antioxidant defence. Silicon 2023, 2, 941. [Google Scholar] [CrossRef]
- Dai, L.Y.; Zhu, H.D.; Yin, K.D.; Du, J.D.; Zhang, Y.X. Seed priming mitigates the effects of saline-alkali stress in soybean seedlings. Chil. J. Agric. Res. 2017, 77, 118–125. [Google Scholar] [CrossRef]
- Ellouzi, H.; Oueslati, O.; Hessini, K.; Rabhi, M.; Abdelly, C. Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci. Hortic. 2021, 288, 110360. [Google Scholar] [CrossRef]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Rajora, N.; Vats, S.; Raturi, G.; Thakral, V.; Kaur, S.; Rachappanavar, V.; Deshmukh, R. Seed priming with melatonin: A promising approach to combat abiotic stress in plants. Plant Stress 2022, 4, 100071. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Ulhassan, Z.; Noman, M.; Zhao, B.; Zhou, W.; Guan, Y. Seed priming with spermine mitigates chromium stress in rice by modifying the ion homeostasis, cellular ultrastructure and phytohormones Balance. Antioxidants 2022, 11, 1704. [Google Scholar] [CrossRef]
- Salemi, F.; Esfahani, M.N.; Tran, L.S.P. Mechanistic insights into enhanced tolerance of early growth of alfalfa (Medicago sativa L.) under low water potential by seed-priming with ascorbic acid or polyethylene glycol solution. Indust. Crops Prod. 2019, 137, 436–445. [Google Scholar] [CrossRef]
- Carvalho, M.E.; Agathokleous, E.; Nogueira, M.L.; Brunetto, G.; Brown, P.H.; Azevedo, R.A. Neutral-to-positive cadmium effects on germination and seedling vigor, with and without seed priming. J. Hazard. Mater. 2023, 448, 130813. [Google Scholar] [CrossRef]
- Muhei, S.H. Seed priming with phytohormones to improve germination under dormant and abiotic stress conditions. Adv. Crop Sci. Technol. 2018, 6, 403–409. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to enhancing antioxidant defense in plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, X.; Zhai, Z.; Ma, S.; Tian, Y.; Cheng, J. Direct evidence of drought stress memory in mulberry from a physiological perspective: Antioxidative, osmotic and phytohormonal regulations. Plant Physiol. Biochem. 2022, 186, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Pandey, S.; Verma, N.; Singh, M.; Pandey, J.; Prasad, S.M. Cereals and Phytohormones Under Salt Stress. In Sustainable Remedies for Abiotic Stress in Cereals; Springer Nature: Singapore, 2022; pp. 291–311. [Google Scholar]
- Oracz, K.; Karpinski, S. Phytohormones signaling pathways and ROS involvement in seed germination. Front. Plant Sci. 2016, 7, 864. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Singh, R.K.; Sharma, S.; Sharma, N.; Panchal, A.; Das, T.; Prasad, A.; Prasad, M. DNA methylation dynamics in response to abiotic and pathogen stress in plants. Plant Cell Rep. 2022, 41, 1931–1944. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ulhassan, Z.; Qi, W.; Lu, H.; AbdElgawad, H.; Minkina, T.; Dawood, M. Association of jasmonic acid priming with multiple defense mechanisms in wheat plants under high salt stress. Front. Plant Sci. 2022, 13, 886862. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, V.; Singh, N.; Pandurangam, V.V.; Shahi, J.P. Ameliorating effect of seed priming by salicylic acid on biochemical traits in Rabi maize (Zea mays L.) genotypes under normal and delayed sowing. J. Pharm. Phytochem. 2018, 7, 2923–2927. [Google Scholar]
- Hatata, M.M.; Hala, M.T.; Dowidar, S.M. Phytohormones effect on growth and certain metabolic aspects of NaCl stressed Barley plant. Egypt. J. Exp. Biol. 2009, 5, 149–155. [Google Scholar]
- Iqbal, H.; Yaning, C.; Waqas, M.; Rehman, H.M.; Sharee, F.M.; Iqbal, S. Hydrogen peroxide application improves quinoa performance by affecting physiological and biochemical mechanisms under water-deficit conditions. J. Agro Crop Sci. 2018, 204, 541–553. [Google Scholar] [CrossRef]
- Rady, M.M.; Talaat, N.B.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.M. Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J. Hortic. Sci. Biotech. 2021, 94, 777–789. [Google Scholar] [CrossRef]
- Al-Harthi, M.M.; Bafeel, S.O.; El-Zohri, M. Gibberellic acid and jasmonic acid improve salt tolerance in summer squash by modulating some physiological parameters symptomatic for oxidative stress and mineral nutrition. Plants 2021, 10, 2768. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y.; et al. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Lone, W.A.; Majeed, N.; Yaqoob, U.; John, R. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep. 2022, 41, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Tuna, A.L.; Kaya, C.; Higgs, D.; Murillo-Amador, B.; Aydemir, S.; Girgin, A.R. Silicon improves salinity tolerance in wheat plants. Environ. Exp. Bot. 2008, 62, 10–16. [Google Scholar] [CrossRef]
- Kaya, G. The efficiency of prechilling and gibberellic acid (GA 3) for breaking thermodormancy in lettuce. J. Seed. Sci. 2022, 44, 1–10. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Zaib-UN-Nisa, N.A.; Shani, M.Y.; Naz, A.; Azmat, M.; Ashraf, I. Salicylic acid seed priming improved dry biomass and ionic efficiency of mungbean [Vigna radiata (l.) wilczek] under salt stress conditions. Pak. J. Bot. 2023, 55, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, M.E.; Zhang, S. Calcium-independent activation of salicylic acid-induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant. Physiol. 2000, 122, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Al-Mudaris, M. Notes on various parameters recording the speed of seed germination. Der Tropenlandwirt. 1998, 99, 147–154. [Google Scholar]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to longterm salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; pp. 350–382. [Google Scholar]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Zorrig, W.; Cornu, J.Y.; Maisonneuve, B.; Rouached, A.; Sarrobert, C.; Shahzad, Z.; Berthomieu, P. Genetic analysis of cadmium accumulation in lettuce (Lactuca sativa). Plant Physiol. Biochem. 2019, 136, 67–75. [Google Scholar] [CrossRef]
- Sergiev, I.; Alexieva, V.; Karnov, E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt. Rend. Acad. Bulg. Sci. 1997, 51, 121–124. [Google Scholar]
- Hodge, D.M.; DeLong, J.M.; Forney, C.F.; Robert, K.P. Improving the thiobarbituric acid reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Wadern, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Mau, J.L.; Chao, G.R.; Wu, K.T. Antioxidantproperties of methanolic extracts from several ear mushrooms. J. Agric. Food Chem. 2001, 49, 5461–5467. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Scebba, F.; Sebastiani, L.; Vitagliano, C. Protective enzymes against activated oxygen species in wheat (Triticumaestivum L.) seedlings: Responses to cold acclimation. J. Plant Physiol. 1999, 155, 762–768. [Google Scholar] [CrossRef]
- Lück, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1965; pp. 885–894. [Google Scholar]
- Fielding, J.L.; Hall, J.L. A Biochemical and Cytochemical Study of Peroxidase Activity in Roots of Pisumsativum: I. a comparison of dab-peroxidase and guaiacol-peroxidase with particular emphasis on the properties of cell wall activity. J. Exp. Bot. 1978, 9, 969–981. [Google Scholar] [CrossRef]
- Berner, M.; Krug, D.; Bihlmaier, C.; Vente, A.; Muller, R.; Bechthold, A. Genes and enzymes involved in caffeic acid biosynthesis in the actinomycete Saccharothrix espanaensis. J. Bacteriol. 2006, 188, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Sharanagat, V.S.; Singh, L.; Mani, S. Effect of germination and roasting on the proximate composition, total phenolics, and functional properties of black chickpea (Cicer arietinum). Legume Sci. 2020, 2, e20. [Google Scholar] [CrossRef]
- Pallaoro, D.S.; Camilli, E.S.; Guimaraes, S.C.; Albuquerque, M.C.F. Methods of priming maize seed. J. Seed. Sci. 2016, 38, 148–154. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Xing, Y.; Jiang, B.; Li, B.; Xu, X.; Zhou, Y. The efficacy of different seed priming agents for promoting sorghum germination under salt stress. PLoS ONE 2021, 16, e0245505. [Google Scholar] [CrossRef]
- Kanjevac, M.; Bojović, B.; Ćirić, A.; Stanković, M.; Jakovljević, D. Seed priming improves biochemical and physiological performance of wheat seedlings under low-temperature conditions. Agriculture 2022, 13, 2. [Google Scholar] [CrossRef]
- Nimir, N.E.A.; Lu, S.; Zhou, G.; Guo, W.; Ma, B.; Wang, Y. Comparative effects of gibberellic acid, kinetin and salicylic acid on emergence, seedling growth and the antioxidant defence system of sweet sorghum (Sorghum bicolor) under salinity and temperature stresses. Crop Pasture Sci. 2015, 66, 145–157. [Google Scholar] [CrossRef]
- Rodríguez, A.; Stella, A.; Storni, M.; Zulpa, G.; Zaccaro, M.C. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Aquat. Biosyst. 2006, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Saeidi-Sar, S.; Khavari-Nejad, R.A.; Fahimi, H.; Ghorbanli, M.; Majd, A. Interactive effects of gibberellin A3 and ascorbic acid on lipid peroxidation and antioxidant enzyme activities in glycine max seedlings under nickel. Russ. J. Plant Physiol. 2007, 54, 74–79. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Inter. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 2016, 86, 76–85. [Google Scholar] [CrossRef]
- Mohammed, A.H.M.A. Physiological aspects of mungbean plant (Vigna radiata L. Wilczek) in response to salt stress and gibberellic acid treatment. Res. J. Agric. Biol. Sci. 2007, 3, 200–213. [Google Scholar]
- Khan, M.M.; Usman, M.; Waseem, R.; Ali, M.A. Role of gibberellic acid (ga~ 3) on citrus seed germination and study of some morphological characteristics. Pak. J. Agric. Sci. 2002, 39, 113–118. [Google Scholar]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Leonard Wijaya, L.; Nasser, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant. 2020, 168, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, K.K.; Singh, S.; Agrawal, M.; Agrawal, S.B. Role of jasmonic and salicylic acid signaling in plants under UV-B stress. In Jasmonates and Salicylates Signaling in Plants; Aftab, T., Yusuf, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 45–63. [Google Scholar]
- Shukry, W.M.; El-Bassiouny, H.M.S. Gibberellic acid effects on protein pattern, hydrolytic enzyme activities and ionic uptake during germination of Vicia faba in sea water. Acta Bot. Hungar. 2002, 44, 145–162. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Farooq, M.A.; Zhang, X.; Zafar, M.M.; Ma, W.; Zhao, J. Roles of reactive oxygen species and mitochondria in seed germination. Front. Plant Sci. 2021, 12, 781734. [Google Scholar] [CrossRef]
- Wiciarz, M.; Niewiadomska, E.; Kruk, J. Effects of salt stress on low molecular antioxidants and redox state of plastoquinone and P700 in Arabidopsis thaliana (glycophyte) and Eutrema salsugineum (halophyte). Photosynthetica 2018, 56, 811–819. [Google Scholar] [CrossRef]
- Yan, Z.; Ming, D.I.A.O.; Cui, J.X.; Chen, X.J.; Wen, Z.L.; Zhang, J.W.; Liu, H.Y. Exogenous GSH protects tomatoes against salt stress by modulating photosystem II efficiency, absorbed light allocation and H2O2-scavenging system in chloroplasts. J. Integ. Agric. 2018, 17, 2257–2272. [Google Scholar]
- Zhu, Z.; Chen, Y.; Shi, G.; Zhang, X. Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem. 2017, 219, 179–184. [Google Scholar] [CrossRef]
- Yan, J.; Tan, Y.; Xu, L.; Lv, Y.; Wang, F.; Shan, W.; Xu, D. Effect of exogenous salicylic acid on salt tolerance of Hosta ensata. Eur. J. Hortic. Sci. 2023, 88, 12. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol. Environ. Saf. 2018, 147, 1010–1016. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, S. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Ellouzi, H.; Sghayar, S.; Abdelly, C. H2O2 seed priming improves tolerance to salinity; drought and their combined effect more than mannitol in Cakile maritima when compared to Eutrema salsugineum. J. Plant Physiol. 2017, 210, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Saxena, P. Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci. 2009, 177, 181–189. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Du, X.; Tang, H.; Shen, C.; Wu, J. Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS ONE 2014, 9, e109492. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2015, 168, 807–815. [Google Scholar] [CrossRef]
- Talaat, N.B.; Abdel Wahab, M.; Alaa, M.; Hanafy, A. Co-application of salicylic acid and spermine alleviates salt stress toxicity in wheat: Growth, nutrient acquisition, osmolytes accumulation, and antioxidant response. Acta Physiol. Plant 2023, 45, 1. [Google Scholar] [CrossRef]
- Jannesar, M.; Seyedi, M.S.; Niknam, V.; Khorzoghi, E.G.; Hassan Ebrahimzadeh, H. Salicylic acid, as a positive regulator of isochorismate synthase, reduces the negative effect of salt stress on Pistacia vera L. by increasing photosynthetic pigments and inducing antioxidant activity. J. Plant Reg. 2022, 41, 1304–1315. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Poor, P.; Janda, T. Salicylic acid: A versatile signaling molecule in plants. J. Plant Growth Regul. 2022, 41, 1887–1890. [Google Scholar] [CrossRef]
- Khalid, M.F.; Saleem, M.S.; Zakir, I.; Khan, R.I.; Sohail, M.; Ejaz, S.; Hussain, S. Salicylic acid induced abiotic stress tolerance in plants. In Plant Stress Mitigators; Academic Press: Cambridge, MA, USA, 2023; pp. 57–67. [Google Scholar]
- Al Mahmud, J.; Bhuyan, M.B.; Anee, T.I.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Cham, Switzerland, 2019; pp. 221–257. [Google Scholar]
- Alamri, S.A.D.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Ali, H.M.; Al-Amri, A.; AlRabiah, H.K. Exogenous application of salicylic acid improves tolerance of wheat plants to lead stress. Adv. Agric. Sci. 2018, 6, 25–35. [Google Scholar]
- Yang, C.; Hu, L.Y.; Ali, B.; Islam, F.; Bai, Q.J.; Yun, X.P.; Zhou, W.J. Seed treatment with salicylic acid invokes defence mechanism of Helianthus annuus against Orobanche cumana. Ann. Appl. Biol. 2016, 169, 408–422. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma 2019, 256, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.M.; Madany, M.M.; González, L. The effect of coumarin application on early growth and some physiological parameters in faba bean (Vicia faba L.). J. Plant Growth Regul. 2015, 34, 233–241. [Google Scholar] [CrossRef]
- Abbaspour, J.; Ehsanpour, A. The impact of salicylic acid on some physiological responses of Artemisia aucheri Boiss. under in vitro drought stress. Acta Agric. Slov. 2016, 107, 287–298. [Google Scholar] [CrossRef]
- Dogbo, D.O.; Gogbeu, S.J.; N’zue, B.; Ya, K.A.; Zohouri, G.P.; Mamyrbekova-Bekro, J.A.; Bekro, Y.A. Comparative activities of phenylalanine ammonia-lyase and tyrosine ammonia-lyase and phenolic compounds accumulated in cassava elicited cell. Afr. Crop Sci. J. 2012, 20, 85–94. [Google Scholar]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. J. Plant Physiol. 2003, 160, 859–863. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellouzi, H.; Zorrig, W.; Amraoui, S.; Oueslati, S.; Abdelly, C.; Rabhi, M.; Siddique, K.H.M.; Hessini, K. Seed Priming with Salicylic Acid Alleviates Salt Stress Toxicity in Barley by Suppressing ROS Accumulation and Improving Antioxidant Defense Systems, Compared to Halo- and Gibberellin Priming. Antioxidants 2023, 12, 1779. https://doi.org/10.3390/antiox12091779
Ellouzi H, Zorrig W, Amraoui S, Oueslati S, Abdelly C, Rabhi M, Siddique KHM, Hessini K. Seed Priming with Salicylic Acid Alleviates Salt Stress Toxicity in Barley by Suppressing ROS Accumulation and Improving Antioxidant Defense Systems, Compared to Halo- and Gibberellin Priming. Antioxidants. 2023; 12(9):1779. https://doi.org/10.3390/antiox12091779
Chicago/Turabian StyleEllouzi, Hasna, Walid Zorrig, Souhir Amraoui, Samia Oueslati, Chedly Abdelly, Mokded Rabhi, Kadambot H. M. Siddique, and Kamel Hessini. 2023. "Seed Priming with Salicylic Acid Alleviates Salt Stress Toxicity in Barley by Suppressing ROS Accumulation and Improving Antioxidant Defense Systems, Compared to Halo- and Gibberellin Priming" Antioxidants 12, no. 9: 1779. https://doi.org/10.3390/antiox12091779
APA StyleEllouzi, H., Zorrig, W., Amraoui, S., Oueslati, S., Abdelly, C., Rabhi, M., Siddique, K. H. M., & Hessini, K. (2023). Seed Priming with Salicylic Acid Alleviates Salt Stress Toxicity in Barley by Suppressing ROS Accumulation and Improving Antioxidant Defense Systems, Compared to Halo- and Gibberellin Priming. Antioxidants, 12(9), 1779. https://doi.org/10.3390/antiox12091779









