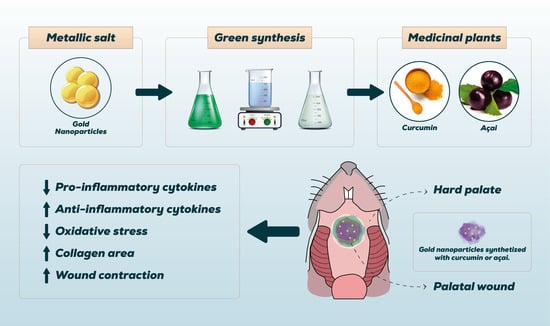
Green Synthesis of Gold Nanoparticles with Curcumin or Açai in the Tissue Repair of Palatal Wounds
Abstract
:1. Introduction
2. Methods
2.1. Green Synthesis of Gold Nanoparticles
2.2. In Vitro Assay
2.2.1. Cell Viability
2.2.2. Cell Viability Test—MTT
2.3. In Vivo Assay
2.3.1. Animals
- Palatal Wound (PW)—without local or systemic treatment;
- PW + Photobiomodulation (PBM)—standard treatment with Laser 660 nm 2 J;
- PW + Omcilon®—standard Omcilon® treatment;
- PW + GNPs-Cur—treatment with gold nanoparticles reduced with curcumin (0.025 mg/mL);
- PW + GNPs-Açai—treatment with gold nanoparticles reduced with açai (0.025 mg/mL).
2.3.2. Palatal Wound Model
2.3.3. Treatment
2.4. Macroscopic Analysis and Inflammatory Score
- No change
- Irritation
- Shallow ulcer (1–2 mm) with clean edges
- Shallow ulcer (1–2 mm) with necrotic edges
- Shallow ulcer (3–4 mm) with clean edges
- Shallow ulcer (3–4 mm) with necrotic edges
- Deep ulcer (1–2 mm) with clean edges
- Deep ulcer (1–2 mm) with necrotic edges
- Deep ulcer (3–4 mm) with clean edges
- Deep ulcer (3–4 mm) with necrotic edges
- Necrotic tissue with increased margins
2.5. Euthanasia
2.6. Wound Size Analysis
2.7. Histomorphometry
2.8. Determination of Cytokine Content Using ELISA
2.9. Biochemical Analysis
- Intracellular determination of reactive oxygen species (ROS) and nitric oxide: The production of hydroperoxides was determined by the intracellular formation of 2′,7′-dichlorofluorescein (DCF) from the oxidation of 2′,7′-dichlorodihydrofluorescein diacetate (DCFHDA) by ROS according to the method previously described by Dong et al. [15] with some modifications. The production of nitric oxide (NO) was evaluated spectrophotometrically through the stable metabolite nitrite. The nitrite content was calculated based on a standard curve from 0 to 100 nM performed with the metabolite sodium nitrite (NaNO2). The results were calculated in μmol Nitrite/mg protein [16].
- Antioxidant defenses: Glutathione (GSH) levels were determined as described by Hissin and Hilf [17], with some adaptations. GSH was measured in the palatal mucosa homogenate after protein precipitation with 1 mL of 10% trichloroacetic acid. An 800 mM phosphate buffer, pH 7.4, and 500 μm DTNB were added to part of the sample. Absorbance was read at 412 nm after 10 min. A reduced glutathione standard curve was used to calculate the GSH levels in the samples.
- Protein Content: The protein content of the palatal mucosa homogenate was assayed using bovine serum albumin as a standard, according to Lowry et al. [18]. Phosphomolybdic phosphotungstic reagent (Folin phenol) was added to bind to the protein. Absorbance was read at 750 nm.
2.10. Statistical Analysis
3. Results
3.1. Characterization of GNPs
3.2. Cell Viability Test
3.3. Macroscopic Analysis and Inflammatory Score
3.4. Wound Contraction
3.5. Histological Analysis
3.6. Pro- and Anti-Inflammatory Cytokines
3.7. Levels of Oxidants and Antioxidants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Leoni, G.; Neumann, P.-A.; Sumagin, R.; Denning, T.L.; Nusrat, A. Wound repair: Role of immune–epithelial interactions. Mucosal Immunol. 2015, 8, 959–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, C.; Felix, D.H. Oral medicine—Update for the dental practitioner Aphthous and other common ulcers. Br. Dent. J. 2005, 199, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Abou Neel, E.A.; Bozec, L.; Perez, R.A.; Kim, H.W.; Knowles, J.C. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int. J. Nanomed. 2015, 10, 6371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, A.; Pillai, S.; Khayambashi, P.; Sabri, H.; Lee, K.T.; Tarar, M.; Zhou, S.; Harb, I.; Tran, S.D. Biomimetic aspects of oral and dentofacial regeneration. Biomimetics 2020, 5, 51. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, J.; Wu, C. Bioactive inorganic/organic nanocomposites for wound healing. J. Appl. Mater. Today 2018, 11, 308–319. [Google Scholar] [CrossRef]
- Mendes, C.; Dos Santos Haupenthal, D.P.; Zaccaron, R.P.; Silveira, G.D.B.; Corrêa, M.E.A.B.; Casagrande, L.D.R.; Mariano, S.D.S.; Silva, J.I.D.S.; de Andrade, T.A.M.; Feuser, P.E.; et al. Effects of the association between photobiomodulation and hyaluronic acid linked gold nanoparticles in wound healing. J. ACS Biomater. Sci. Eng. 2020, 6, 5132–5144. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Asif, M.; Yasmin, R.; Asif, R.; Ambreen, A.; Mustafa, M.; Umbreen, S. Green Synthesis of Silver Nanoparticles (AgNPs), Structural Characterization, and their Antibacterial Potential. Dose-Response 2022, 20, 15593258221088709. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Kang, M.H.; Kim, B.H. Oral wound healing effects of acai berry water extracts in rat oral mucosa. Toxicol. Res. 2018, 34, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percie Du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Marques Neto, R.; Lima Junior, R.; Vale, M.; Souza, M.; Brito, G. Mucosite Oral: Patogênese e manuseio clínico. Rev. Bras. Oncol. Clínica 2008, 5, 18–24. [Google Scholar]
- Singh, A.V.; Bansod, G.; Mahajan, M.; Dietrich, P.; Singh, S.P.; Rav, K.; Thissen, A.; Bharde, A.M.; Rothenstein, D.; Kulkarni, S.; et al. Digital Transformation in Toxicology: Improving Communication and Efficiency in Risk Assessment. ACS Omega 2023, 8, 21377–21390. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sulik, K.K.; Chen, S.-Y. The role of NOX enzymes in ethanol-induced oxidative stress and apoptosis in mouse embryos. Research Support, N I H, Extramural. Toxicol. Lett. 2010, 193, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, S.Y.; Lee, M.; Kim, S.W.; Bae, Y.H. Protection of insulin secreting cells from nitric oxide induced cellular damage by crosslinked hemoglobin. Biomaterials 2004, 25, 843–850. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Callegari-Jacques, S.M. Bioestatística: Princípios e Aplicações; Artmed Editora: Porto Alegre, Brazil, 2009. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS (3. Baskı); Sage Publications Ltd.: London, UK, 2009. [Google Scholar]
- Vieira, S. Bioestatística: Tópicos Avançados, 3rd ed.; RJ; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Zar, J. Biostatistical Analysis, 5th ed.; Prentice-Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-based green synthesis of metallic nanoparticles: Scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol. Environ. Eng. 2016, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Chaushu, L.; Rahmanov Gavrielov, M.; Chaushu, G.; Zar, K.; Vered, M. Curcumin Promotes Primary Oral Wound Healing in a Rat Model. J. Med. Food 2021, 24, 422–430. [Google Scholar] [CrossRef]
- Felin, F.D.; Maia-Ribeiro, E.A.; Felin, C.D.; Bonotto, N.A.C.; Turra, B.O.; Roggia, I.; Azzolin, V.F.; Teixeira, C.F.; Mastella, M.H.; de Freitas, C.R.; et al. Amazonian Guarana-and Açai-Conjugated Extracts Improve Scratched Fibroblast Healing and Eisenia fetida Surgical Tail Amputation by Modulating Oxidative Metabolism. Oxidative Med. Cell. Longev. 2022, 2022, 3094362. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Uehara, A.; Tamai, R.; Takada, H. Innate immune responses in oral mucosa. J. Endotoxin Res. 2002, 8, 465–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetyczka, C.; Hartl, S.; Jeitler, R.; Absenger-Novak, M.; Meindl, C.; Fröhlich, E.; Riedl, S.; Zweytick, D.; Roblegg, E. Cytokine-Mediated Inflammation in the Oral Cavity and Its Effect on Lipid Nanocarriers. Nanomaterials 2021, 11, 1330. [Google Scholar] [CrossRef]
- Freitas, M.O.; Fonseca, A.P.R.; de Aguiar, M.T.; Dias, C.C.; Avelar, R.L.; Sousa, F.B.; Alves, A.P.N.N.; Silva, P.G.d.B. Tumor necrosis factor alpha (TNF-α) blockage reduces acute inflammation and delayed wound healing in oral ulcer of rats. Inflammopharmacology 2022, 30, 1781–1798. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, V.R.N.; Frazão, D.R.; Ferreira, R.d.O.; Mendes, P.F.S.; Baia-Da-Silva, D.C.; Souza-Monteiro, D.; Bittencourt, L.O.; de Moura, J.D.M.; Perdigão, J.M.; Teixeira, B.J.B.; et al. Açaí (Euterpe oleracea Mart.) Attenuates Oxidative Stress and Alveolar Bone Damage in Experimental Periodontitis in Rats. Antioxidants 2022, 11, 1902. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Miyagawa, L.M.; Monteiro, K.M.; Dias, A.L.; Longato, G.B.; Spindola, H.; Vendramini-Costa, D.B.; Quetin-Leclercq, J.; Carvalho, J.E.; Rogez, H. Phenolic composition, antiproliferative and antiulcerogenic activities of a polyphenol-rich purified extract from açai (Euterpe oleracea) fruits. Int. J. Food Sci. Technol. 2021, 56, 6626–6634. [Google Scholar] [CrossRef]
- Brito, C.; Stavroullakis, A.; Ferreira, A.; Li, K.; Oliveira, T.; Nogueira-Filho, G.; Prakki, A. Extract of acai-berry inhibits osteoclast differentiation and activity. Arch. Oral Biol. 2016, 68, 29–34. [Google Scholar] [CrossRef]
- Barbosa, P.O.; Souza, M.O.; Silva, M.P.; Santos, G.T.; Silva, M.E.; Bermano, G.; Freitas, R.N. Açaí (Euterpe oleracea Martius) supplementation improves oxidative stress biomarkers in liver tissue of dams fed a high-fat diet and increases antioxidant enzymes’ gene expression in offspring. Biomed. Pharmacother. 2021, 139, 111627. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef] [Green Version]
- Kwan, K.H.; Liu, X.; To, M.K.; Yeung, K.W.; Ho, C.-M.; Wong, K.K. Modulation of collagen alignment by silver nanoparticles results in better mechanical properties in wound healing. Biol. Med. 2011, 7, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Misof, K.; Zizak, I.; Rapp, G.; Amenitsch, H.; Bernstorff, S. Fibrillar structure and mechanical properties of collagen. J. Struct. Biol. 1998, 122, 119–122. [Google Scholar] [CrossRef]
- BinShabaib, M.S.; Alharthi, S.S.; Helaby, B.S.; AlHefdhi, M.H.; Mohammed, A.E.; Aabed, K. Comparison of the Anti-bacterial Efficacy of Saussurea costus and Melaleuca alternifolia against Porphyromonas gingivalis, Streptococcus mutans, and Enterococcus faecalis: An in-vitro Study. Front. Oral Health 2022, 3, 950840. [Google Scholar] [CrossRef]
- Ning, S.; Zang, J.; Zhang, B.; Feng, X.; Qiu, F. Botanical Drugs in Traditional Chinese Medicine With Wound Healing Properties. Front. Pharmacol. 2022, 13, 885484. [Google Scholar] [CrossRef]
- Pona, A.; Cline, A.; Kolli, S.S.; Taylor, S.L.; Feldman, S.R. Review of future insights of Dragon’s Blood in dermatology. Dermatol. Ther. 2019, 32, e12786. [Google Scholar] [CrossRef]
- Raghuwanshi, N.; Kumari, P.; Srivastava, A.K.; Vashisth, P.; Yadav, T.C.; Prasad, R.; Pruthi, V. Synergistic effects of Woodfordia fruticosa gold nanoparticles in preventing microbial adhesion and accelerating wound healing in Wistar albino rats in vivo. Mater. Sci. Eng. C 2017, 80, 252–262. [Google Scholar] [CrossRef]
- Weinberg, E.; Vered, M.; Atzil, S.; Chaushu, G.; Chaushu, L. The dynamics of closure following excisional mid-palatal mucoperiosteal wound in a rat model. Clin. Oral Investig. 2020, 24, 4385–4393. [Google Scholar] [CrossRef]
- Feugate, J.E.; Li, Q.J.; Wong, L.; Martins-Green, M. The cxc chemokine cCAF stimulates differentiation of fibroblasts into myofibroblasts and accelerates wound closure. J. Cell Biol. 2002, 156, 161–172. [Google Scholar] [CrossRef]
- Chitturi, R.T.; Murali Balasubramaniam, A.; Arjun Parameswar, R.; Kesavan, G.; Muhamed Haris, K.T.; Mohideen, K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J. Int. Oral Heal. 2015, 7, 75–80. [Google Scholar]
- van Beurden, H.; Von den Hoff, J.; Torensma, R.; Maltha, J.; Kuijpers-Jagtman, A. Myofibroblasts in palatal wound healing: Prospects for the reduction of wound contraction after cleft palate repair. J. Dent. Res. 2005, 84, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Vanlancker, E.; Vanhoecke, B.; Sieprath, T.; Bourgeois, J.; Beterams, A.; De Moerloose, B.; De Vos, W.H.; Van de Wiele, T. Oral microbiota reduce wound healing capacity of epithelial monolayers, irrespective of the presence of 5-fluorouracil. Exp. Biol. Med. 2018, 243, 350–360. [Google Scholar] [CrossRef] [PubMed]






| Samples | SD (nm) | Zeta Potential (mV) | Maximum Wavelength |
|---|---|---|---|
| GNPs-Cur | 39 ± 4 | −22 ± 3 | 524 nm |
| GNPs-Açai | 34 ± 2 | −28 ± 3 | 526 nm |
| GNPs | n | Mean ± | Minimum | Maximum | p Value |
|---|---|---|---|---|---|
| PW | 9 | 3.22 ± 1.39 | 1 | 6 | |
| PW + PBM | 12 | 4.42 ± 3.11 | 1 | 10 | |
| PW + Omcilon® | 12 | 3.67 ± 2.06 | 2 | 9 | |
| PW + GNPs-Cur | 12 | 3.33 ± 1.96 | 1 | 7 | |
| PW + GNPs-Açai | 12 | 1.92 ± 0.79 * | 1 | 4 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirupathi, A.; Guzzatti, M.F.M.; Corrêa, M.E.A.B.; Venturini, L.M.; Casagrande, L.d.R.; Lima, I.R.; Da Costa, C.; De Pieri, E.; Tietbohl, L.T.W.; Feuser, P.E.; et al. Green Synthesis of Gold Nanoparticles with Curcumin or Açai in the Tissue Repair of Palatal Wounds. Antioxidants 2023, 12, 1574. https://doi.org/10.3390/antiox12081574
Thirupathi A, Guzzatti MFM, Corrêa MEAB, Venturini LM, Casagrande LdR, Lima IR, Da Costa C, De Pieri E, Tietbohl LTW, Feuser PE, et al. Green Synthesis of Gold Nanoparticles with Curcumin or Açai in the Tissue Repair of Palatal Wounds. Antioxidants. 2023; 12(8):1574. https://doi.org/10.3390/antiox12081574
Chicago/Turabian StyleThirupathi, Anand, Morgana Francisco Machado Guzzatti, Maria Eduarda Anastácio Borges Corrêa, Ligia Milanez Venturini, Laura de Roch Casagrande, Igor Ramos Lima, Camila Da Costa, Ellen De Pieri, Lariani Tamires Witt Tietbohl, Paulo Emilio Feuser, and et al. 2023. "Green Synthesis of Gold Nanoparticles with Curcumin or Açai in the Tissue Repair of Palatal Wounds" Antioxidants 12, no. 8: 1574. https://doi.org/10.3390/antiox12081574
APA StyleThirupathi, A., Guzzatti, M. F. M., Corrêa, M. E. A. B., Venturini, L. M., Casagrande, L. d. R., Lima, I. R., Da Costa, C., De Pieri, E., Tietbohl, L. T. W., Feuser, P. E., Machado-de-Ávila, R. A., Gu, Y., & Silveira, P. C. L. (2023). Green Synthesis of Gold Nanoparticles with Curcumin or Açai in the Tissue Repair of Palatal Wounds. Antioxidants, 12(8), 1574. https://doi.org/10.3390/antiox12081574