Curcumin as a Potential Antioxidant in Stress Regulation of Terrestrial, Avian, and Aquatic Animals: A Review
Abstract
:1. Introduction
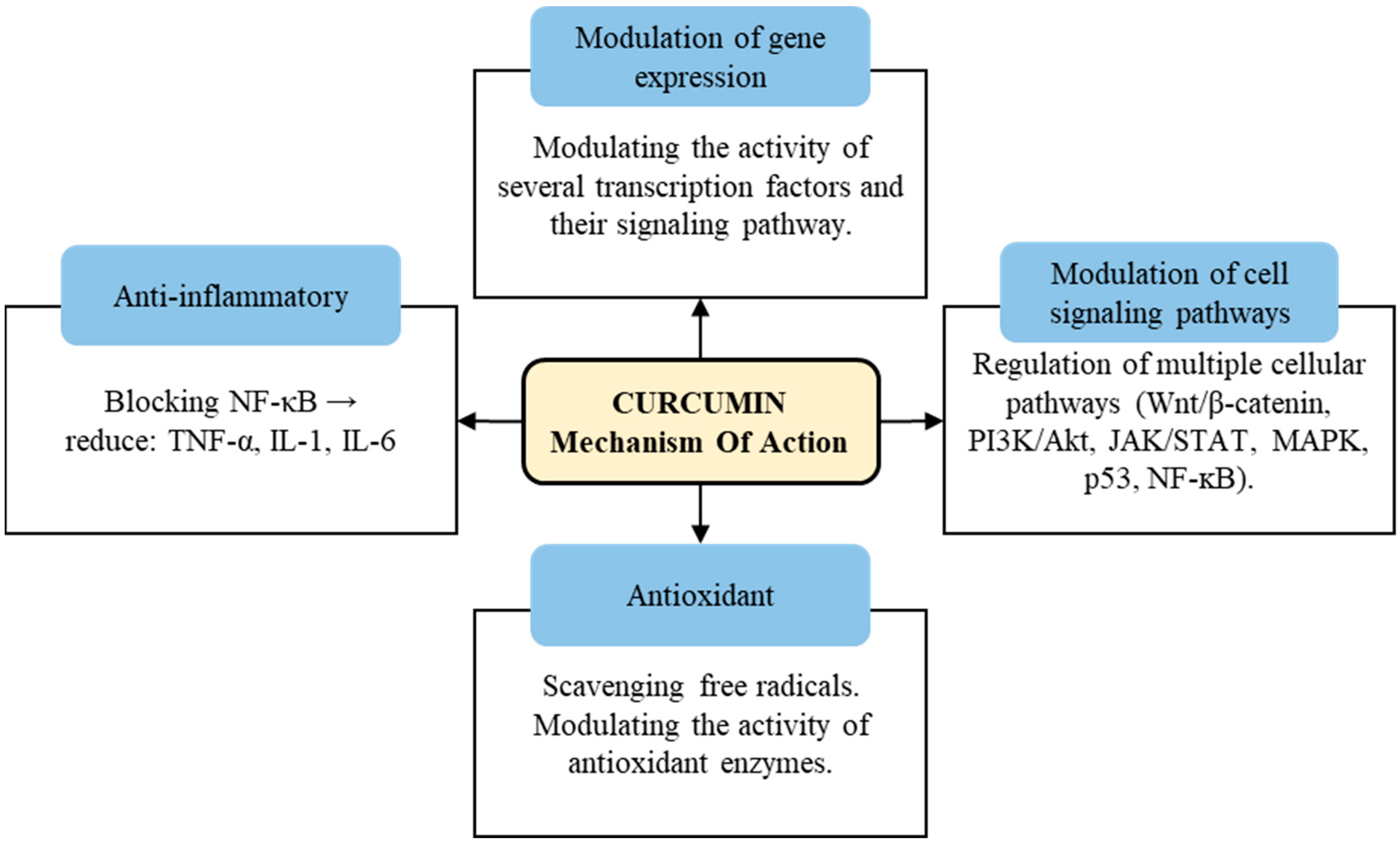
2. Overview of Curcumin
2.1. Biological Activities of Curcumin
2.2. Metabolism of Curcumin
2.3. Other Extractable Components of Turmeric
2.4. Nanoformulation of Curcumin
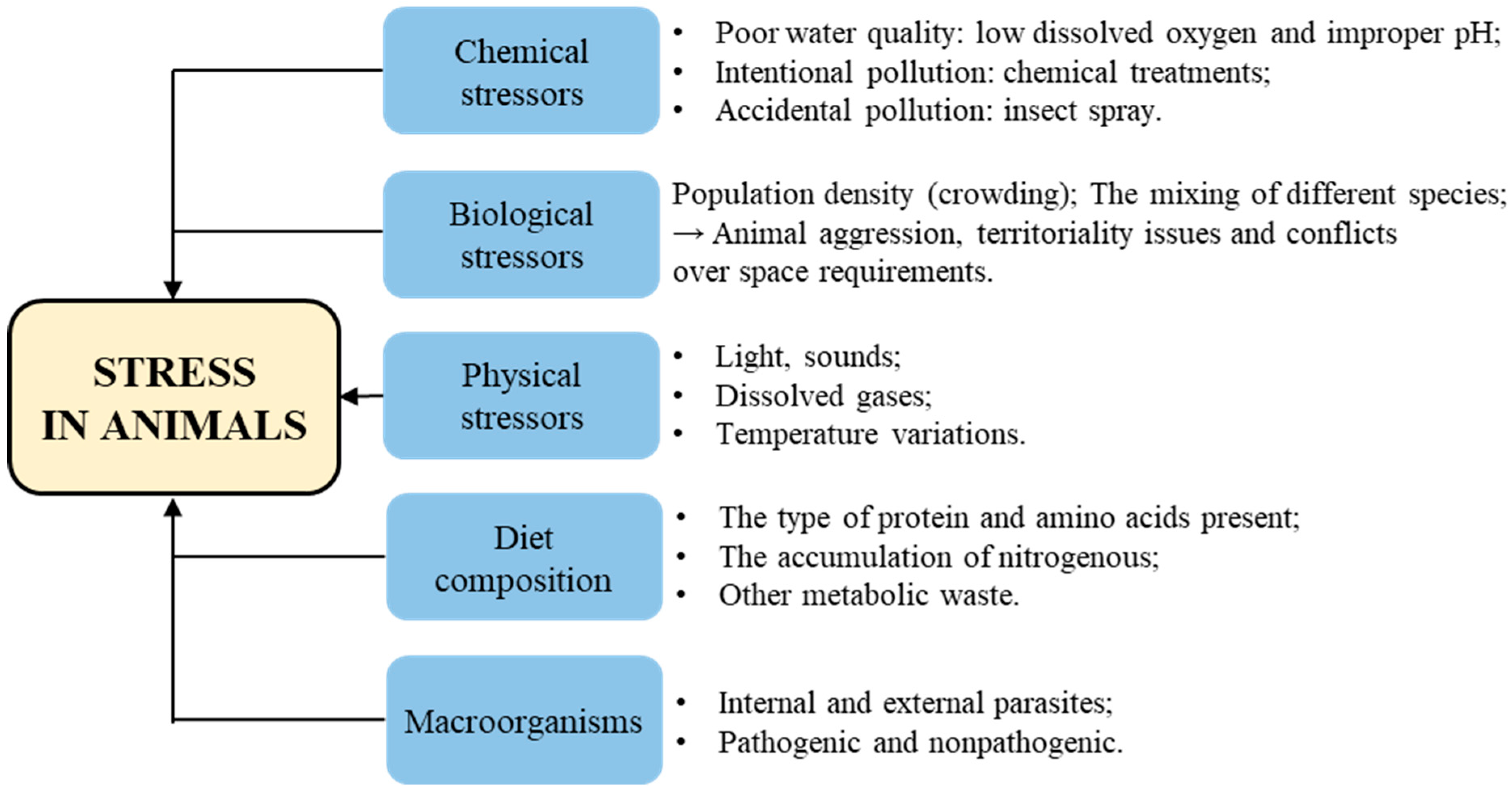
3. Stress in Animals
4. Curcumin in Stress Management of Terrestrial Animals
4.1. Oxidative Stress Management
4.2. Thermal Stress Management
| Animal Category | Experimental Design | Findings (Comparison to Negative Control) | Source |
|---|---|---|---|
| Heat stress | |||
| APRI-line growing/weaned rabbits, aged 5 weeks, weighed 627.11 ± 2.51 g | A total of 100 rabbits were divided into 5 groups: G1 (control), G2 (CUR 20 mg/kg diet), G3 (CUR 25 mg/kg diet), G4 (nanoCUR 2.5 mg/kg diet), and G5 (nanoCUR 5 mg/kg diet). During growing period (8 weeks), ambient temperature relative humidity and temperature–humidity index were 32.77 °C, 43.23%, and 29.54, in turns. | ↔carcass traits. ↔meat composition (moisture, crude protein). ↔Ph values of stomach, intestine, caecum. Caecum activity: ↑NH3-N, ↑VFAs. ↓harmful bacteria, ↓E. coli. Blood hematological parameters: ↑RBCs, ↓WBCs, ↓platelets, ↑HTC (G4, G5) ↔Hb, ↔erythrocytic indices (MCV, MCH, MCHC). | [53] |
| Mature rabbits, aged 6–7 months | A total of 70 male rabbits were divided into 7 groups: G1 (control_CD), G2 (CD + 30 mg/kg diet turmeric), G3 (CD + 60 mg/kg diet turmeric), G4 (CD + 90 mg/kg diet turmeric), G5 (CD + 50 mg/kg diet garlic), G6 (CD + 75 mg/kg diet garlic), and G7 (CD + 100 mg/kg diet garlic). Temperature: 30.45 °C ± 0.32 °C (max) and 26.24 °C ± 0.51 °C (min). Humidity: 75.35% ± 0.64% (max) and 52.10% ± 1.63% (min). The form of turmeric and garlic are in powder form. | ↔final bodyweight, ↔feed intake ↓respiration rate, ↓ear temperature Hematological parameters: ↑Hb, ↑RBCs, ↑WBCs, ↑Platelets, ↑PVC. Serum antioxidants status: ↑TAC, ↓MDA, ↓total CHO, ↓triglyceride. Libido and semen characteristics: ↑mass motility, ↓dead sperm, ↑normal sperm, ↑TFSF, ↑MPS, ↓tail abnormality, ↑initial semen fructose. ↑relative epididymal weight, ↓germ cell apoptotic/seminiferous tubule, ↓relative weight of abdominal fat/kg, ↔relative testicular weight, ↔testicular measurements, ↔hepato-somatic, ↔renal-somatic, ↔spleen-somatic | [54] |
| New Zealand white (NZW) virgin female rabbits | A total of 45 healthy rabbits were divided into 3 groups: G1 (control), G2 (250 mg ginger powder), and G3 (250 mg CUR). The experiment was carried out during summer in Egypt. The powder of ginger/curcumin was mixed with a commercial pelleted diet. | ↑CR, ↑kits born, ↑total kits at weaning, ↑litter size/individual (at birth and weaning), ↑average kit weight and litter weight/individual (at birth and weaning), ↑morality rate. ↑LBW, ↑FI, ↓water consumption. ↑albumin, globulin in blood. ↓urea, creatinine concentrations. ↓cortisol, ↑thyroid hormone (T3 and T4), ↑progesterone. ↓rectal, skin, ear temperature. | [55] |
| C57BL/6J mice, aged 6 weeks, weighed 18–20 g | A total of 48 mice were divided into 6 groups: G1 (no-heat treatment), G2 (HS), G3 (HS + ASA 1 mg/kg b.w.), G4 (HS + CUR 50 mg/kg b.w.), G5 (HS + CUR 100 mg/kg b.w.), G6 (HS + CUR 200 mg/kg b.w.). HS treatment: 41 °C for 20 min. | Indexed: ↓TMs, ↓BP, ↑HR. Serum biochemical parameters: ↓ALT, ↓AST, ↓LDH, ↔TP. Histological integrity: ↓myocardial fibers disorientation, ↓inflammatory cells. Biochemical markers: ↓cTn-I, ↓Ang II. | [56] |
| Sprague–Dawley (SD) rats, aged 65–70 days, weighed 190–220 g | A total of 50 rats were divided into 5 groups: G1 (NT control), G2 (DH control), G3 (CUR 50 mg/kg + DH), G4 (CUR 100 mg/kg + DH), and G5 (CUR 200 mg/kg + DH). CUR was dissolved in 0.5% CMCNa. DH: 41 ± 0.5 °C, 10 ± 1% humidity | ↓creatinine, ↓BUN, ↓KIM-1, ↓NGAL. ↓expression of apoptosis-related proteins (Cyt-c, JNK, caspase-9): G4, G5. | [57] |
| Wistar-strain albino rats, weighed 150–180 g | A total of 24 animals were divided into 4 groups: G1 (DW), G2 (HS + DW), G3 (HS + CUR 0.5 g/kg), and G4 (HS + CUR 2.0 g/kg). CUR: powder (CUR-500™, >95% pure). HS: 37 ± 0.5 °C, 4 h/day | During heat stress: restlessness. After heat stress: - Activity level: G3 (hypoactivity), G4 (hypoactive initially only). - Attitude: G3 (depressed), G4 (near normal). - Provoked behavior: G3 (minimal response), G4 (moderate response). | [58] |
| Damascus goat bucks, aged 12–14 months, weighed 30 ± 1.23 kg | A total of 14 goats were divided into 3 groups: G1 (n = 4, control), G2 (n = 5, PHM 10 gm/head/day DM), and G3 (n = 5, QT 5 gm/head/day DM) | Sperm characteristics: ↑sperm concentration, ↑mass motility score, ↑sperm motility, ↑live spermatozoa, ↑normal spermatozoa, ↔[acrosomal integrity, normal sperm, primary, secondary sperm abnormalities, semen volume]. Hormone and blood biochemical constituents: ↑TST, ↔[TP, ALB, GLU, TG, AST], ↓ALT, ↓CHO. Antioxidant activities: ↓GPx, ↓MDA, ↓TCA. Hematological parameters: ↔[WBC, Hb, PCV, MCHC, MCH, MCV], ↑RBCs. | [59] |
| Hu sheep, aged 4 months, weighed 25.82 ± 0.34 kg | A total of 140 male Hu sheep were housed at temperature (33.32 ± 0.33 °C) and humidity level (70.56% ± 1.26%) and divided into 3 groups: G1 (control), G2 (CUR 450 mg/sheep), and G3 (CUR 900 mg/sheep). | Serum parameters: ↑NEFA (G3), ↔[GLU, TG, LDL, HDL, TC]. Antioxidant enzymes: ↔SOD, ↑GPx Plasma concentration: ↑[IgA, IgM, IgG] ↑TW/BW (G3), ↑TST, ↔testicular hsd3b mRNA. Apoptosis-related genes: ↓caspase-3 (G3) | [60] |
| Buffalo mammary epithelial cells (BuMECs) | Cells were cultured and divided into 7 groups: G1 (control); G2 (HT, 42 °C for 1 h); and G3–7 cultured with 5, 10, 20, 40, and 60 μM CUR, in turns, and then exposed to hyperthermia (42 °C for 1 h). | ↓heat shock on the morphology Cell viability ↑[G3,G4], ↔[G5], ↓[G6,G7]. ↑antioxidant enzymes (SOD, CAT) [G3, G4]. Apoptosis-related genes: ↑BCL2 [G3, G4], ↓Bax, ↓caspase-3. Heat-shock protein: ↑HSP70 [G4], ↑HSP90 [G4, G5]. ↓Inflammatory-response-related genes (TNF-α, NF-κB) [G3, G4]. | [61] |
| Cold Stress | |||
| The female crossbred calves | A total of 24 female crossbred calves were divided into 4 groups: T1 (control), T2 (7.5 g garlic/head), T3 (7.5 g turmeric/head), and T4 (7.5 g garlic + 7.5 g turmeric/head). The experiment was carried out during winter season. | Growth performance, feed intake: highest value in T2. Nutrient utilization: ↑nutrient digestibility, ↓losses of nutrients. | [67] |
| Semen from five mature cattle bulls kept at a semen freezing center | Bull semen was divided into 4 groups: CON (control), TT1, TT2, and TT3 (turmeric extract 100, 200, and 300 µL/5 mL TCFY). Extend semen was cooled slowly to 5 °C and equilibrated for 2 h. | Cattle bull semen quality post-cooling: ↑motility, ↑alive, ↓abnormalities (TT1, TT2), ↑sperm membrane integrity, ↑acrosome (TT1, TT2). The post-thawed extended cattle bull semen: ↑motility, ↑alive, ↓abnormalities (TT2, TT3), ↑sperm membrane integrity, ↑acrosome (TT1, TT2). ↑in vivo fertility rate. | [65] |
| Healthy rabbit bucks, aged 10–12 months, weighed 3.6 ± 0.2 kg | Sperm cryopreservation of bucks was divided into 7 groups: control, CU0.5, CU1.0, and CU1.5 (0.5, 1.0, and 1.5 µg/mL CUR, respectively); CUNPs0.5, CUNPs1.0, and CUNPs1.5 (0.5, 1.0, and 1.5 µg/mL CUNPs, respectively). | Sperm characteristics (%): ↑progressive motility, ↑membrane integrity percentages, ↑viability (CU1.5, CUNPs), ↓abnormality. Sperm apoptosis (%): ↑viable, ↓early apoptosis, ↓late apoptosis, ↓necrosis. Antioxidants indices: ↑TAC, ↑SOD, ↑GPx, ↓MDA, ↓POC. Improve sperm ultrastructure. | [70] |
| Healthy Hariana bulls (Bos indicus), aged 7–8 years, weighed 450–550 kg | The diluted semen samples were divided into five aliquots: G1 (control), G2, G3, G4, and G5 (10, 25, 50, and 75 µM CUR, respectively). The temperature of semen straws reached from 4 °C to −140 °C within 7 min. | Functional sperm attributes: ↑the population (G4, G5), improve intact acrosome, intact membrane (G2, G3). Motility and kinematic: ↓the motile spermatozoa population (G4, G5), ↑total motility, progressive motility, and fast motility (G2, G3). Apoptotic- like changes: ↓DNA fragmentation, ↓deprotamination (G2). ↓carbonylated protein (G2). ↑Vanguard distance (G2, G3, G4). | [68] |
| Three sexually mature Baladi bucks, aged 2–4 years, weighed 50–60 kg | Semen samples were divided into 7 groups: G1 (control), G2 (MENFs 50 μg), G3 (MENFs 100 μg), G4 (TENFs 50 μg), G5 (TENFs 100 μg), G6 (CENFs 50 μg), and G7 (CENFs 100 μg). The diluted semen was cooled to 5 °C for 2 h. | Sperm quality in equilibrated semen: ↑[progressive motility, vitality, plasma membrane integrity] (CENFs). ↑post-thawing sperm quality. Sperm apoptosis and necrosis post-thawing: ↑viable spermatozoa, ↓apoptotic, ↓necrotic. Enzyme activity: ↔AST, ↔ALT. Extender post-thawing on oxidative stress: ↑TAC, ↓MDA. | [69] |
| Mature buffalo bulls | Semen from five bulls was divided into 5 groups: control, TTE1, TTE2, TTE3, and TTE4 (turmeric extract: 100 μL/5 mL, 200 μL/5 mL, 300 μL/5 mL, and 400 μL/5 mL TCFY, respectively). Extended semen was subjected to semen-freezing protocol. | Post-cooling: Sperm motility, alive sperms were significantly higher in TTE1. Sperm abnormalities lower in TTE1. Sperm membrane integrity was higher in TTE1. Acrosome percent was higher in TTE1, TTE2, TTE4. Post-thawing: Sperm motility was higher in TTE1. ↑Sperm membrane integrity (HOST). The conception rate was the best in TTE1. | [71] |
4.3. Nitrosative Stress Management
5. Curcumin in Stress Management of Avian
5.1. Oxidative Stress Management in Birds
| Animal Category | Experimental Design | Findings (Comparison to Negative Control) | Source |
|---|---|---|---|
| commercial Arbor Acres (AA) broilers, aged one day old | A total of 32 broilers were divided into 4 groups: G1 (control group), G2 (1 mg/kg AFB1), G3 (1 mg/kg AFB1 + 300 mg/kg CUR), and G4 (300 mg/kg CUR). | Improving pathological live lesions. ↓[ALT, AST, AKP, γ-GT]. ↓[MDA, ROS]. ↑[GSH, CAT, SOD]. Normal cellular structure. | [76] |
| Ducks, aged one day old | A total of 60 ducks were divided into 3 groups: G1 (control), G2 (0.1 mg/kg AFB1), and G3 (0.1 mg/kg AFB1 + 400 mg/kg CUR). | ↑SOD-1, ↑TRX, ↑HO-1, ↑Nrf2, ↓MDA. ↓P62, ↓LC3B, ↑mTOR, ↑ATG5, ↑LAMP1. ↓Gal3 protein, ↑CTSB. | [77] |
| Broiler chickens (Ross 308), aged 18 days, weighed 751.88 ± 46.28 g | A total of 32 male chickens were divided into 4 groups: G1 (control: BD), G2 (BD + 0.02 mg/kg feed AFB1), G3 (BD + 400 mg/kg feed CUR), and G4 (BD + 0.02 mg/kg feed AFB1 + 400 mg/kg feed CUR). | Enzyme activities: ↑SOD, ↑CAT, ↑GPx. ↑SAC, ↓MDA. Oxidative DNA Damage: ↓8-OHdG mRNA and protein expression: ↓mRNA. NOX4, ↓NOX4 (protein abundance). | [79] |
| Arbor Acres (AA) broilers, aged one day old | A total of 64 broilers were divided into 4 groups: G1 (control: basal diet), G2 (AFB1 5 mg/kg diet), G3 (AFB1 5 mg/kg diet + CUR 300 mg/kg diet), and G4 (CUR 300 mg/kg diet). | Serum enzyme activity: ↓ALT, ↓AST, ↓AKP, ↓GGT. Antioxidant enzymes activity: ↓MDA, ↑SOD, ↑CAT, ↑GSH. Oxidative stress marker (in serum and liver): ↓ROS, ↓8-OHdG. Histopathological observation: hepatic cords and cell structure recovery. The relative mRNA and protein expression: ↑Nrf2, ↑HO-1. | [78] |
| Ducks, aged one day old | A total of 60 ducks were divided into 3 groups: control, AFB1 (AFB1 0.1 mg/kg b.w.), CUR + AFB1 (AFB1 0.1 mg/kg b.w.+ CUR 400 mg/kg feed). | Spleen was smooth and uniform in color. Improve the damage to the spleen and the index of the spleen. Serum immunoglobulin content: ↓lgA, ↑IgG, ↑IgM. Histopathological alterations: ↑the number of ellipsoid lymphatic vessels and sheath-like capillaries, ↓the arterial wall thickening, ↑the count of lymphocytes, neutrophils. Inflammation-related genes: ↑the mRNA expression levels of NF-κB, IκB, TNF-α, IFN-γ, COX2, IL-1β, IL-2, IL-6, IL-18. ↓IL-4 mRNA expression levels. ↓the protein expression levels of p–NF–κB/NF-κB, p-IκB/IκB. ↓TNF-α. ↓p–NF–κB. ↓p-IκB. Nrf2 signaling pathway: ↑[Nrf2, HO-1, SOD-1, GPX2]mRNA expression, ↓keap1 (were returned to the same level as the control group). | [80] |
| Arbor Acres broilers, aged one day old | A total of 120 broilers were divided into 6 groups: C (control group), CC (CUR 450 mg/kg feed), L (AFB1 5 mg/kg + CUR 150 mg/kg feed), M (AFB1 5 mg/kg + CUR 300 mg/kg feed), H (AFB1 5 mg/kg + CUR 400 mg/kg feed), and AFB1 group (AFB1 5 mg/kg feed). | ↓drowsiness, lethargy, and ruffled-feathers symptoms. Duodenum: ↓crypt depth, ↑villo height, ↑V/C. ↓SOD, ↓CAT, ↓8-OHdG ↑ATPase activities. ↓[CYP3A4, CYP2A6, CYP1A2, CYP1A1] ↑the expression of Abcb1 mRNA, P-gp. | [81] |
| Cobb-500 strain chicks, aged one day old | A total of 50 male chicks were divided into 5 groups: CP (positive control), CU (600 mg/kg fumonisin + 50 mg/kg CUR), NC5 (600 mg/kg fumonisin + 5 mg/kg nanoCUR), NC10 (600 mg/kg fumonisin + 10 mg/kg nanoCUR), and NC (negative control). | ↑bodyweight (CU, NC10) Serum biochemistry: ↓glucose, ↓triglycerides (NC10), ↑cholesterol, ↓uric acid, ↓ALT (NC10), ↓AST. Oxidant and antioxidants profile: ↓TBARS, ↓ROS (NC10), ↑SOD, ↑CAT, ↓GST Necropsy and histopathology findings: liver (slightly yellow color), liver and intestines (no histopathological lesions). | [82] |
| White Pekin ducks, aged 1 day old, weighed 43.4 ± 0.1 g | A total of 720 mixed-sex ducks were divided into 4 groups: CON (control group), OTA (2 mg/kg OTA), CUR (400 mg/kg CUR), and OTA + CUR (2 mg/kg OTA + 400 mg/kg CUR). | Serum liver function: ↓AST, ↔[AST, TC, TG, HDL]. Antioxidative capacity: ↔T-AOC, ↑SOD, ↑CAT, ↔GSH-Px, ↓MDA. ↑ACE, ↑Simpson indexes. Recovered the microbiota composition. mRNA expressions: ↓FAS, ↑Nrf2, ↑HMOX1 | [83] |
| White Pekin ducks, aged 1 day old, 43.4 ± 0.1 g | A total of 540 mixed-sex ducks were divided into 3 groups: G1 (control), G2 (2 mg/kg OTA), and G3 (2 mg/kg OTA + 400 mg/kg CUR). | ↑growth performance Antioxidant parameters and jejunal cytokines: ↑GSH-Px, ↑SOD, ↑T-AOC, ↓IL-1β, ↑IL-10, ↓TNF-α, ↓DAO ↑villus height, ↓crypth depth The expression of genes related to apoptosis: ↑Bcl-2, ↓CASP3 Mitochondrial transcription factor: ↓TFAM, ↓TFB1M, ↓TFB2M. | [84] |
| Ducks, aged 1 day old | A total of 75 ducks were divided into 5 groups: CON (control group), LA (low-dose ATO group: 2 mg/kg ATO), MA (medium-dose ATO group: 4 mg/kg ATO), HA (high-dose ATO group: 8 mg/kg ATO), AC (8 mg/kg ATO + 400 mg/kg CUR feed). | ↑bodyweight. ↓muscle arsenic concentration ↑T-AOC, ↓SOD, ↓MDA Improve mitochondrial structure. mRNA expression levels: ↑OPA1, ↑Mfn, ↓Drp1, ↑Nrf1, ↑Nrf2, ↑TFAM. Mitophagy: ↓PINK1, ↓Parkin, ↓LC3-I, ↓LC3-II, ↓p62 Mitochondria-mediated apoptosis: ↓p53, ↓Bax, ↑Bcl-2, ↓Cytc, ↓caspase-3. | [85] |
| Sansui white ducks, aged 1 day, weighed 50–100 g | A total of 32 ducks were 4 groups: G1 (control—deionized water), G2 (4 mg/kg ATO), G3 (8 mg/kg ATO), and G4 (8 mg/kg ATO + 400 mg/kg CUR). | ↑Bodyweight (G4 ducks grew faster). ↓ATO levels in serum and kidney. ↓damage in kidney tissues. ↓Relative mRNA levels (Nrf2, GPX-1, CAT, SOD-1, HO-1). Protein expression levels: ↑Nrf2, ↓Trx, ↑SOD-1, ↓HO-1, ↑T-AOC, MDA. ↓autophagy-related mRNA and protein levels (mTOR, LC3-I, LC3-II, Atg-5, Beclin1, Pink, Parkin)↓apoptosis-related mRNA and protein expression levels (caspase-3, Cytc, p53, Bax). | [86] |
| Specific-pathogen-free Anas platyrhynchos ducks, aged 1 day old, weighed 34.00 ± 0.50 g | A total of 450 male ducks were divided into 3 groups: CON (control: basal diet), LPS (basal diet + LPS 5 mg/kg b.w.), and LPS + CUR (basal diet + LPS 5 mg/kg b.w. + CUR 500 mg/kg b.w.). CUR: powder form. | Repairing the inflammatory manifestation of ling tissues. Antioxidant capacity of the plasma: ↑GSH-Px, ↓MDA, ↑T-SOD. Expression of genes (Nrf2-ARE signaling pathway): ↑Nfr2, ↓Keap1, ↑CAT, ↑HO-1, ↑SOD-1, ↑GCLC, ↑GCLM, ↑NQO-1. Expression of genes (NF-κB signaling pathway): ↓[TLR4, NF-Κb, TNF-α, IL-6, IL-8, NLRP3, caspase-1. | [87] |
| Specific-pathogen-free (SPF) ducks (Anas platyrhynchos), aged 1 day old, weighed 35 ± 1 g | A total of 40 male ducks divided into 4 groups: C0 (corn–soybean basal diet), C0 + LPS (corn–soybean basal diet + 0.5 mg/kg b.w. LPS), C500 (0.5 g/kg b.w. CUR), and C500 + LPS (0.5 g/kg CUR + 0.5 mg/kg b.w. LPS). | Ileum morphology: ↓villus height, crypt depth (highest: C0 + LPS, lowest: C500 + LPS), ↑villus height/crypt depth. mRNA expression of antioxidant genes: ↑Nrf2, ↓Keap1, ↓SOD1 (C0 + LPS: highest), ↔CAT, HO-1 (C500 highest), ↔NQO-1, ↑GCLM, ↑GCLM. mRNA expression of inflammatory-related gene: ↑TLR4, ↑NF-κB, ↑TXNIP, ↑IL-1β, ↑IL-6, ↑TNF-α. Protein expression: ↑Nrf2, ↓HO-1, ↑TXNIP. | [88] |
| Cobb-500 breed broiler chicks, aged 12 days old | A total of 360 male chicks were divided into 6 groups: NCC, NCC + 100 mg/kg CUR, NCC + 200 mg/kg CUR, CC, CC + 100 mg/kg CUR, and CC + 200 mg/kg CUR. | ↔growth parameters. Lesion score: ↔duodenal, ↔jejunum and ileum, ↓cecum. Intestinal permeability: ↓(CC + 100 mg/kg CUR), ↑(CC + 200 mg/kg CUR). Oocyst Shedding: ↓Count of E. maxima. Glutathione: [↑GSH, ↑GSSG, ↑total glutathione] CC + 100 mg/kg CUR. | [89] |
| Cobb-500 chicks, aged 2 weeks | A total of 200 birds were divided into 4 groups: G1 (MSD, negative control), G2 (HSD, positive control), G3 (HSD, CUR 100 mg/kg diet), and G4 (HSD, CUR 200 mg/kg diet). MSD (10 birds/m2) and HSD (20 birds/m2). | Productive performance: ↑bodyweight, ↑food intake, ↑feed conversion ratio. Behavioral observation: enhancement [ingestive behavior, crouching, body care behavior], ↓[walking, standing behavior]. Hematological parameters: ↑PVC, ↑Hb, ↑RBCs, ↔WBCs, ↓ERS, ↓H/L ratio. Immunological parameters: ↑IgG, ↑IgA, ↑IgM, ↓IL-2, ↓IL-6, ↓TNF-a. Hormonal analysis: ↓ALT, ↓AST, ↓total cholesterol. Antioxidant measurements: ↑[SOD, GPx, CAT], ↓MDA. Hormonal concentrations: ↑T3, ↑T4, ↓corticosterone. Gene expression: ↑[GHR, IGF-1] | [91] |
5.2. Thermal Stress Management in Birds
| Animal Category | Experimental Design | Findings (Comparison to Negative Control) | Source |
|---|---|---|---|
| Heat Stress | |||
| Chicks (Ross strain), aged 120 days old | A total of 30 male chicks were divided into 3 groups: T1 (control), T2 (34 °C 8:00–16:00, basal diet), and T3 (34 °C 8:00–16:00, basal diet + CUR 100 mg/kg diet). | Improved the average daily feed intake. ↑Dressing percentage, ↑breast yield, ↓abdominal fat, ↔[leg, liver, heart]. Fatty Acid profile: ↑MUFAs (myristoleic, palmitoleic, oleic), ↑PUFAs (linoleic, docosahexaenoic, eicosapentaenoic), ↓saturated FAs in breast (myristic and palmitic) and thigh (palmitic and stearic) muscles. ↓MDA, ↑ATP, ↓ADP, ↑CoQ10, ↓Na, K-ATPase, ↑5HT, ↓5-HIAA. | [94] |
| Broiler chickens | A total of 100 chickens (maintained in heat stress) were divided into 3 groups: control, ascorbic acid group (dose: 60 mg/tail/day), and turmeric group (dose: 500 mg/kg bodyweight). | ↔Broiler performance: bodyweight gain, feed conversion, feed efficiency. Quality of carcass: ↑carcass percentage, ↑percent of thigh meat, ↑percentage of breast meat, ↑liver weight, ↑gizzard weight. ↓cholesterol. | [96] |
| Ross-308 chicks, aged 50 days old | A total 700 chicks were placed in two halls: normal condition (N) and heat-stressed (S) condition (37 °C). Under each condition, chicks were divided into 5 groups: T1 (basal diet), T2 (CUR 50 g/ton feed), T3 (CUR 75 g/ton feed), T4 (turmeric powder 1.65 kg/ton feed), and T5 (turmeric powder 2.5 kg/ton feed). | Serum lipid profile: ↓cholesterol, ↑HDL, ↓LDL, ↓VLDL, ↓triglyceride. Serum protein profile: ↑albumen, ↑globulin, ↑total protein. Thyroid hormones: ↑T3, ↑T4. ↓ALT, AST enzymes. ↓serum creatin kinase, uric acid, glucose. | [95] |
| Cobb-500 broiler chicks, aged 31 days old | A total of 300 mixed-sex chicks were divided into 5 groups: A (control), B (0.5% turmeric powder), C (0.5% cinnamon powder), D (0.5% ginger powder), and E (0.5% garlic powder). During days 31–42 of the rearing period, the chicks were exposed to environmental temperature (32–34 °C) daily, from 12 a.m. to 16 p.m., to induce heat stress. | ↓bodyweight, ↑average daily gain, ↑average daily feed intake, ↓feed conversion ratio, ↑rectal temperature mean (42-day-old chickens), ↓bursa of Fabricius weight (42-day-old chickens), ↑chickens spleen weight (42-day-old chickens), ↑SOD, ↑GPx, ↓CAT, ↓MDA, ↑total antioxidant capacity, ↑ALP, ↑CE, ↑T3, ↑T4. | [99] |
| Ross chicks, aged one day old | A total of 200 male chicks were divided into 4 groups: G1 (control), G2 (0.5% turmeric), G3 (0.5% cinnamon), and G4 (0.25% cinnamon + 0.25% turmeric). All birds were treated with heat stress (32 °C). | Performance: ↑feed intake, ↑feed intake, ↓feed conversion ratio. Blood, enzyme, and antioxidant parameters: ↓AST, ↓ALT, ↓LDH, ↓uric acid, ↔urea, ↑creatinine, ↓MDA. ↓chlorine, ↔sodium, ↑potassium, ↑hematocrit, ↔rectal temperature. | [98] |
| Ross-308 chicks, aged one day old | A total of 625 mixed-sex chicks were divided into 5 groups: TN-CON (thermoneutral), HS-CON (heat stress-control), HS-Bet (0.1% betaine), HS-TRP (0.2% turmeric rhizome powder), and HS-BT (0.1% betaine + 0.2% turmeric rhizome powder). | ↑bodyweight gain, ↑feed intake, ↓feed-to- gain ratio. Blood leukocyte profile: ↓monocytes, ↓eosinophil, ↓basophils, ↓heterophil, ↑lymphocyte. Antibody titers against SRBC: ↑total antibody, ↑IgM, ↔IgG (28 days of age), ↑IgG (42 days of age). ↔TAC, ↓MDA, ↑GPx, ↑SOD. | [97] |
| Ross-308 chicks, one day old | A total 360 broiler chicks were divided into 6 groups: T0 (control), T1 (3 mg/kg diet of turmeric), T2 (5 mg/kg diet of turmeric), T3 (3 mg/kg diet of carnation flower powder), T4 (5 mg/kg diet of carnation flower powder), and T5 (4 mg/kg mix of turmeric and carnation flower). | ↑WBC, ↓heterophil %, ↑lymphocytes %. The biochemical characteristics: ↓glucose, ↑total protein, ↔albums, ↑globulin, ↓uric acid. The number of bacteria: ↓E. coli, ↓Salmonella, ↑Lactobacillus. | [100] |
| Chick broilers (Marshal), aged one day old | A total 240 chicks were divided into 4 groups: CN (corn-soy based diet), FG (basal diet + 4 g/kg C. longa powder), EG (basal diet + 8 g/kg C. longa powder), and TT (basal diet + 12 g/kg C. longa powder). | The juvenile growth performance: ↑initial weight, ↑final weight, ↑weight gain, ↔feed intake. The villus height: ↑duodenum, ↑jejunum, ↑ileum. The villus width: ↑duodenum, ↑jejunum, ↑ileum. The crypt depth: ↑duodenum, ↑jejunum, ↑ileum. ↓The respiratory rate, ↓breast temperature, ↓comb temperature, ↔heart rate. The hematological parameters: ↔(PCV, hemoglobin, red blood cells, white blood cells, lymphocyte across). Physiological responses: ↓MDA, ↓rectal temperature, ↑T3, ↑uric acid. | [11] |
| Roman egg-laying hens, aged 22 weeks old, weighed 1420 g (start of experiment), aged 31 weeks old, weighed 1940 g (terminated) | A total of 336 hens were divided into 7 groups: TC (thermo-neutral control), HC (heat control), H1 (HC + 100 mg/kg CUR), H2 (HC + 150 mg/kg CUR), H3 (HC + 200 mg/kg CUR), H4 (HC + 250 mg/kg CUR), and H5 (HC + 300 mg/kg CUR). | Serum antioxidant metabolites: ↑SOD, ↑CAT, ↑T-AOC, ↑GSH-Px, ↓MDA. Antioxidant metabolites in liver tissue: ↑SOD, ↓CAT (H5, 6 weeks), ↓T-AOC (H5, 9 weeks), ↓GSH-Px (6 weeks), ↑MDA (H4, 6 weeks; H5, 9 weeks). Antioxidant metabolites in heart tissues: ↑SOD, ↓CAT (H1, 3 weeks), ↑T-AOC, ↓GSH-Px (H2, H5, 6 weeks), ↑MDA (H2, 6 weeks; H3, H4, H5, 9 weeks). Antioxidant metabolites in lung tissues: ↑SOD, ↓CAT (H1, 3 weeks), ↓T-AOC (H1, H4, H5, 3 weeks), ↓GSH-Px (6 weeks), ↓MDA (H3, H4, H5, 3 weeks; 6 weeks; H2, H3, H4, 9 weeks) | [101] |
| Roman egg laying hens, aged 25 weeks old | A total 250 hens were divided into 5 groups: NC (normal temperature control 22–25 °C), HC (high temperature 32 ± 1 °C), HT100 (HC + 100 mg/kg CUR), HT200 (HC + 200 mg/kg CUR), and HT300 (HC + 300 mg/kg CUR). | Corticosterone serum level: ↓(HT100, HT200), ↔HT300. WBC parameters: ↑HT200, ↔(HT100, HT300). Heterophil/lymphocyte (H/L) ratio: ↓(HT100, HT200), ↔HT300. Serum IgG and IgM: ↑(HT100, HT200), ↔HT300. Serum cytokines: ↓(IL-6, IL-1β, TNF-α) HT100, HT200.Liver enzymatic activity: ↓ALT (HT100, HT200). | [102] |
| Chicken embryonic fibroblast cells (CEFs) | A CEF cell line was divided into 6 groups: NC (normal temperature group) H (high-temperature control group), H1(5 μmol/L CUR), H2 (10 μmol/L CUR), H3 (20 μmol/L CUR), and H4 (40 μmol/L CUR). | Cell viability: ↑(H2, H3, H4) after 12 h; ↑H3_after 24 h. ↑cell proliferation. ↓cell apoptosis rate (H3, H4). ↓ROS (H3, H4), ↓MDA. Antioxidant enzyme activity: ↑CAT, ↑SOD, ↑GSH-Px. ↑genes expression (CAT, SOD1, SOD2, GSTO1, GSTT1, GSTA3). ↑MAPK- Nrf2 pathway genes (Nrf2, Jnk, Erk, P38). | [103] |
| Cold Stress | |||
| Ross-308 broiler chicks, aged one day old | A total of 250 male chicks were divided into 5 groups: I (control), II (200 mg/kg CUR), III (400 mg/g CUR), IV (200 mg/g nanocurcumin), and V (400 mg/g nanocurcumin). First week: 32 °C. Second week: 29 °C. Thereafter, the temperature gradually dropped to 15 °C on day 14. | Performance: ↔feed intake, ↑feed conversion ratio (nanocurcumin). ↑weight gain (III, IV). Liver enzyme activities: ↓MDA, ↓LDH, ↓AST. Blood cholesterol: ↓total cholesterol, ↑HDL, ↓LDL, ↓triglycerides. Immuno-function: ↑WBC, ↓heterophils, ↑lymphocytes, ↓heterophils/lymphocytes ratio. | [104] |
| Japanese quails (Coturnix japonica), aged thirty days old | A total of 60 quails were divided into 4 groups: T0 (control), T30 (30 mg/kg diet free CUR), T3 (3 mg/kg diet nanocapsules CUR), and T10 (10 mg/kg diet nanocapsules CUR). The experiment was carried out in a shed, without air-conditioning, during the winter (1 °C–17 °C). | Performance: ↑egg production, ↑egg weight (T30), ↑egg mass, ↔feed intake, ↓feed conversion (g/g), ↓feed conversion (g/dozen_T10). Egg chemical composition: ↔(specific gravity, Haugh unity, yolk index, yolk pH, albumen pH, yolk percentage, eggshell percentage, albumen percentage); ↑(luminosity, yellow intensity). Oxidant/antioxidant status: ↓TBARS, ↑ACAP Fatty acid profile in egg: ↓SFA (T10), ↑MUFA, ↑PUFA (T10) | [105] |
| Hy-Line Brown laying hens, aged 84 weeks old, weighed 1680 ± 10 g | A total of 36 hens were naturally infect with E. coli and divided into 2 groups: T-CON (control) and T-CUR (CUR 200 mg/kg). The experiment was carried out in a shed, without air-conditioning, during the winter (−2.5 °C–19.7 °C). | Egg quality: ↔(Haugh units, albumen pH, yolk weight, egg shell strength, red intensity, shell thickness) in days 21 and 42. ↑(yellow and brightness in fresh eggs). ↓TBARS, ↑ACAP. Antioxidant status: ↓LPO, ↑GPx, ↑GST. ↔(red cell number, hematocrits, hemoglobin concentrations, eosinophil, monocyte). ↓(total protein, alkaline phosphatase, alanine aminotransferase), ↔(globulin, albumin levels). Fecal microbiology: ↓bacterial counts (coliforms and E. coli). | [106] |
6. Curcumin in Management of Aquatic Animals
6.1. Oxidative Stress Management in Aquatic Animals
| Animal Category | Experimental Design | Findings (Comparison to Negative Control) | Source |
|---|---|---|---|
| Spotted seabass (Lateolabrax maculatus) juveniles | A total of 180 fish were divided into 3 groups: Con, TAE2 (2 g/kg diet turmeric aqueous extract), and TAE4 (4 g/kg diet turmeric aqueous extract). H2O2 (600 mM) induced oxidative stress. | Growth performance: ↑weight gain, ↔(survival, feeding rate, feed efficiency, condition factor). Hepatic antioxidant enzymes: ↑T-AOC, ↑SOD, ↑CAT, ↑GPx, ↓MDA. Serum biomarkers: ↓GPT, ↓GOT, ↓LDH. ↑the expression of Nrf2, ho-1, gcl (TAE2, TAE4). ↔keap1 expression. ↑survival (TAE4). ↑the expression of Nrf2, ho-1, gclc. | [109] |
| Adult zebrafish | Embryos of zebrafish were obtained by natural mating and spawning. After 7–9 h post-fertilization, embryos were transferred to a 12-well plate and divided into 4 groups (15 embryos/group): G1 (control), G2 (H2O2 5 mM), G3 (H2O2 5 mM + TLE 100 µg/mL), and G4 (H2O2 5 mM + TLE 200 µg/mL). | ↓the death cell ratio. ↓ROS. ↓lipid peroxidation. | [110] |
| Common carp (Cyprinus carpio) juveniles, weighed 42.3 ± 3.68 g | A total of 540 juveniles were divided into 4 groups: 0TCu (0 g turmeric/kg diet), 0TCu (0 g turmeric/kg diet) 5TCu (5 g turmeric/kg diet), 10TCu (10 g turmeric/kg diet), and 20TCu (20 g turmeric/kg diet). Experiment 1: The fish were exposed to 3.5 mg/L of ambient copper for 24 h. Experiment 2: The fish were exposed to 0.25 mg/L of ambient copper for 3 weeks. | Experiment 1: ↓mortality rate. Experiment 2: ↓cortisol, ↓glucose, ↑T4, ↑T3. ↑Lysozyme, ↑ACH50, ↑bactericidal activity. ↑SOD, ↑CAT, ↑GPx, ↓MDA. ↓TNF-α, ↓IL1-b, ↑IL-10. ↓AST, ↓ALT, ↑red blood cells, ↑hemoglobin, ↑hematocrit. | [111] |
| Healthy Oreochromis niloticus, weighed 36.05 ± 0.31 g | A total of 180 fish were divided into 4 groups: G1 (control, a basal diet), G2 (CUR 200 mg/kg diet), G3 (a basal diet containing 1% MEL), and G4 (CUR 200 mg/kg + MEL 1% diet). | Mortalities and gross changes: normal skin coloration. Growth and whole-body composition: ↑FBW, ↑WG. Hematological variables: ↑WBCs, ↑heterophils, ↑lymphocytes, ↑eosinophils, ↑monocytes. Minimized the reductions in lysozyme activity, NO, C3, IgM. Minimized the reduction of in total protein, globulin, α globulin-1, α globulin-2, γ globulin. Oxidative stress indices: ↑GPx, ↑SOD, ↑MDA. Immune-related genes: ↓TNF-α, ↓IL-1β. | [112] |
| Rainbow trout (Oncorhynchus mykiss W., 1792) | A total of 120 fish were divided into 6 groups: CON (control), CPF (0.04 mg/L CPF), CUR1 (0.5% CUR), CUR2 (1% CUR), CPF + CUR1 (0.04 mg/L CPF + 0.5% CUR), and CPF + CUR2 (0.04 mg/L CPF + 1% CUR). | Blood serum: ↓TOS, ↓OSI, ↑TAC (CFP + CUR2). Liver tissue: ↓TOS, ↓OSI, ↑TAC. Gill tissue: ↓TOS, ↓OSI (CFP + CUR), ↔TAC. | [113] |
| Nile tilapia fish (Oreochromis niloticus), weighed 2.55 ± 0.003 g | A total of 300 fish were divided into 5 groups: control, CUR50, CUR100, CUR150, CUR200 (0, 50, 100, 150, or 200 mg CUR/kg diet, respectively). Injection of both Aeromonas hydrophila and Aeromonas sobria. | ↑antibacterial activity CUR50: The best value of FW, DWG, SGR, FI. Body composition: ↑crude lipid%, ↑crude protein% (max: CUR50). Oxidative status: ↑CAT (max: CUR50), ↑GSH (Max: CUR50). ↓MDA (min: CUR50), ↑Lysozyme activity (max: CUR50). ↑immunoglobin levels (max: CUR50): IgM, IgG. Intestinal microbiota: ↓coliforms, ↓E. coli, ↓Aeromonas spp. ↑survival rate (max: CUR50). | [114] |
| Gilthead seabream larvae of 4 days after hatching | Larvae were distributed in 9 cylindro-conical tanks (100 L) and divided into 3 microdiet groups: CLRL (control), LOW (CUR: 1.5 g/kg feed), and HIGH (CUR: 3.0 g/kg feed). | ↔growth performance. Feeding Incidence: ↔LOW, ↑HIGH. Digestive enzymes: ↑trypsin, ↑chymotrypsin, ↔aminopeptidase, ↔4C-like lipase, 18C-like lipase (↓at 24 DAH, ↔at 31 DAH), alkaline phosphatase (↓at 24 DAH, ↔at 31 DAH), ↔amylase. Antioxidant status: ↑GSH, ↑TAC (24 and 31 DAH). ↓PC (10 and 31 DAH), ↓mtROS (24 and 31 DAH). | [116] |
| Gilthead seabream postlarvae | Postlarvae were kept in 100 L tanks, at an initial density of 2200 individuals (22 postlarvae/L). Treatment diets were divided into 3 groups: CTRL (control), LOW (curcumin: 0.8 g/kg feed), and HIGH (curcumin: 1.5 g/kg feed). | ↔Growth performance. Oxidative status: ↑TAC, ↓PC, ↔the expression of antioxidant defenses (SOD1, CAT, GPx1, GPx3), ↔hsp90aa, ↑GR. Gut morphometry and function: ↔the expression of pept1 and ialp genes. | [117] |
6.2. Ammonia Stress Management in Aquatic Animals
6.3. Thermal Stress Management in Aquatic Animals
| Animal Category | Experimental Design | Findings (Comparison to Negative Control) | Source |
|---|---|---|---|
| Heat Stress | |||
| Nile tilapia (Oreochromis niloticus), weighed 13.54 ± 0.32 g | A total 168 fish were divided into 7 groups: CON, CN50, CN100, and CN200 (nanocurcumin: 50, 100, and 200 mg/kg diet, respectively); C50, C100, and C200 (curcumin: 50, 100, and 200 mg/kg diet). Raising the water temperature from 25 to 40 °C within 3 h, and then 40 °C for 4 h. | Enhancing the growth performance (CN100, CN200). Liver enzymes activities: ↓ALT, ↓AST (CN50, CN100). ↑IgM, ↑C3 (except C50), ↑C4. ↓Cortisol (CN50, CN100). Nanocurcumin is more effective than its free form. | [123] |
| Puntius sophore, a minor carp of the family Cyprinidae | Fish were divided into 4 groups (40 fish/group): A (basal diet), B, C, and D (0.5, 1, and 1.5% curcumin-supplemented feed, respectively → highest CT max value: D. → A and D: heat shocked. → Gene expression analysis in 3 groups: BD (basal diet), BD + HS (basal diet + heat shocked), and 1.5%CUR + HS (1.5% curcumin supplemented + heat shocked). | Gene expression in liver tissues: ↑Nrf2, keap-1: very low, ↑hsp70, ↑hsp110, ↑[hsp27, hsp60] insignificant, hsp90: very low. ↑SOD, ↑CAT, ↔GPx. Gene expression in gill tissues: ↑Nrf2, ↔keap-1, ↑[hsp60, hsp70, hsp90, hsp110], ↑SOD, ↑CAT, ↑GPx. Network analysis: direct binding and interaction between all the hsp, CAT has nonspecific interaction with all of the hsp, nrf-2, and keap-1. GPx has no direct interaction with any genes. | [122] |
| Cold Stress | |||
| The Nile tilapia juveniles, weighed 4.39 ± 0.08 g/fish | A total of 225 fish were divided into 5 groups: T1 (control), T2 (50 ppm nanocurcumin), T3 (100 ppm nanocurcumin), T4 (150 ppm nanocurcumin), and T5 (200 ppm nanocurcumin). Under chronic low temperature (21.02 ± 0.11 °C). | ↔Digestive enzymes Blood health: Ht, Hb, RBCs, and WBCs exposed insignificant alteration. Serum biochemists: ↔[triglyceride, ALT, AST], ↑total protein, ↓[glucose, cortisol, total cholesterol]. ↑lysozyme, bactericidal activities. Antioxidant potency: ↑SOD, ↑GPx, ↑CAT. Gastrointestinal microflora: ↔[TBC, TYMC], ↓coliform. | [125] |
6.4. Stress Due to Stocking Densities in Aquatic Animals
7. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stott, G.H. What is animal stress and how is it measured? J. Anim. Sci. 1981, 52, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Manuja, A.; Aich, P. Stress and its impact on farm animals. Front. Biosci. Elit. 2012, 4E, 1759–1767. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12, S431–S444. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef]
- Lee, H.B.; Yoon, J.H.; Park, J.Y.; Lee, I.Y.; Lim, H.K. A comparison of the physiological responses to heat stress of juvenile and adult starry flounder (Platichthys stellatus). Isr. J. Aquac. Bamidgeh 2021, 73, 1–15. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef]
- Desai, P.P.; Patravale, V.B. Curcumin Cocrystal Micelles—Multifunctional Nanocomposites for Management of Neurodegenerative Ailments. J. Pharm. Sci. 2018, 107, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Awadalla, E.A.; Abd El-Kader, A.E.K.M.; Seifeldin, E.A.; Mahmoud, M.A.; Muddathir, A.R.M.; Abdelsadik, A. Antitoxic Effects of Curcumin against Obesity-Induced Multi-Organs’ Biochemical and Histopathological Abnormalities in an Animal Model. Evid. Based Complement. Altern. Med. 2022, 2022, 9707278. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Brahmachari, G.; Chatterjee, K.; Choudhuri, T. Synthetic antioxidants from a natural source can overtake the oncogenic stress management system and activate the stress-sensitized death of KSHV-infected cancer cells. Int. J. Mol. Med. 2022, 50, 117. [Google Scholar] [CrossRef]
- Stevenson, J.L.; Kalita, G.; Goswami, R.; Das, H.; Sarma, K.; Ali, M.A.; Das, B.K.; Kumar Samanta, A.; Mayengbam, P.; Tolenkhomba, T.C.; et al. Effect of Dietary Supplementation of Turmeric (Curcuma longa) on Health Status of Young Pigs during Pre and Post Weaning Periods. Int. J. Bio-Resour. Stress. Manag. 2023, 14, 279–284. [Google Scholar] [CrossRef]
- Kpomasse, C.C.; Oso, O.M.; Lawal, K.O.; Oke, O.E. Juvenile growth, thermotolerance and gut histomorphology of broiler chickens fed Curcuma longa under hot-humid environments. Heliyon 2023, 9, e13060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, Z.; Zhou, S.; Hu, J.; Yang, R.; Wang, J.; Wang, Y.; Yu, W.; Lin, H.; Ma, Z. The Impacts of Dietary Curcumin on Innate Immune Responses and Antioxidant Status in Greater Amberjack (Seriola dumerili) under Ammonia Stress. J. Mar. Sci. Eng. 2023, 11, 300. [Google Scholar] [CrossRef]
- Oglah, M.K.; Mustafa, Y.F.; Bashir, M.K.; Jasim, M.H. Curcumin and its derivatives: A review of their biological activities. Syst. Rev. Pharm. 2020, 11, 472–481. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. Biofactors 2013, 39, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef] [PubMed]
- Cas, M.D.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of more efficacious curcumin delivery systems using colloid science: Enhanced solubility, stability, and bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Szymusiak, M.; Hu, X.; Leon Plata, P.A.; Ciupinski, P.; Wang, Z.J.; Liu, Y. Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin. Int. J. Pharm. 2016, 511, 415–423. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin-from molecule to biological function. Angew. Chem. Int. Ed. Engl. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Ashida, H.; Tian, X.; Kitakaze, T.; Yamashita, Y. Bisacurone suppresses hepatic lipid accumulation through inhibiting lipogenesis and promoting lipolysis. J. Clin. Biochem. Nutr. 2020, 67, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Min, T. Curcumin, curcumin nanoparticles and curcumin nanospheres: A review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics 2020, 12, 447. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, M.J.; Shim, Y.K.; Yoon, I.; Lee, W.K. Solid Lipid Nanoparticles of Curcumin Designed for Enhanced Bioavailability and Anticancer Efficiency. ACS Omega 2022, 7, 35875–35884. [Google Scholar] [CrossRef]
- Asres, A.; Amha, N. Effect of Stress on Animal Health: A Review. J. Biol. Agric. Healthc. 2014, 4, 116–121. [Google Scholar]
- Gebregeziabhear, E. The Effect of Stress on Productivity of Animals: A review. J. Biol. Agric. Healthc. 2015, 5, 2015. [Google Scholar]
- Yoshikawa, T.; Naito, Y. What Is Oxidative Stress? J. Jpn. Med. Assoc. 2002, 124, 271–276. [Google Scholar]
- Pinto, V.F.; Patriarca, A.; Locani, O.; Vaamonde, G. Natural co-occurrence of aflatoxin and cyclopiazonic acid in peanuts grown in Argentina. Food Addit. Contam. 2001, 18, 1017–1020. [Google Scholar] [CrossRef]
- Kanbur, M.; Eraslan, G.; Sarica, Z.S.; Aslan, Ö. The effects of evening primrose oil on lipid peroxidation induced by subacute aflatoxin exposure in mice. Food Chem. Toxicol. 2011, 49, 1960–1964. [Google Scholar] [CrossRef]
- Atef, H.A.; Mansour, M.K.; Ibrahim, E.M.; Sayed El-Ahl, R.M.H.; Al-Kalamawey, N.M.; El Kattan, Y.A.; Ali, M.A. Efficacy of Zinc Oxide Nanoparticles and Curcumin in Amelioration the Toxic Effects in Aflatoxicated Rabbits. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 795–818. [Google Scholar] [CrossRef]
- Hatipoğlu, D.; Keskin, E. Ameliorative Effects of Curcumin on Aflatoxin B1-Induced Nephrotoxicity in Wistar-Albino Rats. Harran Üniv. Vet. Fakültesi Derg. 2022, 11, 139–145. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of Oral Exposure to Aflatoxin B1-Induced Renal Dysfunction, Oxidative Stress, and Cell Apoptosis in Mice Kidney by Curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Ziada Reem, M.; Nahas, A.A.; Farag, A.A.G.; Kotb, G.A.M. Protective Efficacy of Combined Administration of Vitamins C and Curcumin on Cypermethrin—Induced Oxidative Stress in Male Albino Rats. Egypt. Acad. J. Biol. Sci. 2020, 12, 109–118. [Google Scholar] [CrossRef]
- Yang, S.H.; He, J.B.; Yu, L.H.; Li, L.; Long, M.; Liu, M.D.; Li, P. Protective role of curcumin in cadmium-induced testicular injury in mice by attenuating oxidative stress via Nrf2/ARE pathway. Environ. Sci. Pollut. Res. 2019, 26, 34575–34583. [Google Scholar] [CrossRef]
- Khezri Motlagh, R.; Vahdati, A.; Hosseini, S.E.; Edalatmanesh, M.A. Protective Effects of Gallic Acid and Curcumin on Serum Levels of Hepatic Transaminases, Blood Plasma Parameters and Pituitary-testicular Hormones in Rats Treated Nickel Nanoparticles. J. Ilmu Ternak dan Vet. 2022, 27, 45–56. [Google Scholar] [CrossRef]
- Ishaq, A.; Gulzar, H.; Hassan, A.; Kamran, M.; Riaz, M.; Parveen, A.; Chattha, M.S.; Walayat, N.; Fatima, S.; Afzal, S.; et al. Ameliorative mechanisms of turmeric-extracted curcumin on arsenic (As)-induced biochemical alterations, oxidative damage, and impaired organ functions in rats. Environ. Sci. Pollut. Res. 2021, 28, 66313–66326. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Senbill, H.; Basyony, M.M.; Hassan, A.A. Amelioration of sarcoptic mange-induced oxidative stress and growth performance in ivermectin-treated growing rabbits using turmeric extract supplementation. Animals 2021, 11, 2984. [Google Scholar] [CrossRef]
- Tvrdá, E.; Tušimová, E.; Kováčik, A.; Paál, D.; Greifová, H.; Abdramanov, A.; Lukáč, N. Curcumin has protective and antioxidant properties on bull spermatozoa subjected to induced oxidative stress. Anim. Reprod. Sci. 2016, 172, 10–20. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [PubMed]
- Amin, I.; Rashid, S.M.; Shubeena, S.; Hussain, I.; Ahmad, S.B.; Mir, M.U.R.; Alshehri, S.; Bukhari, S.I.; Mir, T.M.; Rehman, M.U. TLR4/NFκB-Mediated Anti-Inflammatory and Antioxidative Effect of Hexanic and Ethanolic Extracts of Curcuma longa L. in Buffalo Mammary Epithelial Cells. Separations 2022, 9, 414. [Google Scholar] [CrossRef]
- Suresh, S.; Sankar, P.; Kalaivanan, R.; Telang, A.G. Ameliorative effect of nanocurcumin on Staphylococcus aureus-induced mouse mastitis by oxidative stress suppression. Inorg. Nano-Metal. Chem. 2022, 52, 1003–1011. [Google Scholar] [CrossRef]
- Smithyman, M.M.; Gouvêa, V.N.; Oliveira, M.O.; Giacomelli, H.J.M.; Campbell, D.L.; Batistel, F.; Cooke, R.F.; Duff, G.C. Effects of supplemental fat and roughage level on intake, growth performance, and health of newly received feedlot calves. Transl. Anim. Sci. 2021, 5, S25–S29. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Peng, W.; Hu, H.; Cao, T.; Hou, G. Effect of the Single and Combined Use of Curcumin and Piperine on Growth Performance, Intestinal Barrier Function, and Antioxidant Capacity of Weaned Wuzhishan Piglets. Front. Vet. Sci. 2020, 7, 418. [Google Scholar] [CrossRef]
- Ghanem, N.; Amin, A.; Saeed, A.M.; Abdelhamid, S.M.; El-Sayed, A.; Farid, O.A.; Dessouki, S.M.; Faheem, M.S. Effects of curcumin supplementation on viability and antioxidant capacity of buffalo granulosa cells under in vitro culture conditions. World’s Vet. J. 2020, 10, 146–159. [Google Scholar] [CrossRef]
- Rosignoli Da Conceição, A.; Dias, K.A.; Michelin Santana Pereira, S.; Saraiva, L.C.; Alves Oliveira, L.; Gomes De Souza, E.C.; Vilela Gonçalves, R.; Pinto Da Matta, S.L.; Natali, A.J.; Martino, H.S.D.; et al. Protective effects of whey protein concentrate admixtured of curcumin on metabolic control, inflammation and oxidative stress in Wistar rats submitted to exhaustive exercise. Br. J. Nutr. 2022, 127, 526–539. [Google Scholar] [CrossRef]
- Sivaranjani, R.; Nk, L.; Cs, T.; Zachariah, T.J. Dietary supplementation of Cinnamomumverum J. Presl and Curcuma longa L. extract on growth performance, antioxidant and metabolic enzymes activities in experimental rats. Indian J. Exp. Biol. 2020, 58, 242–248. [Google Scholar] [CrossRef]
- Chaidanya, K.A.N.P.; Sejian, V.S.S. Adaptation of Livestock to Environmental Challenges. J. Vet. Sci. Med. Diagn. 2015, 4. [Google Scholar] [CrossRef]
- Keim, S.M.; Guisto, J.A.; Sullivan, J.B. Environmental thermal stress. Ann. Agric. Environ. Med. 2002, 9, 1–15. [Google Scholar]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Most, M.S.; Yates, D.T. Inflammatory mediation of heat stress-induced growth deficits in livestock and its potential role as a target for nutritional interventions: A review. Animals 2021, 11, 3539. [Google Scholar] [CrossRef] [PubMed]
- El-din, T.; Bedier, M.M. Effect of Dietary Addition of Curcumin and Nano-Curcumin on Carcass Traits, Blood Haematology and Caecal Activity of Growing Rabbits Reared Under Heat Stress Conditions. J. Anim. Poult. Prod. Mansoura Univ. 2020, 11, 223–228. [Google Scholar]
- El-Kholy, K.H.; Wafa, W.M.; El-Nagar, H.A.; Aboelmagd, A.M.; El-Ratel, I.T. Physiological Response, Testicular Function, and Health Indices of Rabbit Males Fed Diets Containing Phytochemicals Extract Under Heat Stress Conditions. J. Adv. Vet. Anim. Res. 2021, 8, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Alnaimy Ha, A.; El-Dara, A.A.-H.; Nasr, S.A.-M.; Sharaf, K.A. Impact of Some Medicinal Plants Supplement on Pregnant Rabbits Diet during Hot Summer Season. Res. J. Med. Plants 2019, 13, 145–154. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, W.; Liu, X.; Du, Y.; Liu, L.; Ordovas, J.M.; Lai, C.Q.; Shen, L. Curcumin supplementation improves heat-stress-induced cardiac injury of mice: Physiological and molecular mechanisms. J. Nutr. Biochem. 2020, 78, 108331. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Shen, C.-F.; Kang, Y.; Qi, A.; Xu, W.-J.; Shi, W.-H.; Liu, J.-W. Curcumin prevents renal cell apoptosis in acute kidney injury in a rat model of dry-heat environment heatstroke via inhibition of the mitochondrial apoptotic pathway. Exp. Ther. Med. 2020, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gupta, D. Study of effect of curcumin on heat stress-induced behavioral changes in rats. Int. J. Med. Sci. Public Health 2018, 7, 790. [Google Scholar] [CrossRef]
- El-Hamid, I.S.A.; Rabee, A.E.; Ghandour, M.M.M.A.; Mohammed, R.S.; Sallam, A.M. Influence of Phytochemicals on Haemato-Biochemical Parameters, Oxidative Status, Semen Characteristics and Histological Changes in Damascus Goat Bucks under Heat Stress Conditions. Adv. Anim. Vet. Sci. 2023, 11, 112–123. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, Y.; Li, P.; Xue, Y.; Cui, W.; Chen, Q.; Chen, J.; Wang, F.; Mao, D. Effect of Curcumin Supplement in Summer Diet on Blood Metabolites, Antioxidant Status, Immune Response, and Testicular Gene Expression in Hu Sheep. Animals 2019, 9, 720. [Google Scholar] [CrossRef]
- Grewal, S.; Aggarwal, A.; Vats, P.; Rani, S.; Jaswal, S.; Pal, P.; Senthamilan, S.; Arya, A.; Mohanty, A.K.; Alhussien, M.N. Curcumin induces thermotolerance by reducing oxidative stress, apoptosis, and inflammation in buffalo mammary epithelial cells under heat shock conditions. J. Reprod. Immunol. 2022, 153, 103684. [Google Scholar] [CrossRef] [PubMed]
- Bhimte, A.; Thakur, N.; Lakhani, N.; Yadav, V.; Khare, A.; Lakhani, P. Endocrine changes in livestock during heat and cold stress. J. Pharmacogn. Phytochem. 2018, 7, 127–132. [Google Scholar]
- Toghiani, S.; Hay, E.H.; Roberts, A.; Rekaya, R. Impact of cold stress on birth and weaning weight in a composite beef cattle breed. Livest. Sci. 2020, 236, 104053. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Li, S. Effect of Acute Cold Stress on Neuroethology in Mice and Establishment of Its Model. Animals 2022, 12, 2671. [Google Scholar] [CrossRef]
- Arboud, M.M.; Waheeb, R.S.; El-Sheshtawy, R.I.; Gamal; El-Amrawi, A. Assessment of Cattle Bull Semen Preservability using Tris Extender Enriched with Turmeric Extract. Egypt. J. Vet. Sci. 2020, 51, 357–362. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.O.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2021, 532, 736030. [Google Scholar] [CrossRef]
- Mishra, R.; Singh, S.K.; Palod, J.; Mondal, B.C.; Singh, B.; Singh, V.S. Effect of dietary supplementation of Garlic (Allium sativum) and turmeric (Curcuma longa) powder on growth and nutrient utilization of female crossbred calves during winter season. J. Entomol. Zool. Stud. 2020, 8, 2288–2292. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A.; Mahajan, A.; Sharma, P.; Sachan, V.; Aggrawal, J.; Yadav, S.; Saxena, A.; Kumar Swain, D. Curcumin in a tris-based semen extender improves cryosurvival of Hariana bull spermatozoa. Andrologia 2022, 54, e14255. [Google Scholar] [CrossRef]
- Ismail, A.A.; Abdel-Khalek, A.K.E.; Khalil, W.A.; Yousif, A.I.; Saadeldin, I.M.; Abomughaid, M.M.; El-Harairy, M.A. Effects of mint, thyme, and curcumin extract nanoformulations on the sperm quality, apoptosis, chromatin decondensation, enzyme activity, and oxidative status of cryopreserved goat semen. Cryobiology 2020, 97, 144–152. [Google Scholar] [CrossRef]
- Abdnour, S.A.; Hassan, M.A.E.; Mohammed, A.K.; Alhimaidi, A.R.; Al-Gabri, N.; Al-Khaldi, K.O.; Swelum, A.A. The effect of adding different levels of curcumin and its nanoparticles to extender on post-thaw quality of cryopreserved rabbit sperm. Animals 2020, 10, 1508. [Google Scholar] [CrossRef]
- El-Sheshtawy, R.I. Effect of Tris-extender supplemented with a combination of turmeric and ethylene glycol on buffalo bull semen freezability and in vivo fertility. Trop. Anim. Health Prod. 2021, 53, 238. [Google Scholar] [CrossRef]
- Mozos, I.; Luca, C.T. Crosstalk between Oxidative and Nitrosative Stress and Arterial Stiffness. Curr. Vasc. Pharmacol. 2017, 15, 446–456. [Google Scholar] [CrossRef]
- Longobardi, C.; Damiano, S.; Andretta, E.; Prisco, F.; Russo, V.; Pagnini, F.; Florio, S.; Ciarcia, R. Curcumin modulates nitrosative stress, inflammation and dna damage and protects against ochratoxin a-induced hepatotoxicity and nephrotoxicity in rats. Antioxidants 2021, 10, 1239. [Google Scholar] [CrossRef]
- Ahmed-Farid, O.A.H.; Nasr, M.; Ahmed, R.F.; Bakeer, R.M. Beneficial effects of curcumin nano-emulsion on spermatogenesis and reproductive performance in male rats under protein deficient diet model: Enhancement of sperm motility, conservancy of testicular tissue integrity, cell energy and seminal plasma amino acids content. J. Biomed. Sci. 2017, 24, 66. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Wei, G.; Guo, G.; Yu, H.; Zhang, Y.; Ishfaq, M.; Fazilani, S.A.; Zhang, X. Curcumin protects against Aflatoxin B1-induced liver injury in broilers via the modulation of long non-coding RNA expression. Ecotoxicol. Environ. Saf. 2021, 208, 111725. [Google Scholar] [CrossRef]
- Qiao, B.; He, Y.; Gao, X.; Liu, H.; Rao, G.; Su, Q.; Ruan, Z.; Tang, Z.; Hu, L. Curcumin attenuates AFB1-induced duck liver injury by inhibiting oxidative stress and lysosomal damage. Food Chem. Toxicol. 2023, 172, 113593. [Google Scholar] [CrossRef]
- Li, S.; Muhammad, I.; Yu, H.; Sun, X.; Zhang, X. Detection of Aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotoxicol. Environ. Saf. 2019, 176, 137–145. [Google Scholar] [CrossRef]
- Damiano, S.; Jarriyawattanachaikul, W.; Girolami, F.; Longobardi, C.; Nebbia, C.; Andretta, E.; Lauritano, C.; Dabbou, S.; Avantaggiato, G.; Schiavone, A.; et al. Curcumin Supplementation Protects Broiler Chickens Against the Renal Oxidative Stress Induced by the Dietary Exposure to Low Levels of Aflatoxin B1. Front. Vet. Sci. 2022, 8, 822227. [Google Scholar] [CrossRef]
- Wan, F.; Tang, L.; Rao, G.; Zhong, G.; Jiang, X.; Wu, S.; Huang, R.; Tang, Z.; Ruan, Z.; Chen, Z.; et al. Curcumin activates the Nrf2 Pathway to alleviate AFB1-induced immunosuppression in the spleen of ducklings. Toxicon 2022, 209, 18–27. [Google Scholar] [CrossRef]
- Cheng, P.; Ishfaq, M.; Yu, H.; Yang, Y.; Li, S.; Li, X.; Fazlani, S.A.; Guo, W.; Zhang, X. Curcumin ameliorates duodenal toxicity of AFB1 in chicken through inducing P-glycoprotein and downregulating cytochrome P450 enzymes. Poult. Sci. 2020, 99, 7035–7045. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.M.; Griss, L.G.; Fortuoso, B.F.; Silva, A.D.; Fracasso, M.; Lopes, T.F.; Schetinger, M.R.S.; Gundel, S.; Ourique, A.F.; Carneiro, C.; et al. Feed contaminated by fumonisin (Fusarium spp.) in chicks has a negative influence on oxidative stress and performance, and the inclusion of curcumin-loaded nanocapsules minimizes these effects. Microb. Pathog. 2020, 148, 104496. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.S.; Ruan, D.; Zhu, Y.W.; Li, M.C.; Ye, H.; Wang, W.C.; Yang, L. Protective effect of curcumin on ochratoxin A–induced liver oxidative injury in duck is mediated by modulating lipid metabolism and the intestinal microbiota. Poult. Sci. 2020, 99, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Wang, W.C.; Lin, C.X.; Fouad, A.M.; Chen, W.; Xia, W.G.; Wang, S.; Luo, X.; Zhang, W.H.; Yan, S.J.; et al. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal 2019, 13, 42–52. [Google Scholar] [CrossRef]
- Lan, J.; Tang, L.; Wu, S.; Huang, R.; Zhong, G.; Jiang, X.; Tang, Z.; Hu, L. Curcumin alleviates arsenic-induced injury in duck skeletal muscle via regulating the PINK1/Parkin pathway and protecting mitochondrial function. Toxicol. Appl. Pharmacol. 2022, 434, 115820. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yu, W.; Jiang, X.; Huang, R.; Zhang, X.; Lan, J.; Zhong, G.; Wan, F.; Tang, Z.; Hu, L. Protective effects of curcumin on ATO-induced nephrotoxicity in ducks in relation to suppressed autophagy, apoptosis and dyslipidemia by regulating oxidative stress. Ecotoxicol. Environ. Saf. 2021, 219, 112350. [Google Scholar] [CrossRef]
- Liu, X.; Guan, P.Y.; Yu, C.T.; Yang, H.; Shan, A.S.; Feng, X.J. Curcumin alleviated lipopolysaccharide-induced lung injury via regulating the Nrf2-ARE and NF-κB signaling pathways in ducks. J. Sci. Food Agric. 2022, 102, 6603–6611. [Google Scholar] [CrossRef]
- Yang, H.; Yu, C.; Yin, Z.; Guan, P.; Jin, S.; Wang, Y.; Feng, X. Curcumin: A potential exogenous additive for the prevention of LPS-induced duck ileitis by the alleviation of inflammation and oxidative stress. J. Sci. Food Agric. 2023, 103, 1550–1560. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.Y.; Souza dos Santos, T.; Gould, R.L.; Craig, S.W.; Lorraine Fuller, A.; Pazdro, R.; Kim, W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar] [CrossRef]
- Petrone-Garcia, V.M.; Lopez-Arellano, R.; Patiño, G.R.; Rodríguez, M.A.C.; Hernandez-Patlan, D.; Solis-Cruz, B.; Hernandez-Velasco, X.; Alba-Hurtado, F.; Vuong, C.N.; Castellanos-Huerta, I.; et al. Curcumin reduces enteric isoprostane 8-iso-PGF2α and prostaglandin GF2α in specific pathogen-free Leghorn chickens challenged with Eimeria maxima. Sci. Rep. 2021, 11, 11609. [Google Scholar] [CrossRef]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Sayed, S.; Shukry, M.; El-Sayed, Y.S.; Alshehri, M.A. The Impact of Curcumin on Growth Performance, Growth-Related Gene Expression, Oxidative Stress, and Immunological Biomarkers in Broiler Chickens at Different Stocking Densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef] [PubMed]
- Bilal, R.M.; Hassan, F.; Farag, M.R.; Nasir, T.A.; Ragni, M.; Mahgoub, H.A.M.; Alagawany, M. Thermal stress and high stocking densities in poultry farms: Potential effects and mitigation strategies. J. Therm. Biol. 2021, 99, 102944. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S. Alleviation of heat stress in broiler chicken using turmeric (Curcuma longa)—A short review. J. Anim. Behav. Biometeorol. 2020, 8, 215–222. [Google Scholar] [CrossRef]
- Salah, A.S.; Ahmed-Farid, O.A.; Nassan, M.A.; El-Tarabany, M.S. Dietary curcumin improves energy metabolism, brain monoamines, carcass traits, muscle oxidative stability and fatty acid profile in heat-stressed broiler chickens. Antioxidants 2021, 10, 1265. [Google Scholar] [CrossRef]
- Mustafa, M.M.; Karadas, F.; Tayeb, I.T. Adding Different Levels of Turmeric Powder and Curcumin in the Diet on Some Serum Biochemical of Broiler Reared Under Normal and Heat Stress Conditions. Iraqi J. Agric. Sci. 2021, 52, 10–19. [Google Scholar] [CrossRef]
- Candra, A.A.; Putri, D. Application turmeric as antioxidant for broiler chickens. J. Phys. Conf. Ser. 2020, 1450, 012058. [Google Scholar] [CrossRef]
- Akhavan-Salamat, H.; Ghasemi, H.A. Alleviation of chronic heat stress in broilers by dietary supplementation of betaine and turmeric rhizome powder: Dynamics of performance, leukocyte profile, humoral immunity, and antioxidant status. Trop. Anim. Health Prod. 2016, 48, 181–188. [Google Scholar] [CrossRef]
- Kanani, B.; Daneshyar, P.; Najafi, M. Effects of Cinnamon (Cinnamomum zeylanicum) and Turmeric (Curcuma longa) Powders on Performance, Enzyme Activity, and Blood Parameters of Broiler Chickens Under Heat Stress. Sci. J. 2016, 2016, 47–53. [Google Scholar]
- Sadeghi, A.A.; Moghaddam, M. The effects of turmeric, cinnamon, ginger and garlic powder nutrition on antioxidant enzymes’ status and hormones involved in energy metabolism of broilers during heat stress. Iran. J. Appl. Anim. Sci. 2018, 8, 25–130. [Google Scholar]
- Almayali, A.M.J.; Alshukri, A.Y.A. Effect of Adding Different Levels of Turmeric Root Powder and Carnation Flowers to the Diet on Some Blood and Microorganisms Traits of Broilers under Heat Stress Condition. IOP Conf. Ser. Earth Environ. Sci. 2021, 923, 2–6. [Google Scholar] [CrossRef]
- Nawab, A.; Li, G.; Liu, W.; Lan, R.; Wu, J.; Zhao, Y.; Kang, K.; Kieser, B.; Sun, C.; Tang, S.; et al. Effect of dietary curcumin on the antioxidant status of laying hens under high- temperature condition. J. Therm. Biol. 2019, 86, 102449. [Google Scholar] [CrossRef] [PubMed]
- Nawab, A.; Tang, S.; Li, G.; An, L.; Wu, J.; Liu, W.; Xiao, M. Dietary curcumin supplementation effects on blood immunological profile and liver enzymatic activity of laying hens after exposure to high temperature conditions. J. Therm. Biol. 2020, 90, 102573. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ibtisham, F.; Niu, Y.F.; Wang, Z.; Li, G.H.; Zhao, Y.; Nawab, A.; Xiao, M.; An, L. Curcumin inhibits heat-induced oxidative stress by activating the MAPK-Nrf2/ARE signaling pathway in chicken fibroblasts cells. J. Therm. Biol. 2019, 79, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Golian, A.; Kermanshahi, H.; Bassami, M.R. Effects of curcumin or nanocurcumin on blood biochemical parameters, intestinal morphology and microbial population of broiler chickens reared under normal and cold stress conditions. J. Appl. Anim. Res. 2018, 46, 200–209. [Google Scholar] [CrossRef]
- Marchiori, M.S.; Oliveira, R.C.; Souza, C.F.; Baldissera, M.D.; Ribeiro, Q.M.; Wagner, R.; Gündel, S.S.; Ourique, A.F.; Kirinus, J.K.; Stefani, L.M.; et al. Curcumin in the diet of quail in cold stress improves performance and egg quality. Anim. Feed. Sci. Technol. 2019, 254, 114192. [Google Scholar] [CrossRef]
- da Rosa, G.; Dazuk, V.; Alba, D.F.; Galli, G.M.; Molosse, V.; Boiago, M.M.; Souza, C.F.; Abbad, L.B.; Baldissera, M.D.; Stefani, L.M.; et al. Curcumin addition in diet of laying hens under cold stress has antioxidant and antimicrobial effects and improves bird health and egg quality. J. Therm. Biol. 2020, 91, 102618. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Oxidative Stress in Fish: A Review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Bögner, D.; Bögner, M.; Schmachtl, F.; Bill, N.; Halfer, J.; Slater, M.J. Hydrogen peroxide oxygenation and disinfection capacity in recirculating aquaculture systems. Aquac. Eng. 2021, 92, 102140. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Li, X.; Lu, K.; Wang, L.; Ma, X.; Song, K.; Zhang, C. Antioxidant effects of the aqueous extract of turmeric against hydrogen peroxide-induced oxidative stress in spotted seabass (Lateolabrax maculatus). Aquac. Fish. 2022; in press. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kang, M.C.; Lee, H.H.L.; Cho, C.H.; Choi, I.; Park, Y.; Lee, S.H. Antioxidant effects of turmeric leaf extract against hydrogen peroxide-induced oxidative stress in vitro in vero cells and in vivo in zebrafish. Antioxidants 2021, 10, 112. [Google Scholar] [CrossRef]
- Rajabiesterabadi, H.; Hoseini, S.M.; Fazelan, Z.; Hoseinifar, S.H.; Doan, H. Van Effects of dietary turmeric administration on stress, immune, antioxidant and inflammatory responses of common carp (Cyprinus carpio) during copper exposure. Aquac. Nutr. 2020, 26, 1143–1153. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; El-Houseiny, W.; EL-Murr, A.E.; Ebraheim, L.L.M.; Moustafa, A.A.; Rahman Mohamed, A.A. Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in Oreochromis niloticus. Aquat. Toxicol. 2020, 220, 105406. [Google Scholar] [CrossRef] [PubMed]
- Şahinöz, E.; Aral, F.; Doğu, Z.; Koyuncu, Y. Protective effect of curcumin on different tissues of rainbow trout (Oncorhynchus mykiss W., 1792) against exposition to chlorphyrifos. Appl. Ecol. Environ. Res. 2019, 17, 3371–3385. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Al-Sagheer, A.A.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 2017, 475, 16–23. [Google Scholar] [CrossRef]
- Betancor, M.B.; Caballero, M.J.; Terova, G.; Saleh, R.; Atalah, E.; Benítez-Santana, T.; Bell, J.G.; Izquierdo, M. Selenium inclusion decreases oxidative stress indicators and muscle injuries in sea bass larvae fed high-DHA microdiets. Br. J. Nutr. 2012, 108, 2115–2128. [Google Scholar] [CrossRef]
- Xavier, M.J.; Dardengo, G.M.; Navarro-Guillén, C.; Lopes, A.; Colen, R.; Valente, L.M.P.; Conceição, L.E.C.; Engrola, S. Dietary curcumin promotes gilthead seabream larvae digestive capacity and modulates oxidative status. Animals 2021, 11, 1667. [Google Scholar] [CrossRef]
- Xavier, M.J.; Navarro-Guillén, C.; Lopes, A.; Colen, R.; Teodosio, R.; Mendes, R.; Oliveira, B.; Valente, L.M.P.; Conceição, L.E.C.; Engrola, S. Effects of dietary curcumin in growth performance, oxidative status and gut morphometry and function of gilthead seabream postlarvae. Aquac. Reports 2022, 24, 101128. [Google Scholar] [CrossRef]
- He, K.; Luo, X.; Wen, M.; Wang, C.; Qin, C.; Shao, J.; Gan, L.; Dong, R.; Jiang, H. Effect of acute ammonia toxicity on inflammation, oxidative stress and apoptosis in head kidney macrophage of Pelteobagrus fulvidraco and the alleviation of curcumin. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2021, 248, 109098. [Google Scholar] [CrossRef]
- He, Y.; Fu, Z.; Dai, S.; Yu, G.; Ma, Z. Dietary curcumin supplementation can enhance health and resistance to ammonia stress in the greater amberjack (Seriola dumerili). Front. Mar. Sci. 2022, 9, 961783. [Google Scholar] [CrossRef]
- He, Y.; Fu, Z.; Dai, S.; Yu, G.; Ma, Z.; Wang, X. Dietary curcumin supplementation enhances intestinal immunity and gill protection in juvenile greater amberjack (Seriola dumerili). Heliyon 2022, 8, e11887. [Google Scholar] [CrossRef]
- Sylvester, J.R. Possible Effects of Thermal Effluents on Fish: A Review. Environ. Pollut. 1972, 3, 205–215. [Google Scholar] [CrossRef]
- Mahanty, A.; Mohanty, S.; Mohanty, B.P. Dietary supplementation of curcumin augments heat stress tolerance through upregulation of nrf-2-mediated antioxidative enzymes and hsps in Puntius sophore. Fish. Physiol. Biochem. 2017, 43, 1131–1141. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; El-Sisy, D.M.; Salem, M.E.S. A comparative study of effects of curcumin and its nanoparticles on the growth, immunity and heat stress resistance of Nile tilapia (Oreochromis niloticus). Nat. Portf. 2023, 13, 2523. [Google Scholar] [CrossRef]
- Donaldson, M.R.; Cooke, S.J.; Patterson, D.A.; Macdonald, J.S. Cold shock and fish. J. Fish. Biol. 2008, 73, 1491–1530. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Zaki, M.A.A.; El-Hais, A.M.; Elhanafy, M.G.; El-Bilawy, E.H.; Zaineldin, A.I.; Abdel-Aziz, M.F.A.; Abouelsaad, I.A.; El-Ratel, I.T.; Mzengereza, K.; et al. Microbial, immune and antioxidant responses of Nile tilapia with dietary nano-curcumin supplements under chronic low temperatures. Aquac. Fish. 2022; in press. [Google Scholar] [CrossRef]
- Jia, R.; Liu, B.L.; Feng, W.R.; Han, C.; Huang, B.; Lei, J.L. Stress and immune responses in skin of turbot (Scophthalmus maximus) under different stocking densities. Fish. Shellfish. Immunol. 2016, 55, 131–139. [Google Scholar] [CrossRef]
- Akdemir, F.; Orhan, C.; Tuzcu, M.; Sahin, N.; Juturu, V.; Sahin, K. The efficacy of dietary curcumin on growth performance, lipid peroxidation and hepatic transcription factors in rainbow trout Oncorhynchus mykiss (Walbaum) reared under different stocking densities. Aquac. Res. 2017, 48, 4012–4021. [Google Scholar] [CrossRef]

| Animal Category | Experimental Design | Findings (Comparison to Negative Control) | Source |
|---|---|---|---|
| Toxicity | |||
| Healthy New Zealand rabbits, aged 6–8 weeks, weighed 1–1.5 kg | A total of 35 male rabbits were divided into 5 groups: control, AFB1, AFB1 + CUR (15 mg/kg b.w.), AFB1 + ZnO-NPs (25 µg/kg b.w.), and AFB1 + ZnO-NPs (50 µg/kg b.w.). AFB1 were orally given at a dose of 50 µg dissolved in 0.5 mL of olive oil/animal. | Antioxidant parameters: ↓NO, ↓MDA ↑GSH, ↑SOD, ↑CAT. Serum biochemical parameters: ↓AST, ↓ALT, ↓cholesterol, ↓triglyceride. Genotoxic effect on hepatic cells and renal cells. | [32] |
| BALB/c mice, aged 5 weeks age, weighed 20–22 g | A total of 50 male mice were divided into 5 groups: control, CUR200 (200 mg/kg b.w. CUR), AFB1 (750 µg/kg b.w. AFB1), AFB1 + CUR100 (750 µg/kg b.w. AFB1 + 100 mg/kg b.w. CUR), and AFB1 + CUR200 (750 µg/kg b.w. AFB1 + 200 mg/kg b.w. CUR). These compounds were administered in olive oil (0.2 mL) as a vehicle via oral gavage. | ↑bodyweight. Renal dysfunction: ↓BUN, ↓CREA, ↓UA. Pathological slices: no significant lesions (G5). Kidney oxidative damage: ↓MDA, ↓H2O2, ↑T-AOC, ↑CAT, ↑SOD, ↑GSH. Keap1-Nrf2 signaling pathway: ↑[CAT, SOD1, NQO1, GCLC], ↑the protein expression level of Nrf2 (G5), ↓Keap1, ↓the apoptosis rate of kidney cells, ↓[Bax, caspase-9, caspase-3], ↑Bcl-2. | [34] |
| Wistar albino rats, weighed 34–36 g | A total of 38 male rats were divided into 5 groups: G1 (control), G2 (1 mL 10% DMSO), G3 (300 mg/kg CUR), G4 (250 µg/kg AFB1), and G5 (250 µg/kg AFB1 + 300 mg/kg CUR). AFB1 and CUR were dissolved in 10% DMSO. | ↑bodyweight average after 60 days. Kidney function: ↓BUN, ↓uric acid, ↓creatinine. | [33] |
| Wistar albino rats, aged 12 weeks, weighed 160–180 g | A total of 40 adult male rats were divided into 4 groups and treated orally: control (distilled water), CUR + VitC (200 mg/kg b.w. CUR + 100 mg/kg b.w. VitC), CPM (200 mg/kg b.w.), and CPM + CUR + VitC (200 mg/kg b.w. CPM + 200 mg/kg b.w. CUR + 100 mg/kg b.w. VitC). Curcumin was obtained from the powder of Curcuma longa. | Serum: ↑ATPase, ↓MDA, ↓PC, ↑GSH, ↑SH pt, ↑CAT, ↑SOD, and ↓GST. Brain: ↑ATPase, ↑MDA, ↓PC, ↑GSH, ↑CAT, ↔SOD, and ↓GST. Liver: ↑ATPase, ↓MDA, ↔PC, ↑GSH, ↑CAT, ↓SOD, and ↑GST. | [35] |
| Kunming mice, aged 9 weeks, weighed 45 ± 2 g | A total of 48 male mice divided into 4 groups: control group (distilled water 2 mg/kg), Cd group (CdCl2 2 mg/kg), CUR group (CUR 50 mg/kg), and Cd + CUR group (CdCl2 2 mg/kg + CUR 50 mg/kg). After the mice were injected with CUR solution for 4 h, they were injected with CdCl2 solution. | ↑sperm motility, ↑sperm concentration, ↓abnormal sperm rate, ↑serum testosterone level. ↑GSH-Px, ↑GSH, ↑SOD, ↓MDA. ↑Spermatogenic cells, mature spermatozoa in the testicular seminiferous tubules. ↑the spermatogenic cells, mature spermatozoa ↑mRNA and proteins expression [Nrf2, GSH- Px, γ- GCS]. | [36] |
| Wistar rats, weighed 230 ± 20 g, aged 8 weeks old | A total of 70 adult male rats were divided into 7 groups: control, Ni50 (50 mg/kg NiNPs), Ni50 + GA150 (50 mg/kg NiNPs + 150 mg/kg GA), Ni50 + GA300 (50 mg/kg NiNPs + 300 mg/kg GA), Ni50 + CUR100 (50 mg/kg NiNPs + 100 mg/kg CUR), Ni50 + CUR300 (50 mg/kg NiNPs + 100 mg/kg CUR), and Ni50 + GA300 + CUR300 (50 mg/kg NiNPs + 300 mg/kg GA + 300 mg/kg CUR). | ↓Glucose, ↓triglyceride, ↓cholesterol, ↓LDL, ↓HDL. ↓ALT, ↓AST, ↓ALP, ↓bilirubin, ↑albumin, ↑total protein. ↓BUN and ↓creatinine. ↑LH, ↑FSH, ↑dihydrotestosterone, ↑testosterone. | [37] |
| Male rats, weighed 220–250 g, aged 10 weeks old | A total of 35 male rats were divided into 5 groups: G1 (control), G2 (As 10 mg/L). G3 (As 10 mg/L + CUR 80 mg/kg), G4 (As 10 mg/L + CUR 160 mg/kg), and G5 (As 10 mg/L + CUR 240 mg/kg). Curcumin was extracted from rhizomes of turmeric by a Soxhlet apparatus. | Liver function: ↓ALT, ↓AST, ↓ALP. Kidney function: ↓total bilirubin, ↓urea, ↓creatinine. Serum lipid profile: ↓total cholesterol contents, ↓total triglyceride contents, ↑HDL, ↓LDL. Antioxidant markers (liver and kidney): ↓MDA, ↑SOD, ↑CAT, ↑GPx, ↑GR. | [38] |
| Sarcoptes-infested rabbits, aged 60 days, weighed 869.5 ± 94.27 g | A total of 83 rabbits were divided into 3 groups: G1 (control), G2 (IVM 0.2 mg/kg b.w.), G3 (IVM 0.2 g/kg b.w. + TE 1 mg/kg diet), and G4 (IVM 0.2 g/kg b.w. + TE 2 mg/kg diet). The aqueous extract of turmeric was obtained from the rhizome of Curcuma longa. | Growth performance: ↑final weight, ↑wt. gain, ↓mortality rate. Improved nutrient digestibility of DM, CP, NDF, ADF. Day 30: ↓ALT, ↓AST. ↓TBARS (Day 30), ↑T-AOC, ↑SOD (Day 30), ↑GSH-Px. | [39] |
| Adult Holstein Friesian breeding bulls | Ejaculates were collected from 5 adults bulls and divided into 10 groups: -Groups untreated with FeAA: G1 (2.9% SC control), G2, G3, G4, and G5 (2.9%SC + 5, 10, 25, and 50 µmol/L CUR, respectively). -Groups treated with FeAA (150 µmol/L FeSO4 + 750 µmol/L ascorbic acid): G6 (FeAA control), G7, G8, G9, and G10 (FeAA + 5, 10, 25, and 50 µmol/L CUR, respectively). | ↑MOT, ↑PROG. ↑mitochondrial activity. ↓ROS, ↓superoxide generation (25, 50 µmol/L CUR). ↑SOD, ↑CAT (FeAA treatment), ↑GSH. ↓MDA (FeAA treatment). ↔MDA (FeAA untreated). ↑GPx. | [40] |
| RAW264.7 cells | Cells were stimulated with curcumin (0, 5, 10, and 20 μM) for 20 h. The positive control group and the curcumin-treated groups were then exposed to H2O2 (500 μM) for 4 h or 8 h, respectively. | ↑cell viability (5 μM). ↑CAT, ↑SOD, ↑GSH-Px. ↓MDA, ↓ROS (5 and 10 μM). Protecting cells from apoptosis. Active Nrf2 (5 and 10 μM). ↑the expression of hemoxygenase-1. ↑the expression of glutamate–cysteine ligase. ↓transcription of glutamate–cysteine ligase. | [41] |
| Inflammation and Diseases | |||
| Buffalo mammary epithelial cells (BuMECs) | Cells were seeded in 6-well plates in DMEM and divided into 4 groups: G1 (control), G2 (LPS: 5 µg/mL, 6 h), G3 (HECl 50 µg/mL 24 h + LPS 5 µg/mL 6 h), and G4 (EECl 50 µg/mL 24 h + LPS 5 µg/mL 6 h). The powder of the rhizome of C. longa was dissolved in the two solvents (hexane and ethanol). | The highest DPPH at the doses of 50 and 100 µg/mL. ↓TLR4 expression (G3 and G4) Inflammatory gene expression - G3: ↓TNFα, ↓IL-6, ↓NFκB - G4: ↓TNFα, ↔IL-6, ↓NFκB ↑Nrf2 expression (G3 and G4). | [42] |
| Swiss albino mice, weighed 25–35 g | A total 75 adult female mice were divided into 5 groups: G1 (healthy control), G2 (S. aureus infected), G3 (Vehicle control: 0.1 mL/10 g b.w. gum acacia), G4 (CUR 100 mg/kg b.w.), and G5 (CUR-NP: 10 mg/kg b.w. CUR in nanoparticle-encapsulated form) for 24 h, 48 h, and 72 h. | ↔lymphocytes, ↓neutrophils ↓LPO, ↑SOD (24 h, 48 h), ↓SOD (72 h) ↓CAT (G4-24 h, 72 h, G5-48 h, 72 h), ↑CAT (G5-24 h), ↓GST (24 h, G5-48 h), ↑GST (72 h, G4-48 h). ↓congestion, inflammatory cellular infiltration, and edema. Normal secretion of milk. | [43] |
| Crossbreed calves, weighed 201 ± 14 kg, aged 8 months | A total of 331 crossbreed calves were divided into 4 groups: control, MON (monensin: 22 mg/kg d.m.), TUR100, and TUR200 (100 and 200 mg/kg d.m. TUR, respectively). TUR extract, 95%. Treatment: bovine respiratory disease (BRD). | Decreased the number of calves that required a third medication for BRD compared with MON. | [44] |
| Weaned | |||
| Wuzhishan piglets, aged 35 days, weighed 3.54 ± 0.28 kg | A total of 50 weaned piglets were divided into 5 groups: G1 (control), G2 (50 mg/kg PIP), G3 (200 mg/kg CUR), G4 (200 mg/kg CUR + 50 mg/kg PIP), and G5 (300 mg/kg CUR). | ↔initial weight, ↔final weight F/G (G4 and G5): lower than others. In the jejunal and ileum mucosa: ↔IL-1β, ↔TNF- α, ↔IL-6, ↔IL-10. G4 and G5: ↑SOD, ↑GSH-Px, ↓MDA, ↔T-AOC. | [45] |
| Buffalo granulosa cells (GCs) | GCs were divided into 6 groups: G1 (control), G2 (DMSO), G3 (1 μM CUR), G4 (2.5 μM CUR), G5 (5 μM CUR), and G6 (10 μM CUR) for 24 h and 48 h at 37 °C. | ↓viability (in vitro). ↑mitochondrial activity (G4, G5). ↑ROS (G6). Enzyme activity (CAT, SOD, GSH, DPPH): 24 h (↑G5), 48 h (↓all groups). | [46] |
| Excessive Physical Activity | |||
| Wistar rats, aged 12 weeks | A total of 48 male rats were divided into 6 groups: G1 (AIN-93M: standard diet), G2 (AIN-93M + ET), G3 (WPC + CUR), G4 (WPC + CUR + ET), G5 (CUR), and G6 (CUR + ET). WPC (44.0 g/kg diet), CUR (1.2 g/kg diet). | Gene expression: ↓TNF-α, ↓IL-6, ↑IL-10. Biomarker for oxidative stress: ↓MDA, ↓PC, ↓NO (G4). Antioxidant enzymes: ↑CAT (ET), ↑SOD, ↑GST (G5). | [47] |
| Growth Performance | |||
| Wistar rats, weighed 150–230 g | A total of 18 male rats were divided into 3 groups: control, T1 (2.5% cinnamon + turmeric extract), and T2 (5% cinnamon + turmeric extract). The extract of cinnamon and turmeric mixture was incorporated at 2.5 and 5% concentration into powdered diet. | % weight gain: T1 (31.8%), control (38.17%), and T2 (38.24%). Organ weight: ↑liver, ↔kidney, ↔heart. Enzyme assays: ↑CAT (in liver), ↓SOD (in liver and muscles_T2), ↔GST, ↔LDH, ↔MDH, ↔AST, ↔ALT. ↔Blood parameters (Hb, RCB, PCV, MCV, MHC, MCHC, PLT). Serum liquid profile: The animals in T2 have high cholesterol. | [48] |
| Animal Category | Experimental Design | Findings (Comparison to Negative Control) | Source |
|---|---|---|---|
| Yellow catfish (Pelteobagrus fulvidraco). Weighted 100 ± 50 g | The kidney cells of fish were divided into 6 groups: CON (control), AM (ammonia, 0.23 mg/L), CUR (curcumin, 45 μmol/L), 5A (ammonia + curcumin 5 μmol/L), 25A (ammonia + curcumin 25 μmol/L), and 45A (ammonia + curcumin 45 μmol/L). | ↑cell viability, ↓ROS. ↓mRNA levels of SOD and GPx genes. ↓expression of IL-1, IL-6, NF-κB, TNF-α, COX-2 genes. ↑expression of Arg-1 and Bcl-2. ↓number of apoptotic cells. | [118] |
| The wild-caught juvenile of the greater amberjack (Seriola dumerili) | A total of 135 juveniles were divided into 3 groups: CUR0%, CUR0.01% (100 mg/kg curcumin), and CUR0.02% (200 mg/kg curcumin). Ammonia challenge: NH4Cl (1 g/L farming environment). | Antioxidant capacity of the liver: the relative expression ↑CAT (CUR0.02%) after recovery, ↓GSH-Px, ↓GR, ↓Keap1, ↔Mn-SOD. Enzyme activity (SOD, GSH): ↓[after challenge], ↑[after recovery]. Antioxidant capacity of the spleen: the relative expression CAT [↑after challenge, ↓after recover in CUR0.01%], ↓GR, ↑Keap1, ↑Mn-SOD. Enzyme activity: ↑SOD, ↑GSH. | [119] |
| The greater amberjack (Seriola dumerili), weighed 151.44 ± 7.16 g | A total of 135 juveniles were divided into 3 groups: CUR0%, CUR0.01% (100 mg/kg curcumin), and CUR0.02% (200 mg/kg curcumin). Ammonia challenge: NH4Cl (1 g/L farming environment). | Survival and growth performance: ↑final bodyweight, ↑weight gain, ↑survival rate (CUR0.01%). Intestinal histology structure: ↑fold height (CUR0.01%), ↑muscular thickness, ↑enterocyte height. Intestinal immune enzyme activity: ↑ALP, ↑ACP, ↑LZM (CUR0.01%). Intestinal immune gene expression: ↓[C3, C4, IgT, NF-κB1], ↔[Hepc, IL-1β, TGF-β], ↑[IFN-γ, IL-10, TNF-α, MX]_CUR0.01%], ↓Il-8_CUR0.01%. ↑activity of antioxidant enzymes in the gill (SOD, GSH-Px) Relative expression of immune-related genes in the gill: ↓Keap1, ↑Hsp70 (CUR0.01%), ↑Cu-SOD, ↑GSH-Px. | [120] |
| Greater amberjack (Seriola dumerili), initial weight: 100.90 ± 0.03 g | A total of 225 fish were divided into 3 groups: CON (control), CUR75 (75 mg/kg curcumin), and CUR150 (150 mg/kg curcumin). Ammonium chloride (NH4Cl) was used as an ammonia source. | Intestinal and hepatic: ↑ALP, ↑ACP, ↑SOD, ↑T-AOC, ↓GSH, ↓GSH-Px. Liver, spleen, head kidney, and brain tissues after post-recovery: ↑[SOD, T-AOC, GSH, GSH-Px, CAT], ↓MDA. | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuong, D.T.C.; Moniruzzaman, M.; Smirnova, E.; Chin, S.; Sureshbabu, A.; Karthikeyan, A.; Min, T. Curcumin as a Potential Antioxidant in Stress Regulation of Terrestrial, Avian, and Aquatic Animals: A Review. Antioxidants 2023, 12, 1700. https://doi.org/10.3390/antiox12091700
Tuong DTC, Moniruzzaman M, Smirnova E, Chin S, Sureshbabu A, Karthikeyan A, Min T. Curcumin as a Potential Antioxidant in Stress Regulation of Terrestrial, Avian, and Aquatic Animals: A Review. Antioxidants. 2023; 12(9):1700. https://doi.org/10.3390/antiox12091700
Chicago/Turabian StyleTuong, Do Thi Cat, Mohammad Moniruzzaman, Elena Smirnova, Sungyeon Chin, Anjana Sureshbabu, Adhimoolam Karthikeyan, and Taesun Min. 2023. "Curcumin as a Potential Antioxidant in Stress Regulation of Terrestrial, Avian, and Aquatic Animals: A Review" Antioxidants 12, no. 9: 1700. https://doi.org/10.3390/antiox12091700
APA StyleTuong, D. T. C., Moniruzzaman, M., Smirnova, E., Chin, S., Sureshbabu, A., Karthikeyan, A., & Min, T. (2023). Curcumin as a Potential Antioxidant in Stress Regulation of Terrestrial, Avian, and Aquatic Animals: A Review. Antioxidants, 12(9), 1700. https://doi.org/10.3390/antiox12091700








