Predominantly Pro-Inflammatory Phenotype with Mixed M1/M2 Polarization of Peripheral Blood Classical Monocytes and Monocyte-Derived Macrophages among Patients with Excessive Ethanol Intake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Subjects
2.2. Experimental Design
2.3. CD14+ Monocyte Isolation and Polarization into Monocyte-Derived Macrophages
2.4. Analysis of M1/M2-Related Markers and TNFa-Producing Cells after Short-Term M1/M2 Stimulation
2.5. Analysis of M1/M2 Phenotype in Monocyte-Derived Macrophages (MDMs) after 6-Day In Vitro Culture
2.6. Quantitation of Soluble Levels of Inflamatory Cytokines
2.7. RNA Isolation, cDNA Synthesis, and Real-Time PCR
2.8. miRNA Expression Analysis
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Study Cohort
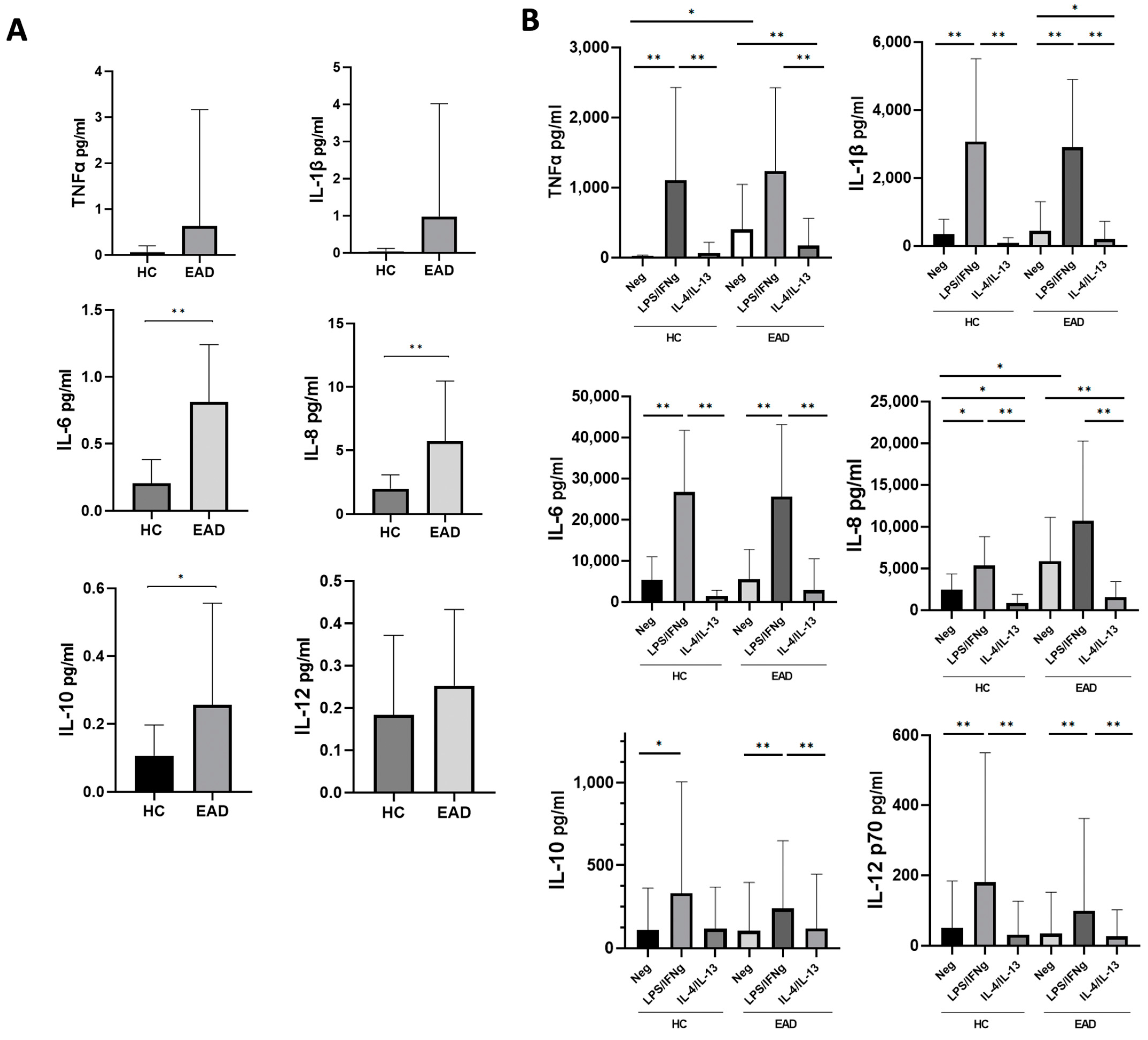
3.2. Levels of Soluble Inflammatory Cytokines and the M1/M2 Phenotype and Production of Cytokines at the mRNA Level of CD14+ PB Monocytes after Short-Term Culture
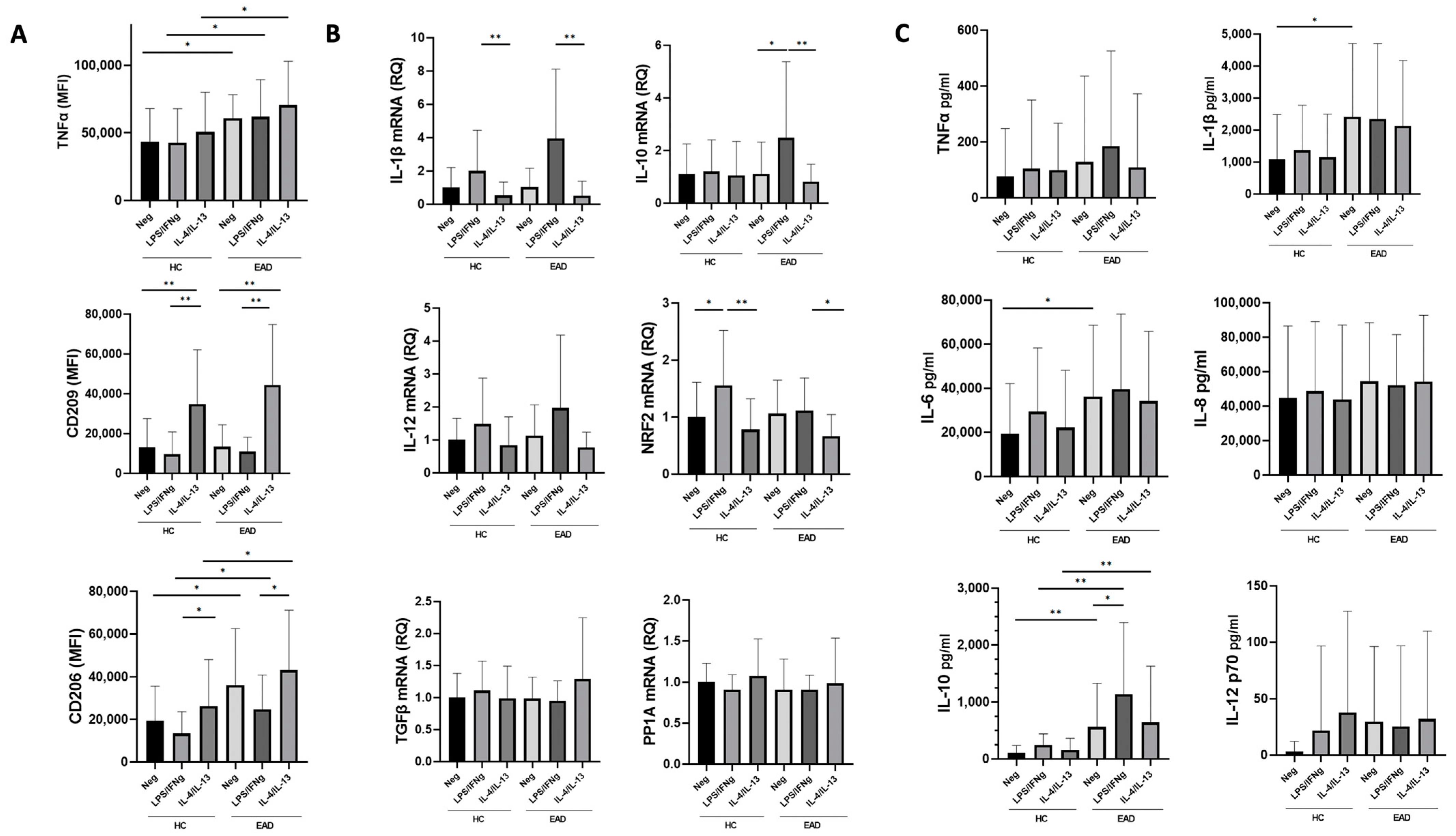
3.3. M1/M2 Phenotype and Production of Inflammatory Cytokines at the mRNA Level by MDMs after Stimulation with LPS/IFNγ and IL-4/IL-13
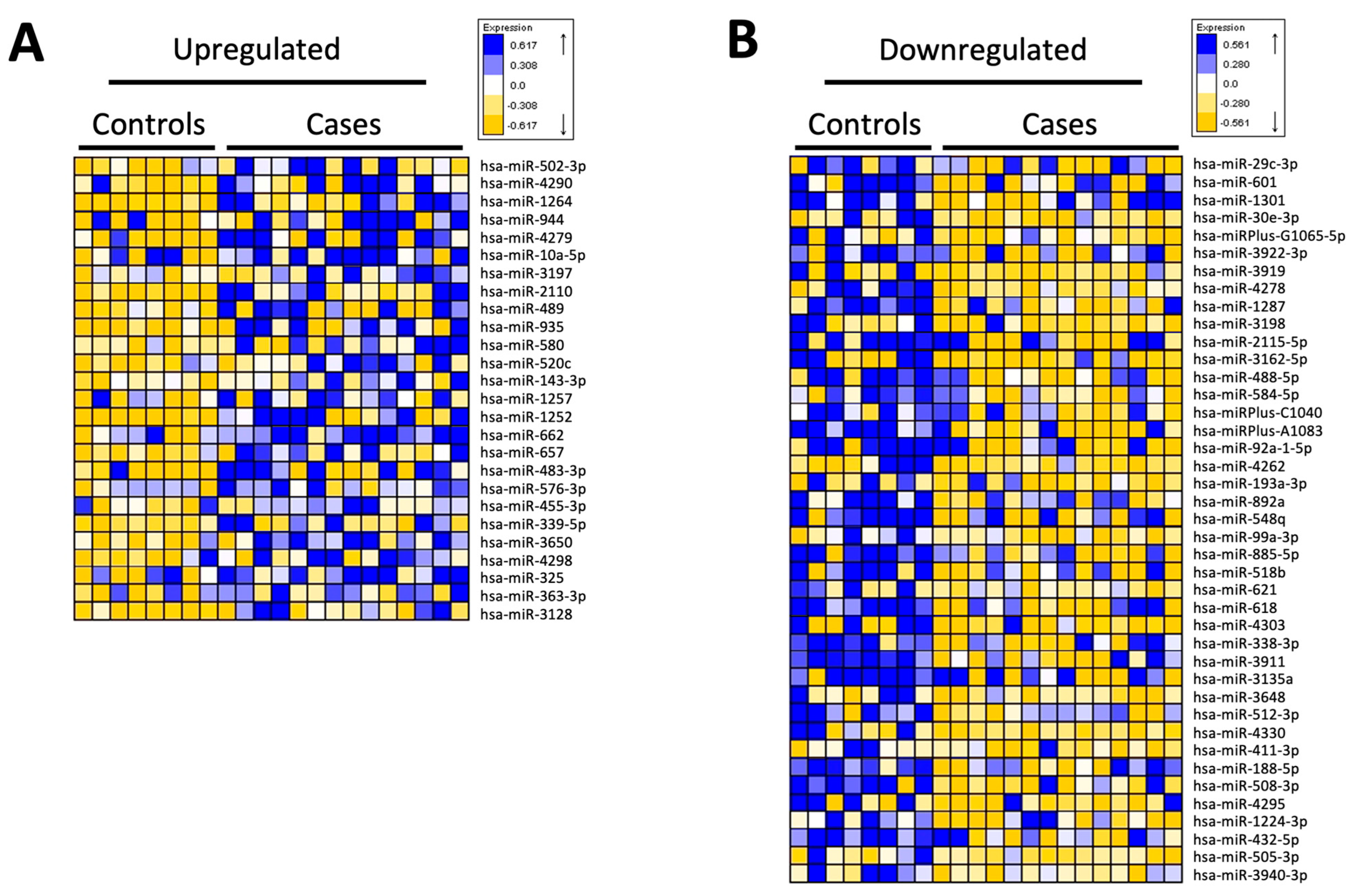
3.4. miRNA Analysis of CD14+ Monocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szabo, G.; Saha, B. Alcohol’s Effect on Host Defense. Alcohol Res. Curr. Rev. 2015, 37, 159–170. [Google Scholar]
- Adams, C.; Conigrave, J.H.; Lewohl, J.; Haber, P.; Morley, K.C. Alcohol Use Disorder and Circulating Cytokines: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2020, 89, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Laso, F.J.; Vaquero, J.M.; Almeida, J.; Marcos, M.; Orfao, A. Chronic Alcohol Consumption Is Associated With Changes in the Distribution, Immunophenotype, and the Inflammatory Cytokine Secretion Profile of Circulating Dendritic Cells. Alcohol Clin. Exp. Res. 2007, 31, 846–854. [Google Scholar] [CrossRef]
- Laso, F.J.; Vaquero, J.M.; Almeida, J.; Marcos, M.; Orfao, A. Production of Inflammatory Cytokines by Peripheral Blood Monocytes in Chronic Alcoholism: Relationship with Ethanol Intake and Liver Disease. Cytom. B Clin. Cytom. 2007, 72B, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Mandrekar, P. Macrophages and Alcohol-Related Liver Inflammation. Alcohol Res. Curr. Rev. 2015, 37, 251–262. [Google Scholar]
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal Role of TLR4 Receptors in Alcohol-Induced Neuroinflammation and Brain Damage. J. Neurosci. 2010, 30, 8285–8295. [Google Scholar] [CrossRef]
- Bala, S.; Marcos, M.; Kodys, K.; Csak, T.; Catalano, D.; Mandrekar, P.; Szabo, G. Up-Regulation of MicroRNA-155 in Macrophages Contributes to Increased Tumor Necrosis Factor α (TNFα) Production via Increased MRNA Half-Life in Alcoholic Liver Disease. J. Biol. Chem. 2011, 286, 1436–1444. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 Suppresses Macrophage Inflammatory Response by Blocking Proinflammatory Cytokine Transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.-C. MiRNA-Mediated Macrophage Polarization and Its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.-N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 Receptors Protect against Alcoholic Liver Disease by Regulating Kupffer Cell Polarization in Mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef]
- Xu, J.; Chi, F.; Guo, T.; Punj, V.; Lee, W.N.P.; French, S.W.; Tsukamoto, H. NOTCH Reprograms Mitochondrial Metabolism for Proinflammatory Macrophage Activation. J. Clin. Investig. 2015, 125, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, L.; You, H.; Cheng, M.; Yang, Y.; Huang, C.; Li, J. Alternative Activation of Macrophages by Prostacyclin Synthase Ameliorates Alcohol Induced Liver Injury. Lab. Investig. J. Tech. Methods Pathol. 2021, 101, 1210–1224. [Google Scholar] [CrossRef]
- Walline, C.C.; Blum, J.S.; Linton, T.; Mangiacarne, D.; Liangpunsakul, S. Early Activation of Peripheral Monocytes with Hallmarks of M1 and M2 Monocytic Cells in Excessive Alcohol Drinkers: A Pilot Study. J. Investig. Med. 2018, 66, 1–4. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Bueno, C.; Almeida, J.; Alguero, M.C.; Sánchez, M.L.; Vaquero, J.M.; Laso, F.J.; San Miguel, J.F.; Escribano, L.; Orfao, A. Flow Cytometric Analysis of Cytokine Production by Normal Human Peripheral Blood Dendritic Cells and Monocytes: Comparative Analysis of Different Stimuli, Secretion-Blocking Agents and Incubation Periods. Cytometry 2001, 46, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Caballero, A.; García-Montero, A.C.; Bueno, C.; Almeida, J.; Varro, R.; Chen, R.; Pandiella, A.; Orfao, A. A New Simple Whole Blood Flow Cytometry-Based Method for Simultaneous Identification of Activated Cells and Quantitative Evaluation of Cytokines Released during Activation. Lab. Investig. J. Tech. Methods Pathol. 2004, 84, 1387–1398. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- García-Calvo, X.; Bolao, F.; Sanvisens, A.; Zuluaga, P.; Tor, J.; Muga, R.; Fuster, D. Significance of Markers of Monocyte Activation (CD163 and SCD14) and Inflammation (IL-6) in Patients Admitted for Alcohol Use Disorder Treatment. Alcohol. Clin. Exp. Res. 2020, 44, 152–158. [Google Scholar] [CrossRef]
- Pascual, M.; Montesinos, J.; Marcos, M.; Torres, J.-L.; Costa-Alba, P.; García-García, F.; Laso, F.-J.; Guerri, C. Gender Differences in the Inflammatory Cytokine and Chemokine Profiles Induced by Binge Ethanol Drinking in Adolescence. Addict. Biol. 2017, 22, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Janicova, A.; Haag, F.; Xu, B.; Garza, A.P.; Dunay, I.R.; Neunaber, C.; Nowak, A.J.; Cavalli, P.; Marzi, I.; Sturm, R.; et al. Acute Alcohol Intoxication Modulates Monocyte Subsets and Their Functions in a Time-Dependent Manner in Healthy Volunteers. Front. Immunol. 2021, 12, 652488. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Richards, S.; Mann, D.; Cross, A.; Smith, G.B.; Netzer, G.; Kovacs, E.; Hasday, J. Acute Immunomodulatory Effects of Binge Alcohol Ingestion. Alcohol 2015, 49, 57–64. [Google Scholar] [CrossRef]
- McKim, S.E.; Gäbele, E.; Isayama, F.; Lambert, J.C.; Tucker, L.M.; Wheeler, M.D.; Connor, H.D.; Mason, R.P.; Doll, M.A.; Hein, D.W.; et al. Inducible Nitric Oxide Synthase Is Required in Alcohol-Induced Liver Injury: Studies with Knockout Mice. Gastroenterology 2003, 125, 1834–1844. [Google Scholar] [CrossRef]
- Feng, R.; Morine, Y.; Ikemoto, T.; Imura, S.; Iwahashi, S.; Saito, Y.; Shimada, M. Nrf2 Activation Drive Macrophages Polarization and Cancer Cell Epithelial-Mesenchymal Transition during Interaction. Cell Commun. Signal. CCS 2018, 16, 54. [Google Scholar] [CrossRef]
- González-Reimers, E.; Santolaria-Fernández, F.; Medina-García, J.A.; González-Pérez, J.M.; de la Vega-Prieto, M.J.; Medina-Vega, L.; Martín-González, C.; Durán-Castellón, M.C. TH-1 and TH-2 Cytokines in Stable Chronic Alcoholics. Alcohol Alcohol. 2012, 47, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J.; Fahlman, C.S. Redox- and Oxidant-Mediated Regulation of Interleukin-10: An Anti-Inflammatory, Antioxidant Cytokine? Biochem. Biophys. Res. Commun. 2002, 297, 163–176. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Wang, X.-Z. Interleukin-10 and Chronic Liver Disease. World J. Gastroenterol. 2006, 12, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Saikia, P.; Nagy, L.E. MiRNAs Involved in M1/M2 Hyperpolarization Are Clustered and Coordinately Expressed in Alcoholic Hepatitis. Front. Immunol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Thevenot, P.; Saravia, J.; Giaimo, J.; Happel, K.I.; Dugas, T.R.; Cormier, S.A. Chronic Alcohol Induces M2 Polarization Enhancing Pulmonary Disease Caused by Exposure to Particulate Air Pollution. Alcohol. Clin. Exp. Res. 2013, 37, 10–1111. [Google Scholar] [CrossRef]
- Saha, B.; Bruneau, J.C.; Kodys, K.; Szabo, G. Alcohol-Induced MiR-27a Regulates Differentiation and M2 Macrophage Polarization of Normal Human Monocytes. J. Immunol. Baltim. Md 1950 2015, 194, 3079–3087. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, X.; Sun, Z.; Yang, Y.X.; Guo, H.; Li, J.; Sun, K.; Wu, R.; Xu, J.; Jiang, Q.; et al. TRPV1 Alleviates Osteoarthritis by Inhibiting M1 Macrophage Polarization via Ca2+/CaMKII/Nrf2 Signaling Pathway. Cell Death Dis. 2021, 12, 504. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ. Res. 2016, 119, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.Y.S.; Glim, J.E.; Stavenuiter, A.W.D.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H.J. Human Macrophage Polarization in Vitro: Maturation and Activation Methods Compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Ascoli, B.M.; Parisi, M.M.; Bristot, G.; Antqueviezc, B.; Géa, L.P.; Colombo, R.; Kapczinski, F.; Guma, F.T.C.R.; Brietzke, E.; Barbé-Tuana, F.M.; et al. Attenuated Inflammatory Response of Monocyte-Derived Macrophage from Patients with BD: A Preliminary Report. Int. J. Bipolar Disord. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.B.; Swardfager, W.; Maurya, P.K.; Graiff, M.Z.; Pedrini, M.; Asevedo, E.; Cassinelli, A.C.; Bauer, M.E.; Cordeiro, Q.; Scott, J.; et al. An Immunological Age Index in Bipolar Disorder: A Confirmatory Factor Analysis of Putative Immunosenescence Markers and Associations with Clinical Characteristics. Int. J. Methods Psychiatr. Res. 2018, 27, e1614. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Abshire, K.M.; Farokhnia, M.; Akhlaghi, F.; Leggio, L. Effect of Oral Alcohol Administration on Plasma Cytokine Concentrations in Heavy Drinking Individuals. Drug Alcohol Depend. 2021, 225, 108771. [Google Scholar] [CrossRef]
- Karabegović, I.; Abozaid, Y.; Maas, S.C.E.; Labrecque, J.; Bos, D.; De Knegt, R.J.; Ikram, M.A.; Voortman, T.; Ghanbari, M. Plasma MicroRNA Signature of Alcohol Consumption: The Rotterdam Study. J. Nutr. 2022, 152, 2677–2688. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Li, Y.-S.; Wu, C.-C.; Wang, K.-C.; Huang, T.-C.; Chen, Z.; Chien, S. Extracellular MicroRNA-92a Mediates Endothelial Cell–Macrophage Communication. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2492–2504. [Google Scholar] [CrossRef]
- Solé, C.; Domingo, S.; Penzo, E.; Moliné, T.; Porres, L.; Aparicio, G.; Ferrer, B.; Cortés-Hernández, J. Downregulation of MiR-885-5p Promotes NF-ΚB Pathway Activation and Immune Recruitment in Cutaneous Lupus Erythematosus. J. Investig. Dermatol. 2023, 143, 209–219. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, G.; Di, Z.; Zhao, Q. MiR-339-5p Inhibits Alcohol-Induced Brain Inflammation through Regulating NF-ΚB Pathway. Biochem. Biophys. Res. Commun. 2014, 452, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, P.; Zhou, F. MiR-489-3p Inhibits TLR4/NF-ΚB Signaling to Prevent Inflammation in Psoriasis. Exp. Ther. Med. 2021, 22, 744. [Google Scholar] [CrossRef] [PubMed]
- Pallarès-Albanell, J.; Zomeño-Abellán, M.T.; Escaramís, G.; Pantano, L.; Soriano, A.; Segura, M.F.; Martí, E. A High-Throughput Screening Identifies MicroRNA Inhibitors That Influence Neuronal Maintenance and/or Response to Oxidative Stress. Mol. Ther. Nucleic Acids 2019, 17, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, X.; Quan, X.; Li, X.; Zhou, B. MiR-92a Family: A Novel Diagnostic Biomarker and Potential Therapeutic Target in Human Cancers. Front. Mol. Biosci. 2019, 6, 98. [Google Scholar] [CrossRef]

| Variable | EAD Patients (n = 20) | Controls (n = 22) | p-Value |
|---|---|---|---|
| Age (years) Sex (M/F) * | 46.6 (10.5) 15/5 (75%/25%) | 42.3 (14.1) 15/7 (68.2%/31.8%) | 0.27 0.63 |
| Total Bilirubin (mg/dL) | 0.7 (0.8) | 0.6 (0.3) | 0.48 |
| AST (U/L) | 43.0 (38.0) | 20.4 (8.5) | 0.02 |
| ALT (U/L) | 49.3 (56.2) | 23.3 (8.2) | 0.05 |
| ALP (U/L) | 86.0 (35.7) | 52.3 (12.8) | 0.001 |
| LDH (U/L) | 171.4 (42.9) | 185.6 (64.4) | 0.42 |
| GGT (U/L) | 194.3 (303.8) | 23.5 (20.7) | 0.02 |
| Proteins (g/dL) | 7.3 (0.6) | 7.5 (0.4) | 0.24 |
| Albumin (g/dL) | 6.0 (1.4) | 4.7 (0.2) | 0.001 |
| Ferritin (ng/mL) | 498.9 (562.4) | 151.3 (112.7) | 0.01 |
| Hemoglobin (g/dL) | 15.2 (1.5) | 15.3 (1.3) | 0.74 |
| Hematocrit (%) | 42.0 (9.9) | 43.5 (7.4) | 0.57 |
| Erythrocyte MCV (fL) | 95.5 (6.8) | 89.2 (4.0) | 0.001 |
| Erythrocyte MCH (pg) | 33.1 (2.8) | 30.5 (1.6) | 0.001 |
| Leukocytes (×103 cells/μL) | 7.6 (1.9) | 6.3 (1.4) | 0.001 |
| Neutrophils (×103 cells/μL) | 4.4 (1.7) | 3.3 (1.0) | 0.008 |
| Lymphocytes (×103 cells/μL) | 2.2 (0.6) | 2.3 (0.6) | 0.47 |
| Platelets (×103 cells/μL) | 214.9 (73.9) | 262.5 (67.8) | 0.03 |
| Total cholesterol (mg/dL) | 212.9 (41.0) | 220.8 (35.7) | 0.51 |
| Triglycerides (mg/dL) | 126.4 (68.6) | 115.4 (50.4) | 0.55 |
| Prothrombin activity (%) | 90.8 (9.3) | 94.9 (6.1) | 0.11 |
| APTT (seconds) | 37.3 (5.1) | 34.7 (2.2) | 0.07 |
| Fibrinogen (mg/dL) | 372.1 (111.8) | 311.0 (57.8) | 0.05 |
| D-Dimer (μg/mL) | 0.5 (0.3) | 0.4 (0.7) | 0.70 |
| miRNAs | Fold-Change |
|---|---|
| hsa-miR-483-3p | +2.906 |
| hsa-miR-95 | +1.834 |
| hsa-miR-339-5p | +1.498 |
| hsa-miR-143-3p | +1.293 |
| hsa-miR-489 | +1.219 |
| hsa-miR-501-3p | +1.198 |
| hsa-miR-92a-3p | +1.128 |
| hsa-miR-10a-5p | +1.093 |
| hsa-miR-885-5p | −2.364 |
| hsa-miR-3176 | −1.721 |
| hsa-miR-188-5p | −1.610 |
| hsa-miR-512-3p | −1.560 |
| hsa-miR-515 | −1.427 |
| hsa-miR-455-3p | −1.422 |
| hsa-miR-30a-3p | −1.374 |
| hsa-miR-1224-3p | −1.370 |
| hsa-miR-421-3p | −1.361 |
| hsa-miR-584-5p | −1.333 |
| Category | Functions Annotation | p-Value | Molecules | # Molecules |
|---|---|---|---|---|
| Hepatocellular carcinoma | Liver cancer | 3.45 × 10−5 | hsa-miR-143-3p hsa-miR-192-5p hsa-miR-193a-3p hsa-miR-29b-3p hsa-miR-483-3p hsa-miR-515 hsa-miR-92a-3p | 7 |
| Liver hyperplasia /Hyperproliferation | Liver cancer | 3.45 × 10−5 | hsa-miR-143-3p hsa-miR-192-5p hsa-miR-193a-3p hsa-miR-29b-3p hsa-miR-483-3p hsa-mir-515 hsa-miR-92a-3p | 7 |
| Liver hepatitis | Chronic hepatitis B | 5.83 × 10−2 | hsa-miR-143-3p | 1 |
| Renal inflammation | lupus nephritis | 1.47 × 10−1 | hsa-miR-92a-3p | 1 |
| Renal nephritis | lupus nephritis | 1.47 × 10−1 | hsa-miR-92a-3p | 1 |
| Liver cirrhosis | Cirrhosis of liver | 2.22 × 10−1 | hsa-miR-143-3p | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Regueras, M.; Carbonell, C.; Salete-Granado, D.; García, J.-L.; Gragera, M.; Pérez-Nieto, M.-Á.; Morán-Plata, F.-J.; Mayado, A.; Torres, J.-L.; Corchete, L.-A.; et al. Predominantly Pro-Inflammatory Phenotype with Mixed M1/M2 Polarization of Peripheral Blood Classical Monocytes and Monocyte-Derived Macrophages among Patients with Excessive Ethanol Intake. Antioxidants 2023, 12, 1708. https://doi.org/10.3390/antiox12091708
Fernández-Regueras M, Carbonell C, Salete-Granado D, García J-L, Gragera M, Pérez-Nieto M-Á, Morán-Plata F-J, Mayado A, Torres J-L, Corchete L-A, et al. Predominantly Pro-Inflammatory Phenotype with Mixed M1/M2 Polarization of Peripheral Blood Classical Monocytes and Monocyte-Derived Macrophages among Patients with Excessive Ethanol Intake. Antioxidants. 2023; 12(9):1708. https://doi.org/10.3390/antiox12091708
Chicago/Turabian StyleFernández-Regueras, María, Cristina Carbonell, Daniel Salete-Granado, Juan-Luis García, Marcos Gragera, María-Ángeles Pérez-Nieto, Francisco-Javier Morán-Plata, Andrea Mayado, Jorge-Luis Torres, Luis-Antonio Corchete, and et al. 2023. "Predominantly Pro-Inflammatory Phenotype with Mixed M1/M2 Polarization of Peripheral Blood Classical Monocytes and Monocyte-Derived Macrophages among Patients with Excessive Ethanol Intake" Antioxidants 12, no. 9: 1708. https://doi.org/10.3390/antiox12091708
APA StyleFernández-Regueras, M., Carbonell, C., Salete-Granado, D., García, J.-L., Gragera, M., Pérez-Nieto, M.-Á., Morán-Plata, F.-J., Mayado, A., Torres, J.-L., Corchete, L.-A., Usategui-Martín, R., Bueno-Martínez, E., Rojas-Pirela, M., Sabio, G., González-Sarmiento, R., Orfao, A., Laso, F.-J., Almeida, J., & Marcos, M. (2023). Predominantly Pro-Inflammatory Phenotype with Mixed M1/M2 Polarization of Peripheral Blood Classical Monocytes and Monocyte-Derived Macrophages among Patients with Excessive Ethanol Intake. Antioxidants, 12(9), 1708. https://doi.org/10.3390/antiox12091708







