Protecting the Eye Lens from Oxidative Stress through Oxygen Regulation
Abstract
1. Introduction
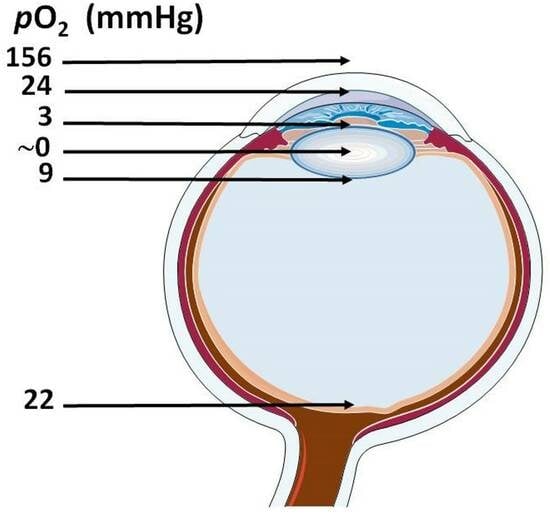
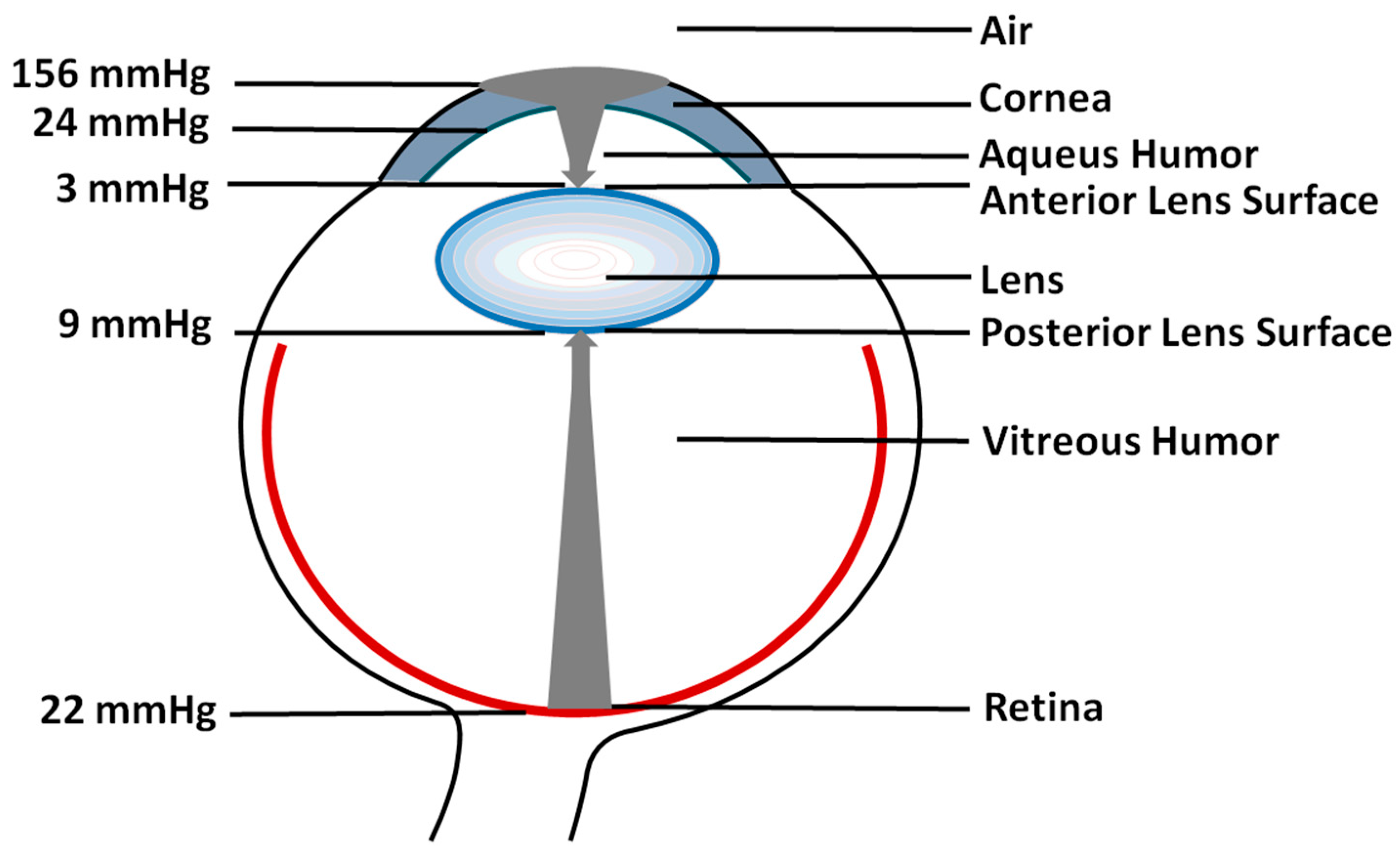
2. Oxygen Partial Pressure and Oxygen Concentration
3. Oxygen Partial Pressure Outside the Lens (First Mechanism)
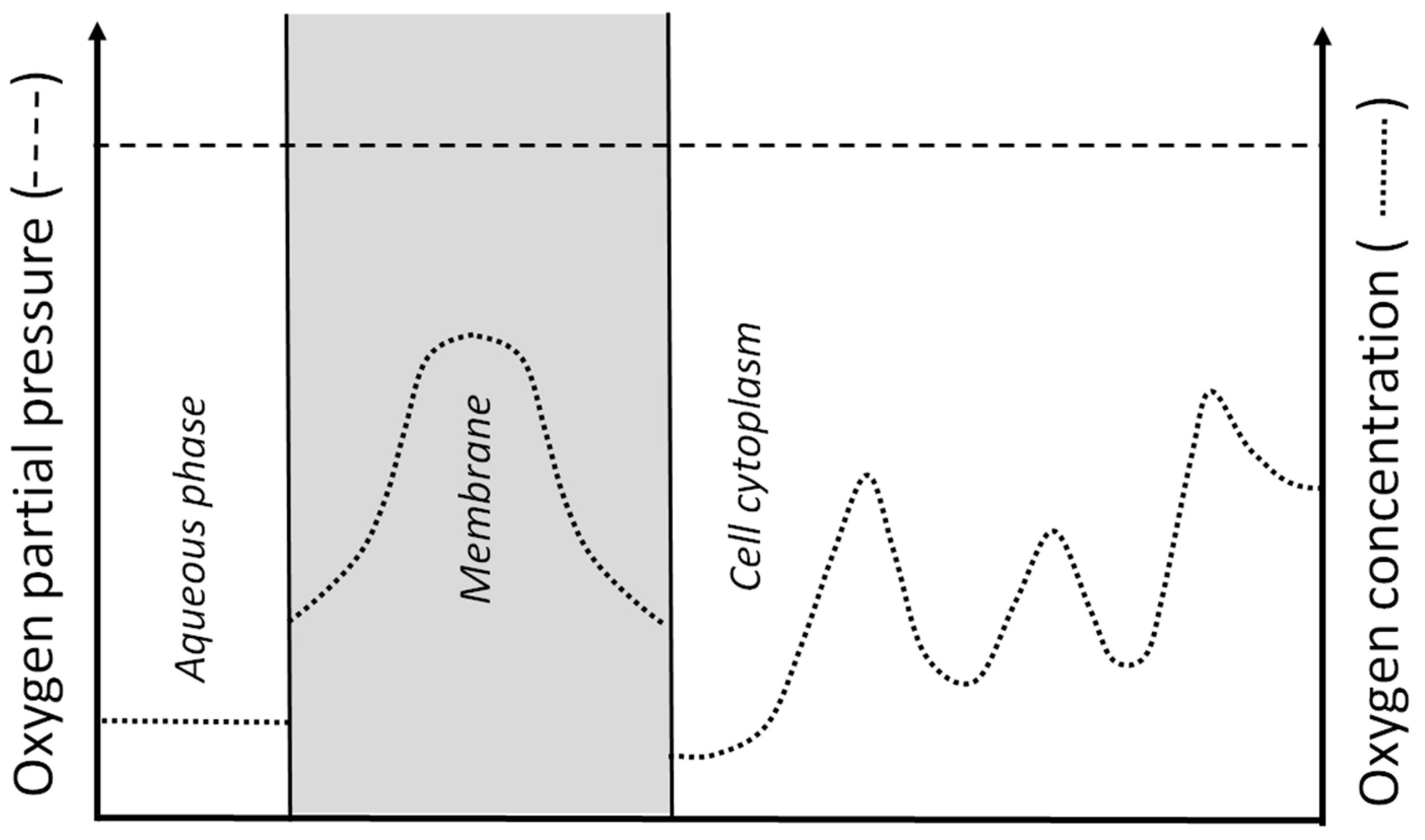
4. Oxygen Consumption within the Lens (Second Mechanism)
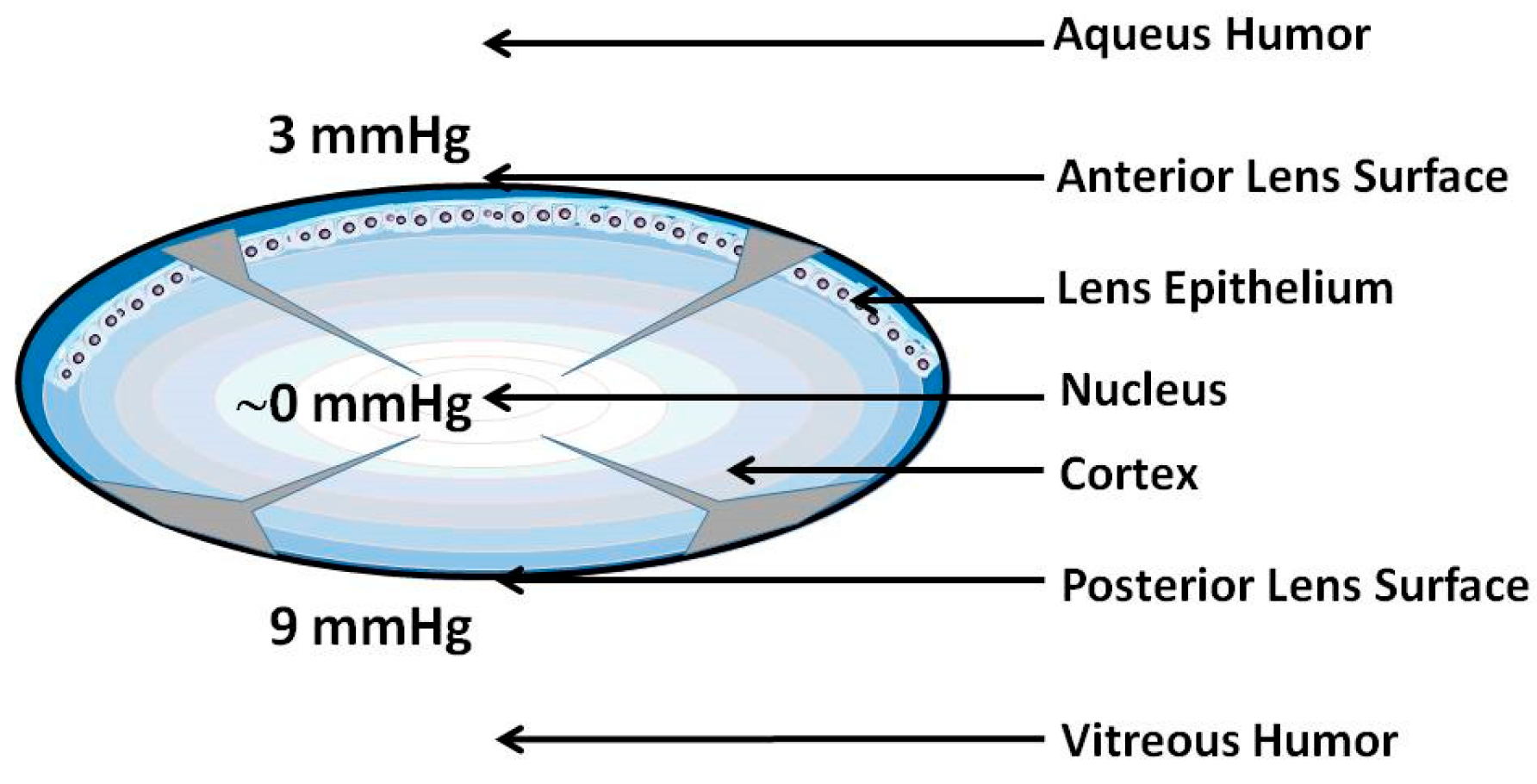
5. Barriers for Oxygen Transport into the Lens Center (Third Mechanism)
6. Resistance of Lens Components to Oxidation
7. Disturbing the Oxygen Partial Pressure around the Lens Promotes Cataract Development
8. Conclusions
- 1.
- Oxygen partial pressure (oxygen concentration) within the lens is very low. Oxygen is delivered to the lens through diffusion. The retina is a very well oxygenated system, with oxygen delivered constantly through the blood vessels.
- 2.
- Regulation of the oxygen partial pressure is the major mechanism protecting the lens against oxidative stress (as was discussed throughout the review). In the retina, molecular oxygen is involved in the creation of all vulnerable conditions for retinal elements, indicated below, with protective mechanisms developed during evolution to diminish the harmful effects of high oxygen partial pressure in the retina.
- 3.
- The lens is avascular with minimal metabolism, and metabolites are delivered to the center of the lens through diffusion. The very high metabolism in the retina requires continuous and intensive delivery of metabolites from the blood. Two major factors protect the lens against the harmful effects of the inflammatory cascade, which can be initiated by cholesterol microcrystals in the cells of other tissue and organs [68,69,70]: First, lens fiber cells lose their intracellular organelles (including inflammasomes) soon after they are formed [1,3], and cholesterol microcrystals cannot activate inflammasomes. Second, the lens is avascular; so, development of an inflammatory cascade is not possible. Thus, cholesterol crystals that are formed in the aged lenses [27] do not disturb lens homeostasis. Although the inflammatory cascade is directly connected with oxidative stress [71], we do not discuss it in this paper. In the retina, the inflammatory cascade can be harmful, as in the case of wet age-related macular degeneration [72,73].
- 4.
- Both the lens and the retina are exposed to light. This can create strongly damaging oxidative stress conditions. A healthy lens is transparent and does not contain light-absorbing molecules, especially photosensitizers. Conversely, the function of the photoreceptors in the retina is to absorb incoming light. The retina also contains a number of photosensitizers such as all-trans retinal, cytochrome c oxidase, and porphyrins [74,75,76,77]. They absorb light and consequently can generate reactive oxygen species and free radicals that can start a damaging oxidative cascade. To decrease the exposure of the retina to the most damaging blue light, macular carotenoids evolved as a blue light filter [78,79]. To increase the effectiveness of this indirect antioxidation action, the pre-receptoral layers of the retina contain a high concentration of macular carotenoids [78,79,80].
- 5.
- The lipids of the lens membranes are highly saturated to resist oxidation (see Section 5). In contrast, to maintain the proper functioning of the photoreceptor machinery, photoreceptor membranes are highly unsaturated with high amounts of easily oxidized polyunsaturated phospholipids.
- 6.
- To protect the retina, in the membranes of retinal pigment epithelium and photoreceptors, raft domains enriched in saturated lipids and cholesterol are present [81,82,83,84]. Raft domains are surrounded by the bulk domain enriched in long-chain (C18–C24) [85,86,87] and very-long-chain (>C24) polyunsaturated phospholipids with 3–9 double bonds [88,89]. Rhodopsin is also located in the bulk domain of the photoreceptor outer segment membrane [81,82,86,90]. Interestingly, macular carotenoids are essentially excluded from raft domains and concentrate in bulk lipid [91,92]. In this location, they can effectively protect vulnerable polyunsaturated phospholipids and rhodopsin through their antioxidant action, according to the most accepted mechanism through which macular carotenoids, lutein and zeaxanthin, protect the retina from age-related macular degeneration [93,94,95,96].
- 7.
- Fiber cells (especially those in the lens nucleus) are considered the longest living cells in the human body (see Section 5). In contrast, the lifespan of photoreceptors is only a few weeks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wride, M.A. Lens fibre cell differentiation and organelle loss: Many paths lead to clarity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1219–1233. [Google Scholar] [CrossRef] [PubMed]
- Bassnett, S. On the mechanism of organelle degradation in the vertebrate lens. Exp. Eye Res. 2009, 88, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bassnett, S. Lens organelle degradation. Exp. Eye Res. 2002, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. Prog. Mol. Biol. Transl. Sci. 2015, 134, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.K.; Bonanno, J.A.; Radke, C.J. Oxygen-deficient metabolism and corneal edema. Prog. Retin. Eye Res. 2011, 30, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Swartz, H.M. EPR Oximetry in Biological and Model Samples. In Biomedical EPR, Part A: Free Radicals, Metals, Medicine, and Physiology; Eaton, S.R., Eaton, G.R., Berliner, L.J., Eds.; Springer: Boston, MA, USA, 2005; pp. 229–282. [Google Scholar] [CrossRef]
- Siegfried, C.J.; Shui, Y.B.; Holekamp, N.M.; Bai, F.; Beebe, D.C. Oxygen distribution in the human eye: Relevance to the etiology of open-angle glaucoma after vitrectomy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5731–5738. [Google Scholar] [CrossRef]
- Beebe, D.C.; Holekamp, N.M.; Siegfried, C.; Shui, Y.B. Vitreoretinal influences on lens function and cataract. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1293–1300. [Google Scholar] [CrossRef]
- Helbig, H.; Hinz, J.P.; Kellner, U.; Foerster, M.H. Oxygen in the anterior chamber of the human eye. Ger. J. Ophthalmol. 1993, 2, 161–164. [Google Scholar]
- McNulty, R.; Wang, H.; Mathias, R.T.; Ortwerth, B.J.; Truscott, R.J.; Bassnett, S. Regulation of tissue oxygen levels in the mammalian lens. J. Physiol. 2004, 559, 883–898. [Google Scholar] [CrossRef]
- Hill, R.M.; Fatt, I. Oxygen uptake from a reservoir of limited volume by the human cornea in vivo. Science 1963, 142, 1295–1297. [Google Scholar] [CrossRef]
- Larke, J.R.; Parrish, S.T.; Wigham, C.G. Apparent human corneal oxygen uptake rate. Am. J. Optom. Physiol. Opt. 1981, 58, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Shui, Y.B.; Liu, M.; Bai, F.; Huang, A.J.; Ma, N.; Beebe, D.C.; Siegfried, C.J. Mitochondrial oxygen metabolism in primary human lens epithelial cells: Association with age, diabetes and glaucoma. Free Radic. Biol. Med. 2016, 97, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yappert, M.C.; Jumblatt, M.M.; Borchman, D. Hyperoxia and thyroxine treatment and the relationships between reactive oxygen species generation, mitochondrial membrane potential, and cardiolipin in human lens epithelial cell cultures. Curr. Eye Res. 2008, 33, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Bantseev, V.L.; Herbert, K.L.; Trevithick, J.R.; Sivak, J.G. Mitochondria of rat lenses: Distribution near and at the sutures. Curr. Eye Res. 1999, 19, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar] [PubMed]
- Seylaz, J.; Pinard, E.; Meric, P.; Correze, J.L. Local cerebral PO2, PCO2, and blood flow measurements by mass spectrometry. Am. J. Physiol. 1983, 245, H513–H518. [Google Scholar] [CrossRef]
- Vaupel, P.; Höckel, M.; Mayer, A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid. Redox Signal. 2007, 9, 1221–1235. [Google Scholar] [CrossRef]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Beebe, D.C.; Shui, Y.B.; Siegfried, C.J.; Holekamp, N.M.; Bai, F. Preserve the (intraocular) environment: The importance of maintaining normal oxygen gradients in the eye. Jpn. J. Ophthalmol. 2014, 58, 225–231. [Google Scholar] [CrossRef]
- Eaton, J.W. Is the lens canned? Free Radic. Biol. Med. 1991, 11, 207–213. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of membranes derived from the total lipids extracted from clear and cataractous lenses of 61–70-year-old human donors. Eur. Biophys. J. 2015, 44, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Byrdwell, W.C.; Yappert, M.C. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3938–3942. [Google Scholar]
- Deeley, J.M.; Mitchell, T.W.; Wei, X.; Korth, J.; Nealon, J.R.; Blanksby, S.J.; Truscott, R.J. Human lens lipids differ markedly from those of commonly used experimental animals. Biochim. Biophys. Acta 2008, 1781, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Yappert, M.C.; Borchman, D. Sphingolipids in human lens membranes: An update on their composition and possible biological implications. Chem. Phys. Lipids 2004, 129, 1–20. [Google Scholar] [CrossRef]
- Yappert, M.C.; Rujoi, M.; Borchman, D.; Vorobyov, I.; Estrada, R. Glycero- versus sphingo-phospholipids: Correlations with human and non-human mammalian lens growth. Exp. Eye Res. 2003, 76, 725–734. [Google Scholar] [CrossRef]
- Raguz, M.; Mainali, L.; O’Brien, W.J.; Subczynski, W.K. Lipid domains in intact fiber-cell plasma membranes isolated from cortical and nuclear regions of human eye lenses of donors from different age groups. Exp. Eye Res. 2015, 132, 78–90. [Google Scholar] [CrossRef][Green Version]
- Li, L.K.; So, L.; Spector, A. Age-dependent changes in the distribution and concentration of human lens cholesterol and phospholipids. Biochim. Biophys. Acta 1987, 917, 112–120. [Google Scholar] [CrossRef]
- Rujoi, M.; Jin, J.; Borchman, D.; Tang, D.; Yappert, M.C. Isolation and Lipid Characterization of Cholesterol-Enriched Fractions in Cortical and Nuclear Human Lens Fibers. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1634–1642. [Google Scholar] [CrossRef]
- Zelenka, P.S. Lens lipids. Curr. Eye Res. 1984, 3, 1337–1359. [Google Scholar] [CrossRef]
- Bassnett, S.; Shi, Y.; Vrensen, G.F. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1250–1264. [Google Scholar] [CrossRef]
- Gonen, T.; Cheng, Y.; Kistler, J.; Walz, T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. J. Mol. Biol. 2004, 342, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Kistler, J.; Bullivant, S. Lens gap junctions and orthogonal arrays are unrelated. FEBS Lett. 1980, 111, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Buzhynskyy, N.; Hite, R.K.; Walz, T.; Scheuring, S. The supramolecular architecture of junctional microdomains in native lens membranes. EMBO Rep. 2007, 8, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Dunia, I.; Cibert, C.; Gong, X.; Xia, C.H.; Recouvreur, M.; Levy, E.; Kumar, N.; Bloemendal, H.; Benedetti, E.L. Structural and immunocytochemical alterations in eye lens fiber cells from Cx46 and Cx50 knockout mice. Eur. J. Cell Biol. 2006, 85, 729–752. [Google Scholar] [CrossRef] [PubMed]
- Zampighi, G.A.; Eskandari, S.; Hall, J.E.; Zampighi, L.; Kreman, M. Micro-domains of AQP0 in lens equatorial fibers. Exp. Eye Res. 2002, 75, 505–519. [Google Scholar] [CrossRef]
- Costello, M.J.; McIntosh, T.J.; Robertson, J.D. Distribution of gap junctions and square array junctions in the mammalian lens. Investig. Ophthalmol. Vis. Sci. 1989, 30, 975–989. [Google Scholar]
- Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Widomska, J.; Stein, N. Chapter 3—Role of cholesterol in maintaining the physical properties of the plasma membrane. In Cholesterol; Bukiya, A.N., Dopico, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 41–71. [Google Scholar] [CrossRef]
- Widomska, J.; Subczynski, W.K.; Mainali, L.; Raguz, M. Cholesterol Bilayer Domains in the Eye Lens Health: A Review. Cell Biochem. Biophys. 2017, 75, 387–398. [Google Scholar] [CrossRef]
- Altenbach, C.; Greenhalgh, D.A.; Khorana, H.G.; Hubbell, W.L. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: Application to spin-labeled mutants of bacteriorhodopsin. Proc. Natl. Acad. Sci. USA 1994, 91, 1667–1671. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Renk, G.E.; Crouch, R.K.; Hyde, J.S.; Kusumi, A. Oxygen diffusion-concentration product in rhodopsin as observed by a pulse ESR spin labeling method. Biophys. J. 1992, 63, 573–577. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Mainali, L.; Raguz, M.; O’Brien, W.J. Organization of lipids in fiber-cell plasma membranes of the eye lens. Exp. Eye Res. 2017, 156, 79–86. [Google Scholar] [CrossRef]
- Nealon, J.R.; Blanksby, S.J.; Abbott, S.K.; Hulbert, A.J.; Mitchell, T.W.; Truscott, R.J. Phospholipid composition of the rat lens is independent of diet. Exp. Eye Res. 2008, 87, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Deeley, J.M.; Blanksby, S.J.; Leisch, F.; Ellis, S.R.; Truscott, R.J.; Mitchell, T.W. Instability of the cellular lipidome with age. Age 2012, 34, 935–947. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.C.; Vermeer, M.A.; Hendriks, A.L.; Bloemendal, H.; Cohen, L.H. Biosynthetic capacity of the human lens upon aging. Exp. Eye Res. 1991, 53, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Lynnerup, N.; Kjeldsen, H.; Heegaard, S.; Jacobsen, C.; Heinemeier, J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS ONE 2008, 3, e1529. [Google Scholar] [CrossRef]
- Huang, L.; Grami, V.; Marrero, Y.; Tang, D.; Yappert, M.C.; Rasi, V.; Borchman, D. Human lens phospholipid changes with age and cataract. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Yappert, M.C.; Afzal, M. Lens lipids and maximum lifespan. Exp. Eye Res. 2004, 79, 761–768. [Google Scholar] [CrossRef]
- Li, L.K.; So, L.; Spector, A. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J. Lipid Res. 1985, 26, 600–609. [Google Scholar] [CrossRef]
- Ravandeh, M.; Coliva, G.; Kahlert, H.; Azinfar, A.; Helm, C.A.; Fedorova, M.; Wende, K. Protective Role of Sphingomyelin in Eye Lens Cell Membrane Model against Oxidative Stress. Biomolecules 2021, 11, 276. [Google Scholar] [CrossRef]
- Paterson, C.A.; Zeng, J.; Husseini, Z.; Borchman, D.; Delamere, N.A.; Garland, D.; Jimenez-Asensio, J. Calcium ATPase activity and membrane structure in clear and cataractous human lenses. Curr. Eye Res. 1997, 16, 333–338. [Google Scholar] [CrossRef]
- Harding, J.J. The biochemical organization of the lens. Trans. Ophthalmol. Soc. 1982, 102 Pt 3, 310–313. [Google Scholar]
- Babizhayev, M.A.; Deyev, A.I.; Linberg, L.F. Lipid peroxidation as a possible cause of cataract. Mech. Ageing Dev. 1988, 44, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Zigman, S.; Paxhia, T.; Marinetti, G.; Girsch, S. Lipids of human lens fiber cell membranes. Curr. Eye Res. 1984, 3, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Mainali, L. Association of Alpha-Crystallin with Fiber Cell Plasma Membrane of the Eye Lens Accompanied by Light Scattering and Cataract Formation. Membranes 2021, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M.; Shui, Y.B.; Beebe, D.C. Vitrectomy surgery increases oxygen exposure to the lens: A possible mechanism for nuclear cataract formation. Am. J. Ophthalmol. 2005, 139, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Bantseev, V.; Oriowo, O.M.; Giblin, F.J.; Leverenz, V.R.; Trevithick, J.R.; Sivak, J.G. Effect of hyperbaric oxygen on guinea pig lens optical quality and on the refractive state of the eye. Exp. Eye Res. 2004, 78, 925–931. [Google Scholar] [CrossRef]
- Palmquist, B.M.; Philipson, B.; Barr, P.O. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br. J. Ophthalmol. 1984, 68, 113–117. [Google Scholar] [CrossRef]
- Giblin, F.J.; Padgaonkar, V.A.; Leverenz, V.R.; Lin, L.R.; Lou, M.F.; Unakar, N.J.; Dang, L.; Dickerson, J.E., Jr.; Reddy, V.N. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp. Eye Res. 1995, 60, 219–235. [Google Scholar] [CrossRef]
- Quiram, P.A.; Leverenz, V.R.; Baker, R.M.; Dang, L.; Giblin, F.J.; Trese, M.T. Microplasmin-induced posterior vitreous detachment affects vitreous oxygen levels. Retina 2007, 27, 1090–1096. [Google Scholar] [CrossRef]
- Giblin, F.J.; Quiram, P.A.; Leverenz, V.R.; Baker, R.M.; Dang, L.; Trese, M.T. Enzyme-induced posterior vitreous detachment in the rat produces increased lens nuclear pO2 levels. Exp. Eye Res. 2009, 88, 286–292. [Google Scholar] [CrossRef]
- Li, Q.; Yan, H.; Ding, T.B.; Han, J.; Shui, Y.B.; Beebe, D.C. Oxidative responses induced by pharmacologic vitreolysis and/or long-term hyperoxia treatment in rat lenses. Curr. Eye Res. 2013, 38, 639–648. [Google Scholar] [CrossRef]
- Michael, R.; Bron, A.J. The ageing lens and cataract: A model of normal and pathological ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1278–1292. [Google Scholar] [CrossRef]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J.W.; Friedrich, M.G. Molecular Processes Implicated in Human Age-Related Nuclear Cataract. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5007–5021. [Google Scholar] [CrossRef] [PubMed]
- Pau, H.; Graf, P.; Sies, H. Glutathione levels in human lens: Regional distribution in different forms of cataract. Exp. Eye Res. 1990, 50, 17–20. [Google Scholar] [CrossRef]
- Xie, P.Y.; Kanai, A.; Nakajima, A.; Kitahara, S.; Ohtsu, A.; Fujii, K. Glutathione and glutathione-related enzymes in human cataractous lenses. Ophthalmic. Res. 1991, 23, 133–140. [Google Scholar] [CrossRef]
- Tulenko, T.N.; Chen, M.; Mason, P.E.; Mason, R.P. Physical effects of cholesterol on arterial smooth muscle membranes: Evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J. Lipid. Res. 1998, 39, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.F.; Mason, R.P. Lipid peroxidation induces cholesterol domain formation in model membranes. J. Biol. Chem. 2005, 280, 39380–39387. [Google Scholar] [CrossRef]
- Preston Mason, R.; Tulenko, T.N.; Jacob, R.F. Direct evidence for cholesterol crystalline domains in biological membranes: Role in human pathobiology. Biochim. Biophys. Acta 2003, 1610, 198–207. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Pasenkiewicz-Gierula, M. Hypothetical Pathway for Formation of Cholesterol Microcrystals Initiating the Atherosclerotic Process. Cell Biochem. Biophys. 2020, 78, 241–247. [Google Scholar] [CrossRef]
- Telander, D.G. Inflammation and age-related macular degeneration (AMD). Semin. Ophthalmol. 2011, 26, 192–197. [Google Scholar] [CrossRef]
- Donoso, L.A.; Kim, D.; Frost, A.; Callahan, A.; Hageman, G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2006, 51, 137–152. [Google Scholar] [CrossRef]
- Boulton, M.; Rózanowska, M.; Rózanowski, B. Retinal photodamage. J. Photochem. Photobiol. B 2001, 64, 144–161. [Google Scholar] [CrossRef]
- Pautler, E.L.; Morita, M.; Beezley, D. Hemoprotein(s) mediate blue light damage in the retinal pigment epithelium. Photochem. Photobiol. 1990, 51, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Gorgels, T.G.; van Norren, D. Ultraviolet and green light cause different types of damage in rat retina. Investig. Ophthalmol. Vis. Sci. 1995, 36, 851–863. [Google Scholar] [CrossRef][Green Version]
- Grimm, C.; Wenzel, A.; Williams, T.; Rol, P.; Hafezi, F.; Remé, C. Rhodopsin-mediated blue-light damage to the rat retina: Effect of photoreversal of bleaching. Investig. Ophthalmol. Vis. Sci. 2001, 42, 497–505. [Google Scholar]
- Junghans, A.; Sies, H.; Stahl, W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 2001, 391, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.; Wooten, B.R.; Engles, M.; Wong, J.C. The influence of filtering by the macular carotenoids on contrast sensitivity measured under simulated blue haze conditions. Vis. Res. 2012, 63, 58–62. [Google Scholar] [CrossRef]
- Machida, N.; Kosehira, M.; Kitaichi, N. Clinical Effects of Dietary Supplementation of Lutein with High Bio-Accessibility on Macular Pigment Optical Density and Contrast Sensitivity: A Randomized Double-Blind Placebo-Controlled Parallel-Group Comparison Trial. Nutrients 2020, 12, 2966. [Google Scholar] [CrossRef] [PubMed]
- Seno, K.; Kishimoto, M.; Abe, M.; Higuchi, Y.; Mieda, M.; Owada, Y.; Yoshiyama, W.; Liu, H.; Hayashi, F. Light- and guanosine 5’-3-O-(thio)triphosphate-sensitive localization of a G protein and its effector on detergent-resistant membrane rafts in rod photoreceptor outer segments. J. Biol. Chem. 2001, 276, 20813–20816. [Google Scholar] [CrossRef]
- Boesze-Battaglia, K.; Dispoto, J.; Kahoe, M.A. Association of a photoreceptor-specific tetraspanin protein, ROM-1, with triton X-100-resistant membrane rafts from rod outer segment disk membranes. J. Biol. Chem. 2002, 277, 41843–41849. [Google Scholar] [CrossRef]
- Nair, K.S.; Balasubramanian, N.; Slepak, V.Z. Signal-dependent translocation of transducin, RGS9-1-Gbeta5L complex, and arrestin to detergent-resistant membrane rafts in photoreceptors. Curr. Biol. 2002, 12, 421–425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, R.E.; Elliott, M.H.; Brush, R.S.; Anderson, R.E. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Rapp, L.M.; Maple, S.S.; Choi, J.H. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1200–1209. [Google Scholar]
- Stinson, A.M.; Wiegand, R.D.; Anderson, R.E. Fatty acid and molecular species compositions of phospholipids and diacylglycerols from rat retinal membranes. Exp. Eye Res. 1991, 52, 213–218. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Liu, A.; Chang, J.; Lin, Y.; Shen, Z.; Bernstein, P.S. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J. Lipid Res. 2010, 51, 3217–3229. [Google Scholar] [CrossRef]
- Rezanka, T. Very-long-chain fatty acids from the animal and plant kingdoms. Prog. Lipid Res. 1989, 28, 147–187. [Google Scholar] [CrossRef]
- Polozova, A.; Litman, B.J. Cholesterol dependent recruitment of di22:6-PC by a G protein-coupled receptor into lateral domains. Biophys. J. 2000, 79, 2632–2643. [Google Scholar] [CrossRef]
- Wisniewska, A.; Subczynski, W.K. Distribution of macular xanthophylls between domains in a model of photoreceptor outer segment membranes. Free Radic. Biol. Med. 2006, 41, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Wisniewska, A.; Widomska, J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch. Biochem. Biophys. 2010, 504, 61–66. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A. Mechanistic evidence for eye diseases and carotenoids. In Carotenoids in Health and Disease, 1st ed.; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Possible biologic mechanisms for a protective role of xanthophylls. J. Nutr. 2002, 132, 540s–542s. [Google Scholar] [CrossRef] [PubMed]
- Cullen, A.P. Photokeratitis and other phototoxic effects on the cornea and conjunctiva. Int. J. Toxicol. 2002, 21, 455–464. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Widomska, J. Protecting the Eye Lens from Oxidative Stress through Oxygen Regulation. Antioxidants 2023, 12, 1783. https://doi.org/10.3390/antiox12091783
Subczynski WK, Pasenkiewicz-Gierula M, Widomska J. Protecting the Eye Lens from Oxidative Stress through Oxygen Regulation. Antioxidants. 2023; 12(9):1783. https://doi.org/10.3390/antiox12091783
Chicago/Turabian StyleSubczynski, Witold Karol, Marta Pasenkiewicz-Gierula, and Justyna Widomska. 2023. "Protecting the Eye Lens from Oxidative Stress through Oxygen Regulation" Antioxidants 12, no. 9: 1783. https://doi.org/10.3390/antiox12091783
APA StyleSubczynski, W. K., Pasenkiewicz-Gierula, M., & Widomska, J. (2023). Protecting the Eye Lens from Oxidative Stress through Oxygen Regulation. Antioxidants, 12(9), 1783. https://doi.org/10.3390/antiox12091783