Natural Products as Dietary Agents for the Prevention and Mitigation of Oxidative Damage and Inflammation in the Intestinal Barrier
Abstract
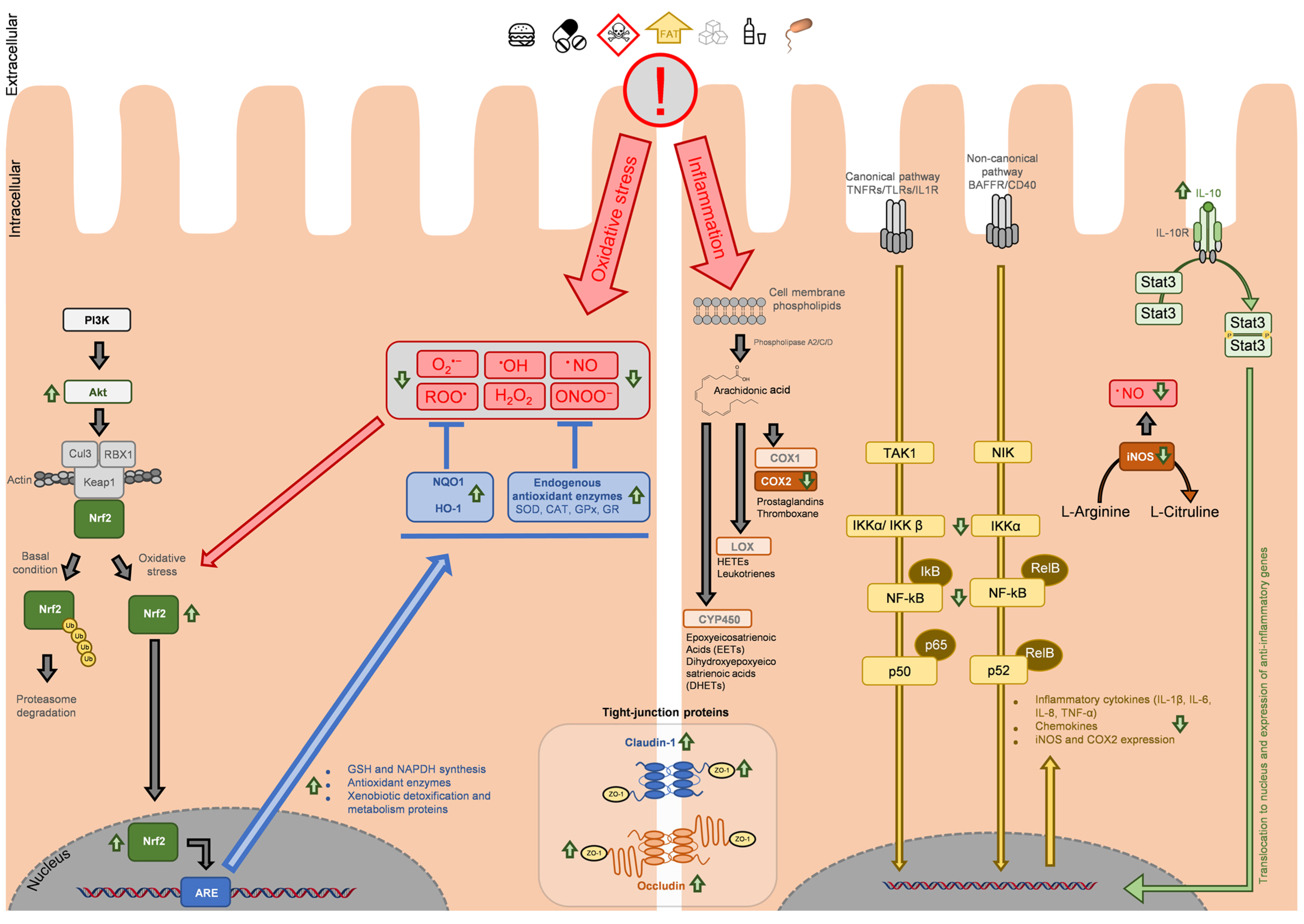
:1. Introduction
2. Antioxidant and Anti-Inflammatory Activity of Natural Compounds at Intestinal Level
2.1. Antioxidant Potential of Extracts against Xenobiotics and Contaminants Resulting from Food Processing
2.2. Antioxidant Potential of Extracts against High Caloric Diet
2.3. Antioxidant Potential of Extracts against Commonly Used Food Additives
2.4. Antioxidant Potential of Extracts against Other Diet or Ingested Components
2.5. Antioxidant Potential of Phytochemicals Commonly Found in the Diet
| Compound | Concentration | Experimental Model | Observations | Ref. |
|---|---|---|---|---|
| Caffeic acid | 250 mg/kg | Intestinal samples from Wistar rat | Decreased cisplatin-induced lipid peroxidation Increased SOD, GST, GR, GPx, and CAT activities | [78] |
| Crocin | 50 mg/kg | Ileum and colon samples from Wistar Rats | Reduced acrylamide-induced oxidative stress Reduced lipid peroxidation Normalized SOD and CAT levels Increased GSH levels Prevented villi degradation | [37] |
| Caffeic acid | 60 and 120 mg/kg | Intestinal sample from Sprague Dawley rats | Reduced ketoprofen-induced oxidative damage Increased GPx and GR activities Increased GSH content HO-1 upregulation | [79] |
| Carvacrol-thymol mixture | 100 mg/kg (1:1) | Jejunum samples from swine | Decreased weaning-induced intestinal oxidative stress Decreased ROS levels and lipid peroxidation Increased SOD and GPx activity | [77] |
| Ellagic acid | 10 mg/kg | Jejunum samples from BALB/c mice | Reduced oxidative stress induced by oxidized fish oil Reduced lipid peroxidation Increased SOD and GPx activity | [80] |
| Punicalin | ||||
| Punicalagin | ||||
| Puerarin | 10 and 50 mg/kg | Colon samples from BALB/c mice | Reduced dextran sulphate sodium-induced oxidative stress Reduced lipid peroxidation Prevented GSH depletion Normalized SOD and CAT activity Normalized Nfr2, HO-1, and NQO1 expression | [81] |
| Eriodictyol | 20 and 50 mg/kg | Colon samples from Wistar rats | Prevented 2,4,6-trinitrobenzenesulfonic acid-induced reduction in SOD, CAT, and GPx levels Increased IL-10 levels Reduced lipid peroxidation | [82] |
| Epigallocatechin gallate | 50 mg/kg | Colon samples from C57BL/6J mice | Reduced dextran sulphate sodium-induced oxidative damage Reduced lipid peroxidation Increased SOD and GPx levels | [83] |
| 25 mg/kg | Small intestine samples from C57BL/6J mice | Prevented morphological alterations induced by ionizing radiation | [73] | |
| 2 µM | Human intestinal epithelial cells (HIEC) | Reduced ROS induced by ionizing radiation Upregulated Nrf2 and HO-1 | [73] | |
| Chlorogenic acid | 25 µM | Porcine intestinal epithelial cells (IPEC-J2) | Reduced extracellular H2O2 content and intracellular ROS levels induced by LPS | [84] |
| 3-Acetyl-11-keto-β-boswellic acid | 27 ng/mL | Human colorectal adenocarcinoma cells (Caco-2) | Reduced H2O2-induced ROS increase and NF-kB expression Prevented downregulation of tight-junction proteins (ZO-1 and occludin) | [59] |
| Resveratrol | 50 µM | Porcine intestinal epithelial cells (IPECJ2 cells) | Reduced H2O2-induced cell death Reduced oxidative stress Increased CAT, GPx, and SOD expression and activities Reversion of H2O2-induced downregulation of claudin-1, occludin and ZO-1 Upregulation of Nrf2, Akt, and Keap1 | [75] |
| Curcumin | 50 µM | Porcine intestinal epithelial cells (IPECJ2 cells) | Reduced H2O2-induced cell death Decreased ROS and lipid peroxidation Increased SOD and CAT levels Increased SOD and GPx expression | [76] |
| Carvacrol | 53.5–214 µM | Human colorectal adenocarcinoma cells (Caco-2) | Decreased H2O2-induced oxidative stress Avoided GSH depletion | [72] |
| Thymol | 62.5–250 µM | |||
| Procyanidin B2 | 10 µM | Human colorectal adenocarcinoma cells (Caco-2) | Reduced acrylamide-induced oxidative stress and cell death Prevented GSH depletion Increased GST and GCL levels | [42] |
| Epicatechin | 10 µM | Human colorectal adenocarcinoma cells (Caco-2) | Reduced acrylamide-induced oxidative stress and cell death Prevented GSH depletion and decreased GST and GCL levels | [42] |
| Caffeic acid | 50 µM | Human intestinal epithelial cells (Int-407) | Reduced ketoprofen-induced ROS Increased GPx and GR activities Nrf2, DJ-1, and HO-1 upregulation | [79] |
| Schisandrin A | 10 µM | Human colorectal adenocarcinoma cells (HT-29) | Reduced deoxynivalenol-induced oxidative stress Increased CAT, SOD, GPx, and GST activity Increased GSH content | [85] |
2.6. Anti-Inflammatory Potential of Plant-Derived Extracts
2.7. Anti-Inflammatory Potential of Phytochemicals Commonly Found in the Diet
| Phytochemical | Concentration | Experimental Model | Observations | Ref. |
|---|---|---|---|---|
| Carvacrol-thymol mixture | 100 mg/kg (1:1) | Jejunum samples from swine | Decreased TNF-α and IL-1β mRNA levels in weaning piglets | [77] |
| Ellagic acid | 10 mg/kg | Jejunum samples from BALB/c mice | Decreased TNF-α, IFN-γ, and IL-6 mRNA expression induced by oxidized fish oil | [80] |
| Punicalin | Decreased TNF-α, IFN-γ, and IL-6 mRNA expression induced by oxidized fish oil | |||
| Punicalagin | Decreased IFN-γ mRNA expression induced by oxidized fish oil | |||
| Puerarin | 10 and 50 mg/kg | Colon samples from BALB/c mice | Reduced DSS-induced TNF-α, IFN-γ, IL-1β, and IL-6 mRNA expression Reduced NO and PGE2 production Reduced COX-2 and iNOS protein and mRNA expression | [81] |
| Curcumin | 100 mg/kg | Colon samples from BALB/c mice | Reduced DSS-induced inflammation Reduced iNOS expression and NO production Decreased TNF-α, IL-1β, and IL-6 mRNA expression Reduced NF-kB activation | [88] |
| 100 mg/kg | Colon samples from Sprague Dawley Rats | Reduce 2,4,6-trinitrobenzenesulfonic acid-induced colitis Reduced expression of NF-kB and IL-27 mRNA Decreased protein expression of TLR4, NF-kB, and IL-27 | [89] | |
| Epigallocatechin gallate | 50 mg/kg | Colon samples from C57BL/6J mice | Reduced DSS-induced inflammation Decreased IL-6 and TNF-α levels | [83] |
| Allicin | 25 and 50 mg/kg | Colon samples from Sprague Dawley Rats | Decreased acrylamide-induced LPS levels Decreased levels of IL-1β, IL-18, TNF-α, and IL-6 Increased IL-10 levels Upregulated tight-junction proteins expression | [43] |
| Berberine | 10 and 20 mg/kg | Colon samples from C3H/HeN mice | Reduced 2,4,6-trinitrobenzenesulfonic acid-induced colitis Decreased IL-1β, TNF-α, and IL-6 levels Increased IL-10 levels Inhibited TLR4, iNOS and COX-2 | [90] |
| Caffeic acid | 60 and 120 mg/kg | Intestinal samples from Sprague Dawley Rats | Reduced ketoprofen-induced NO levels and COX-2 expression | [79] |
| Kaempferol | 50 mg/kg | Colon samples from C57BL/6J mice | Reduced DSS-induced colitis Reduced serum levels of IL-1β, IL-6, and TNF-α Increased IL-10 mRNA expression Decreased mRNA expression of IL-1β, IL-6, COX-2, iNOS, TLR4, NLRP3, MAPK1, and NF-kB Increased mRNA expression of ZO-1, occludin and claudin-1 | [91] |
| Eriodictyol | 20 and 50 mg/kg | Colon samples from Wistar rats | Reduced 2,4,6-trinitrobenzenesulfonic acid-induced colitis Increased IL-10 levels Decreased levels of IL-1β, IL-12, IL-2, TNF-α, and IL-6 Reduced TLR4 expression and NF-kB activation | [82] |
| Naringin | 25–100 mg/kg | Colon samples from C57BL/6J mice | Reduced DSS-induced colitis Reduced IL-1β, TNF-α, and IL-6 levels Decreased NF-kB activation | [92] |
| Chlorogenic acid | 25 and 50 µM | Porcine intestinal epithelial cells (IPEC-J2) | Reduction in LPS-induced TNF-α, IL-8, and IL-6 encoding genes expression and IL-8 and IL-6 levels Reduced COX-2 expression | [84] |
| Resveratrol | 10–50 µM | Human colorectal adenocarcinoma cells (Caco-2) | Reduced LPS-induced COX-2 protein and mRNA expression Reduced PGE2 production Inhibited NF-kB pathway | [93] |
| 3-Acetyl-11-keto-β-boswellic acid | 27 ng/mL | Human colorectal adenocarcinoma cells (Caco-2) | Reduced TNF-α/IFN-γ-induced downregulation of tight-junction proteins (ZO-1 and occludin) Downregulated NF-kB expression Reduced paracellular permeability induced by inflammatory stimuli | [59] |
| Schisandrin A | 10 µM | Human colorectal adenocarcinoma cells (HT-29) | Reduced deoxynivalenol-induced inflammation Decreased COX-2, NF-kB, and MAPK expression Reduced NO, IL-8, and PGE2 levels | [85] |
| Cyanidin-3-O-glucoside | 0.05–0.2 µM | Human colorectal adenocarcinoma cells (Caco-2)/mouse macrophages (RAW 264.7) co-culture | Reduced LPS-induced TNF-α, IL-1β, IL-6, and IL-8 levels in the apical side of transwell model | [94] |
3. Main Molecular Targets in Antioxidant and Anti-Inflammatory Response
4. The Role of Macrophages in Oxidative Stress and Inflammation Management in the Intestinal Barrier
4.1. Inflammatory Bowel Diseases (IBD), Current Available Treatments, and the Use of Phytochemicals in Preventing and Mitigating the Symptoms
4.2. Limitations in Using In Vitro vs. In Vivo Models
5. The Need to Find Correlations between Experimental Data and Clinical Effect and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Stark, C. Guidelines for food and nutrient intake. In Biochemistry, Physiology and Molecular Aspects of Human Nutrition, 3rd ed.; Stipanuk, M.H., Caudill, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 34–47. [Google Scholar]
- Martins-Gomes, C.; Silva, T.L.; Andreani, T.; Silva, A.M. Glyphosate vs. Glyphosate-Based Herbicides Exposure: A Review on Their Toxicity. J. Xenobiotics 2022, 12, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Luo, S.; Deng, J.; Yang, H. Phytochemicals in Chronic Disease Prevention. Nutrients 2023, 15, 4933. [Google Scholar] [CrossRef] [PubMed]
- Cintoni, M.; Palombaro, M.; Maramao, F.S.; Raoul, P.; Egidi, G.; Leonardi, E.; Bianchi, L.; Campione, E.; Rinninella, E.; Gasbarrini, A.; et al. Metabolic Disorders and Psoriasis: Exploring the Role of Nutritional Interventions. Nutrients 2023, 15, 3876. [Google Scholar] [CrossRef] [PubMed]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Fung, F.; Wang, H.-S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Biolato, M.; Manca, F.; Marrone, G.; Cefalo, C.; Racco, S.; Miggiano, G.A.; Valenza, V.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal permeability after Mediterranean diet and low-fat diet in non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and Function of Intestinal Macrophages. Front. Immunol. 2018, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. The Anatomy of the Large Intestine. In Lower Gastrointestinal Tract Surgery: Vol.1, Laparoscopic Procedures; Parker, M., Hohenberger, W., Eds.; Springer Nature: Berlin, Germany, 2019; pp. 27–89. [Google Scholar]
- Ma, Z.F.; Lee, Y.Y. Chapter 7—Small intestine anatomy and physiology. In Clinical and Basic Neurogastroenterology and Motility; Rao, S.S.C., Lee, Y.Y., Ghoshal, U.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 101–111. [Google Scholar]
- Nguyen, H.D.; Aljamaei, H.M.; Stadnyk, A.W. The Production and Function of Endogenous Interleukin-10 in Intestinal Epithelial Cells and Gut Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Fujishima, K.; Kengaku, M. Modeling Intestinal Stem Cell Function with Organoids. Int. J. Mol. Sci. 2021, 22, 10912. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Sheng, Y.H.; Hasnain, S.Z. Mucus and Mucins: The Underappreciated Host Defence System. Front. Cell. Infect. Microbiol. 2022, 12, 744. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef]
- Michael, C. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Mantzouranis, G.; Fafliora, E.; Saridi, M.; Tatsioni, A.; Glanztounis, G.; Albani, E.; Katsanos, K.H.; Christodoulou, D.K. Alcohol and narcotics use in inflammatory bowel disease. Ann. Gastroenterol. 2018, 31, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Marley, A.R.; Nan, H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016, 7, 105–114. [Google Scholar] [PubMed]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Rattan, S.I. Biology of ageing: Principles, challenges and perspectives. Rom. J. Morphol. Embryol.=Rev. Roum. Morphol. Embryol. 2015, 56, 1251–1253. [Google Scholar]
- Francesco, C.; Margherita, M.; Francesca, R.; Abdo, J.; Alice Gerges, G.; Sahar Al, K.; Tarek, B.-A.; Rosalyn, J.; Provvidenza, D.; Angelo, L.; et al. Colorectal Carcinogenesis: Role of Oxidative Stress and Antioxidants. Anticancer Res. 2017, 37, 4759. [Google Scholar]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef]
- Gedik, S.; Erdemli, M.E.; Gul, M.; Yigitcan, B.; Gozukara Bag, H.; Aksungur, Z.; Altinoz, E. Investigation of the protective effects of crocin on acrylamide induced small and large intestine damage in rats. Biotech. Histochem. 2018, 93, 267–276. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 1677–1692. [Google Scholar] [CrossRef]
- Yan, F.; Wang, L.; Zhao, L.; Wang, C.; Lu, Q.; Liu, R. Acrylamide in food: Occurrence, metabolism, molecular toxicity mechanism and detoxification by phytochemicals. Food Chem. Toxicol. 2023, 175, 113696. [Google Scholar] [CrossRef] [PubMed]
- Amirshahrokhi, K. Acrylamide exposure aggravates the development of ulcerative colitis in mice through activation of NF-κB, inflammatory cytokines, iNOS, and oxidative stress. Iran. J. Basic Med. Sci. 2021, 24, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Lu, J.; Nie, C.; Guo, Z.; Li, C.; Yu, Q.; Xie, J.; Chen, Y. Combined Effects of Acrylamide and Ochratoxin A on the Intestinal Barrier in Caco-2 Cells. Foods 2023, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martín, M.Á. Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway. J. Nutr. Biochem. 2011, 22, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lu, L.; Bo, N.; Chaoyue, Y.; Haiyang, Y. Allicin Ameliorates Intestinal Barrier Damage via Microbiota-Regulated Short-Chain Fatty Acids-TLR4/MyD88/NF-κB Cascade Response in Acrylamide-Induced Rats. J. Agric. Food Chem. 2021, 69, 12837–12852. [Google Scholar] [CrossRef] [PubMed]
- Chudy, S.; Teichert, J. Oxysterols in stored powders as potential health hazards. Sci. Rep. 2021, 11, 21192. [Google Scholar] [CrossRef] [PubMed]
- Incani, A.; Serra, G.; Atzeri, A.; Melis, M.P.; Serreli, G.; Bandino, G.; Sedda, P.; Campus, M.; Tuberoso, C.I.G.; Deiana, M. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem. Toxicol. 2016, 90, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.P.; Tuberoso, C.I.G.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols -induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-k.; Chen, G.; Wang, R.-J.; Peng, J. Oregano essential oil decreased susceptibility to oxidative stress-induced dysfunction of intestinal epithelial barrier in rats. J. Funct. Foods 2015, 18, 1191–1199. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano Essential Oil Induces SOD1 and GSH Expression through Nrf2 Activation and Alleviates Hydrogen Peroxide-Induced Oxidative Damage in IPEC-J2 Cells. Oxidative Med. Cell. Longev. 2016, 2016, 5987183. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, H.; Zhao, Q.; Wang, X.; Zhang, J.; Zhao, X. Polyphenol-Rich Loquat Fruit Extract Prevents Fructose-Induced Nonalcoholic Fatty Liver Disease by Modulating Glycometabolism, Lipometabolism, Oxidative Stress, Inflammation, Intestinal Barrier, and Gut Microbiota in Mice. J. Agric. Food Chem. 2019, 67, 7726–7737. [Google Scholar] [CrossRef]
- Fernando, F.A.; Denis, R.; Geneviève, P.; Stéphanie, D.; Sébastien, M.; Thibault, V.V.; Carole, G.; Quentin, M.; Yves, D.; Emile, L.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Y.-L.; Zhang, X.; Wang, K.-B.; Huang, J.-A.; Liu, Z.-H.; Zhu, M.-Z. Polyphenols from Fu Brick Tea Reduce Obesity via Modulation of Gut Microbiota and Gut Microbiota-Related Intestinal Oxidative Stress and Barrier Function. J. Agric. Food Chem. 2021, 69, 14530–14543. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT–mTOR–S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Zhang, G.; Sun, M.; He, S.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020, 11, 7817–7829. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Teng, X.; Guo, P.; Zuo, Y.; Zhao, H.; Wang, P.; Liang, H. Garlic oil alleviates high triglyceride levels in alcohol-exposed rats by inhibiting liver oxidative stress and regulating the intestinal barrier and intestinal flora. Food Sci. Nutr. 2022, 10, 2479–2495. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus Extract Attenuates Inflammation and Oxidative Stress in Intestinal Epithelial Cells via NF-κB Activation and Nrf2 Response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef] [PubMed]
- Shil, A.; Olusanya, O.; Ghufoor, Z.; Forson, B.; Marks, J.; Chichger, H. Artificial Sweeteners Disrupt Tight Junctions and Barrier Function in the Intestinal Epithelium through Activation of the Sweet Taste Receptor, T1R3. Nutrients 2020, 12, 1862. [Google Scholar] [CrossRef]
- Santos, P.S.; Caria, C.R.P.; Gotardo, E.M.F.; Ribeiro, M.L.; Pedrazzoli, J.; Gambero, A. Artificial sweetener saccharin disrupts intestinal epithelial cells’ barrier function in vitro. Food Funct. 2018, 9, 3815–3822. [Google Scholar] [CrossRef]
- Hanawa, Y.; Higashiyama, M.; Kurihara, C.; Tanemoto, R.; Ito, S.; Mizoguchi, A.; Nishii, S.; Wada, A.; Inaba, K.; Sugihara, N.; et al. Acesulfame potassium induces dysbiosis and intestinal injury with enhanced lymphocyte migration to intestinal mucosa. J. Gastroenterol. Hepatol. 2021, 36, 3140–3148. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress–Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Wheildon, N.; Ishikawa, S. Food Additive P-80 Impacts Mouse Gut Microbiota Promoting Intestinal Inflammation, Obesity and Liver Dysfunction. SOJ Microbiol. Infect. Dis. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Fu, G.; Xuan, R.; Zhai, L.; Lu, Y.; Tang, M.; Liu, J.; Zhang, C.; Chen, H.; Wang, F. Food additive sodium bisulfite induces intracellular imbalance of biothiols levels in NCM460 colonic cells to trigger intestinal inflammation in mice. Toxicol. Lett. 2022, 359, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhakim, Y.M.; Hashem, M.M.M.; Abo-El-Sooud, K.; Ali, H.A.; Anwar, A.; El-Metwally, A.E.; Mahmoud, E.A.; Moustafa, G.G. Involvement of tumor necrosis factor-α, interferon gamma-γ, and interleukins 1β, 6, and 10 in immunosuppression due to long-term exposure to five common food preservatives in rats. Gene 2020, 742, 144590. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Toratani, S.; Shea-Donohue, T.; Kashiwabara, Y.; Vogel, S.N.; Metcalf, E.S. Pro- and Anti-Inflammatory Gene Expression in the Murine Small Intestine and Liver After Chronic Exposure to Alcohol. Alcohol. Clin. Exp. Res. 2001, 25, 579–589. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Farhadi, A.; Forsyth, C.B.; Rangan, J.; Jakate, S.; Shaikh, M.; Banan, A.; Fields, J.Z. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 2009, 50, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Kitts, D.D. Flavonoid composition of orange peel extract ameliorates alcohol-induced tight junction dysfunction in Caco-2 monolayer. Food Chem. Toxicol. 2017, 105, 398–406. [Google Scholar] [CrossRef]
- Xia, T.; Duan, W.; Zhang, Z.; Li, S.; Zhao, Y.; Geng, B.; Zheng, Y.; Yu, J.; Wang, M. Polyphenol-rich vinegar extract regulates intestinal microbiota and immunity and prevents alcohol-induced inflammation in mice. Food Res. Int. 2021, 140, 110064. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. Vitr. 2015, 29, 647–656. [Google Scholar] [CrossRef]
- Xie, L.-W.; Cai, S.; Zhao, T.-S.; Li, M.; Tian, Y. Green tea derivative (−)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radic. Biol. Med. 2020, 161, 175–186. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol–thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2016, 11, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Arivarasu, N.A.; Priyamvada, S.; Mahmood, R. Oral administration of caffeic acid ameliorates the effect of cisplatin on brush border membrane enzymes and antioxidant system in rat intestine. Exp. Toxicol. Pathol. 2013, 65, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-T.; Ho, C.-Y.; Jhang, J.-J.; Lu, C.-C.; Yen, G.-C. DJ-1 plays an important role in caffeic acid-mediated protection of the gastrointestinal mucosa against ketoprofen-induced oxidative damage. J. Nutr. Biochem. 2014, 25, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-q.; Tao, X.; Men, X.-m.; Xu, Z.-w.; Wang, T. In vitro and in vivo antioxidant activities of three major polyphenolic compounds in pomegranate peel: Ellagic acid, punicalin, and punicalagin. J. Integr. Agric. 2017, 16, 1808–1818. [Google Scholar] [CrossRef]
- Jeon, Y.-D.; Lee, J.-H.; Lee, Y.-M.; Kim, D.-K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef]
- Hu, L.-H.; Liu, J.-Y.; Yin, J.-B. Eriodictyol attenuates TNBS-induced ulcerative colitis through repressing TLR4/NF-kB signaling pathway in rats. Kaohsiung J. Med. Sci. 2021, 37, 812–818. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Palócz, O.; Pászti-Gere, E.; Gálfi, P.; Farkas, O. Chlorogenic Acid Combined with Lactobacillus plantarum 2142 Reduced LPS-Induced Intestinal Inflammation and Oxidative Stress in IPEC-J2 Cells. PLoS ONE 2016, 11, e0166642. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Turner, P.C.; Co, V.A.; Wang, M.F.; Amiri, K.M.A.; El-Nezami, H. Schisandrin A protects intestinal epithelial cells from deoxynivalenol-induced cytotoxicity, oxidative damage and inflammation. Sci. Rep. 2019, 9, 19173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-T.; Lu, C.-C.; Yen, G.-C. Phytochemicals enhance antioxidant enzyme expression to protect against NSAID-induced oxidative damage of the gastrointestinal mucosa. Mol. Nutr. Food Res. 2017, 61, 1600659. [Google Scholar] [CrossRef] [PubMed]
- Romier-Crouzet, B.; Van De Walle, J.; During, A.; Joly, A.; Rousseau, C.; Henry, O.; Larondelle, Y.; Schneider, Y.-J. Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem. Toxicol. 2009, 47, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Kao, N.-J.; Hu, J.-Y.; Wu, C.-S.; Kong, Z.-L. Curcumin represses the activity of inhibitor-κB kinase in dextran sulfate sodium-induced colitis by S-nitrosylation. Int. Immunopharmacol. 2016, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhan, L.; Liao, H.; Chen, L.; Lv, X. Curcumin Improves TNBS-Induced Colitis in Rats by Inhibiting IL-27 Expression via the TLR4/NF-κB Signaling Pathway. Planta Med. 2013, 29, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-A.; Hyun, Y.-J.; Kim, D.-H. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur. J. Pharmacol. 2010, 648, 162–170. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Xu, F.; Zhao, S.; Wu, X.; Wang, Y.; Xie, J. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-κB Axis. Front. Immunol. 2021, 12, 679897. [Google Scholar] [CrossRef]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. Vitr. 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Yang, M.; Lu, X.; Xu, J.; Liu, X.; Zhang, W.; Guan, R.; Zhong, H. Cellular uptake, transport mechanism and anti-inflammatory effect of cyanidin-3-glucoside nanoliposomes in Caco-2/RAW 264.7 co-culture model. Front. Nutr. 2022, 9, 995391. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Nakkarach, A.; Foo, H.L.; Song, A.A.-L.; Mutalib, N.E.A.; Nitisinprasert, S.; Withayagiat, U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb. Cell Factories 2021, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio Tejo, F.; Quintanilla, R.A. Contribution of the Nrf2 Pathway on Oxidative Damage and Mitochondrial Failure in Parkinson and Alzheimer’s Disease. Antioxidants 2021, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z.-y.; Kong, A.-N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.P. The antioxidant response element and oxidative stress modifiers in airway diseases. Curr. Mol. Med. 2008, 8, 376–383. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Sternberg, P.; Cai, J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1671–1678. [Google Scholar] [CrossRef]
- Eggler, A.L.; Liu, G.; Pezzuto, J.M.; van Breemen, R.B.; Mesecar, A.D. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA 2005, 102, 10070–10075. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ouyang, Y.; Lu, N.; Li, N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front. Immunol. 2020, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.L.; Rios-Arce, N.D.; Atkinson, S.; Bierhalter, H.; Schoenherr, D.; Bazil, J.N.; McCabe, L.R.; Parameswaran, N. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol. Rep. 2017, 5, e13263. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-X.; Wang, B.; Li, B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front. Immunol. 2020, 11, 1315. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, H.; Fu, Y.; Gu, Y.; Zou, H.; Yuan, Y.; Gu, J.; Liu, Z.; Bian, J. Role of PI3K/Akt-Mediated Nrf2/HO-1 Signaling Pathway in Resveratrol Alleviation of Zearalenone-Induced Oxidative Stress and Apoptosis in TM4 Cells. Toxins 2022, 14, 733. [Google Scholar] [CrossRef]
- Zhan, X.; Li, J.; Zhou, T. Targeting Nrf2-Mediated Oxidative Stress Response Signaling Pathways as New Therapeutic Strategy for Pituitary Adenomas. Front. Pharmacol. 2021, 12, 565748. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Ramkumar, K.M. Chapter 12—Redox Sensitive Transcription via Nrf2-Keap1 in Suppression of Inflammation. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 149–161. [Google Scholar]
- Souto, E.B.; Sampaio, A.C.; Campos, J.R.; Martins-Gomes, C.; Aires, A.; Silva, A.M. Chapter 2—Polyphenols for skin cancer: Chemical properties, structure-related mechanisms of action and new delivery systems. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 63, pp. 21–42. [Google Scholar]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A Review on its Anti-Inflammatory Properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef]
- Shin, H.S.; Satsu, H.; Bae, M.-J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, H. Predisposition of Inflammatory Bowel Disease Is Influenced by IL-8, IL-10, and IL-18 Polymorphisms: A Meta-Analysis. Int. Arch. Allergy Immunol. 2020, 181, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Dabkeviciene, D.; Jonusiene, V.; Zitkute, V.; Zalyte, E.; Grigaitis, P.; Kirveliene, V.; Sasnauskiene, A. The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116. Med. Oncol. 2015, 32, 258. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Gu, C.; Zhou, C.; Yang, Y.; Chen, C.; Zeng, B.; Wu, J.; Lu, W.; Wang, W.; Sun, Z.; et al. Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. Cell Commun. Signal. 2020, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Ferretti, G.; Cognetti, F.; Milella, M.; Ciuffreda, L. Colorectal cancer stem cells properties and features: Evidence of interleukin-8 involvement. Cancer Drug Resist. (Alhambra Calif.) 2019, 2, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Papoutsopoulou, S.; Pollock, L.; Walker, C.; Tench, W.; Samad, S.S.; Bergey, F.; Lenzi, L.; Sheibani-Tezerji, R.; Rosenstiel, P.; Alam, M.T.; et al. Impact of Interleukin 10 Deficiency on Intestinal Epithelium Responses to Inflammatory Signals. Front. Immunol. 2021, 12, 690817. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Romero, L.; Riveron, R.; Flores, C.; Kanagavelu, S.; Chung, K.D.; Alonso, A.; Sotolongo, J.; Ruiz, J.; Manukyan, A.; et al. Human intestinal epithelial cells express interleukin-10 through Toll-like receptor 4-mediated epithelial-macrophage crosstalk. J. Innate Immun. 2015, 7, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Shouval, D.S.; Ouahed, J.; Biswas, A.; Goettel, J.A.; Horwitz, B.H.; Klein, C.; Muise, A.M.; Snapper, S.B. Chapter Five—Interleukin 10 Receptor Signaling: Master Regulator of Intestinal Mucosal Homeostasis in Mice and Humans. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 122, pp. 177–210. [Google Scholar]
- Denning, T.L.; Campbell, N.A.; Song, F.; Garofalo, R.P.; Klimpel, G.R.; Reyes, V.E.; Ernst, P.B. Expression of IL-10 receptors on epithelial cells from the murine small and large intestine. Int. Immunol. 2000, 12, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the Crosstalk between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.-Y.; Kim, J.H.; Kang, J.-E.; Park, M.H.; Kim, E.-J.; et al. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, W.; Li, X.; Chen, W.; Liu, Z.; Wen, J.; Liu, Z. A Protective Role of the NRF2-Keap1 Pathway in Maintaining Intestinal Barrier Function. Oxidative Med. Cell. Longev. 2019, 2019, 1759149. [Google Scholar] [CrossRef]
- Brahmi, F.; Nury, T.; Debbabi, M.; Hadj-Ahmed, S.; Zarrouk, A.; Prost, M.; Madani, K.; Boulekbache-Makhlouf, L.; Lizard, G. Evaluation of Antioxidant, Anti-Inflammatory and Cytoprotective Properties of Ethanolic Mint Extracts from Algeria on 7-Ketocholesterol-Treated Murine RAW 264.7 Macrophages. Antioxidants 2018, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Han, S.-D.; Kim, M.; Mony, T.J.; Lee, E.-S.; Kim, K.-M.; Choi, S.-H.; Hong, S.H.; Choi, J.W.; Park, S.J. Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice. Antioxidants 2021, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Ma, A.; Bao, Y.; Wang, M.; Sun, Z. In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Sousa, C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants 2022, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Balatinácz, A.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Anti-inflammatory effect of lavender (Lavandula angustifolia Mill.) essential oil prepared during different plant phenophases on THP-1 macrophages. BMC Complement. Med. Ther. 2021, 21, 287. [Google Scholar] [CrossRef]
- Zuzarte, M.; Francisco, V.; Neves, B.; Liberal, J.; Cavaleiro, C.; Canhoto, J.; Salgueiro, L.; Cruz, M.T. Lavandula viridis L’Hér. Essential Oil Inhibits the Inflammatory Response in Macrophages Through Blockade of NF-KB Signaling Cascade. Front. Pharmacol. 2022, 12, 695911. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.B.; Rahali, F.Z.; Nehme, R.; Falleh, H.; Jemaa, M.B.; Sellami, I.H.; Ksouri, R.; Bouhallab, S.; Ceciliani, F.; Abdennebi-Najar, L.; et al. Anti-inflammatory activity of essential oils from Tunisian aromatic and medicinal plants and their major constituents in THP-1 macrophages. Food Res. Int. 2023, 167, 112678. [Google Scholar] [CrossRef] [PubMed]
- González-Chávez, M.M.; Ramos-Velázquez, C.S.; Serrano-Vega, R.; Pérez-González, C.; Sánchez-Mendoza, E.; Pérez-Gutiérrez, S. Anti-inflammatory activity of standardized dichloromethane extract of Salvia connivens on macrophages stimulated by LPS. Pharm. Biol. 2017, 55, 1467–1472. [Google Scholar] [CrossRef]
- Sudaramoorthy, A.; Shanmugam, G.; Shanmugam, N. Inhibitory effect of Salvia coccinea on inflammatory responses through NF-κB signaling pathways in THP-1 cells and acute rat diabetes mellitus. Acta Histochem. 2021, 123, 151735. [Google Scholar] [CrossRef]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Cappello, M.S.; Dugay, A.; Deguin, B.; Lauria, G.; Cappello, A.R.; Tundis, R. New Insights into the Antioxidant and Anti-Inflammatory Effects of Italian Salvia officinalis Leaf and Flower Extracts in Lipopolysaccharide and Tumor-Mediated Inflammation Models. Antioxidants 2021, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Félix, L.M.; Teixeira, I.; Martins-Gomes, C.; Schäfer, J.; Souto, E.B.; Santos, D.J.; Bunzel, M.; Nunes, F.M. Orange thyme: Phytochemical profiling, in vitro bioactivities of extracts and potential health benefits. Food Chem. X 2021, 12, 100171. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Taghouti, M.; Schäfer, J.; Bunzel, M.; Silva, A.M.; Nunes, F.M. Chemical characterization and bioactive properties of decoctions and hydroethanolic extracts of Thymus carnosus Boiss. J. Funct. Foods 2018, 43, 154–164. [Google Scholar] [CrossRef]
- Oliveira, J.R.d.; de Jesus Viegas, D.; Martins, A.P.R.; Carvalho, C.A.T.; Soares, C.P.; Camargo, S.E.A.; Jorge, A.O.C.; de Oliveira, L.D. Thymus vulgaris L. extract has antimicrobial and anti-inflammatory effects in the absence of cytotoxicity and genotoxicity. Arch. Oral Biol. 2017, 82, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Marrelli, M.; Menichini, F.; Tundis, R.; Statti, G.A.; Solimene, U.; Menichini, F. Chemical composition and protective effect of oregano (Origanum heracleoticum L.) ethanolic extract on oxidative damage and on inhibition of NO in LPS-stimulated RAW 264.7 macrophages. J. Enzym. Inhib. Med. Chem. 2011, 26, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Menichini, F.; Conforti, F.; Tundis, R.; Bonesi, M.; Saab, A.M.; Statti, G.A.; Cindio, B.D.; Houghton, P.J.; Menichini, F.; et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem. 2009, 117, 174–180. [Google Scholar] [CrossRef]
- Mir, R.H.; Sawhney, G.; Verma, R.; Ahmad, B.; Kumar, P.; Ranjana, S.; Bhagat, A.; Madishetti, S.; Ahmed, Z.; Jachak, S.M.; et al. Origanum vulgare L.: In vitro Assessment of Cytotoxicity, Molecular Docking Studies, Antioxidant and Anti-inflammatory Activity in LPS Stimulated RAW 264.7 Cells. Med. Chem. 2021, 17, 983–993. [Google Scholar] [CrossRef]
- Pepe, G.; Sommella, E.; Manfra, M.; De Nisco, M.; Tenore, G.C.; Scopa, A.; Sofo, A.; Marzocco, S.; Adesso, S.; Novellino, T.; et al. Evaluation of anti-inflammatory activity and fast UHPLC–DAD–IT-TOF profiling of polyphenolic compounds extracted from green lettuce (Lactuca sativa L.; var. Maravilla de Verano). Food Chem. 2015, 167, 153–161. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kim, Y.; Ryu, S.I.; Lee, M.; Lee, H.-J.; Lim, Y.P.; Paik, J.K. Anti-inflammatory effect from extracts of Red Chinese cabbage and Aronia in LPS-stimulated RAW 264.7 cells. Food Sci. Nutr. 2020, 8, 1898–1903. [Google Scholar] [CrossRef]
- Jung, H.A.; Karki, S.; Ehom, N.Y.; Yoon, M.H.; Kim, E.J.; Choi, J.S. Anti-Diabetic and Anti-Inflammatory Effects of Green and Red Kohlrabi Cultivars (Brassica oleracea var. gongylodes). Prev. Nutr. Food Sci. 2014, 19, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Marzocco, S.; Adesso, S.; Zarrouk, M.; Guerfel, M. Olive oil polyphenols extracts inhibit inflammatory markers in J774A.1 murine macrophages and scavenge free radicals. Acta Histochem. 2018, 120, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pannee, C.; Chandhanee, I.; Wacharee, L. Antiinflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide-stimulated J774A.1 cells. J. Adv. Pharm. Technol. Res. 2014, 5, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; García-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kumar, A.; Zhang, J.Y.; Johnson, S.A.; Chai, S.C.; Arjmandi, B.H. Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food Funct. 2015, 6, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.B.; Debnath, T.; Ye, M.; Hasnat, M.A.; Lim, B.O. In vitro antioxidant and anti–inflammatory activities of Korean blueberry (Vaccinium corymbosum L.) extracts. Asian Pac. J. Trop. Biomed. 2014, 4, 807–815. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. X 2022, 15, 100437. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Tenore, G.C.; Marzocco, S.; Manfra, M.; Calabrese, G.; Aquino, R.P.; Campiglia, P. UHPLC profiling and effects on LPS-stimulated J774A.1 macrophages of flavonoids from bergamot (Citrus bergamia) juice, an underestimated waste product with high anti-inflammatory potential. J. Funct. Foods 2014, 7, 641–649. [Google Scholar] [CrossRef]
- Lee, D.; Yu, J.S.; Huang, P.; Qader, M.; Manavalan, A.; Wu, X.; Kim, J.-C.; Pang, C.; Cao, S.; Kang, K.S.; et al. Identification of Anti-Inflammatory Compounds from Hawaiian Noni (Morinda citrifolia L.) Fruit Juice. Molecules 2020, 25, 4968. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular Antioxidant and Anti-Inflammatory Effects of Coffee Extracts with Different Roasting Levels. J. Med. Food 2017, 20, 626–635. [Google Scholar] [CrossRef]
- Lee, I.C.; Lee, J.S.; Lee, J.H.; Kim, Y.; So, W.Y. Anti-Oxidative and Anti-Inflammatory Activity of Kenya Grade AA Green Coffee Bean Extracts. Iran. J. Public Health 2019, 48, 2025–2034. [Google Scholar] [CrossRef]
- Antonietti, S.; Silva, A.M.; Simões, C.; Almeida, D.; Félix, L.M.; Papetti, A.; Nunes, F.M. Chemical Composition and Potential Biological Activity of Melanoidins From Instant Soluble Coffee and Instant Soluble Barley: A Comparative Study. Front. Nutr. 2022, 9, 825584. [Google Scholar] [CrossRef] [PubMed]
- Novilla, A.; Djamhuri, D.S.; Nurhayati, B.; Rihibiha, D.D.; Afifah, E.; Widowati, W. Anti-inflammatory properties of oolong tea (Camellia sinensis) ethanol extract and epigallocatechin gallate in LPS-induced RAW 264.7 cells. Asian Pac. J. Trop. Biomed. 2017, 7, 1005–1009. [Google Scholar] [CrossRef]
- Hossen, I.; Kaiqi, Z.; Hua, W.; Junsong, X.; Mingquan, H.; Yanping, C. Epigallocatechin gallate (EGCG) inhibits lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells via modulating nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) signaling pathway. Food Sci. Nutr. 2023, 11, 4634–4650. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, W. Anti-Inflammatory Effect of Quercetin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2016, 21, 450. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Yang, M.; Liu, M. Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB signalling pathway. J. Pharm. Pharmacol. 2020, 73, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Hauck, C.; Yum, M.-Y.; Rizshsky, L.; Widrlechner, M.P.; McCoy, J.-A.; Murphy, P.A.; Dixon, P.M.; Nikolau, B.J.; Birt, D.F. Rosmarinic Acid in Prunella vulgaris Ethanol Extract Inhibits Lipopolysaccharide-Induced Prostaglandin E2 and Nitric Oxide in RAW 264.7 Mouse Macrophages. J. Agric. Food Chem. 2009, 57, 10579–10589. [Google Scholar] [CrossRef]
- Park, C.M.; Song, Y.S. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar] [CrossRef]
- Cho, Y.-C.; Park, J.; Cho, S. Anti-Inflammatory and Anti-Oxidative Effects of luteolin-7-O-glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation. Int. J. Mol. Sci. 2020, 21, 2007. [Google Scholar] [CrossRef]
- Lee, J.K. Anti-inflammatory effects of eriodictyol in lipopolysaccharidestimulated raw 264.7 murine macrophages. Arch. Pharmacal Res. 2011, 34, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Búfalo, M.C.; Ferreira, I.; Costa, G.; Francisco, V.; Liberal, J.; Cruz, M.T.; Lopes, M.C.; Batista, M.T.; Sforcin, J.M. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J. Ethnopharmacol. 2013, 149, 84–92. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Song, J.; Kim, H.R.; Hwang, K.A. Oleanolic acid regulates NF-κB signaling by suppressing MafK expression in RAW 264.7 cells. BMB Rep. 2014, 47, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-X.; Wink, M. Evidence for Anti-Inflammatory Activity of Isoliquiritigenin, 18β Glycyrrhetinic Acid, Ursolic Acid, and the Traditional Chinese Medicine Plants Glycyrrhiza glabra and Eriobotrya japonica, at the Molecular Level. Medicines 2019, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; de Souza Araújo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef]
- Mowat, A.M.; Bain, C.C. Mucosal Macrophages in Intestinal Homeostasis and Inflammation. J. Innate Immun. 2011, 3, 550–564. [Google Scholar] [CrossRef]
- Kühl, A.A.; Erben, U.; Kredel, L.I.; Siegmund, B. Diversity of Intestinal Macrophages in Inflammatory Bowel Diseases. Front. Immunol. 2015, 6, 613. [Google Scholar] [CrossRef]
- Qualls, J.E.; Kaplan, A.M.; Van Rooijen, N.; Cohen, D.A. Suppression of experimental colitis by intestinal mononuclear phagocytes. J. Leukoc. Biol. 2006, 80, 802–815. [Google Scholar] [CrossRef]
- Hine, A.M.; Loke, P.n. Intestinal Macrophages in Resolving Inflammation. J. Immunol. 2019, 203, 593–599. [Google Scholar] [CrossRef]
- Ma, C.; Dutton, S.J.; Cipriano, L.E.; Singh, S.; Parker, C.E.; Nguyen, T.M.; Guizzetti, L.; Gregor, J.C.; Chande, N.; Hindryckx, P.; et al. Systematic review with meta-analysis: Prevalence, risk factors and costs of aminosalicylate use in Crohn’s disease. Aliment. Pharmacol. Ther. 2018, 48, 114–126. [Google Scholar] [CrossRef]
- Luzentales-Simpson, M.; Pang, Y.C.F.; Zhang, A.; Sousa, J.A.; Sly, L.M. Vedolizumab: Potential Mechanisms of Action for Reducing Pathological Inflammation in Inflammatory Bowel Diseases. Front. Cell Dev. Biol. 2021, 9, 612830. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Mocciaro, F.; Civitavecchia, G.; Scimeca, D.; Cottone, M. Minimizing infliximab toxicity in the treatment of inflammatory bowel disease. Dig. Liver Dis. 2008, 40, S236–S246. [Google Scholar] [CrossRef] [PubMed]
- Allgayer, H. Review article: Mechanisms of action of mesalazine in preventing colorectal carcinoma in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 18, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ascoytia, C.; McCarrier, K.P.; Martin, M.; Feagan, B.G.; Jairath, V. Physicians’ Perspectives on Cost, Safety, and Perceived Efficacy Determine Aminosalicylate Use in Crohn’s Disease. Dig. Dis. Sci. 2018, 63, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Ouyang, Z.-J.; Feng, L.-L.; Chen, G.; Guo, W.-J.; Shen, Y.; Wu, X.-D.; Sun, Y.; Xu, Q. Suppression of NF-κB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol. Appl. Pharmacol. 2014, 281, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Li, S.; Xin, D.; Li, S.; Yan, B.; Wang, S.; Liu, C. Procyanidin improves experimental colitis by regulating macrophage polarization. Biomed. Pharmacother. 2023, 165, 115076. [Google Scholar] [CrossRef]
- Fan, H.; Chen, W.; Zhu, J.; Zhang, J.; Peng, S. Toosendanin alleviates dextran sulfate sodium-induced colitis by inhibiting M1 macrophage polarization and regulating NLRP3 inflammasome and Nrf2/HO-1 signaling. Int. Immunopharmacol. 2019, 76, 105909. [Google Scholar] [CrossRef]
- Xiang, C.; Liu, M.; Lu, Q.; Fan, C.; Lu, H.; Feng, C.; Yang, X.; Li, H.; Tang, W. Blockade of TLRs-triggered macrophage activation by caffeic acid exerted protective effects on experimental ulcerative colitis. Cell. Immunol. 2021, 365, 104364. [Google Scholar] [CrossRef]
- Han, D.; Wu, Y.; Lu, D.; Pang, J.; Hu, J.; Zhang, X.; Wang, Z.; Zhang, G.; Wang, J. Polyphenol-rich diet mediates interplay between macrophage-neutrophil and gut microbiota to alleviate intestinal inflammation. Cell Death Dis. 2023, 14, 656. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kim, Y.; Jin, S.G.; Kim, J.Y. Acai berry extract as a regulator of intestinal inflammation pathways in a Caco-2 and RAW 264.7 co-culture model. J. Food Biochem. 2021, 45, e13848. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, Q.; Hou, K.; Ding, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Regulatory effects of Ganoderma atrum polysaccharides on LPS-induced inflammatory macrophages model and intestinal-like Caco-2/macrophages co-culture inflammation model. Food Chem. Toxicol. 2020, 140, 111321. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Chen, X. Investigation into the anti-inflammatory mechanism of coffee leaf extract in LPS-induced Caco-2/U937 co-culture model through cytokines and NMR-based untargeted metabolomics analyses. Food Chem. 2023, 404, 134592. [Google Scholar] [CrossRef] [PubMed]
- Asfaha, S.; Dubeykovskiy, A.N.; Tomita, H.; Yang, X.; Stokes, S.; Shibata, W.; Friedman, R.A.; Ariyama, H.; Dubeykovskaya, Z.A.; Muthupalani, S.; et al. Mice That Express Human Interleukin-8 Have Increased Mobilization of Immature Myeloid Cells, Which Exacerbates Inflammation and Accelerates Colon Carcinogenesis. Gastroenterology 2013, 144, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Patera, A.C.; Pong-Kennedy, A.; Deno, G.; Gonsiorek, W.; Manfra, D.J.; Vassileva, G.; Zeng, M.; Jackson, C.; Sullivan, L.; et al. Murine CXCR1 Is a Functional Receptor for GCP-2/CXCL6 and Interleukin-8/CXCL8. J. Biol. Chem. 2007, 282, 11658–11666. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Souto, E.B.; Silva, A.M. Chapter 15—Nanophytosomes: A novel approach for the delivery of herbal drugs. In Systems of Nanovesicular Drug Delivery; Nayak, A.K., Hasnain, M.S., Aminabhavi, T.M., Torchilin, V.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 239–257. [Google Scholar]
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic compounds of blackthorn (Prunus spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef]

| Plant/Extract | Concentration | Experimental Model | Observations | Ref. |
|---|---|---|---|---|
| Grape pomace aqueous extract | 5 g extract/100 g diet | Duodenum lysate from swine | Reduced lipid peroxidation Increased antioxidant potential Increased CAT and GPx activities | [55] |
| Colon lysate from swine | Reduced lipid peroxidation Increased antioxidant potential Increased SOD activity | |||
| Grape seed proanthocyanidin extract | 50 mg/kg | Colon samples from male C57BL/6J mice | Reduced dextran sulphate sodium-induced oxidative stress Decreased malondialdehyde production Normalized SOD activity Prevented GSH depletion | [56] |
| Origanum vulgare L. essential oil | 5 and 20 mg/kg | Jejunum samples from Wistar rats | Reduced diquat-induced oxidative stress Decreased ROS and TBARS levels Normalized SOD and GPx activities | [47] |
| Fu brick tea (fermented tea; Camellia sinensis L.) | 100 mg/kg | Colon samples from Sprague Dawley rats | Reduced oxidative stress induced by high-fat diet Decreased lipid peroxidation Increased SOD and CAT levels Reversed the downregulation of ZO-1, occludin, and claudin-1 | [51] |
| Garlic oil | 20 and 40 mg/kg | Colon samples from Sprague Dawley rats | Reduced alcohol-induced oxidative stress and lipid peroxidation Increased SOD and GPx levels Upregulated ZO-1 and Claudin-1 expression | [57] |
| Astragalus membranaceus dried root extract (Axtragyl®) | 50 and 100 µg/mL | Rat small intestine epithelial cells (IEC-6 cells) | Reduced H2O2-induced ROS increase Activation of Nrf2 to nuclei Increased expression of HO-1 and NQO1 | [58] |
| Origanum vulgare L. essential oil | 10 µg/mL | Porcine small intestinal epithelial cells (IPEC-J2) | Reduced H2O2-induced intracellular and extracellular ROS increase Reduced lipid peroxidation Increased GSH and upregulated SOD, CAT, GCL, and Nrf2 expression | [48] |
| Cranberry (Vaccinium macrocarpon Aiton) extract * | 200 mg/kg | Jejunum samples from male C57BL/6J mice | Reduced oxidative stress induced by high-fat/high-sucrose diet Increased SOD2 levels | [50] |
| Olive oil phenolic extract | 25 µg/mL | Human colorectal adenocarcinoma cells (Caco-2) | Decreased oxysterols-induced ROS increase Prevented GSH depletion | [46] |
| Boswellia serrata resin (hydroethanolic extract) | 1 µg/mL | Human colorectal adenocarcinoma cells (Caco-2) | Reduced H2O2-induced ROS increase and NF-kB expression Prevented downregulation of tight-junction proteins (ZO-1 and occludin) | [59] |
| Cocoa extract | 10 µg/mL | Human colorectal adenocarcinoma cells (Caco-2) | Reduced acrylamide-induced oxidative stress and cell death Prevented GSH depletion Increased GST and GCL levels | [42] |
| Plant/Extract | Concentration | Experimental Model | Observations | Ref. |
|---|---|---|---|---|
| Fu brick tea (fermented tea; Camellia sinensis L.) | 100 mg/kg | Colon samples from Sprague Dawley rats | Decreased LPS in serum induced by high-fat diet Reduced IL-6, TNF-α, and MCP-1 expression Increased IL-10 levels | [51] |
| Grape seed proanthocyanidin extract | 50 mg/kg | Colon samples from C57BL/6J mice | Reduced dextran sulphate sodium-induced inflammation Decreased TNF-α and IL-1β levels and respective mRNA expression Restored IL-10 level and increased its mRNA expression Reduced mRNA expression of NLRP3, ASC, and caspase-1 | [56] |
| Origanum vulgare L. essential oil | 5 and 20 mg/kg | Jejunum samples from Wistar rats | Reduced diquat-induced TNF-α, IL-1β and IL-6 mRNA expression | [47] |
| Cranberry (Vaccinium macrocarpon Aiton) extract * | 200 mg/kg | Jejunum samples from C57BL/6J mice | Reduced inflammation induced by high-fat/high-sucrose diet Reduced COX-2 and NF-kB expression | [50] |
| Polyphenol-rich extract of Zhenjiang aromatic vinegar | Colon samples from ICR mice | Reduced alcohol-induced inflammation Increased IL-10 and IL-22 levels Reduced TNF-α, IL-6, IL-1β, and LPS levels | [71] | |
| Astragalus membranaceus Bunge. dried root hydroalcoholic extract (Axtragyl®) | 50 and 100 µg/mL | Rat small intestine epithelial cells (IEC-6 cells) | Reduced IFN-γ/LPS-induced TNF-α release Inhibited nitrotyrosine formation Reduced iNOS and COX-2 expression Decreased NF-kB activation | [58] |
| Punica granatum L. fruit peel aqueous extract | 50 µM GAE | Human colorectal adenocarcinoma cells (Caco-2) | Decreased ERK 1/2 activation Decreased NO release, IL-1β-induced NF-kB activation and IL-8 and PGE2 secretion | [87] |
| Saccharum officinarum L. stem aqueous extract | 50 µM GAE | Human colorectal adenocarcinoma cells (Caco-2) | Decreased IL-1β-induced NF-kB activation, IL-8 secretion and PGE2 secretion | [87] |
| Quercus robur L. duramen aqueous extract | 50 µM GAE | Human colorectal adenocarcinoma cells (Caco-2) | Decreased NO release, IL-1β-induced NF-kB activation and IL-8 secretion | [87] |
| Vitis vinifera L. seeds extract * | 50 µM GAE | Human colorectal adenocarcinoma cells (Caco-2) | Decreased IL-1β-induced IL-8 secretion | [87] |
| Theobroma cacao L. extract * | 50 µM GAE | Human colorectal adenocarcinoma cells (Caco-2) | Decreased NO release and IL-1β-induced PGE2 secretion | [87] |
| Olive oil phenolic extract | 25 µg/mL | Human colorectal adenocarcinoma cells (Caco-2) | Decreased oxysterols-induced NO, IL-8 and IL-6 increase Reduced JNK and IkB phosphorylation Decreased iNOS expression | [46] |
| Boswellia serrata resin (hydroethanolic extract) | 1 µg/mL | Human colorectal adenocarcinoma cells (Caco-2) | Reduced TNF-α/IFN-γ-induced downregulation of tight-junction proteins (ZO-1 and occluding) Downregulated NF-kB expression Reduced paracellular permeability induced by inflammatory stimuli | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Natural Products as Dietary Agents for the Prevention and Mitigation of Oxidative Damage and Inflammation in the Intestinal Barrier. Antioxidants 2024, 13, 65. https://doi.org/10.3390/antiox13010065
Martins-Gomes C, Nunes FM, Silva AM. Natural Products as Dietary Agents for the Prevention and Mitigation of Oxidative Damage and Inflammation in the Intestinal Barrier. Antioxidants. 2024; 13(1):65. https://doi.org/10.3390/antiox13010065
Chicago/Turabian StyleMartins-Gomes, Carlos, Fernando M. Nunes, and Amélia M. Silva. 2024. "Natural Products as Dietary Agents for the Prevention and Mitigation of Oxidative Damage and Inflammation in the Intestinal Barrier" Antioxidants 13, no. 1: 65. https://doi.org/10.3390/antiox13010065






