Abstract
Anthocyanins, a class of flavonoid compounds responsible for the vibrant colors of many fruits and vegetables, have received considerable attention in recent years due to their potential health benefits. This review, focusing on evidence from both in vitro and in vivo studies, provides a comprehensive overview of the current state of knowledge regarding the health-promoting properties of anthocyanins. The chemical structure and diversity of anthocyanins, their bioavailability, and their mechanisms of action at the cellular and molecular level are examined. Research on the antioxidant, anti-inflammatory, anticancer, and neuroprotective effects of anthocyanins is critically reviewed. Special emphasis is placed on the role of anthocyanins in the prevention and treatment of chronic diseases such as cardiovascular diseases, diabetes, and neurodegenerative diseases. This review also discusses the challenges of translating in vitro findings to in vivo and highlights the importance of considering dose, bioavailability, and metabolism when assessing the therapeutic potential of anthocyanins. This review concludes with the identification of gaps in current research and suggestions for future directions for anthocyanin studies, including the need for more long-term clinical trials and investigations into potential synergistic effects with other phytochemicals. This comprehensive analysis highlights the promising role of anthocyanins in promoting human health and provides valuable insights for researchers, health professionals, and the nutraceutical industry. This study provides new insights, as it comprehensively investigates the dual anti-inflammatory and anticancer effects of anthocyanins in both in vitro and in vivo models. By uncovering the biological properties of anthocyanins from a variety of natural sources, this research not only expands our knowledge of the action of these compounds at the cellular level, but also enhances their clinical relevance through in vivo validation. Furthermore, the innovative use of anthocyanins may lead to important advances in their therapeutic application in the future.
1. Introduction
Plant-derived bioactive compounds represent a vast and diverse group of molecules that have evolved over millions of years as part of plants’ adaptive strategies. These compounds, while not essential for basic plant metabolism, play critical roles in plant survival, reproduction, and environmental interactions. The biosynthesis of these secondary metabolites is often triggered by specific environmental stresses or developmental stages, leading to a complex and dynamic phytochemical profile within each plant species [1,2]. This natural diversity offers an extensive reservoir of potentially beneficial compounds for human use. The study of plant bioactive compoun encompasses not only their identification and characterization but also the investigation of their biosynthetic pathways, genetic regulation, and ecological functions. Understanding these aspects can lead to the development of enhanced cultivation practices, breeding programs, and biotechnological approaches to optimize the production of desired compounds. In addition, research into traditional medicinal plants continues to uncover new bioactive compounds, combining ethnobotanical knowledge with modern scientific research [3,4].
For centuries, plants have been the primary source of chemical compounds with various health-promoting properties [5]. They are called phytochemicals. Substances contained in plants play a key role in the prevention and treatment of numerous diseases. Initially, their properties were studied organoleptically through experiments conducted in folk medicine. Over the years, and with the progress of science, it became possible to conduct research on specific substances contained in, among others, extracts or essential oils of plant origin. Chemical knowledge was also deepened, allowing these key compounds to be divided into classes. The most important groups of compounds contained in plants include polyphenols [6], carotenoids [7], glucosinolates, saponins, phytosterols, and terpenoids [8]. Each of these groups has unique properties that contribute to the improvement of human health.
This paper will be mainly devoted to anthocyanins—a group of natural plant dyes that belong to the flavonoid family. Anthocyanins (from the Greek anthos = flower and kianos = blue) [9] are found in many fruits, vegetables, flowers, and leaves, giving them characteristic red, purple, and blue colors (Figure 1). These pigments occur in different combinations and proportions, which affects the richness of the colors [10]. Anthocyanin pigments are easily degraded during food processing and storage. This affects the color, quality, and nutritional properties [11]. At low pH (acidic conditions), anthocyanins are more stable, resulting in a red pigment, while higher pH values will cause the blue color to fade [12]. It should be emphasized that acetylation and glycosylation significantly affect the biological function of anthocyanins, increasing, among other things, their stability, solubility, and bioavailability. Acetylation increases the stability of anthocyanins, especially against environmental factors such as heat, light, and pH changes. This makes them more effective as pigments and more resistant to degradation, potentially prolonging their biological activity. Glycosylation increases the solubility and bioavailability of anthocyanins. It also influences the way they are absorbed, transported, and metabolized in the body, affecting their antioxidant activity and potential health benefits. In conclusion, both modifications enhance the functional and biological properties of anthocyanins, improving their anticancer and antioxidant properties. In recent years, there has been a growing interest in the health-promoting properties of anthocyanins, which is due to both their potential health benefits and growing awareness of the importance of a healthy diet. The introduction of anthocyanins into the daily diet can have a number of benefits for human health. Numerous studies have suggested that anthocyanins have powerful antioxidant properties, which means that they can neutralize harmful free radicals in the body and protect cells from oxidative damage [13,14,15,16]. In addition, anthocyanins have anti-inflammatory effects [13,17,18,19], which may be important in preventing and treating chronic inflammatory diseases. These compounds can also be used to help treat conditions such as heart disease [20,21,22], type 2 diabetes [19,23,24,25], and some cancers [26]. Anthocyanins also have beneficial effects on eye health [27,28], especially considering the complications resulting from elevated blood glucose levels—diabetic retinopathy and prevention of macular degeneration in eye-related diseases. Additionally, studies indicate that consuming foods rich in anthocyanins can support cognitive function, improve memory, and also have a supportive effect on some neurodegenerative diseases, which is particularly important in the context of an aging society [29,30,31,32,33].

Figure 1.
Natural anthocyanins and their sources in the diet.
This paper will discuss the main sources of anthocyanins, some mechanisms of their health-promoting effects with specific examples, and the results of the latest scientific research on their impact on human health. We will also present an analysis of the potential applications of anthocyanins in the prevention and therapy of various diseases, as well as the prospects for further research in this field. We will mainly emphasize the anti-inflammatory and anticancer effects of anthocyanins in scientific works, both in vitro and in vivo.
2. Study Design
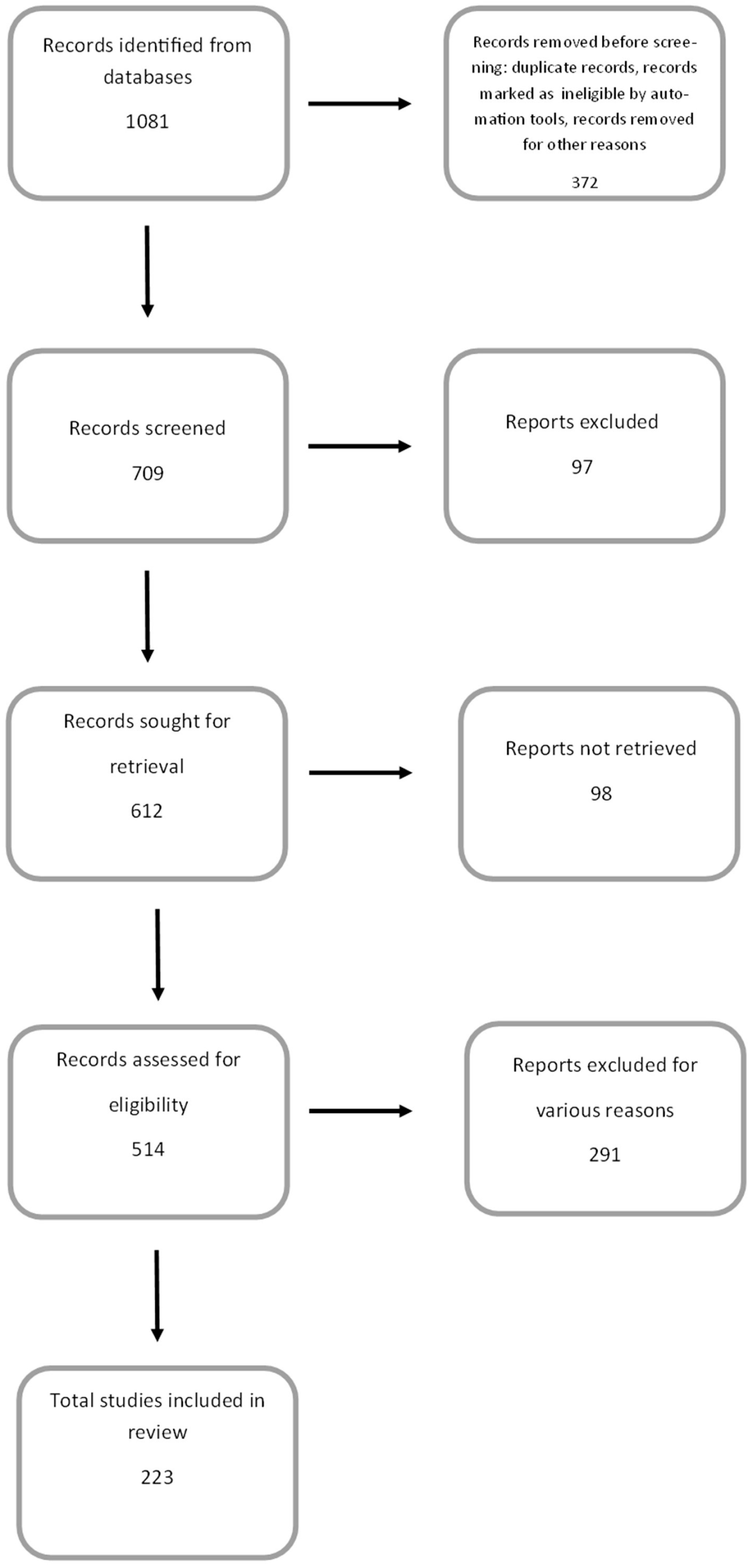
A search of appropriate literature, i.e., health benefits of anthocyanins in in vitro and in vivo studies, was performed using Google Scholar and NCBI-PubMed. The following keywords were used: anthocyanins, anthocyanidins, cyanidin, delphinidin, peonidin, petunidin, malvidin, anti-inflammatory/anticancer/antiproliferative/antioxidant/cardioprotective/neuroprotective/ properties of anthocyanins, in vitro models, and in vivo models. While the search term “health benefits of anthocyanins” identified more than a thousand articles in the PubMed database dating back to 1920, this review primarily included articles from 2000 onwards. These accounted for 94.6% of all articles used in this review. During the course of the work, some articles were rejected due to their inconsistency with the topic, lack of reliable data, or the presence of general information that did not specify the relationship being studied (Figure 2).

Figure 2.
PRISMA flow diagram demonstrating the screening method for the article.
Chemical Structure of Anthocyanins
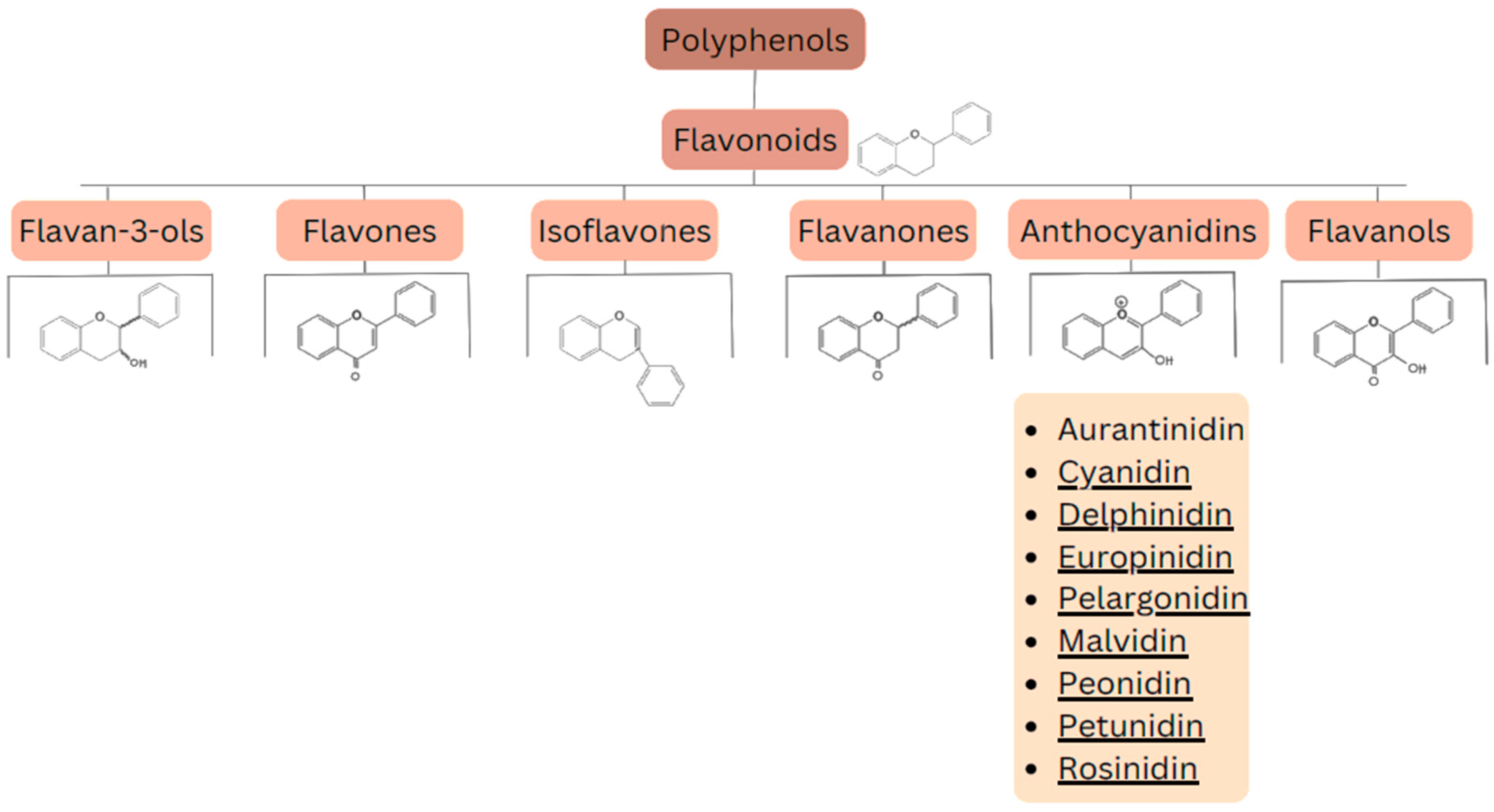
Flavonoids are a group of naturally occurring chemical compounds found in plants [34]. They are polyphenols that play an important role in defending plants against pathogens [35] and UV radiation [36,37,38] (this protective effect has also been analyzed for the prevention and treatment of skin disorders). Flavonoids also have numerous health benefits for humans, including antioxidant effects: they neutralize free radicals [39], which can help prevent cell damage; anti-inflammatory effects [40]: they can reduce inflammation in the body; anticancer effects: research suggests that flavonoids may inhibit growth of cancer cells [41]; and cardioprotective effects: they can improve the function of blood vessels and lower blood pressure [42,43]. Flavonoids can be divided into several main groups, including: flavan-3-ols (catechins) (e.g., epicatechin, epigallocatechin), flavones (e.g., apigenin, luteolin), isoflavones (e.g., genistein, daidzein), flavanones (e.g., naringenin, hesperidin), anthocyanins (e.g., cyanidin, delphinidin), and flavonols (e.g., quercetin, kaempferol). The division of these compounds is presented in Figure 3.

Figure 3.
Division of polyphenols into different classes of compounds.
A very interesting group among the above-mentioned is the anthocyanins. Anthocyanins are a group of flavonoids responsible for the red, purple, and blue colors of many fruits, vegetables, and flowers [44]. They have a wide range of health benefits and play an important role in protecting plants from harmful environmental factors. These natural pigments of plant origin are found in vacuoles (mainly in flowers and fruits) in the form of granules of various sizes around 3 to 10 μm in diameter [45]. Anthocyanins are anthocyanidin glycosides, which means that they consist of an aglycone (anthocyanidin) molecule connected to a sugar molecule [46]. Their chemical structure is crucial for their color and biological properties.
Anthocyanins can be divided into their three most important parts:
- Anthocyanidins: Aglycones, which are the basic chemical structure of anthocyanins. They have a ion flavylium structure (2-phenylchromene), which is responsible for their color. It contains seven different chemical side groups, which can be composed of hydrogen atoms, hydroxyl groups, and methoxy groups. The most common anthocyanidins are cyanidin, delphinidin, pelargonidin, peonidin, petunidin, and malvidin.
- Sugar part: Anthocyanidins are linked to one or more sugars through glycosidic bonds. The most common sugars are glucose, galactose, and rhamnose. This combination increases the solubility of anthocyanins in water and the stability of the dye [47].
- Structural modifications: Anthocyanins can have various functional groups, such as hydroxyl (-OH) and methoxyl (-OCH3) groups, which influence their chemical properties and color [10]. These modifications can affect the intensity and range of the light absorption spectrum, which is responsible for the variety of colors they can take on.
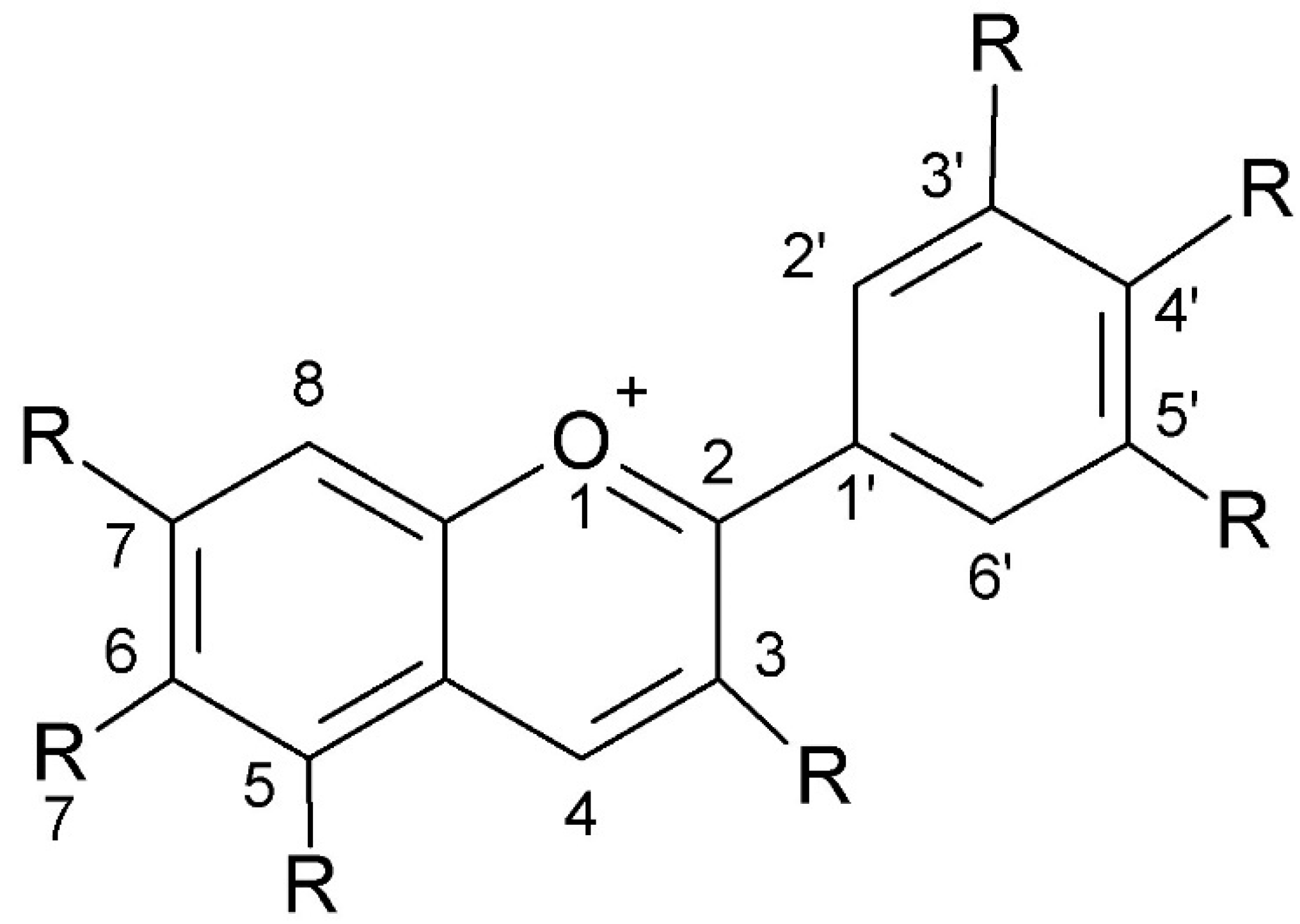
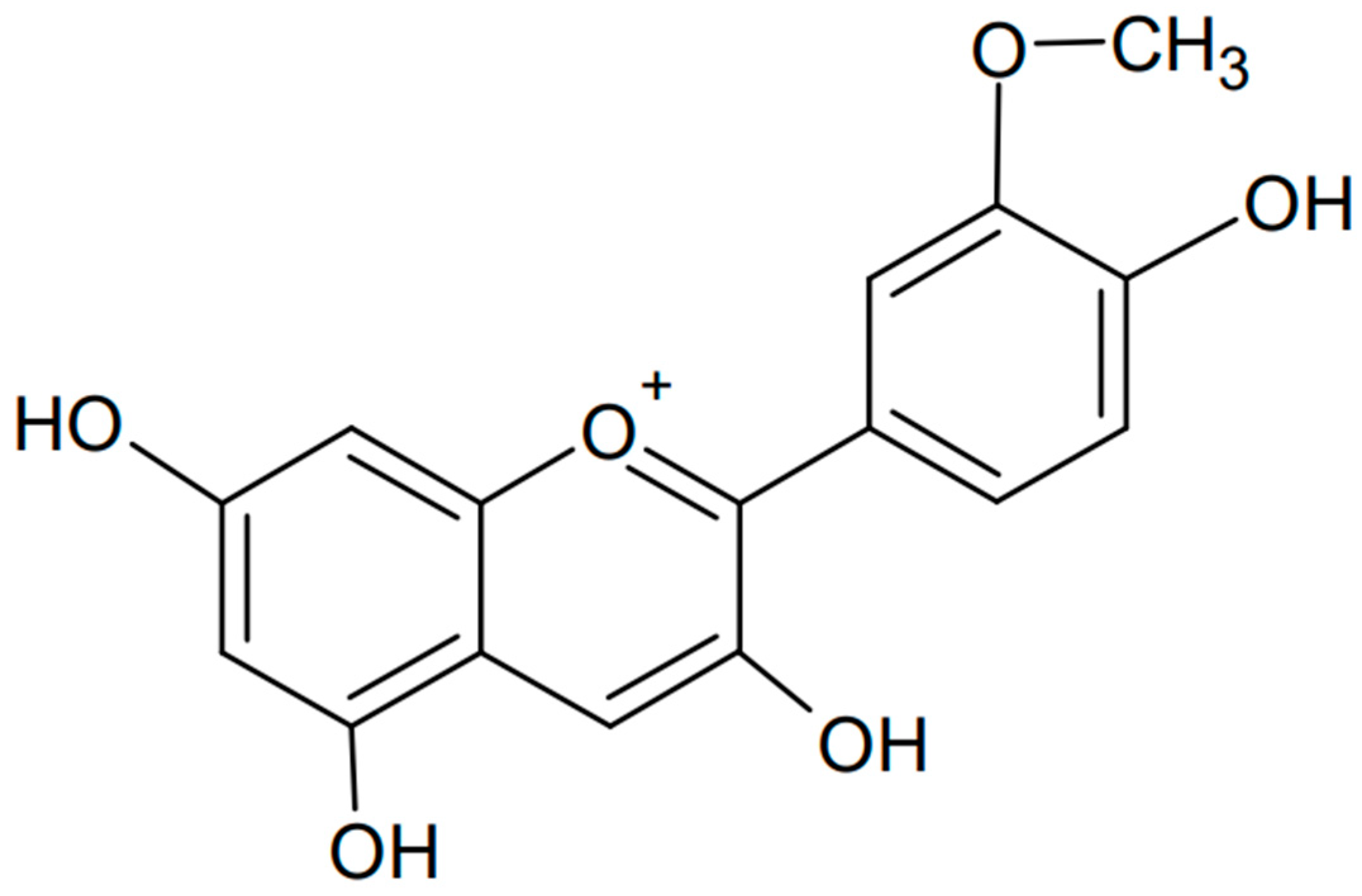
Anthocyanins have a complex chemical structure consisting of three rings: two aromatic rings (A and B) and one heterocyclic ring with oxygen (C) of cationic nature, often occurring as flavylium (2-phenylchromene). The C15 skeleton is based on a chromate ring bearing a second aromatic ring B in position 2 (C6-C3-C6). The parent structure of anthocyanidins is the flavylium cation [48]. The model is shown in Figure 4.

Figure 4.
The chemical structure of anthocyanins (created with ChemSketch).
High anthocyanin content is found in blueberries, raspberries, black currants, elderberries, blackberries, chokeberries, red cabbage, grapes, and eggplants [49,50,51,52,53,54,55,56]. For industrial purposes, anthocyanins are obtained by extraction from red cabbage leaves or grape skins [57,58]. Some species and examples of anthocyanins found in them are presented in Table 1.

Table 1.
Examples of plants containing significant amounts of anthocyanins.
3. Anthocyanins and Their Biological Activity
3.1. Cyanidin
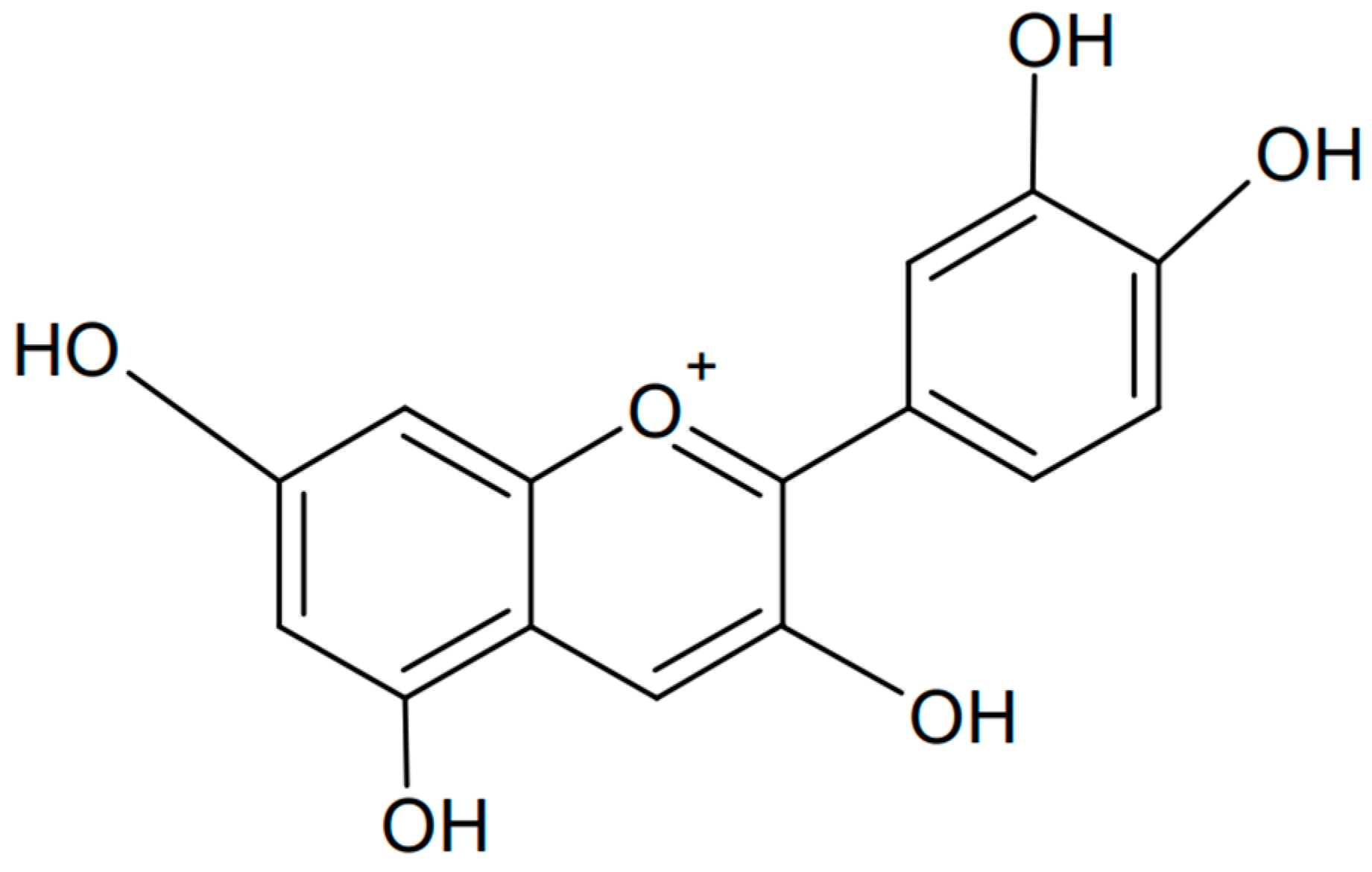
Cyanidin is one of the anthocyanins that has been most extensively investigated. It has a flavanol-based chemical structure with hydroxyl and methoxyl groups. Chemically, cyanidin is known as the 3,3′,4′,5,7-pentahydroxyflavyl cation. The formula of cyanidin is presented in Figure 5. This organic compound is found in the largest amounts in the skin of fruits, especially red ones such as apples, plums, and hawthorn [64,65,66]. Its color depends on the pH value: it appears red in acidic conditions and blue in alkaline conditions.

Figure 5.
Chemical formula of cyanidin (created with ChemSketch).
Cyanidin has many health benefits, including its use in the treatment of pathologies associated with reactive oxygen species and their neutralization. Cyanidin and cyanidin 3-O-β-D-glucoside have shown a protective effect on DNA cleavage and a dose-dependent activity of scavenging free radicals. In one study, the inhibition of xanthine oxidase (XO) activity was also examined [67]. Anthocyanins are powerful antioxidants that neutralize free radicals and protect cells from oxidative damage. Cyanidin has better antioxidant properties than even vitamin E [68]. One study on pigmented oranges showed that cyanidin-3-O-β-glucopyranoside should be considered one of the most effective antioxidants in dietary plants [69]. Cyanidin glucoside is a highly efficient scavenger of free oxygen radicals in direct interaction with reactive oxygen species (H2O2, -O2, OH·).
The effect of cyanidin on the oxidation of low-density lipoproteins (LDLs) is important in the pathogenesis of atherosclerosis. Studies suggest that anthocyanins may have anticancer properties, helping to prevent the development of cancer. This is supported by the work of Feng et al., who studied the effect of cyanidin-3-rutinoside in several leukemia and lymphoma cell lines. Cyanidin induced the accumulation of superoxides, which are involved in the induction of apoptosis in HL-60 cells. Furthermore, the activation of p38 MAPK and JNK contributed to cell death via activation of the mitochondrial pathway, mediated by Bim [70]. An antitumor effect was also confirmed in lung large-cell carcinoma in nude mice [71]. The study showed impaired tumor growth, increased tumor apoptosis, reduced levels of inflammatory cytokines (IL-1β, TNF-α, C-reactive protein, and IL-6), reduced factors associated with inflammation (cyclooxygenase-2 protein and nuclear factor-κB (NF-κB) mRNA), increased inhibition of NF-κB kinase mRNA, and downregulation of metastasis-related factors (transforming growth factor-β, CD44, epidermal growth factor receptor, and vascular endothelial growth factor) after cyanidin-3-O-glucoside treatment. Studies have also confirmed inhibition of the proliferation of B16-F10 murine melanoma cells. The results suggest that anthocyanins derived from elderberry may be used as local adjuvant agents in skin cancer therapy [72]. Caco-2 cells in vitro were the next line tested for responses after cyanidin-3-O-glucoside administration [73]. This confirmed inhibition of the proliferation of human tumor cells. Cyanidin-glucoside also modulated cell cycle progression in a liver precancerous lesion in vivo study and additionally influenced the induction of apoptosis in prostate cancer [74], in the induction of apoptosis in colorectal cancer cells via the NF-κB signaling pathway [75], and in renal cell carcinoma tumorigenesis [76]. An extremely important and relatively newly discovered correlation is between anthocyanin consumption and eye health. Inhibitors of diabetic cataracts were studied among anthocyanin monomers isolated from grape skin extract. Using rat lens organ cultures, malvidin 3-glucoside, delphinidin 3-glucoside, cyanidin 3-glucoside, petunidin 3-glucoside, and peonidin 3-glucoside showed an inhibitory effect on lens opacity [77]. Another disease entity in which anthocyanins have been successfully used is the inhibition of the dangerous consequences of diabetes, specifically diabetic retinopathy [25]. Anthocyanins can support heart health by lowering blood pressure, improving blood vessel function, and reducing the risk of atherosclerosis. The results showed that cyanidin glucosides reduced the infarct area, alleviated pathological changes, inhibited ST-segment elevation, and attenuated oxidative stress, ferroptosis-related protein expression, USP19, Beclin1, NCOA4, and LC3II/LC3I expression. The compound could potentially be used as a myocardial protection agent against ischemia-induced damage [78].
3.2. Peonidin
Another type of anthocyanin is peonidin, which is known for its red and purple hues and is found in various fruits and flowers [79,80,81]. The formula of peonidin is presented in Figure 6. Like other anthocyanins, peonidin has antioxidant properties and potential health benefits, including anti-inflammatory and anticancer effects [82]. One study showed the anticancer effects of peonidin 3-glucoside and cyanidin 3-glucoside in the highly sensitive HS578T cell line. The compounds were found to cause cell cycle arrest in the G2/M phase, reducing the levels of cyclin-dependent kinase (CDK)-1, CDK-2, cyclin B1, and cyclin E proteins. In addition, anthocyanins induced caspase-3 activation, chromatin condensation, and cell death [83]. Another study showed that peonidin 3-glucoside inhibits lung cancer metastasis via the MAPK pathway. It has been shown that anthocyanin can significantly inhibit invasion, motility, and secretion of matrix metalloproteinase (MMP)-2, MMP-9, and urokinase-type plasminogen activator (u-PA) in lung cancer cells. Phosphorylation of extracellular signal-regulated kinase (ERK)1/2, a member of the mitogen-activated protein kinase (MAPK) family involved in the upregulation of MMP and u-PA, was attenuated. Activation of activating protein-1 (AP-1) was also inhibited. The study was conducted in the H1299 cell line, and it was very important to perform complementary in vivo studies and find that peonidin inhibits lung cancer cell metastasis [84].

Figure 6.
Chemical formula of peonidin (created with ChemSketch).
In addition to its anticancer properties, peonidin has antioxidant properties, helping to neutralize free radicals and protect cells from damage. This theory is supported by the results of Nas et al., in which peonidin-3-glucoside (P3G) was tested for its antioxidant properties and administered to a nematode species under various forms of stress (ultraviolet, heat, and oxidative stress). Peonidin glucoside showed free radical scavenging activity, which extended life and improved the health of the organism in the presence of UV radiation, heat, and oxidative stress, although these mechanisms may differ from its antioxidant activity [85]. This is important for deepening further research.
Other scientists analyzed twelve anthocyanins with a structure analogous to peonidin from the Chinese purple sweet potato (Ipomoea batatas (L.)). They were identified by LC-MS/MS. The functional properties of the anthocyanin monomers were investigated: antioxidant activity, proliferative effects on probiotics, and inhibition of harmful bacteria in vitro. Peonidin removes 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals and superoxide anions and has the potential to reduce energy consumption and Fe2+ chelating capacity. Anthocyanins have been shown to induce the proliferation of beneficial bacterial flora and inhibit the growth of harmful intestinal microorganisms such as Staphylococcus aureus and Salmonella typhimurium. The ability to prebiotically modulate intestinal microbiota is the basis for pharmaceutical development and the introduction of health-promoting foods to the industry [86].
3.3. Delphinidin
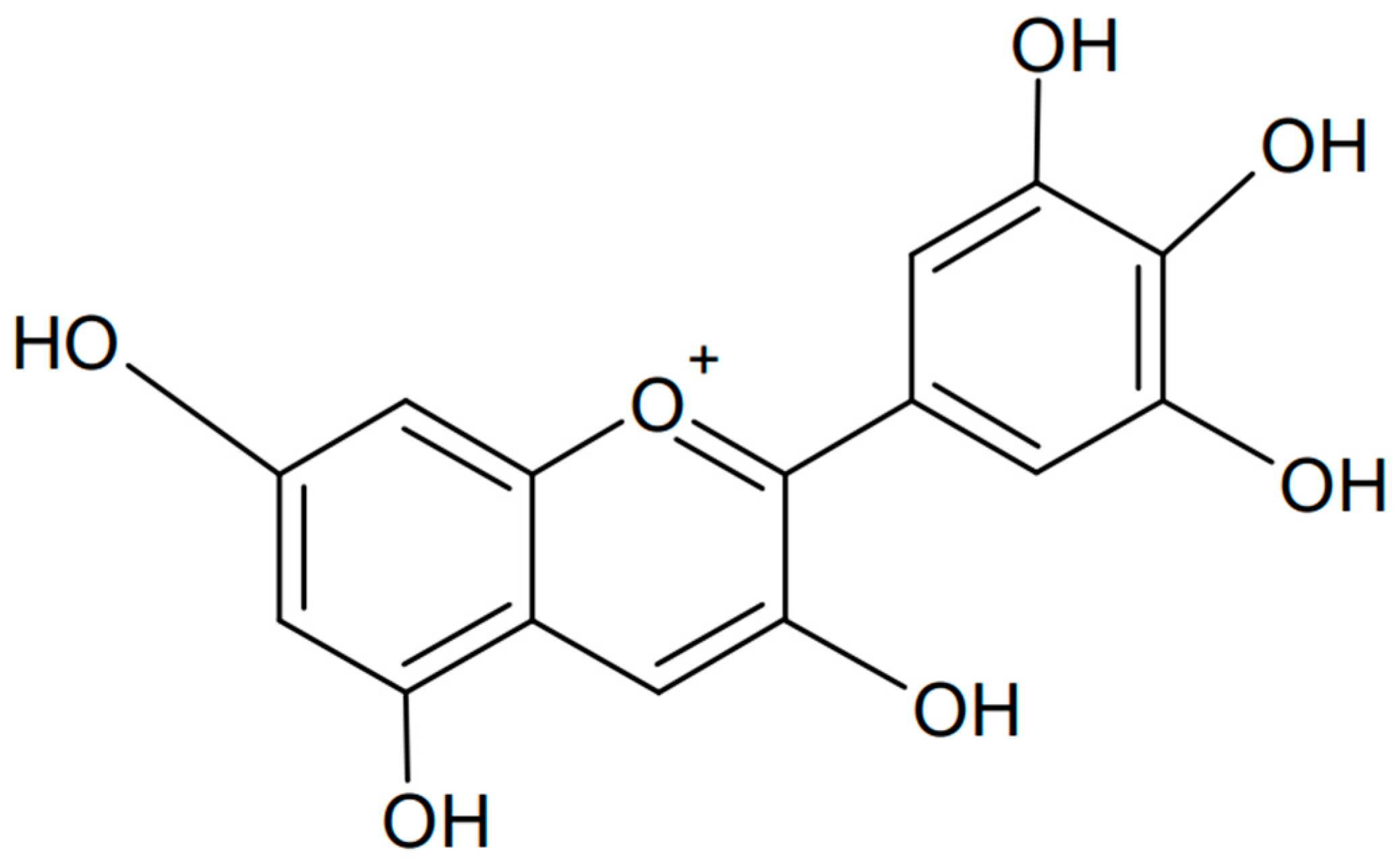
Delphinidin is a polyphenolic anthocyanidin found in many plants that gives them a blue, purple, or red color, depending on the pH of the environment. The name “delphinidin” comes from the Greek word “delphinion”, which means “dolphin” (possibly for the fancied resemblance of flowers of some species to classical sculptures of dolphins), because this compound was first isolated from a plant called Delphinium [87]. The chemical formula of delphinidin is presented in Figure 7. Delphinidin has many beneficial health properties, including antioxidant, anti-inflammatory, and anticancer properties. It can be found in fruits such as blueberries, grapes (especially dark varieties), cranberries, and elderberries [88,89,90].

Figure 7.
Chemical formula of delphinidin (created with ChemSketch).
This anthocyanin is the subject of numerous scientific studies aimed at better understanding its mechanisms of action and full health potential. Much of this research focuses on its role in preventing and treating various diseases. One of the health benefits is its antioxidant effect. Delphinidin is a powerful antioxidant that helps neutralize free radicals in the body, which can protect cells from oxidative damage [91]. This is confirmed by a study carried out on pomegranate extract (Punica granatum L.), which contained the anthocyanins delphinidin, cyanidin, and pelargonidin. These anthocyanins exhibited scavenging activity against ·OH and O2·-, inhibited a Fenton reagent ·OH-generating system, possibly by chelating with ferrous ion, and inhibited H2O2-induced lipid peroxidation in rat brain homogenates [92]. Another study compared the antioxidant mechanism of delphinidin and pelargonidin using quantum chemical density functional theory and in vitro chemical antioxidant tests [93]. The geometric configuration, bond dissociation energy, and PCM (polarizable continuum) solvent model of the reaction enthalpy change were analyzed, and the reaction enthalpy change value was calculated to determine the active site (delphinidin’s C4’ site and pelargonidin’s C3 site were the free radical scavenging active sites). The results showed that the ability of delphinidin to scavenge free radicals was slightly higher than that of pelargonidin.
The antioxidant and anti-inflammatory properties of delphinidin may also support brain health, potentially reducing the risk of neurodegenerative diseases such as Alzheimer’s disease. This was demonstrated by Heysieattalab et al. [94], who showed that a high dose of delphinidin reduced acetylcholinesterase, amyloid precursor protein (APP), and amyloid beta protein (Aβ) levels in an Alzheimer’s disease model. Delphinidin treatment reduced amyloid plaque formation in rats with lesions of the nucleus basalis of Meynert (NBM). The scientific work suggested that delphinidin is a plate-like molecule sandwiched between β-plates, related to Aβ molecules, and inhibiting the formation of amyloid fibrils.
Further research shows that delphinidin can inhibit the growth of cancer cells and induce their apoptosis (programmed cell death), making it a potential agent in the fight against cancer. An experiment performed in human colon cancer HCT116 cells found that delphinidin caused a decrease in cell viability, induction of apoptosis, cleavage of PARP, activation of caspases-3, -8, and -9, an increase in Bax with a simultaneous decrease in Bcl-2, and arrest of the cell cycle in the G2/M phase. Inhibition of IKKα, phosphorylation and degradation of IκBα, phosphorylation of NF-κB/p65 at Ser536, and nuclear translocation of NF-κB/p65 were confirmed by immunoblot analysis. This suggests that delphinidin has the potential to inhibit colon cancer development [95]. In contrast, other studies performed in the human osteosarcoma-derived U2OS cell line and the breast cancer cell line MCF7 showed cytotoxic effects and induction of apoptosis via sigmoidal pathways [96,97].
In conclusion, delphinidin and delphinidin-rich fruits may be suggested as supportive treatments for a wide variety of diseases and other related disorders. These include cardiovascular diseases [98], thrombosis [99], psoriatic disease [100], hepatitis [101], and even obesity [102].
3.4. Petunidin
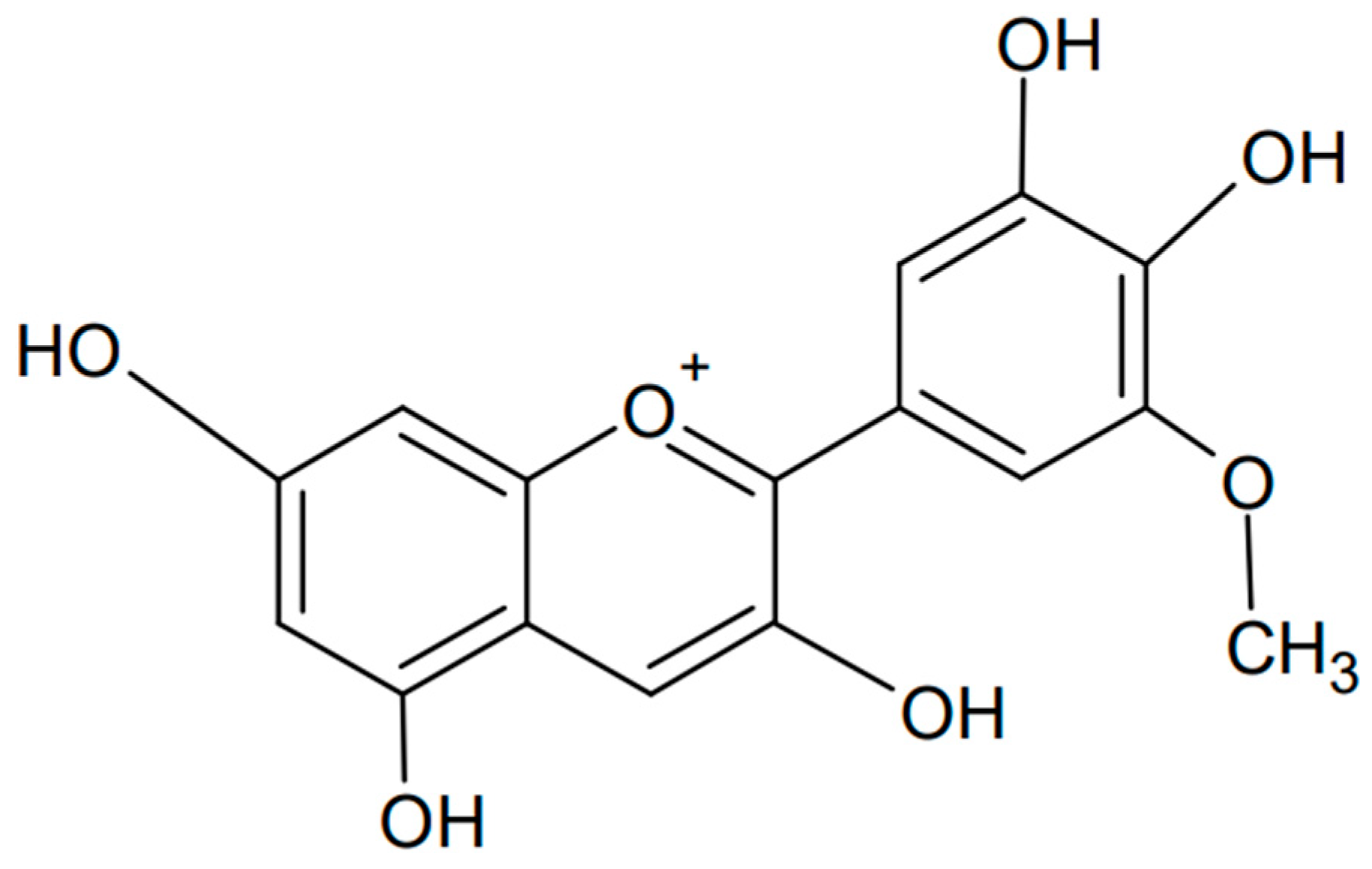
Petunidin is another example of an anthocyanidin, a type of plant pigment responsible for the red, purple, and blue colors of many fruits, vegetables, and flowers. Chemically known as 3,5-dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)chromenylium, it is found in various fruits such as grapes, berries (e.g., blackcurrants), and flowers such as petunias, from which the compound is named [103,104]. The chemical formula of petunidin is presented in Figure 8. Petunidin, like other anthocyanins, has strong antioxidant properties that help protect cells from damage caused by free radicals. This has been confirmed not only experimentally but also computationally by studying the structure and antioxidant properties of the natural food color [105]. Electron-releasing substituents, pKa, atomic charge, and bond order were examined, finding ease of hydrogen atom transfer and occurrence in the deprotonated form in blood. In addition, significant bioactivity of petunidin against nuclear receptor ligands, significant activity as a kinase inhibitor, and moderate activity as an enzyme and protease inhibitor were found. Petunidin may be a potential antioxidant used in pharmacology.

Figure 8.
Chemical formula of petunidin (created with ChemSketch).
A study of the correlation of petunidin with carotenoids was also conducted using lycopene as an example and possible interactions that occur between them [106]. A model of a cardiac myofibroblast cell line (H9c2) induced by hydrogen peroxide (H2O2) was used. Petunidin synergistically interacted with lycopene, and each of them significantly protected against the loss of cell viability and activity of intracellular antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase). The health-promoting effect is mainly based on the activation of the Nrf2 antioxidant response pathway.
In addition to its antioxidant activity, petunidin also has anti-inflammatory effects. The combination of these two properties and their mutual interpenetration are well reflected in a study on Hashimoto’s thyroiditis—a common autoimmune thyroid disease [107]. The two main pathophysiological factors of the disease are inflammation and oxidative stress. C57BL/6N mice treated with petunidin had reduced levels of TPOAb, TgAb, T3, T4, IgG, IgA, and IgM, which were accompanied by significant changes in the shape of follicles and increased lymphocyte infiltration. Anthocyanin improved thyroid dysfunction by inhibiting apoptosis and reducing ROS levels, MDA content, CD4+ levels, IFN-γ and IL-17A levels, and Th1/Th17 differentiation by regulating the NOX4/PKM2 axis.
Petunidin can also significantly improve isolated heart function in rats with anoxia/reoxygenation-induced myocardium injury [108]. It inhibits cardiomyocyte apoptosis, increases Bcl-2 protein expression, decreases NOX4 and Bax expression, and reduces cytoplasmic cytochrome c levels. Another impressive and research-proven property of petunidin is its antiproliferative effect. It has also been used to improve sRANKL-induced osteopenia in mice, increasing osteoid formation and inhibiting bone resorption. Petunidin reduced the expression of c-fos, Nfatc1, Mmp9, Ctsk, and Dc-stamp mRNA in RAW264.7 cells [109].
In studies on nematodes, it was found that petunidin glucoside significantly extended the average lifespan by 19.50%, increased motility, and reduced lipofuscin accumulation, while maintaining fertility [110]. Petunidin has many properties that may contribute to its anticancer effects, such as antioxidant, anti-inflammatory, antiproliferative, and apoptosis-inducing effects on cancer cells [111]. However, further research is needed to fully understand its potential and safety of use.
3.5. Malvidin
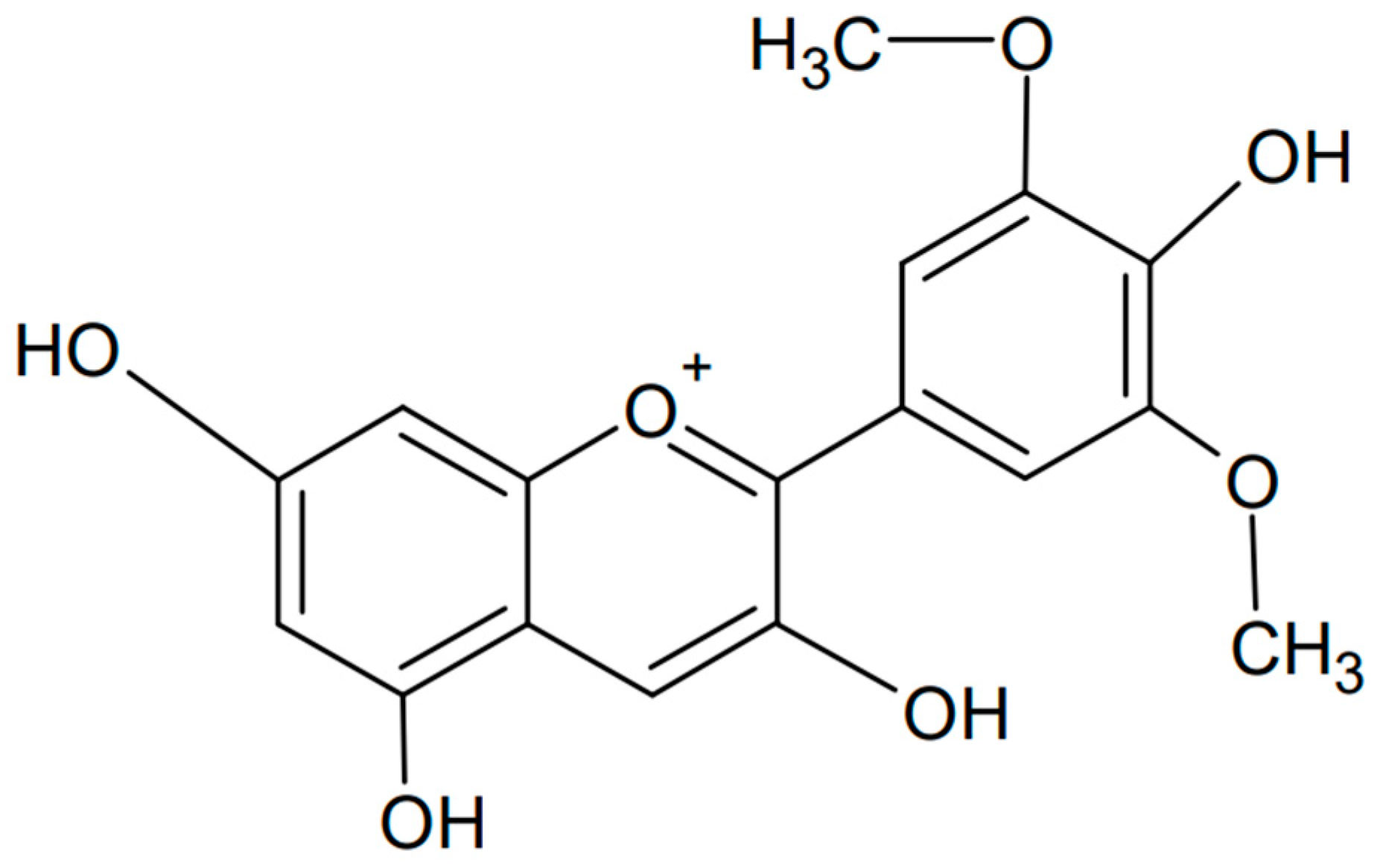
Malvidin is a type of anthocyanin, a compound with antioxidant properties that contribute to the color of many fruits, flowers, and vegetables. Malvidin is particularly known for imparting the blue or purple hue often found in grapes, berries, and other dark fruits [112,113,114,115]. Chemically known as 3′,5′-dimethoxy-3,4′,5,7-tetrahydroxyflavylium, it is an anthocyanidin cation whose structure is similar to delphinidin but has methyl groups attached to positions 3′ and 5′ (PubChem CID: 159287). The chemical formula of malvidin is presented in Figure 9. Like other anthocyanins, malvidin acts as an antioxidant, helping to neutralize free radicals and reduce oxidative stress. This was confirmed when malvidin glycosides found in blueberries were tested [116]. The anthocyanins reduced levels of reactive oxygen species (ROS) and xanthine oxidase-1 (XO-1) and increased superoxide dismutase (SOD) and heme oxygenase-1 (HO-1). Such properties may protect cells from oxidative degradation and offer the possibility of using malvidin as a potential functional food to prevent oxidative stress-related diseases. It has also been shown that malvidin is the most abundant polyphenol in red wine, and that its moderate consumption may impact chronic inflammatory diseases such as obesity, diabetes, high blood pressure, and cardiovascular diseases [117]. Attenuation of lipopolysaccharide-induced nuclear factor-kappa B, poly-ADP-ribose polymerase, and mitogen-activated protein kinase activation, reactive oxygen species production, and mitochondrial depolarization, along with a concomitant increase in mitogen-activated protein kinase phosphatase-1 expression and Akt activation, are examples of parameters influenced by malvidin.

Figure 9.
Chemical formula of malvidin (created with ChemSketch).
The antitumor potential of malvidin is another area of interest in medicine. Its activity has been confirmed in various types of cancer, such as leukemia, colon/rectal cancer, stomach cancer, liver cancer, lung cancer, oral cancer, and breast cancer in both in vivo and in vitro studies [118]. Malvidin and its glycosides have been shown to induce apoptosis, autophagy, cell cycle arrest, suppression of cell proliferation, and prevention of metastasis by modulating different signaling pathways. For example, a study on hepatocellular carcinoma found that malvidin glucoside significantly regulated the microbial TCA cycle of the KEGG pathway by improving the expression of key proteins (porA, DLAT, aceE, PC, and OGDH) [119]. Another experiment on gastric and esophageal adenocarcinomas resulted in significant cell arrest in the G0–G1 phase of the cell cycle [120]. Other studies have highlighted the importance of the JAK/STAT-3 pathway in oral cancer, where malvidin acts as a STAT-3 inhibitor in the SCC13 oral cancer cell line, inhibiting STAT-3 phosphorylation and nuclear translocation, thereby inducing cell cycle arrest and mitochondrial-mediated apoptosis [121].
These examples confirm the importance of further scientific research into the potential therapeutic applications of malvidin due to its health benefits.
4. Anti-Inflammatory Properties of Anthocyanins
Inflammation is a generalized reaction of the body that occurs as a result of the action of a damaging factor [122]. It can be a physical, mechanical, chemical, or biological factor [123]. The causes can also be divided into endogenous and exogenous. The purpose of inflammation is to mobilize the body, the cells, and mediators produced by the cells to neutralize the threat and fight it. Inflammation is characterized by redness, swelling, fever, loss of function, and pain [124]. The mechanism of inflammation is quite complex and can be divided into three main phases: initiation, propagation, and termination. In the first phase, tissue damage or infection leads to the release of inflammatory mediators (e.g., histamine, prostaglandins, leukotrienes, tumor necrosis factor (TNF), interleukin-1 (IL-1), and interleukin-6 (IL-6)). Blood vessels dilate, and their permeability increases. Immune system cells are recruited to the site of damage (e.g., leukocytes). In the next stage, immune system cells are activated and migrate: neutrophils, basophils, eosinophils, lymphocytes, and monocytes [125]. The release of additional inflammatory mediators and increased vascular permeability leads to edema. The final phase occurs after the inflammatory factor is removed. The body mobilizes reserves to repair damaged tissues, which contributes to the end of the inflammatory response and the return to homeostasis [126]. Freeing the host organism from the primary cause of inflammation protects it from the development of many diseases and illnesses. It is said that inflammation is a reaction necessary for survival [127].
Recent studies have reported that anthocyanins are becoming increasingly popular due to their numerous health benefits, including their anti-inflammatory properties. Due to their strong antioxidant properties, anthocyanins may play an important role in the treatment and prevention of inflammation [128]. In addition to studies confirming the anti-inflammatory effect of anthocyanins conducted in in vitro cell lines and in vivo animal studies, which are presented in Table 2, there are also clinical trials of the use of plant-derived compounds in humans. Although studies in humans are less numerous, there is evidence that anthocyanin consumption may be beneficial in the context of inflammatory diseases such as arthritis, heart disease, and inflammatory bowel disease.
A study conducted on a population of 2375 Framingham Heart Study Offspring Cohort participants showed that higher anthocyanin intake was inversely correlated with all twelve individual biomarkers of inflammation considered in the study [129]. Another randomized controlled trial focused on the problem of hypercholesterolemia based on the inflammatory disease atherosclerosis. Anthocyanin intake significantly reduced levels of high-sensitivity C-reactive protein (hsCRP), soluble vascular cell adhesion molecule-1 (sVCAM-1), plasma IL-1β, and LDL cholesterol compared with placebo [130]. Anthocyanins have significant potential as anti-inflammatory agents due to their antioxidant properties and ability to modulate inflammatory pathways.

Table 2.
Anti-inflammatory properties of anthocyanins in in vitro and in vivo studies.
Table 2.
Anti-inflammatory properties of anthocyanins in in vitro and in vivo studies.
| Plant/Source | Compounds | Cell Line/Model | Effect | In Vitro/In Vivo | Ref. |
|---|---|---|---|---|---|
| Petunidin chloride with a purity of 99%, | petunidin (anthocyanins) and lycopene (carotenoids) | hydrogen peroxide (H2O2)-induced heart myofibroblast cell (H9c2) line model | protected against the loss of the cell viability, increased: superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) induced: expression of (mRNA), protein of NAD(P)H quinone reductase (NQO1) and heme oxygenase (HO-1) of the nuclear factor erythrocyte 2-related factor 2 (Nrf2) | In vitro | [106] |
| Syzygium cumini | delphinidin 3,5-diglucoside (DDG),petunidin-3,5-diglucoside (PDG), malvidin 3,5-diglucoside (MDG) | lipopolysaccharide (LPS)-induced RAW264.7 macrophages | inhibition of nitric oxide release and pro-inflammatory mediators: mouse interleukin 6 (IL-6), mouse interleukin (IL-1β) and mouse tumor necrosis factor (TNF-α) | In vitro | [131] |
| Solanum lycopersicum | approximately 11 to over 23 different kinds of anthocyanins, including 3 anthocyanidins (delphinidin, petunidin (petunidin-3-glucoside), and malvidin) | 661W a mouse photoreceptor cell line | inhibition of H2O2-induced cell death, inhibition of ROS production, suppression of apoptosis | In vitro | [132] |
| Chrysobalanus icaco L. | delphinidin-3-glucoside, cyanidin 3-glucoside, petunidin 3-glucoside, delphinidin 3-(6″-acetoyl) galactoside, delphinidin 3-(6″-oxaloyl) arabinoside, peonidin 3-glucoside, petunidin 3-(6″-acetoyl) galactoside or petunidin 3-(6″-oxaloyl) arabinoside, peonidin 3-(6″-acetoyl) glucoside, or peonidin 3-(6″-oxaloyl) arabinoside | TNF-α induced non-malignant colonic fibroblasts CCD-18Co and HT-29 colorectal adenocarcinoma cells | decreased intracellular ROS production in CCD-18Co, decreased TNF-α, IL-1β, IL-6 expression | In vitro | [133] |
| Purple carrots (Purple Haze) or potatoes (MacIntosh) | cyanidin-3-O-(2″-xylosyl-6″-(6‴-feruloyl-glucosyl)-galactoside and petunidin-3-O-(p-coumaroyl)-rutinoside-5-Oglucoside | Caco-2 BBe1/THP-1 co-culture cell model, gastrointestinal model | inhibited cellular inflammation, inhibited IL-8 and TNF-α secretion and expression of pro-inflammatory cytokines by blocking NF-κB, and MAPK mediated inflammatory cellular signaling cascades | In vitro | [134] |
| Commercial | delphinidin and petunidin | rat cardiomyocytes line H9c2 | DFT calculation- the antioxidant activity from chemical mechanism | In vitro | [135] |
| Bilberry (Vaccinium myrtillus L.) | delphinidin-3-galactoside chloride, delphinidin 3- glucoside chloride, cyanidin-3-galactoside, cyanidin-3-glucoside, petunidin-3-glucoside, and cyanidin-3-arabinoside | RAW 264.7 cell line | anti-inflammatory and antioxidant activity, inhibited oxidation of linoleic acid, suppressed nitric oxide (NO) generation, pro-inflammatory cytokines iNOS, COX-2, TNF-α, and IL-6 in LPS-induced cells | In vitro | [136] |
| Anthocyanidins purified by HPLC (Irvine, CA, USA) | petunidin, delphinidin, cyanidin, pelargonidin, malvidin, and peonidin | human aortic smooth muscle cells (HASMCs) | inhibited PDGF-BB-induced phosphorylation of focal adhesion kinase (FAK), suppressed FAK activity by binding in an ATP, reduced HASMC migration, inhibited PDGF-BB-induced FAK phosphorylation, F-actin reduction, and FAK activity, protected against atherosclerosis | In vitro | [137] |
| Plant source | cyanidin (Cy), peonidin (Pn), pelargonidin (Pg), malvidin (Mv), delphinidin (Dp), and petunidin (Pt) | referenced cell lines, ulcerative colitis | reduced expression levels of TNF-α and IL-1β, reduced serum levels of IL-12 and IFN-γ, down-regulated IFN-γ, and the inflammation-associated ROS-producing enzyme myeloperoxidase (MPO), NF-κB signaling pathway, prevented increase of IL-6, and nitric oxide synthase (iNOS) | In vitro | [138] |
| Four varieties of beans: Negro 8025, Bayo Victoria, Pinto Durango, and Pinto Saltillo | delphinidin-3-glucoside, malvidin-3-glucoside, petunidin-3-glucoside, pelargonidin-3-glucoside, and cyanidin-3-glucoside | human intestinal cell model | antioxidant activities, inhibited lipid peroxidation, chelating capacities, deoxy-D-ribose degradation, decreased interleukin-8 (IL-8), modulated interleukin-10 (IL-10), inhibited tumor necrosis factor alpha (TNFα), NF-κβ, cyclooxygenase-2 (COX-2) | In vitro | [139] |
| Red clover (Trifolium pratense) | delphinidin-3, 5-O-diglucoside, Cyanidin-3-O-galactoside, Cyanidin-3-O-glucoside, Petunidin-3-O-galactoside, Peonidin-3-O-galactoside, Malvidin-3-O-galactoside, Petunidin-3-O-rutinoside | mouse monocyte RAW 264.7 cells | anti-inflammatory and antioxidant effects, suppressed expression of genes: TNFα, interleukin (IL)1β, inducible nitric oxide synthase (iNOS), monocyte chemoattractant protein (MCP)1, and cyclooxygenase (COX)2, stimulated intracellular reactive oxygen species (ROS), increased NADPH oxidase 1 (NOX1) and phosphorylation of p47phox, nuclear factor erythroid 2-related factor 2 (NRF2), factor kappa B (NF-kB), reduced iNOS, COX2 | In vitro | [140] |
| Red grape skin | delphinidin 3-glucoside, Cyanidin 3-glucoside, Petunidin 3-glucoside, Peonidin 3-glucoside, Malvidin 3-glucoside, Peonidin 3-(6″-acetyl)-glucoside, Malvidin 3-(6″-acetyl)-glucoside, Delphinidin 3-(6″-coumaroyl)-glucoside, Malvidin 3-(6″-caffeoyl)-glucoside, Petunidin 3-(6″-coumaroyl)-glucoside, Peonidin 3-(6″-coumaroyl)-glucoside, Malvidin 3-(6″-coumaroyl)-glucoside | R3/1 cell line | anti-inflammatory activity, decreased NF-kb reporter and IL-1α | In vitro | [141] |
| Rabbiteye blueberry (Vaccinium ashei) | delphindin-3-galactoside, delphindin-3-glucoside, cyaniding-3-galactoside, petunidin-3-galactoside, cyaniding-3-glucoside, cyaniding-3-arabinoside, petunidin-3-glucoside, peonidin-3-galactoside, petunidin-3-arabinoside, peonidin-3-glucosidea, malvidin-3-galactoside, malvidin-3-glucoside, malvidin-3-arabinose | endothelial cell culture-HRCECs human retinal capillary endothelial cells | antioxidant and anti-inflammatory, decreased the reactive oxygen species ROS, increased the enzyme activity of catalase (CAT) and superoxide dismutase (SOD), inhibited: intercellular adhesion molecule-1 (ICAM-1), nuclear factor-kappa B (NF-κB), Akt pathway, Nox4 expression, nitric oxide (NO) levels, decreased vascular endothelial cell growth factor (VEGF) | In vitro | [142] |
| Commercial | cyanidin-3-O-glucoside chloride, delphinidin -3-O-glucoside cholride, pelargonidin-3-O-glucoside chloride, malvidin-3-O-glucoside chloride, peonidin-3-O-glucoside chloride, and petunidin-3-O-glucoside chloride combined with lutein | Caco-2 cell model | suppression of pro-inflammatory mediators IL-8, NO, antioxidant effects, antagonistic interaction on lipid peroxidation in a phosphatidylcholine liposome membrane with lutein | In vitro | [143] |
| Purple tomato (Solanum lycopersicum L.) line V118 and red tomato H5108 F1 | petunidin-3-O-caffeoyl-rutinoside-5-O-glucoside, petunidin-3-O-(p-coumaroyl)-rutinoside-5-O-glucoside, and malvidin-3-O-(p-coumaroyl)-rutinoside-5-O-glucoside | MCF-10A breast epithelial cells, simulated gastrointestinal digestion model | anti-inflammatory effect, reduced MDA and NO production, increased GPx, SOD, protection against oxidative stress | In vitro | [144] |
| Extract from banana bract | malvidin, petunidin, delphinidin-3-O-galactoside, peonidin-3-O-beta-galactopyranoside, cyanidin 3-O-xylosyl-rutinoside, cyanidin 3-O-rutinoside, malvidin-3-rutinoside, pelargonidin-3-O-glucoside, delphinidin-3-O-rutinoside | LPS-stimulated murine macrophages– RAW 264.7 | anti-inflammatory properties, antioxidant activity, suppressed the NO, pro-inflammatory and PGE2 production, increased the endogenous antioxidants substances level, inhibited the nuclear translocation of NF-κB | In vitro | [145] |
| Purple yam, Dioscorea alata L. | cyanidin-3,5-diglucoside, cyanidin-3-diglucoside-5-glycosides, delphinidin-3-glucose-5-rutinoside, delphinidin-3-glucoside, delphinidin-3,5-diglucoside | trinitrobenzenesulfonic acid (TNBS)-induced colitis mouse model | reduced MPO concentrations, tumor necrosis factor α, interferon γ, iNOS, decreased expression of proteins ZO-1, claudin-1, occludin, mucin-1, and mucin-2 | In vitro | [146] |
| Commercial | cyanidin-3-glucoside, delphinidin-3-glucoside, malvidin-3-glucoside, peonidin-3-glucoside, pelargonidin-3-glucoside, petunidin-3-glucoside | human carcinogenic colon Caco-2 cells | antioxidant and anti-inflammatory effects, modulated the biomarkers: reactive oxygen species (ROS), reactive nitrogen species (RNS), pro-inflammatory cytokines and chemokines: IL-8, IL-6, IL-1β, PGE2; defensive enzymes: catalase, superoxide dismutase; intracellular signaling pathways NF-κB, mitogen-activated protein kinase | In vitro | [147] |
| Gynura bicolor DC. | cyanidin, keracyanin, kuromanin, malvidin, pelargonidin, peonidin, and petunidin | human umbilical vein endothelial (HUVE) cells | antioxidative and anti-inflammatory potentials, decreased reactive oxygen species formation, preserved glutathione content and retained glutathione peroxide and catalase activities, down regulated interleukin-6, tumor necrosis factor-alpha and prostaglandin E2 production, reduced cyclooxygenase-2 activity | In vitro | [148] |
| Red (RO) and yellow (YO) onion peel extracts | cyanidin-O-malonylhexoside, peonidin-O-hexoside, delphinidin-O-hexoside acetate, cyanidin-O-hexoside acetate, petunidin-O-hexoside | human adenocarcinoma cells from the MCF-7 and HT-29 cell line | suppressed NLRP3/caspase-1 signaling, decreased inflammatory cytokines, Notch-1 levels, boosted VEGF-mediated angiogenesis, reduced microbial infection, inflammation, and promoted tissue regeneration | In vitro | [149] |
| Plant materials: two fruits (mahaleb cherry and blackcurrant) and two vegetables (black carrot and “Sun Black” tomato) | pelargonidin, cyanidin, delphinidin, peonidin, petunidin, and malvidin and their mono-, di-, or tri-glycosides | human microvascular endothelial cell line (HMEC-1) | antioxidant effects, cardiovascular protection, expression of endothelial adhesion molecules VCAM-1 and ICAM-1 | In vitro | [150] |
| Northern highbush blueberry extract | delphinidin, cyanidin, petunidin, peonidin, and malvidin with variation existing in the number of hydroxyl groups, methylation, location of sugar molecules | colon epithelial cell lines, NCM 356 and CCD 841 CoN | decreased in nuclear and cytoplasmic generated reactive oxygen species (ROS), increased cell viability, inhibited IL-1b, NF-jB, COX-2 expression | In vitro | [151] |
| Bilberry (Vaccinium myrtillus L.) | delphinidin, cyanidin, petunidin, peonidin and malvidin | HeLa-TLR4, THP-1, and human embryonic kidney (HEK)TLR2/HEK-TLR4 cell lines | anti-inflammatory effect, decreased tumor necrosis factor-α, interleukin (IL)-6, IL-1β expression, induced nitric oxide synthases, cyclooxygenases, nuclear factor kappa B, and Janus kinase-signal transducer and activator of transcription signaling pathways | In vitro | [152] |
| Grape (Vitis vinifera L.)-16 grape varieties | the 3-O-monoglucosides of delphinidin, cyanidin, petunidin, peonidin, and malvidin | human epithelial gastric cells (AGS) | reduced IL-8, TNFα, alleviated inflammatory processes | In vitro | [153] |
| Red wine extract | delphinidin, petunidin, peonidin, malvidin | murine macrophage cell line J774A.1 and Raw 264.7 | prevented the carcinogenesis process, modulated IL-1β secretion, NLRP3 inflammasome pathway, increased the synthesis of NLRP3 and pro-IL-1β proteins, inhibited express the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD), inflammasome complexes | In vitro | [154] |
| Black soybean | three major anthocyanins (cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, and petunidin3-O-glucoside) ~ about 90% of the total peak area and six minor anthocyanins cyanidin3-O-glucoside, delphinidin-3-O-glucoside, and petunidin-3-O-glucoside | 3T3-L1 mouse embryo fibroblasts and RAW264.7 macrophage cells | anti-inflammatory, antidiabetic effect, decreased the production of reactive oxygen species and inflammatory mediators and cytokines (NO, MCP-1, PGE2, TNFα, and IL-6) and the release of free fatty acids, increased anti-inflammatory adiponectin secretion | In Vitro | [155] |
| Vitis labrusca extract | 3-O-glucosides: peonidin, delphinidin, petunidin, and malvidin; 3-p-coumaroyl-glucosides: cyanidin, peonidin, petunidin and malvidin, and malvidin-3,5-diglucoside | Swiss mice | reduced carrageenan-induced mechanical and thermal hyperalgesia, paw edema, and neutrophil recruitment, anti-inflammatory properties | In vivo | [156] |
| Vaccinium myrtillus-bilberry | delphidin-3-O-galactoside, delphidin-3-O-glucoside, delphidin-3-O-arabinoside, cyanidin-3-O- galactoside, cyanidin-3-O-glucoside, petunidin-3-O-galactoside, cyanidin-3-O-arabinoside, petunidin-3-O-glucoside, peonidin-3-O-galactoside, petunidin-3-O-arabinoside, peonidin-3-O-glucoside, malvidin-3-O-galactoside, malvidin-3-O-glucoside, malvidin-3-O-arabinoside | mouse model | anti-inflammatory properties, decreased lipid peroxidation and mucosal injury in the ileum, directly reduced ROS, decrease of Myeloperoxidase | In vivo | [157] |
| Black soybean | glycosides of cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin | rat model with Peyronie disease | anti-inflammatory and antifibrosis activities, decreased TGF-b1 expression, decrease activity of fibrin injections | In vivo | [158] |
| Substance obtained commercially | Petunidin chloride (purity ≥ 98%) | adult male Sprague–Dawley (SD) rats | improved isolated heart function, reduced oxidative stress, up-regulated Bcl-2 protein expression, down-regulated NOX4 and Bax expression, reduced the level of cytoplasmic cytochrome c | In vivo | [108] |
| Substance purified by HPLC | Petunidin, delphinidin, cyanidin, pelargonidin, malvidin, and peonidin | rat in vivo and rat aorta ex vivo | reduced (HASMC) human aortic smooth muscle cell migration, inhibited PDGF-BB-induced FAK phosphorylation, F-actin reduction, and FAK activity | In vivo | [137] |
| Solanum lycopersicum L. | petunidin-3-O-caffeoyl-rutinoside-5-O-glucoside, petunidin-3-O-(p-coumaroyl)-rutinoside-5-O-glucoside and malvidin-3-O-(p-coumaroyl)-rutinoside-5-O-glucoside | carrageenan-induced paw edema rat | reduced MDA and NO production, increased GPx and SOD activities in edematous tissue, inhibited paw edema formation | In vivo | [144] |
| Red onion (RO) and yellow (YO) onion peel extracts | cyanidin-O-malonylhexoside, peonidin-O-hexoside, delphinidin-O-hexoside acetate, cyanidin-O-hexoside acetate, petunidin-O-hexoside | rat | suppressed NLRP3/caspase-1 signaling, decreased inflammatory cytokines, Notch-1 levels, boosted VEGF-mediated angiogenesis, reduced microbial infection, inflammation, and promoted tissue regeneration | In vivo | [149] |
| Bilberry anthocyanin extract | delphinidin-3-galactoside, delphinidin-3-glucoside, cyanidin-3-galactoside, delphinidin-3-arabinoside, cyanidin-3-glucoside, petunidin-3-galactoside, cyanidin-3-arabinoside, petunidin-3-glucoside, peonidin-3-galactoside, petunidin-3-arabinoside, peonidin-3-glucoside, malvidin-3-galactoside, malvidin-3-glucoside, malvidin-3-arabinoside | model of phototoxicity in pigmented rabbits | reduced changes in apoptotic proteins (Bax, Bcl-2, and caspase-3), increased the levels of superoxide dismutase, glutathione peroxidase, and catalase, decreased the malondialdehyde level, inhibited the levels of pro-inflammatory cytokines and angiogenic parameters (IL-1β and VEGF) | In vivo | [159] |
| Commercial compounds | canidin, delphinidin, malvidin, pelargonidin and petunidin | the blood-–rain barrier in vivo and human U-87 MG cell line | anti-inflammatory, cardioprotective, anti-angiogenic, and anticarcinogenic properties, activation TGF-β Smad and non-Smad signaling pathways | In vivo | [160] |
| Lycium ruthenicum Murray | petunidin 3-O-[rhamnopyranosyl-(trans-p-coumaroyl)]-5-O-[β-D-glucopyranoside] | dextran sodium sulfate-induced colitis in mice | anti-inflammatory effects, reduction of the expression of pro-inflammatory cytokines and related mRNA: TNF-α, IL-6, IL-1β, and IFNγ, and promotion of the intestinal barrier function by histological and immunofluorescence analysis, increased proteins such as ZO-1, occludin, and claudin-1, blocked pro-inflammatory cytokines | In vivo | [161] |
| Portuguese blueberries (Vaccinium corymbosum L.) | malvidin, petunidin, peonidin, delphinidin, and cyanidin | 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis rat model | reduction in leukocyte infiltration, increased antioxidant defenses, downregulated nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), anti-inflammatory mechanism | In vivo | [162] |
| Lycium ruthenicum Murray (LR) | delphinidin-3-glu, cyanidin-3-glu, petunidin-3-glu, peonidin-3-glu, malvidin-3-glu, delphinidin, cyanidin, petunidin, pelargonidin and malvidin | rat model involving gouty arthritis induced by monosodium urate | decreased tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-18 (IL-18), prostaglandin E2 (PE2), cyclooxygenase-1 (COX-1) enzymes, paw volume, reduced inflammation | In vivo | [162] |
| Black soybean | delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, petunidin-3-O-glucoside | adult male Sprague–Dawley rats–the chronic bacterial prostatitis (CBP) rat model | anti-inflammatory and antimicrobial effects, decreased bacterial growth, reduction of prostatic inflammation compared with the control group | In vivo | [163] |
5. Anticancer Effect of Anthocyanins
In this paper, we also want to draw attention to a key problem of modern times: the increasing incidence of various cancers in the population and the potential use of anthocyanins as antiproliferative drugs. Anthocyanins, which are natural plant pigments, have strong anticancer effects, which is the subject of numerous scientific studies. Cancer is an abnormal and uncontrolled growth of cells in the body, which can lead to the formation of tumors (tissue masses) and metastases (spread of cancer cells to other parts of the body). Cells breaking away from the regulation of the cell cycle result in a cascade of unnatural multiplication and division. Cancers can be benign or malignant [164,165,166].
Cancers can have many causes, including genetic factors (inheritance of mutations in genes that increase the risk of cancer, e.g., BRCA1 and BRCA2 in the case of breast cancer) [167,168], environmental factors (exposure to carcinogens, e.g., tobacco smoke, asbestos, UV radiation) [169,170,171], lifestyle factors (a diet low in vegetables and fruits, lack of physical activity, excessive alcohol consumption, smoking) [172,173], infections (oncogenic viruses, e.g., human papillomavirus HPV, hepatitis B and C virus) [174,175], and chronic diseases (chronic inflammatory conditions, such as intestinal inflammation, may increase the risk of developing cancer). It turns out that anthocyanins have significant potential as anticancer agents due to their antioxidant properties, ability to induce apoptosis, inhibit cancer cell proliferation, inhibit angiogenesis, and reduce metastasis [176]. Studies carried out in A549 and H1299 lung cancer cells, as well as in vivo in mice, confirm the antiproliferative effects on lung cancer cells, reduction of tumor size, and suppression of cancer migration. The key proteins in these studies were MMP-2, MMP-9, COX-2, C-myc, cyclin D1, β-catenin, cyclin B, pERK, and VEGF. Apoptosis was promoted by Bcl-2 and PARP [177,178,179]. Another cancer for which anthocyanin extract was used as a supportive treatment is breast cancer. Different cell lines, such as BT474, MDA-MB-453, MDA-MB-231, A17, N202/1A, and N202/1E, confirmed the downregulation of the Akt/mTOR pathway, pro-survival Sirt1/survivin pathways, antiproliferation, apoptosis, and reduction of the metastatic markers Sp1, Sp4, and VCAM-1 [180,181,182]. Liver cancer and stomach cancer are other examples of the use of anthocyanins for anticancer activity. Among anthocyanin extracts from blueberries, blackberries, and grapes, the action of cyanidin in digestive system cancers is prominent. It induces apoptosis via the Bax, cytochrome C, caspase 3, and Akt pathways [183,184]. Another study highlights the induction of apoptosis via the p38/Fas/FasL/caspase 8 and p38/p53/Bax signaling pathways [185]. Anthocyanins have also been used to inhibit the growth and division of prostate cancer cells (22Rv1, PC-3, C4-2) [186]. In laboratory studies on cell lines of various cancers (e.g., breast, colon, prostate cancer), anthocyanins have demonstrated the ability to inhibit the growth of cancer cells and induce apoptosis. In animal studies (e.g., mice), anthocyanin supplementation led to a reduction in the size of tumors and the number of metastases. Examples of both in vitro and in vivo studies of the anticancer effects of anthocyanins are presented in Table 3.

Table 3.
Anticancer properties of anthocyanins in vitro and in vivo studies.
6. Conclusions and Future Perspectives
The significant potential of anthocyanins as bioactive compounds with multiple health benefits is highlighted by the extensive body of research reviewed in this review. Both in vitro and in vivo studies have consistently demonstrated the antioxidant, anti-inflammatory, anticarcinogenic, and neuroprotective properties of these flavonoid pigments. The mechanistic insights gained from these studies indicate that anthocyanins exert their beneficial effects through multiple pathways. These include modulation of oxidative stress, regulation of inflammatory mediators, influence on gene expression, and interaction with various cellular signaling cascades. Importantly, while in vitro studies provide valuable insights into the molecular mechanisms of anthocyanin action, translating these findings to in vivo efficacy faces several challenges. The ultimate health effects of anthocyanins in living organisms are significantly influenced by factors such as bioavailability, metabolism, and the complex interactions within biological systems. The need for more comprehensive and integrated research approaches is highlighted by the discrepancies sometimes observed between in vitro and in vivo results. Future research directions should be focused on the conduct of long-term clinical trials to establish the efficacy and safety of anthocyanin supplementation in humans. It is also important to investigate the potential synergistic effects of anthocyanins with other phytochemicals and nutrients. Valuable insights will also be gained from elucidating the role of the gut microbiome in anthocyanin metabolism and bioactivity. Another important area for future study is the development of novel delivery systems to improve the bioavailability and stability of anthocyanins. New avenues for the application of anthocyanins in health care may be opened by exploring their potential in personalized nutrition and medicine. It is, of course, worth noting that there are many different compounds in nature that exhibit strong anticancer and anti-inflammatory effects, including quercetin, curcumin, resveratrol, sulforaphane, and lycopene. Each of these compounds has a different effect in different concentrations, which is related to a number of different factors ranging from bioavailability through synergistic effects to long-term effects.
In conclusion, further research is needed to fully understand their mechanisms of action and optimize their use in disease prevention and treatment, although current evidence strongly supports the health-promoting properties of anthocyanins. Anthocyanins promise to be valuable tools in the quest for improved human health and well-being as our understanding of these complex compounds increases. Ongoing scientific research into anthocyanins not only advances our knowledge of plant-derived bioactive compounds; it also contributes to the broader field of preventive medicine and nutritional science.
Author Contributions
Conceptualization, P.S. and T.K.; writing—original draft preparation, M.M., A.M.-S., P.S., T.K. and J.S.; writing—review and editing, L.P., P.S. and T.K.; supervision, P.S., T.K. and J.S.; visualization, T.K., A.M.-S. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Riaz, M.; Khalid, R.; Afzal, M.; Anjum, F.; Fatima, H.; Zia, S.; Rasool, G.; Egbuna, C.; Mtewa, A.G.; Uche, C.Z.; et al. Phytobioactive compounds as therapeutic agents for human diseases: A review. Food Sci. Nutr. 2023, 11, 2500–2529. [Google Scholar] [CrossRef]
- Guo, M.; Lv, H.; Chen, H.; Dong, S.; Zhang, J.; Liu, W.; He, L.; Ma, Y.; Yu, H.; Chen, S.; et al. Strategies on biosynthesis and production of bioactive compounds in medicinal plants. Chin. Herb. Med. 2023, 16, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Merecz-Sadowska, A.; Picot, L.; Brčić Karačonji, I.; Wieczfinska, J.; Śliwiński, T.; Sitarek, P. Genetic Manipulation and Bioreactor Culture of Plants as a Tool for Industry and Its Applications. Molecules 2022, 27, 795. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, A.J.A.B. A brief review on bioactive compounds in plants. Bioact. Compd. Plants Benefits Risks Man Anim. 2010, 50, 11–17. [Google Scholar]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- International Food Information Council (IFIC); Foundation US Food and Drug Administration (FDA). Food Ingredients and Colors; IFIC Foundation: Washington, DC, USA, 2004. [Google Scholar]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Marquart, L.C. Di e Farben der Bluthen, eine Chemisch-Physiologische Abhandlung; Kessinger Publishing: Bonn, Germany, 1835. [Google Scholar]
- Strack, D.; Wray, V. Anthocyanins. In Methods in Plant Biochemistry; Academic Press: Cambridge, MA, USA, 1989; Volume 1, pp. 325–356. [Google Scholar]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.W.; Munawaroh, H.; Ramelan, A.H. The effect of pH and color stability of anthocyanin on food colorant. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 193, p. 012047. [Google Scholar]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An insight into anti-inflammatory activities and inflammation related diseases of anthocyanins: A review of both in vivo and in vitro investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef] [PubMed]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and anti-inflammatory properties of anthocyanins extracted from Oryza sativa L. in primary dermal fibroblasts. Oxidative Med. Cell. Longev. 2019, 1, 2089817. [Google Scholar] [CrossRef]
- Nikbakht, E.; Singh, I.; Vider, J.; Williams, L.T.; Vugic, L.; Gaiz, A.; Kundur, A.R.; Colson, N. Potential of anthocyanin as an anti-inflammatory agent: A human clinical trial on type 2 diabetic, diabetic at-risk and healthy adults. Inflamm. Res. 2021, 70, 275–284. [Google Scholar] [CrossRef]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins and heart health. Ann. Ist. Super. Di Sanita 2007, 43, 369. [Google Scholar]
- Pascual-Teresa, D.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef]
- Sancho, R.A.S.; Pastore, G.M. Evaluation of the effects of anthocyanins in type 2 diabetes. Food Res. Int. 2012, 46, 378–386. [Google Scholar] [CrossRef]
- Różańska, D.; Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018, 27, 135–142. [Google Scholar] [CrossRef]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic effects of anthocyanins for vision and eye health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef]
- Khoo, H.E.; Ng, H.S.; Yap, W.S.; Goh, H.J.H.; Yim, H.S. Nutrients for prevention of macular degeneration and eye-related diseases. Antioxidants 2019, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Chung, J.I.; Kim, M.O. Anthocyanins improve hippocampus-dependent memory function and prevent neurodegeneration via JNK/Akt/GSK3β signaling in LPS-treated adult mice. Mol. Neurobiol. 2019, 56, 671–687. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, H.; Zhang, G.; Meng, J.; Deng, K.; Zhou, W.; Wang, H.; Wang, Z.; Hu, N.; Suo, Y. Anthocyanins from Lycium ruthenicum Murr. ameliorated d-galactose-induced memory impairment, oxidative stress, and neuroinflammation in adult rats. J. Agric. Food Chem. 2019, 67, 3140–3149. [Google Scholar] [CrossRef]
- Varadinova, M.G.; Docheva-Drenska, D.I.; Boyadjieva, N.I. Effects of anthocyanins on learning and memory of ovariectomized rats. Menopause. 2009, 16, 345–349. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Farjadmand, F.; Karimpour-Razkenari, E.; Nabavi, S.M.; Ardekani, M.R.; Saeedi, M. Plant polyphenols: Natural and potent UV-protective agents for the prevention and treatment of skin disorders. Mini Rev. Med. Chem. 2021, 21, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.; Potapovich, A.; Suhan, T.; De Luca, C.; Pressi, G.; Dal Toso, R.; Korkina, L. Plant polyphenols against UV-C-induced cellular death. Planta Medica 2008, 74, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, X.; Chen, F.; Wang, M. Dietary polyphenols as photoprotective agents against UV radiation. J. Funct. Foods 2017, 30, 108–118. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Cristofaro, M.; Breschi, M.C.; Calderone, V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J. Pharm. Pharmacol. 2013, 65, 750–756. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Chanoca, A.; Kovinich, N.; Burkel, B.; Stecha, S.; Bohorquez-Restrepo, A.; Ueda, T.; Eliceiri, K.W.; Grotewold, E.; Otegui, M.S. Anthocyanin vacuolar inclusions form by a microautophagy mechanism. Plant Cell 2015, 27, 2545–2559. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61. [Google Scholar] [CrossRef]
- Cabrita, L.; Fossen, T.; Andersen, Ø.M. Colour and stability of the six common anthocyanidin 3-glucosides in aqueous solutions. Food Chem. 2000, 68, 101–107. [Google Scholar] [CrossRef]
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J. Plant Biochem. Physiol. 2017, 5, 2. [Google Scholar] [CrossRef]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-gut microbiota-health axis: A review. Crit. Rev. Food Sci. Nutr. 2023, 64, 7563–7588. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Blueberries and their anthocyanins: Factors affecting biosynthesis and properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Slimestad, R.; Solheim, H. Anthocyanins from black currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef]
- Fan-Chiang, H.J.; Wrolstad, R.E. Anthocyanin pigment composition of blackberries. J. Food Sci. 2005, 70, C198–C202. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Mazza, G.; Francis, F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef] [PubMed]
- Demirdöven, A.; Özdoğan, K.; Erdoğan-Tokatlı, K. Extraction of anthocyanins from red cabbage by ultrasonic and conventional methods: Optimization and evaluation. J. Food Biochem. 2015, 39, 491–500. [Google Scholar] [CrossRef]
- Ananga, A.; Georgiev, V.; Ochieng, J.; Phills, B.; Tsolova, V. Production of anthocyanins in grape cell cultures: A potential source of raw material for pharmaceutical, food, and cosmetic industries. Mediterr. Genet. Code-Grapevine Olive 2013, 1, 247–287. [Google Scholar]
- Baldi, A.; Romani, A.; Mulinacci, N.; Vincieri, F.F.; Casetta, B. HPLC/MS application to anthocyanins of Vitis vinifera L. J. Agric. Food Chem. 1995, 43, 2104–2109. [Google Scholar] [CrossRef]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of apoptosis in cancer cells by bilberry (Vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem. 2003, 51, 68–75. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Gil, M.I.; Tomás-Barberán, F.A. Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. J. Agric. Food Chem. 2005, 53, 9544–9552. [Google Scholar] [CrossRef]
- Kim, M.J.; Hyun, J.N.; Kim, J.A.; Park, J.C.; Kim, M.Y.; Kim, J.G.; Lee, S.J.; Chun, S.C.; Chung, I.M. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef]
- Veigas, J.M.; Narayan, M.S.; Laxman, P.M.; Neelwarne, B. Chemical nature, stability and bioefficacies of anthocyanins from fruit peel of Syzygium cumini Skeels. Food Chem. 2007, 105, 619–627. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Dougall, D.K. Regulation of skin color in apples. Crit. Rev. Plant Sci. 1992, 10, 487–502. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Wu, P.; Li, F.; Zhang, J.; Yang, B.; Ji, Z.; Chen, W. Phytochemical compositions of extract from peel of hawthorn fruit, and its antioxidant capacity, cell growth inhibition, and acetylcholinesterase inhibitory activity. BMC Complement. Altern. Med. 2017, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Russo, A.; Galvano, F.; Galvano, G.; Barcellona, M.L.; Li Volti, G.; Vanella, A. Cyanidin and cyanidin 3-O-β-D-glucoside as DNA cleavage protectors and antioxidants. Cell Biol. Toxicol. 2003, 19, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Amorini, A.M.; Fazzina, G.; Lazzarino, G.; Tavazzi, B.; Di Pierro, D.; Santucci, R.; Federica, S.; Fabio, G.; Galvano, G. Activity and mechanism of the antioxidant properties of cyanidin-3-O-β-glucopyranoside. Free. Radic. Res. 2001, 35, 953–966. [Google Scholar] [CrossRef]
- Feng, R.; Ni, H.M.; Wang, S.Y.; Tourkova, I.L.; Shurin, M.R.; Harada, H.; Yin, X.M. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J. Biol. Chem. 2007, 282, 13468–13476. [Google Scholar] [CrossRef]
- Wu, C.F.; Wu, C.Y.; Lin, C.F.; Liu, Y.W.; Lin, T.C.; Liao, H.J.; Chang, G.R. The anticancer effects of cyanidin 3-O-glucoside combined with 5-fluorouracil on lung large-cell carcinoma in nude mice. Biomed. Pharmacother. 2022, 151, 113128. [Google Scholar] [CrossRef]
- Rugină, D.; Hanganu, D.; Diaconeasa, Z.; Tăbăran, F.; Coman, C.; Leopold, L.; Bunea, A.; Pintea, A. Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 949. [Google Scholar] [CrossRef]
- Matboli, M.; Hasanin, A.H.; Hussein, R.; El-Nakeep, S.; Habib, E.K.; Ellackany, R.; Saleh, L.A. Cyanidin 3-glucoside modulated cell cycle progression in liver precancerous lesion, in vivo study. World J. Gastroenterol. 2021, 27, 1435. [Google Scholar] [CrossRef]
- Sorrenti, V.; Vanella, L.; Acquaviva, R.; Cardile, V.; Giofrè, S.; Di Giacomo, C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015, 47, 1303–1310. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yun, S.M.; Song, M.Y.; Jung, K.; Kim, E.H. Cyanidin chloride induces apoptosis by inhibiting NF-κB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, D.; Hao, Y.; Liu, Q.; Wu, Y.; Liu, X.; Luo, J.; Zhou, T.; Sun, B.; Luo, X.; et al. Cyanidin curtails renal cell carcinoma tumorigenesis. Cell. Physiol. Biochem. 2018, 46, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Morimitsu, Y.; Kubota, K.; Tashiro, T.; Hashizume, E.; Kamiya, T.; Osawa, T. Inhibitory effect of anthocyanins and colored rice on diabetic cataract formation in the rat lenses. In International Congress Series; Elsevier: Amsterdam, The Netherlands, 2002; Volume 1245, pp. 503–508. [Google Scholar] [CrossRef]
- Shan, X.; Lv, Z.Y.; Yin, M.J.; Chen, J.; Wang, J.; Wu, Q.N. The protective effect of Cyanidin-3-Glucoside on myocardial Ischemia-Reperfusion injury through ferroptosis. Oxidative Med. Cell. Longev. 2021, 1, 8880141. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Carle, R.; Schieber, A. Detection of peonidin and pelargonidin glycosides in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Tatsuzawa, F.; Toya, Y.; Watanabe, H.; Hirayama, Y.; Shinoda, K.; Hara, R.; Seki, H.; Ando, T. Acylated peonidin 3-rutinoside-5-glucosides from commercial petunia cultivars with pink flowers. Heterocycles 2004, 63, 509–517. [Google Scholar] [CrossRef]
- Wojdyło, A.; Samoticha, J.; Nowicka, P.; Chmielewska, J. Characterisation of (poly) phenolic constituents of two interspecific red hybrids of Rondo and Regent (Vitis vinifera) by LC–PDA–ESI-MS QTof. Food Chem. 2018, 239, 94–101. [Google Scholar] [CrossRef]
- Rajan, V.K.; Hasna, C.K.; Muraleedharan, K. The natural food colorant Peonidin from cranberries as a potential radical scavenger–A DFT based mechanistic analysis. Food Chem. 2018, 262, 184–190. [Google Scholar] [CrossRef]
- Chen, P.N.; Chu, S.C.; Chiou, H.L.; Chiang, C.L.; Yang, S.F.; Hsieh, Y.S. Cyanidin 3-Glucoside and Peonidin 3-Glucoside Inhibit Tumor Cell Growth and Induce Apoptosis in Vitro and Suppress Tumor Growth in Vivo. Nutr. Cancer 2005, 53, 232–243. [Google Scholar] [CrossRef]
- Ho, M.L.; Chen, P.N.; Chu, S.C.; Kuo, D.Y.; Kuo, W.H.; Chen, J.Y.; Hsieh, Y.S. Peonidin 3-Glucoside Inhibits Lung Cancer Metastasis by Downregulation of Proteinases Activities and MAPK Pathway. Nutr. Cancer 2010, 62, 505–516. [Google Scholar] [CrossRef]
- Nas, J.S.B.; Manalo, R.V.M.; Medina, P.M.B. Peonidin-3-glucoside extends the lifespan of Caenorhabditis elegans and enhances its tolerance to heat, UV, and oxidative stresses. ScienceAsia 2021, 47, 457–468. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, F.; Tanaka, M.; Maeda, H.; Shimizu, K.; Sakata, Y. Characterization of cyanic flower color of Delphinium cultivars. J. Jpn. Soc. Hortic. Sci. 2000, 69, 428–434. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Tanzawa, F.; Kobayashi, H.; Suzuki, S.; Takata, R.; Saito, H. Leaf removal accelerated accumulation of delphinidin-based anthocyanins in ‘Muscat Bailey A’ [Vitis× labruscana (Bailey) and Vitis vinifera (Muscat Hamburg)] grape skin. J. Jpn. Soc. Hortic. Sci. 2014, 83, 17–22. [Google Scholar] [CrossRef]
- Krupa, T.; Tomala, K. Antioxidant capacity, anthocyanin content profile in ‘Bluecrop’blueberry fruit. J. Fruit. Ornam. Plant Res. 2007, 66, 129–141. [Google Scholar] [CrossRef]
- Estévez, L.; Mosquera, R.A. Molecular structure and antioxidant properties of delphinidin. J. Phys. Chem. A 2008, 112, 10614–10623. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Deng, Z.; Zhang, B.; Li, H. Antioxidant activity of delphinidin and pelargonidin: Theory and practice. J. Food Biochem. 2022, 46, e14192. [Google Scholar] [CrossRef]
- Heysieattalab, S.; Sadeghi, L. Effects of delphinidin on pathophysiological signs of nucleus basalis of Meynert lesioned rats as animal model of Alzheimer disease. Neurochem. Res. 2020, 45, 1636–1646. [Google Scholar] [CrossRef]
- Yun, J.M.; Afaq, F.; Khan, N.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2009, 48, 260–270. [Google Scholar] [CrossRef]
- Lee, D.Y.; Park, Y.J.; Hwang, S.C.; Kim, K.D.; Moon, D.K.; Kim, D.H. Cytotoxic effects of delphinidin in human osteosarcoma cells. Acta Orthop. Et Traumatol. Turc. 2018, 52, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Oroudjev, E.; Wilson, L.; Ayoub, G. Delphinidin and cyanidin exhibit antiproliferative and apoptotic effects in MCF7 human breast cancer cells. Integr. Cancer Sci. Ther. 2015, 2, 82–86. [Google Scholar] [CrossRef]
- Dayoub, O.; Andriantsitohaina, R.; Clere, N. Pleiotropic beneficial effects of epigallocatechin gallate, quercetin and delphinidin on cardiovascular diseases associated with endothelial dysfunction. Cardiovasc. Hematol. Agents Med. Chem. Former. Curr. Med. Chem. -Cardiovasc. Hematol. Agents 2013, 11, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Z.; Reheman, A.; Jin, J.W.; Li, C.; Wang, Y.; Andrews, M.C.; Chen, P.; Zhu, G.; Ling, W.; et al. Plant food delphinidin-3-glucoside significantly inhibits platelet activation and thrombosis: Novel protective roles against cardiovascular diseases. PLoS ONE 2012, 7, e37323. [Google Scholar] [CrossRef]
- Tsiogkas, S.G.; Mavropoulos, A.; Skyvalidas, D.N.; Patrikiou, E.; Ntavari, N.; Daponte, A.I.; Grammatikopoulou, M.G.; Dardiotis, E.; Roussaki-Schulze, A.V.; Sakkas, L.I.; et al. Delphinidin diminishes in vitro interferon-γ and interleukin-17 producing cells in patients with psoriatic disease. Immunol. Res. 2022, 70, 161–173. [Google Scholar] [CrossRef]
- Cremonini, E.; Iglesias, D.E.; Matsukuma, K.E.; Hester, S.N.; Wood, S.M.; Bartlett, M.; Fraga, C.G.; Oteiza, P.I. Supplementation with cyanidin and delphinidin mitigates high fat diet-induced endotoxemia and associated liver inflammation in mice. Food Funct. 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef]
- Tsuda, S.; Fukui, Y.; Nakamura, N.; Katsumoto, Y.; Yonekura-Sakakibara, K.; Fukuchi-Mizutani, M.; Ohira, K.; Ueyama, Y.; Ohkawa, H.; Holton, T.A.; et al. Flower color modification of Petunia hybrida commercial varieties by metabolic engineering. Plant Biotechnol. 2004, 21, 377–386. [Google Scholar] [CrossRef]
- Ando, T.; Saito, N.; Tatsuzawa, F.; Kakefuda, T.; Yamakage, K.; Ohtani, E.; Koshi-ishi, M.; Matsusake, Y.; Kokubun, H.; Watanabe, H.; et al. Floral anthocyanins in wild taxa of Petunia (Solanaceae). Biochem. Syst. Ecol. 1999, 27, 623–650. [Google Scholar] [CrossRef]
- Rajan, V.K.; Ragi, C.; Muraleedharan, K. A computational exploration into the structure, antioxidant capacity, toxicity and drug-like activity of the anthocyanidin “Petunidin”. Heliyon 2019, 5, e02115. [Google Scholar] [CrossRef]
- Zheng, S.; Deng, Z.; Chen, F.; Zheng, L.; Pan, Y.; Xing, Q.; Rong, T.; Li, H. Synergistic antioxidant effects of petunidin and lycopene in H9c2 cells submitted to hydrogen peroxide: Role of Akt/Nrf2 pathway. J. Food Sci. 2020, 85, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, L.; Wang, X. Petunidin suppresses Hashimoto’s thyroiditis by regulating Th1/Th17 homeostasis and oxidative stress. Cell. Immunol. 2024, 403, 104858. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, C.; Shao, L.; Zhu, H.; Wang, Y.; Huang, X.; Wang, S.; Hong, L. Targeting NOX 4 by petunidin improves anoxia/reoxygenation-induced myocardium injury. Eur. J. Pharmacol. 2020, 888, 173414. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Maeda, T.; Moriwaki, S.; Nomura, A.; Kato, Y.; Niida, S.; Kruger, M.C.; Suzuki, K. Petunidin, a b-ring 5′-O-methylated derivative of delphinidin, stimulates osteoblastogenesis and reduces srankl-induced bone loss. Int. J. Mol. Sci. 2019, 20, 2795. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, J.; Liu, P.; Guo, R.; Yan, Y.; Mi, J.; Lu, L.; Cao, Y.; Zeng, X. Petunidin-3-O-[rhamnopyranosyl-(trans-p-coumaroyl)]-5-O-(β-D-glucopyranoside), the main anthocyanin from the fruits of Lycium ruthenicum murray, enhances the lifespan of Caenorhabditis elegans by activating DAF-16 and improving the gut microbiota. Food Biosci. 2024, 61, 104642. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Akgun, B. Petunidin: Advances on Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology. In Handbook of Dietary Flavonoids; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Mendoza, J.; Pina, F.; Basilio, N.; Guimaraes, M.; de Freitas, V.; Cruz, L. Extending the stability of red and blue colors of malvidin-3-glucoside-lipophilic derivatives in the presence of SDS micelles. Dye. Pigment. 2018, 151, 321–326. [Google Scholar] [CrossRef]
- Hurst, R.D.; Wells, R.W.; Hurst, S.M.; McGhie, T.K.; Cooney, J.M.; Jensen, D.J. Blueberry fruit polyphenolics suppress oxidative stress-induced skeletal muscle cell damage in vitro. Mol. Nutr. Food Res. 2010, 54, 353–363. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Santos-Buelga, C.; Alonso, G.L.; Salinas, M.R. Anthocyanin composition of the fruit of Coriaria myrtifolia L. Phytochem. Anal. An. Int. J. Plant Chem. Biochem. Tech. 2002, 13, 354–357. [Google Scholar] [CrossRef]
- Li, Y.; Fanli, M.; Yinan, Z. Study of anthocyanins in fruit of different Vaccinium genotypes. In VIII International Symposium on Vaccinium Culture; ISHS: Leuven, Belgium, 2004; Volume 715, pp. 589–594. [Google Scholar]
- Huang, W.; Zhu, Y.; Li, C.; Sui, Z.; Min, W. Effect of blueberry anthocyanins malvidin and glycosides on the antioxidant properties in endothelial cells. Oxidative Med. Cell. Longev. 2016, 1, 1591803. [Google Scholar] [CrossRef]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F., Jr. Antioxidant and anti-inflammatory effects in RAW264. 7 macrophages of malvidin, a major red wine polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar] [CrossRef]
- Sood, R.; Choi, H.K.; Lee, H.J. Potential anti-cancer properties of malvidin and its glycosides: Evidence from in vitro and in vivo studies. J. Funct. Foods 2024, 116, 106191. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, J.; Gao, N.; Sun, X.; Meng, X.; Liu, R.; Liu, Y.; Wang, W.; Li, B.; Wang, Y. Blueberry malvidin-3-galactoside modulated gut microbial dysbiosis and microbial TCA cycle KEGG pathway disrupted in a liver cancer model induced by HepG2 cells. Food Sci. Human. Wellness 2020, 9, 245–255. [Google Scholar] [CrossRef]
- Kamrani, Y.Y.; Esmaeelian, B.; Jabbari, M.; Tabaraei, B.; Yazdanyar, A.; Ebrahimi, S.N. Anti-cancer effects of malvidin-3, 5-diglucoside from Alcea longipedicellata, on gastric cancer cell line (AGS). Planta Medica 2008, 74, PA174. [Google Scholar] [CrossRef]
- Baba, A.B.; Nivetha, R.; Chattopadhyay, I.; Nagini, S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial-mediated apoptosis by abrogating the JAK/STAT-3 signalling pathway. Food Chem. Toxicol. 2017, 109, 534–543. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation–cause or consequence of heart failure or both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W. Analysis of inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–151. [Google Scholar] [CrossRef]
- Stankov, S.V. Definition of inflammation, causes of inflammation and possible anti-inflammatory strategies. Open Inflamm. J. 2012, 5, 1–9. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Cassidy, A.; Rogers, G.; Peterson, J.J.; Dwyer, J.T.; Lin, H.; Jacques, P.F. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am. J. Clin. Nutr. 2015, 102, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Abdin, M.; Hamed, Y.S.; Akhtar, H.M.S.; Chen, D.; Chen, G.; Wan, P.; Zeng, X. Antioxidant and anti-inflammatory activities of target anthocyanins di-glucosides isolated from Syzygium cumini pulp by high speed counter-current chromatography. J. Food Biochem. 2020, 44, e13209. [Google Scholar] [CrossRef]
- Ooe, E.; Ogawa, K.; Horiuchi, T.; Tada, H.; Murase, H.; Tsuruma, K.; Shimazawa, M.; Hara, H. Analysis and characterization of anthocyanins and carotenoids in Japanese blue tomato. Biosci. Biotechnol. Biochem. 2016, 80, 341–349. [Google Scholar] [CrossRef]
- Venancio, V.P.; Cipriano, P.A.; Kim, H.; Antunes, L.M.G.; Talcott, S.T.; Mertens-Talcott, S.U. Cocoplum (Chrysobalanus icaco L.) anthocyanins exert anti-inflammatory activity in human colon cancer and non-malignant colon cells. Food Funct. 2017, 8, 307–314. [Google Scholar] [CrossRef]
- Zhang, H.; Hassan, Y.I.; Renaud, J.; Liu, R.; Yang, C.; Sun, Y.; Tsao, R. Bioaccessibility, bioavailability, and anti-inflammatory effects of anthocyanins from purple root vegetables using mono-and co-culture cell models. Mol. Nutr. Food Res. 2017, 61, 1600928. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Y.; Li, H.; Chen, X.; Zhang, C.; Wang, H.; Deng, Z. The antioxidant activity and active sites of delphinidin and petunidin measured by DFT, in vitro chemical-based and cell-based assays. J. Food Biochem. 2019, 43, e12968. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Chun, E.M.; Al Mijan, M.; Park, S.H.; Moon, S.K.; Lim, B.O. Anthocyanins profiling of bilberry (Vaccinium myrtillus L.) extract that elucidates antioxidant and anti-inflammatory effects. Food Agric. Immunol. 2021, 32, 713–726. [Google Scholar] [CrossRef]
- Son, J.E.; Lee, E.; Jung, S.K.; Kim, J.E.; Oak, M.H.; Lee, K.W.; Lee, H.J. Anthocyanidins, novel FAK inhibitors, attenuate PDGF-BB-induced aortic smooth muscle cell migration and neointima formation. Cardiovasc. Res. 2014, 101, 503–512. [Google Scholar] [CrossRef]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, M.R.; Cervantes-Cardoza, V.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Estrella, I.; García-Gasca, T.D.J.; Herrera-Carrera, E.; Díaz-Rivas, J.O.; Rocha-Guzmán, N.E. Phenolic composition changes of processed common beans: Their antioxidant and anti-inflammatory effects in intestinal cancer cells. Food Res. Int. 2015, 76, 79–85. [Google Scholar] [CrossRef]
- Lee, S.G.; Brownmiller, C.R.; Lee, S.O.; Kang, H.W. Anti-inflammatory and antioxidant effects of anthocyanins of Trifolium pratense (red clover) in lipopolysaccharide-stimulated RAW-267.4 macrophages. Nutrients 2020, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of extraction solvent and temperature on polyphenol profiles, antioxidant and anti-inflammatory effects of red grape skin by-product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxidative Med. Cell. Longev. 2018, 1, 1862462. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Bucknall, M.; Arcot, J. Effect of different anthocyanidin glucosides on lutein uptake by Caco-2 cells, and their combined activities on anti-oxidation and anti-inflammation in vitro and ex vivo. Molecules 2018, 23, 2035. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Bioaccessibility, in vitro antioxidant activities and in vivo anti-inflammatory activities of a purple tomato (Solanum lycopersicum L.). Food Chem. 2014, 159, 353–360. [Google Scholar] [CrossRef]
- Al-Masri, A.A.; Ameen, F. Anti-inflammatory effect of anthocyanin-rich extract from banana bract on lipopolysaccharide-stimulated raw 264.7 macrophages. J. Funct. Foods 2023, 107, 105628. [Google Scholar] [CrossRef]
- Chen, T.; Hu, S.; Zhang, H.; Guan, Q.; Yang, Y.; Wang, X. Anti-inflammatory effects of Dioscorea alata L. anthocyanins in a TNBS-induced colitis model. Food Funct. 2017, 8, 659–669. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Bucknall, M.; Arcot, J. Interactive effects of β-carotene and anthocyanins on cellular uptake, antioxidant activity and anti-inflammatory activity in vitro and ex vivo. J. Funct. Foods 2018, 45, 129–137. [Google Scholar] [CrossRef]
- Chao, C.Y.; Liu, W.H.; Wu, J.J.; Yin, M.C. Phytochemical profile, antioxidative and anti-inflammatory potentials of Gynura bicolor DC. J. Sci. Food Agric. 2015, 95, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Mounir, R.; Alshareef, W.A.; El Gebaly, E.A.; El-Haddad, A.E.; Ahmed, A.M.S.; Mohamed, O.G.; Enan, E.T.; Mosallam, S.; Tripathi, A.; Selim, H.M.R.M.; et al. Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals 2023, 16, 1379. [Google Scholar] [CrossRef]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical scavenging and anti-inflammatory activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, K.; Deshpande, A.; Datta, R.; Ramakrishna, W. Anti-inflammatory effects of northern highbush blueberry extract on an in vitro inflammatory bowel disease model. Nutr. Cancer 2020, 72, 1178–1190. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.J. Anti-inflammatory activity of bilberry (Vaccinium myrtillus L.). Curr. Issues Mol. Biol. 2022, 44, 4570–4583. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Di Lorenzo, C.; Regazzoni, L.; Fumagalli, M.; Sangiovanni, E.; de Sousa, L.P.; Bavaresco, L.; Tomasi, D.; Bosso, A.; Aldini, G.; et al. Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties. Food Funct. 2019, 10, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Chalons, P.; Amor, S.; Courtaut, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schni-Kerth, V.; Aires, V.; Delmas, D. Study of potential anti-inflammatory effects of red wine extract and resveratrol through a modulation of interleukin-1-beta in macrophages. Nutrients 2018, 10, 1856. [Google Scholar] [CrossRef]
- Kim, J.N.; Han, S.N.; Kim, H.K. Anti-inflammatory and anti-diabetic effect of black soybean anthocyanins: Data from a dual cooperative cellular system. Molecules 2021, 26, 3363. [Google Scholar] [CrossRef]
- Silva, C.F.; Fattori, V.; Tonetti, C.R.; Ribeiro, M.A.; Matos, R.L.; Carra, J.B.; Meurer, E.C.; Hirooka, E.Y.; Rafael, J.A.; Georgetti, S.R.; et al. Hydroethanolic extract of grape peel from Vitis labrusca winemaking waste: Antinociceptive and anti-inflammatory activities. Food Technol. Biotechnol. 2022, 60, 21–28. [Google Scholar] [CrossRef]
- Jakesevic, M.; Xu, J.; Aaby, K.; Jeppsson, B.; Ahrné, S.; Molin, G. Effects of Bilberry (Vaccinium myrtillus) in Combination with Lactic Acid Bacteria on Intestinal Oxidative Stress Induced by Ischemia–Reperfusion in Mouse. J. Agric. Food Chem. 2013, 14, 3468–3478. [Google Scholar] [CrossRef]
- Sohn, D.W.; Bae, W.J.; Kim, H.S.; Kim, S.W.; Kim, S.W. The Anti-inflammatory and Antifibrosis Effects of Anthocyanin Extracted From Black Soybean on a Peyronie Disease Rat Model. Urolog 2014, 84, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective Effects of Bilberry Anthocyanins via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef] [PubMed]
- Ouanouki, A.; Lamy, S.; Annabi, B. Anthocyanidins inhibit epithelial–mesenchymal transition through a TGFβ/Smad2 signaling pathway in glioblastoma cells. Mol. Carcinog. 2017, 56, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free. Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, S.; Zhou, W.U.; Meng, J.; Deng, K.; Zhou, H.; Hu, N.; Suo, Y. Anthocyanin composition of fruit extracts from Lycium ruthenicum and their protective effect for gouty arthritis. Ind. Crops Prod. 2019, 129, 414–423. [Google Scholar] [CrossRef]
- Bae, W.J.; Yoon, B.I.; Kim, S.; Choi, Y.S.; Kim, S.J.; Cho, H.J.; Ha, U.S.; Hong, S.-H.; Sohn, D.W.; Kim, S.W. The anti-inflammatory and anti-microbial effects of anthocyanin extracted from black soybean on chronic bacterial prostatitis rat model. J. Sex. Med. 2013, 10, 245. [Google Scholar]
- Weinberg, R.A. How cancer arises. Sci. Am. 1996, 275, 62–70. [Google Scholar] [CrossRef]
- Bailar, J.C.; Gornik, H.L. Cancer undefeated. N. Engl. J. Med. 1997, 336, 1569–1574. [Google Scholar] [CrossRef]
- Cairns, J. The cancer problem. Sci. Am. 1975, 233, 64–79. [Google Scholar] [CrossRef]
- Welcsh, P.L.; King, M.C. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Human. Mol. Genet. 2001, 10, 705–713. [Google Scholar] [CrossRef]
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B Biol. 2001, 63, 8–18. [Google Scholar] [CrossRef] [PubMed]
- LaDou, J. The asbestos cancer epidemic. Environ. Health Perspect. 2004, 112, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Willett, W.C. Dietary factors and risk of colon cancer. Ann. Med. 1994, 26, 443–452. [Google Scholar] [CrossRef]
- Brown, J.C.; Winters-Stone, K.; Lee, A.; Schmitz, K.H. Cancer, physical activity, and exercise. Compr. Physiol. 2012, 2, 2775. [Google Scholar]
- Muñoz, N.; Castellsagué, X.; de González, A.B.; Gissmann, L. HPV in the etiology of human cancer. Vaccine 2006, 24, S1–S10. [Google Scholar] [CrossRef]
- Petry, K.U. HPV and cervical cancer. Scand. J. Clin. Lab. Investig. 2014, 74, 59–62. [Google Scholar] [CrossRef]
- Chen, J.; Xu, B.; Sun, J.; Jiang, X.; Bai, W. Anthocyanin supplement as a dietary strategy in cancer prevention and management: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7242–7254. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Kausar, H.; Munagala, R.; Singh, I.P.; Gupta, R. Ramesh, Lung cancer inhibitory activity of dietary berries and berry polyphenolics. J. Berry Res. 2016, 6, 105–114. [Google Scholar] [CrossRef]
- Lu, J.N.; Panchanathan, R.; Lee, W.S.; Kim, H.J.; Kim, D.H.; Choi, Y.H.; Kim, G.S.; Shin, S.C.; Hong, S.C. Anthocyanins from the fruit of vitis coignetiae pulliat inhibit tnf-augmented cancer proliferation, migration, and invasion in a549 cells. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 2919–2923. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Lage, N.N.; Layosa, M.A.A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 103710. [Google Scholar] [CrossRef]
- Mazzoni, L.; Giampieri, F.; Suarez, J.M.A.; Gasparrini, M.; Mezzetti, B.; Hernandez, T.Y.F.; Battino, M.A. Isolation of strawberry anthocyanin-rich fractions and their mechanisms of action against murine breast cancer cell lines. Food Funct. 2019, 10, 7103–7120. [Google Scholar] [CrossRef] [PubMed]
- Amatori, S.; Mazzoni, L.; Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Provenzano, A.E.; Persico, G.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract (PRSE) shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 2016, 6, 30917. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Platini, F.; Scardino, A.; Alabiso, O.; Vasapollo, G.; Tessitore, L. Autophagy inhibition enhances anthocyanininduced apoptosis in hepatocellular carcinoma. Mol. Cancer Ther. 2008, 7, 2476–2485. [Google Scholar] [CrossRef]
- Lu, J.N.; Lee, W.S.; Nagappan, A.; Chang, S.H.; Choi, Y.H.; Kim, H.J.; Kim, G.S.; Ryu, C.H.; Shin, S.C.; Jung, J.M.; et al. Anthocyanins from the fruit of vitis coignetiae pulliat potentiate the cisplatin activity by inhibiting PI3K/Akt signaling pathways in human gastric cancer cells. J. Cancer Prev. 2015, 20, 50–56. [Google Scholar] [CrossRef]
- Huang, H.P.; Chang, Y.C.; Wu, C.H.; Hung, C.N.; Wang, C.J. Anthocyanin-rich mulberry extract inhibit the gastric cancer cell growth in vitro and xenograft mice by inducing signals of p38/ p53 and c-jun. Food Chem. 2011, 129, 1703–1709. [Google Scholar] [CrossRef]
- Eskra, J.N.; Schlicht, M.J.; Bosland, M.C. Effects of black raspberries and their ellagic acid and anthocyanin constituents on taxane chemotherapy of castration-resistant prostate cancer cells. Sci. Rep. 2019, 9, 4367. [Google Scholar] [CrossRef]
- Shin, D.Y.; Ryu, C.H.; Lee, W.S.; Kim, D.C.; Kim, S.H.; Hah, Y.-S.; Lee, S.J.; Shin, S.C.; Kang, H.S.; Choi, Y.H. Induction of Apoptosis and Inhibition of Invasion in Human Hepatoma Cells by Anthocyanins from Meoru. Ann. N. Y. Acad. Sci. 2009, 1171, 137–148. [Google Scholar] [CrossRef]
- Zu, X.Y.; Zhang, Z.Y.; Zhang, X.W.; Yoshioka, M.; Yang, Y.N.; Li, J. Anthocyanins extracted from Chinese blueberry (Vaccinium uliginosum L.) and its anticancer effects on DLD-1 and COLO205 cells. Chin. Med. J. 2010, 123, 2714–2719. [Google Scholar] [PubMed]
- Lamy, S.; Lafleur, R.; Bédard, V.; Moghrabi, A.; Barrette, S.; Gingras, D.; Béliveau, R. Anthocyanidins inhibit migration of glioblastoma cells: Structure-activity relationship and involvement of the plasminolytic system. J. Cell. Biochem. 2007, 100, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Charepalli, V.; Reddivari, L.; Vadde, R.; Walia, S.; Radhakrishnan, S.; Vanamala, J.K.P. Eugenia jambolana (Java plum) fruit extract exhibits anti-cancer activity against early stage human HCT-116 colon cancer cells and colon cancer stem cells. Cancers 2016, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, D.; Hayouni, E.A.; Valentin, A.; Cazaux, S.; Moukarzel, B.; Hamdi, M.; Bouajila, J. LC–MS analysis, anticancer, antioxidant and antimalarial activities of Cynodon dactylon L. extracts. Ind. Crops Prod. 2013, 45, 240–247. [Google Scholar] [CrossRef]
- Duan, J.; Guo, H.; Fang, Y.; Zhou, G. The mechanisms of wine phenolic compounds for preclinical anticancer therapeutics. Food Nutr. Res. 2021, 65. [Google Scholar]
- Bontempo, P.; Carafa, V.; Grassi, R.; Basile, A.; Tenore, G.C.; Formisano, C.; Rigano, D.; Altucci, L. Antioxidant, antimicrobial and anti-proliferative activities of Solanum tuberosum L. var. Vitelotte. Food Chem. Toxicol. 2013, 55, 304–331. [Google Scholar]
- Zhou, F.; Wang, T.; Zhang, B.; Zhao, H. Addition of sucrose during the blueberry heating process is good or bad? Evaluating the changes of anthocyanins/anthocyanidins and the anticancer ability in HepG-2 cells. Food Res. Int. 2018, 107, 509–517. [Google Scholar]
- Wang, G.; Fu, X.L.; Wang, J.J.; Guan, R.; Sun, Y.; Tony To, S.S. Inhibition of glycolytic metabolism in glioblastoma cells by Pt3glc combinated with PI3K inhibitor via SIRT3-mediated mitochondrial and PI3K/Akt–MAPK pathway. J. Cell. Physiol. 2019, 234, 5888–5903. [Google Scholar]
- Pan, F.; Liu, Y.; Liu, J.; Wang, E. Stability of blueberry anthocyanin, anthocyanidin and pyranoanthocyanidin pigments and their inhibitory effects and mechanisms in human cervical cancer HeLa cells. RSC Adv. 2019, 9, 10842–10853. [Google Scholar]
- Olejnik, A.; Kaczmarek, M.; Olkowicz, M.; Kowalska, K.; Juzwa, W.; Dembczyński, R. ROS-modulating anticancer effects of gastrointestinally digested Ribes nigrum L. fruit extract in human colon cancer cells. J. Funct. Foods 2018, 42, 224–236. [Google Scholar]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the genus Vitis: Phenolic compounds, anticancer properties and clinical relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Visvanathan, R.; Jayathilake, C.; Chaminda Jayawardana, B.; Liyanage, R. Health-beneficial properties of potato and compounds of interest. J. Sci. Food Agric. 2016, 96, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Yao, L.; Lu, C.; Li, C.; Zhou, Y.; Su, C.; Chen, B.; Shen, Y. Phenolic composition, antioxidant and antibacterial properties, and in vitro anti-HepG2 cell activities of wild apricot (Armeniaca Sibirica L. Lam) kernel skins. Food Chem. Toxicol. 2019, 129, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, A.; Gismondi, A.; Chirico, R.; Di Marco, G.; Petrone, V.; Fanelli, M.; D’Agostino, A.; Canini, A.; Grelli, S.; Albanese, L.; et al. Antioxidant Phytocomplexes Extracted from Pomegranate (Punica granatum L.) Using Hydrodynamic Cavitation Show Potential Anticancer Activity in Vitro. Antioxidants 2023, 12, 1560. [Google Scholar] [CrossRef]
- Jabamalairaj, A.; Priatama, R.A.; Heo, J.; Park, S.J. Medicinal metabolites with common biosynthetic pathways in Solanum nigrum. Plant Biotechnol. Rep. 2019, 13, 315–327. [Google Scholar] [CrossRef]
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J. Nutr. Sci. 2014, 3, e46. [Google Scholar] [CrossRef]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and proapoptotic activities of anthocyanin and anthocyanidin extracts from blueberry fruits on B16-F10 melanoma cells. Food Nutr. Res. 2017, 61, 1325308. [Google Scholar] [CrossRef]
- Zhang, Y.; Vareed, S.K.; Nair, M.G. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005, 76, 1465–1472. [Google Scholar] [CrossRef]
- Aqil, F.; Gupta, A.; Munagala, R.; Jeyabalan, J.; Kausar, H.; Sharma, R.J.; Pal Singh, I.; Gupta, R.C. Antioxidant and Antiproliferative Activities of Anthocyanin/Ellagitannin-Enriched Extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr. Cancer 2012, 64, 428–438. [Google Scholar] [CrossRef]
- Han, L.; Yan, Y.; Fan, M.; Gao, S.; Zhang, L.; Xiong, X.; Li, R.; Xiao, X.; Wang, X.; Ni, l.; et al. Pt3R5G inhibits colon cancer cell proliferation through inducing ferroptosis by down-regulating SLC7A11. Life Sci. 2022, 306, 120859. [Google Scholar] [CrossRef]
- Kim, M.J.; Paramanantham, A.; Lee, W.S.; Yun, J.W.; Chang, S.H.; Kim, D.C.; Park, H.S.; Choi, Y.H.; Kim, G.S.; Ryu, C.H.; et al. Anthocyanins derived from vitis coignetiae pulliat contributes anti-cancer effects by suppressing NF-κB pathways in Hep3B human hepatocellular carcinoma cells and in vivo. Molecules 2020, 25, 5445. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, N.; Kaur, R.; Jaglan, S.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds and pharmacological and food applications of Syzygium cumini—A review. Food Funct. 2018, 9, 6096–6115. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yan, Y.; Fan, M.; Gao, S.; Zhang, L.; Xiong, X.; Li, R.; Xiao, X.; Wang, X.; Ni, L.; et al. Anthocyanin Inhibits Colon Cancer Cell Proliferation Through Inducing Ferroptosis by Down-Regulating SLC7A11. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).