Abstract
Chemotherapy (CT) and radiation therapy (RT), while effective against cancer, often cause severe side effects, such as oral mucositis and other oral diseases. Oral mucositis, characterized by inflammation and ulceration of the oral mucosa, is one of the most painful side effects that can reduce quality of life and limit cancer treatment. Curcumin, a polyphenol from Curcuma longa, has garnered attention for its anti-inflammatory, antioxidant, and anti-carcinogenic properties, which protect the oral mucosa by reducing oxidative stress and modulating inflammation. This study reviews the therapeutic potential of curcumin in preventing and managing oral mucositis caused by CT and RT. Clinical trials show curcumin’s effectiveness in reducing the incidence and severity of oral mucositis. Although curcumin supplementation appears to be a promising and cost-effective approach for mitigating oral complications in cancer patients, further clinical trials are needed to confirm its efficacy and optimize dosing strategies.
1. Introduction
Today, according to estimates reported by the International Agency for Research on Cancer as part of the GLOBOCAN project, one in five people will be affected by cancer during their lifetime, with a higher mortality prevalence in the male sex compared to the female sex (4:3) [1]. Worldwide, lung cancer is the cancer with the highest incidence, exceeding in percentage those of breast, colorectal, prostate, stomach, head, and neck cancers [1].
Because of the world’s growing and aging population, it is estimated that there will be 35 million new cases of cancer in 2050, up from 20 million in 2022 [1,2].
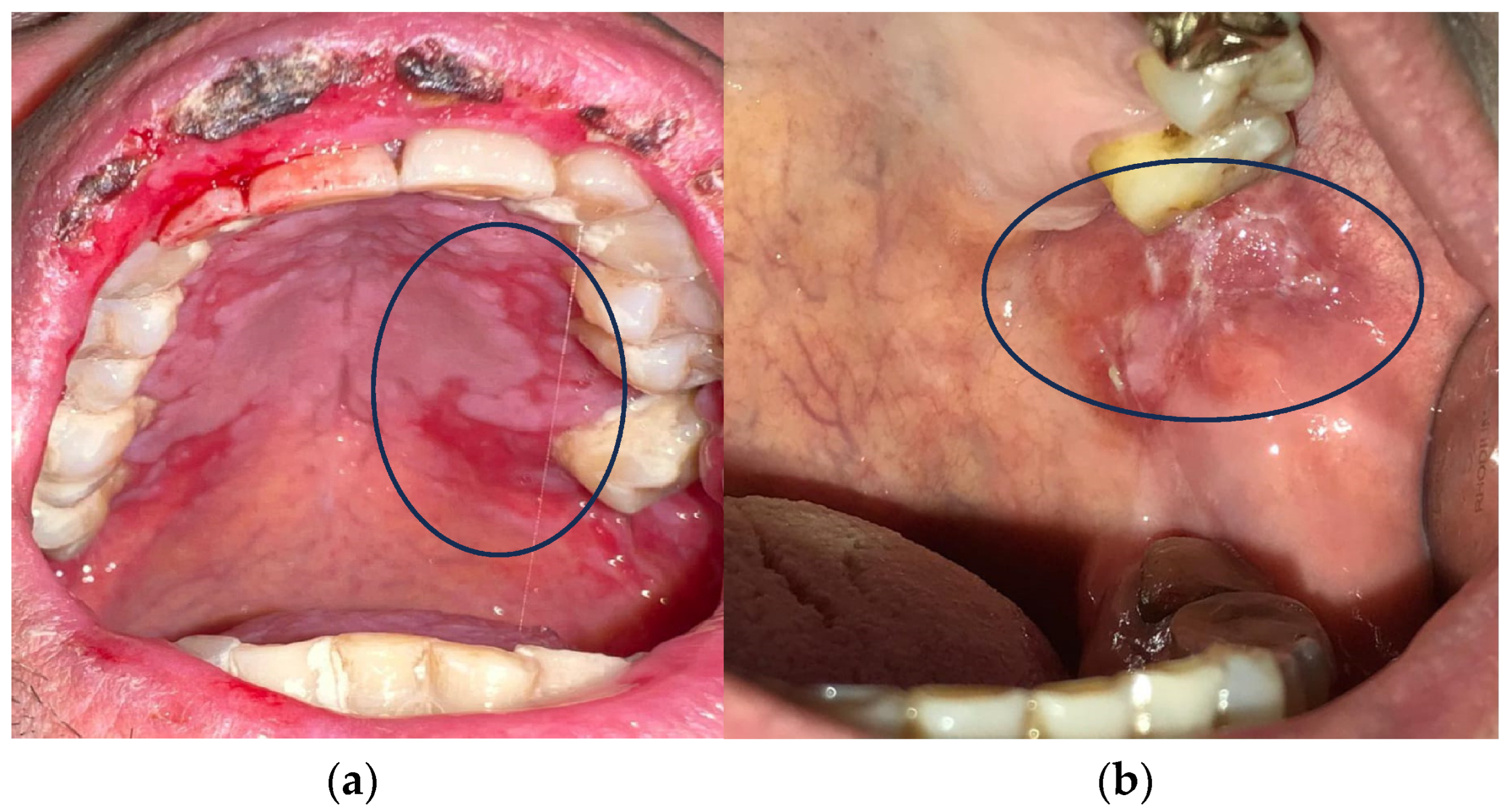
Cancer treatment is a major challenge in modern medicine. Based on different parameters, there are many therapeutic treatment options [3,4,5,6]. Systemic CT and RT are the most popular because they are most effective in highly invasive cancers [7]. The occurrence of oral mucositis (OM) and gastrointestinal mucositis is one of the side effects of CT and RT (Figure 1) [8,9,10].
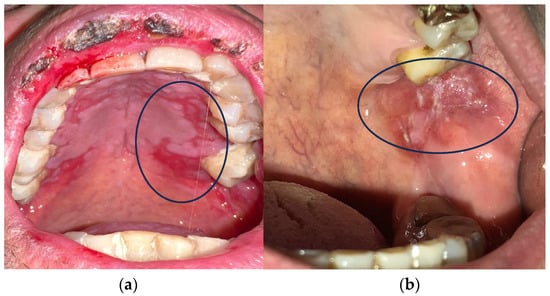
Figure 1.
(a,b) Oral mucositis induced by radiotherapy and chemotherapy.
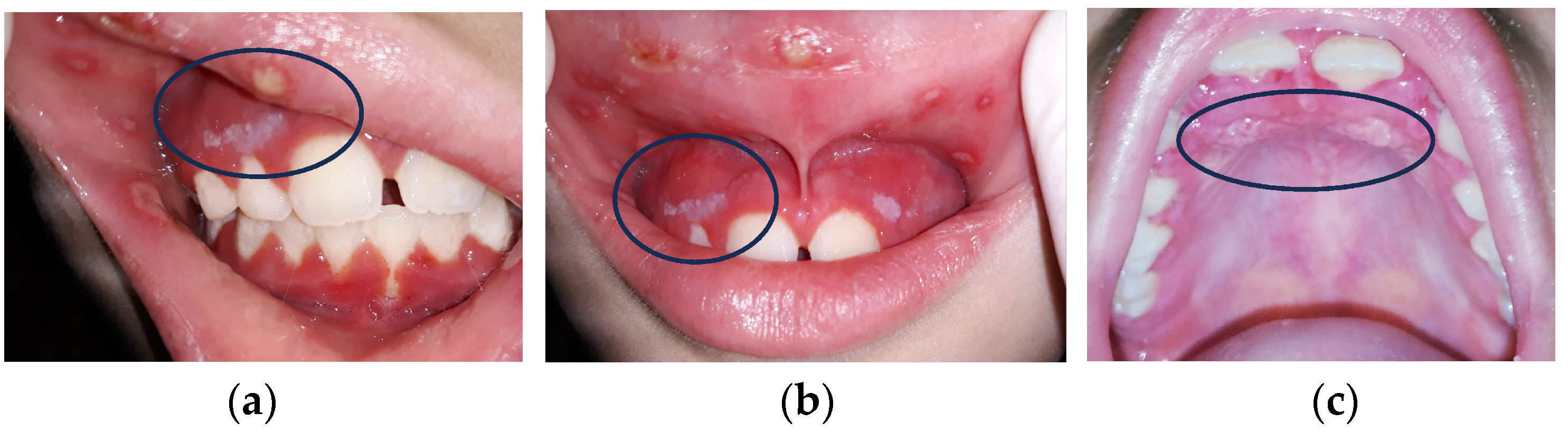
OM incidence rates in CT patients are variable, depending on chemotherapy protocols (20–40% in conventional CHT, 80% in high-dose CT, 75–100% in HSCT, and consistently after RT in head and neck squamous cell cancer patients) (Figure 2a–c) [11,12,13,14,15].
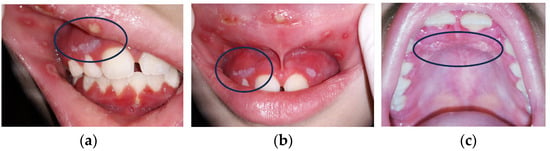
Figure 2.
(a–c) Three kinds of oral lesions in immunodepressed girl with neck cancer.
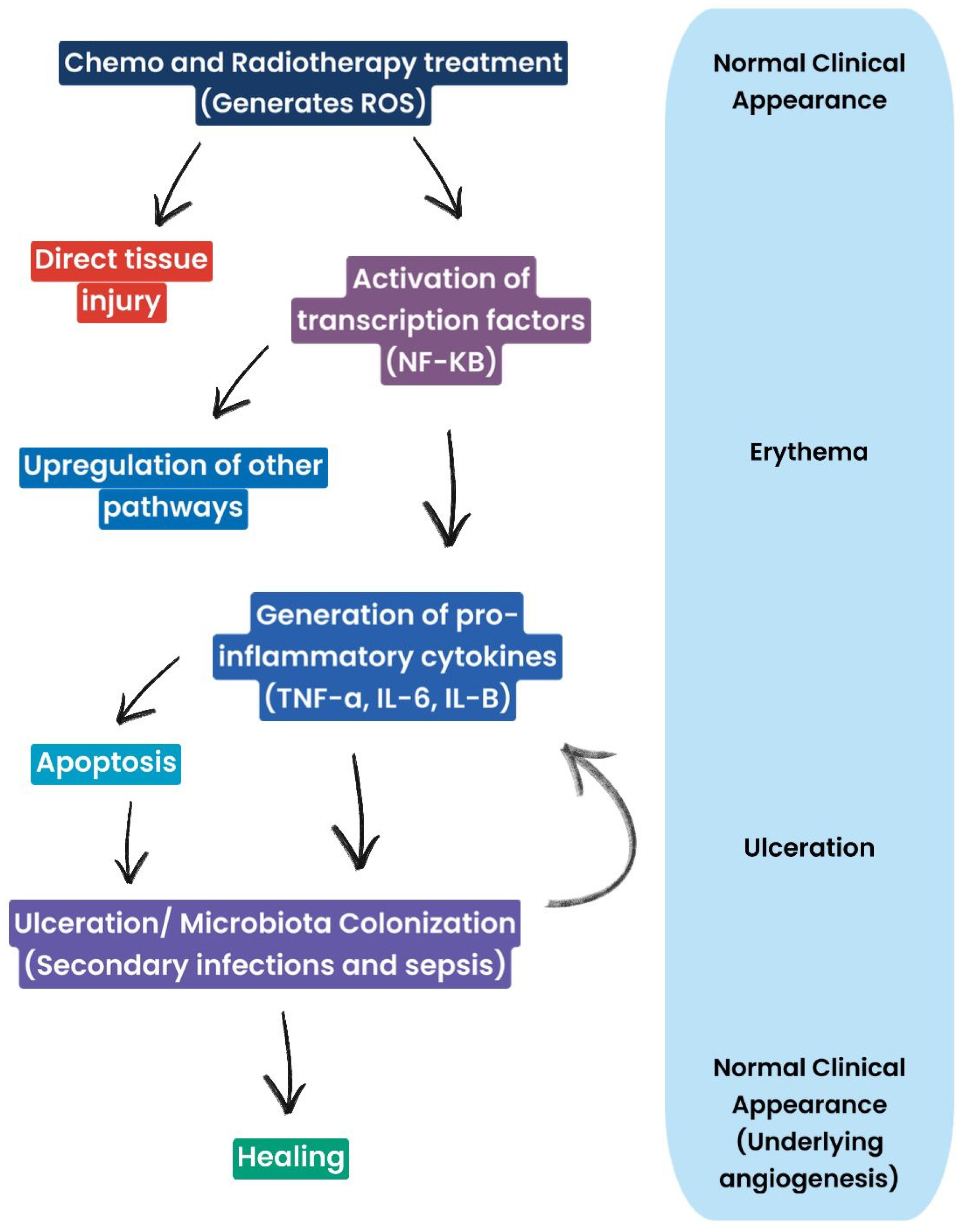
Oral CT mucositis (OCT) occurs after 4–5 days and disappears approximately one month after cessation of administration, depending on the severity of the mucosal lesions and the patient’s overall health [16,17] (Figure 2). OM from RT (RTMO) also occurs a few days later and becomes chronic, as it can persist for 3–4 weeks after treatment [18,19,20,21]. Healing occurs 6–8 weeks after the end of therapy [8,22,23,24]. OM is highly disabling and has a significant impact on the patient’s quality of life. It manifests itself with painful lesions that make feeding and oral hygiene difficult and promotes the onset of local and systemic bacterial infections in immunodepressed and immunocompromised cancer patients [25]. It leads to the reduction or even discontinuation of CT and RT, reducing the effectiveness of antineoplastic therapy (Figure 3).
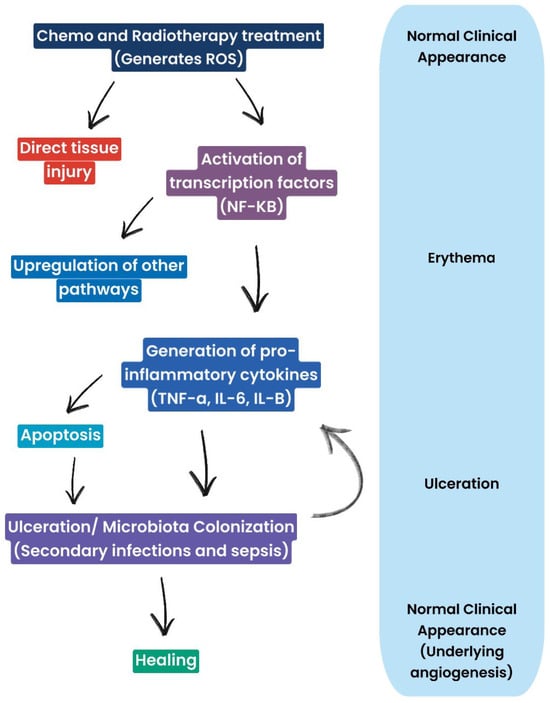
Figure 3.
Diagram representing mucosal cells and clinical manifestations of oral mucositis: ROS generation leads, on the one hand, to tissue damage and inflammation and, on the other hand, triggers pathways involved in repair and healing. Therefore, the healing process is not strictly linear, but it involves a balance between damage and healing processes.
Clinical manifestations of CT- and RT-induced inflammation follow a typical sequence. Initially, erythema is observed, indicating inflammation and increased vascular permeability. This may progress to ulceration, which represents more advanced damage, with impairment of oral mucosal integrity. In the final stage, healing is observed, with restoration of mucosal integrity and angiogenesis. Ulcerations are often complicated by microbial colonization, which can lead to secondary infections and worsen the overall clinical picture.
Nutritional and psychological status are impaired, as there is also an alteration of taste [26]. To date, there are no real guidelines for the OM treatment [27,28]. Synthetic mouthwashes (chlorhexidine and mucosamine) are commonly prescribed [29,30]. Chlorhexidine gluconate, the most prescribed mouthwash for the OM treatment, although having good potential for resolution of CT and RT lesions, has manifested side effects, such as dehydration and burning, which increase the likelihood of bacterial infection of the affected mucosa itself. In addition, the presence of alcohol in mouthwashes increases and/or stimulates pain [7,31,32,33]. A new therapeutic approach is the use of mouthwashes based on hyaluronic acid supplemented with amino acids such as mucosamine. They have shown antioxidant properties, acting directly on reactive oxygen species (ROS), scavenging and blocking them. They have shown good ability to reduce pain and inflammation and to regenerate damaged oral mucosa [18,34]. In more advanced stages, cryotherapy and photobiomodulation (PBM) protocols, as well as topical analgesics based on morphine, benzocaine, menthol, and cortisone, are also used to reduce pain symptoms [35,36,37].
1.1. Curcumin: Mechanisms of Action
The use of herbal medicines is a focus in the OM treatment [22,38,39]. In particular, recent studies have provided information on the complex biological mechanisms of phytotherapies, confirming their prophylactic and therapeutic properties in various diseases of the oral cavity (mucositis, gingivitis, aphthous stomatitis, caries, periodontitis, oral cancer, oral candidiasis, oral submucosal fibrosis, precancerous lesions, etc.) [40,41,42,43]. One of the most researched substances is curcumin, as a phytotherapeutic agent, and has gained special prominence. Curcumin (diferuloylmethane) is derived from the root of Curcuma longa, an Asian plant of the Zingiberaceae family, and is considered the most active component of turmeric [44,45]. Since ancient times, curcumin has been recognized for its therapeutic properties (healing, antioxidant, antifungal, anti-inflammatory, antidepressant, adjuvant to chemo and radiation therapies, hypoglycemic), supported today by multiple scientific studies [46,47,48,49,50,51]. Its antioxidant role is particularly important in the context of oral mucositis, where curcumin helps mitigate oxidative stress, one of the key factors in mucosal damage caused by chemotherapy and radiation therapy. Curcumin’s ability to neutralize free radicals and reduce oxidative damage plays a critical role in preserving the integrity of the oral mucosa, thereby reducing the severity and duration of mucositis.
Using curcumin showed an interesting phytotherapeutic result in CTOM and RTOM, with excellent tolerance also shown in pediatric cancer patients [40,52,53].
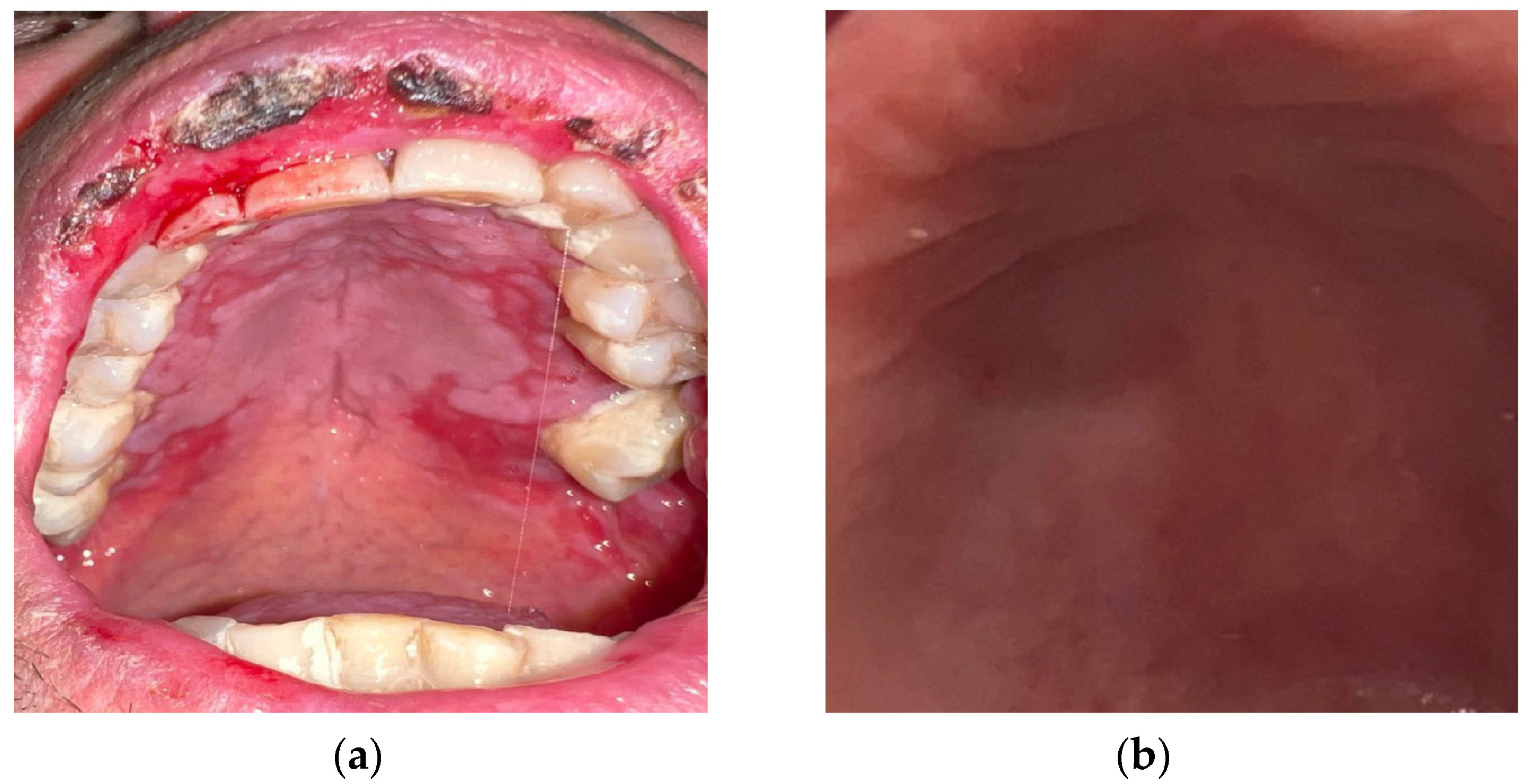
A variety of formulations have been used (tablets, films, mouthwashes, gels, sprays, pastes, and chewing gum) [54]. Topical therapy, especially gel formulation, is better tolerated and has fewer side effects compared to systemic administration, is easier to apply, increases contact time and thus absorption, enhancing the soothing and re-epithelializing effects [55,56,57] (Figure 4a,b). In all cases, patients with CTOM and RTOM who underwent curcumin therapy manifested lower exposure to mucositis onset, reduced pain symptoms, and faster recovery, without side effects [54,58,59,60,61]. In patients treated with curcumin, the mean visual analog scale (VAS) scores were significantly lower after 3–4 weeks of radiotherapy and at post-radiotherapy visits compared to patients treated with other therapies [22,62,63].
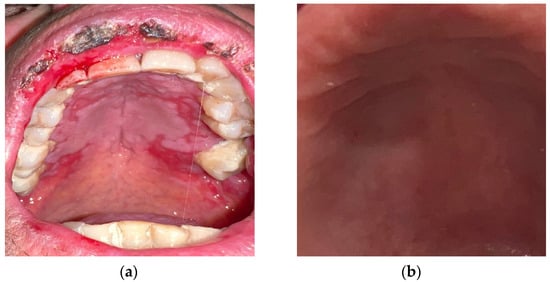
Figure 4.
(a) Post-chemotherapy palatal mucositis; (b) detail of the palatal mucosa after treatment with curcumin gel.
The reduction in pain symptoms is due not only to better and faster tissue healing, but also, at high doses, to the release of serotonin, dopamine, and norepinephrine, with reduced pain transmission pathways and increased morphine efficacy [64,65,66]. Its pleiotropic nature ensures phytotherapeutic properties. In fact, by interfering with several biological processes and the release of inflammatory factors, including ROS, C-reactive proteins, interleukins (ILs), cytokines, transforming growth factor-β (TGF-β), cyclooxygenase-2 (COX-2), nuclear factor kappa B (NF-κB), tumor necrosis factor-α (TNF-α), it modulates the oxidation-reduction phenomena [67,68,69,70]. Thus, by acting on the various factors involved in the complex mechanism of chain reactions now recognized in the onset of OM, it ensures their control and therapeutic action [22,52,71,72].
Moreover, curcumin’s role in activating nuclear factor erythroid 2-related factor 2 (Nrf2) further enhances its antioxidant defense, boosting the expression of endogenous antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-px). This activation not only shields the mucosal cells from oxidative damage but also accelerates the healing process by reducing inflammation and promoting cellular repair mechanisms.
By inhibiting fibroblasts and myoblasts (collagen types I and III) of the oral mucosa, it reduces the onset of scar fibrosis. In combination with capsaicin, it induces cell apoptosis with increased CD8 T cells [73]. Because of the way it photosensitizes when oxygen is present, it can support photodynamic therapy in analgesic therapy [74]. Curcumin, by promoting the release of TGF-β-1, stimulates fibronectin and collagen production by fibroblasts, thereby facilitating re-epithelialization and mucosal healing. Curcumin activates Nrf2 and promotes the release of antioxidant enzymes such as SOD, catalase (CAT), glutathione (GSH), and GSH-px [45,75,76,77,78].
Curcumin has been observed to stimulate vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), both of which are responsible for cell proliferation and activity, thus promoting faster tissue healing [76,79,80,81].
1.2. Limitations of Curcumin
Despite its promising therapeutic potential, curcumin suffers from certain limitations, primarily related to its bioavailability. Curcumin is not hydrophilic and is poorly bioavailable. It is poorly absorbed from the gastrointestinal tract and is rapidly metabolized and eliminated in the gastro intestinal tract and liver [82,83,84]. To improve absorption and bioavailability, different formulations were used (emulsions, nanoparticles, hydrogels, ointments, tablets, chewing gum, powder) or it was used combined with other substances (piperine, honey, triamcinolone hyaluronidase gel, green tea, photodynamic therapy, tulsi). No side effects were observed even at high doses. A total of 12 g/day for 3 months was discovered to be a well-tolerated dosage [85,86,87,88,89,90]. The purpose of this review was to confirm the therapeutic properties of curcumin in RTMO and CTMO. However, further research is needed to better study its properties and develop proper protocols to better enhance its efficacy.
2. Materials and Methods
2.1. Search Strategy Literature
Our systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The literature search was performed using three major databases: PubMed, Web of Science, and Scopus. We searched for studies published within the last ten years, using the following key-words: “curcumin AND (oral mucositis OR oral disease) AND (radiotherapy OR chemotherapy).” The search focused on clinical trials and studies examining curcumin’s role in managing oral mucositis in cancer patients undergoing chemotherapy or radiotherapy. The final search was completed on 16 June 2024, and the review protocol was registered on PROSPERO with code ID “CDR 575864” [91].
2.2. Eligibility Criteria
The eligibility of the studies was determined by the following factors:
Population: oncology patients receiving chemotherapy and/or radiotherapy and experiencing oral mucositis.
Intervention: the use of curcumin or its derivatives for the prevention or treatment of oral mucositis.
Comparators: placebo, other standard treatments, or no treatment.
Outcomes: severity and duration of oral mucositis, pain relief, healing time, and adverse effects.
Study design: randomized controlled trials (RCTs), clinical trials, and pilot studies.
2.3. Inclusion Criteria
To be included in the review, studies had to meet the following criteria:
Full-text articles published in English.
Clinical trials with human participants, including randomized controlled trials, pilot studies, and comparative studies.
Studies specifically addressing the effects of curcumin on oral mucositis related to cancer treatments (chemotherapy or radiotherapy).
Studies providing quantitative outcomes related to mucositis severity, healing time, or pain management.
2.4. Exclusion Criteria
Studies were excluded if they met any of the following criteria:
In vitro or animal model studies.
Literature reviews, meta-analyses, and case reports.
Studies without clear quantitative data or that did not focus on the use of curcumin for oral mucositis in cancer patients.
Non-English articles or studies lacking a full-text version.
2.5. Selection of Studies
The selection process followed a step-by-step review of all identified articles. After an initial screening based on titles and abstracts, duplicate articles were removed. The remaining articles were assessed for relevance and eligibility through full-text reviews. Two authors (I.T. and L.F.) independently evaluated the studies, and any disagreements were resolved through discussion. The references of the included studies were also reviewed to identify additional relevant papers.
2.6. Statistical Analysis
A descriptive synthesis was used to analyze the included studies. Where applicable, data from the studies were summarized and compared regarding the effectiveness of curcumin on oral mucositis, its safety profile, and patient outcomes. Given the variability in the designs and interventions of the included studies, a meta-analysis was not performed. However, the findings were synthesized narratively, focusing on the key results and methodological differences [92].
3. Results
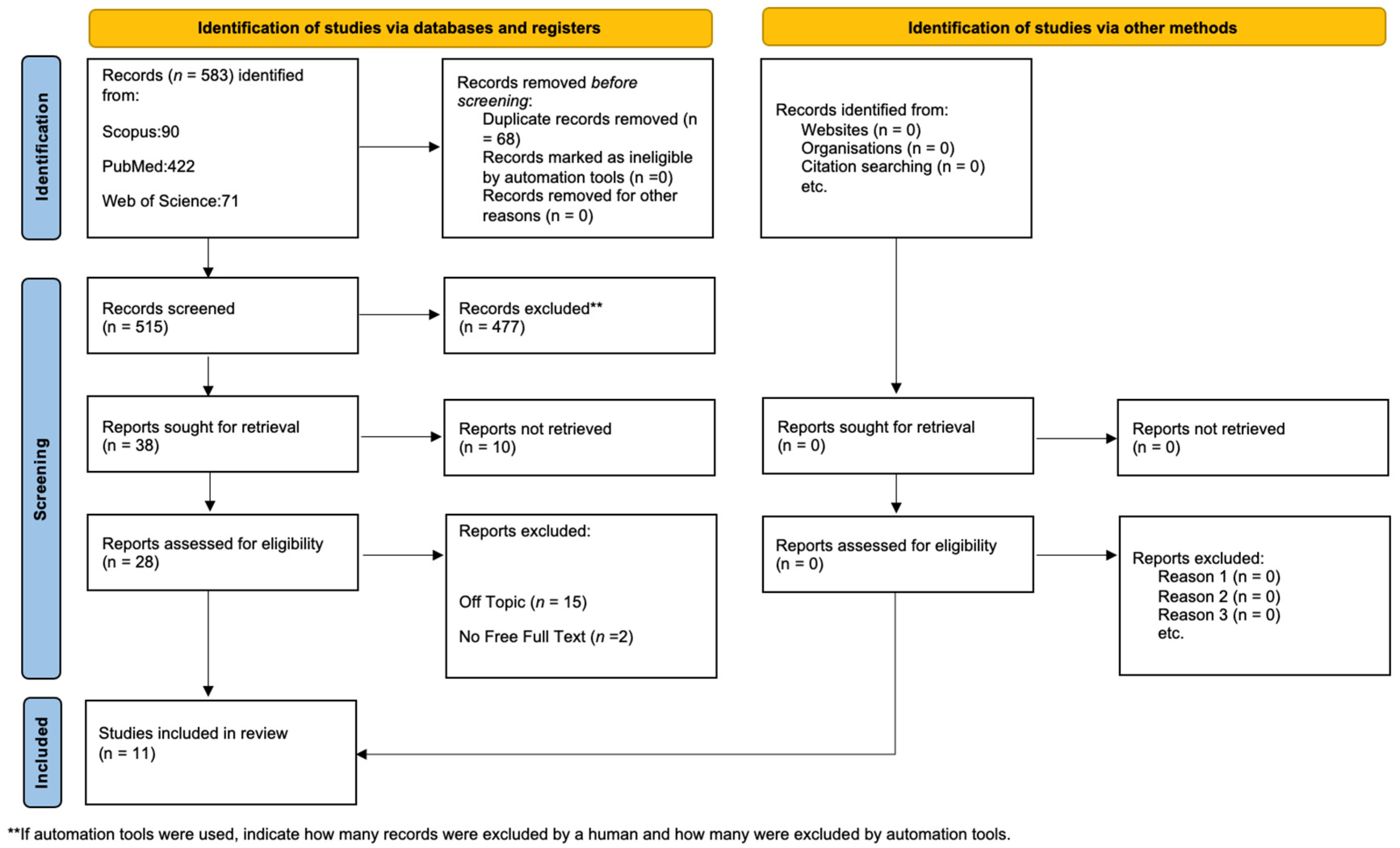
Initially, the literature search turned up 583 documents: 422 from PubMed, 71 from WOS, and 90 from Scopus. After having removed 68 duplicates, 515 articles remained for assessment. Following the evaluation for eligibility and inclusion criteria, 17 papers were included in the final analysis. Figure 5 provides a summary of the study procedure and PRISMA flowchart, while Table 1 contains in-depth descriptions of the included papers.
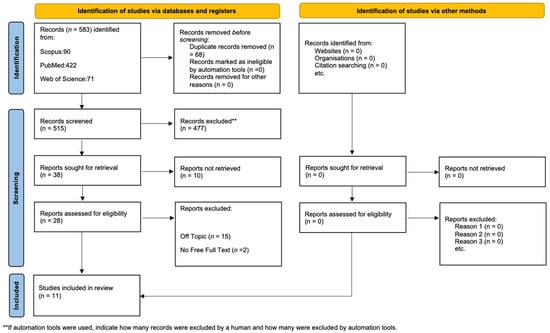
Figure 5.
PRISMA flowchart.

Table 1.
Examined articles.
Quality Assessment and Risk of Bias of Included Articles
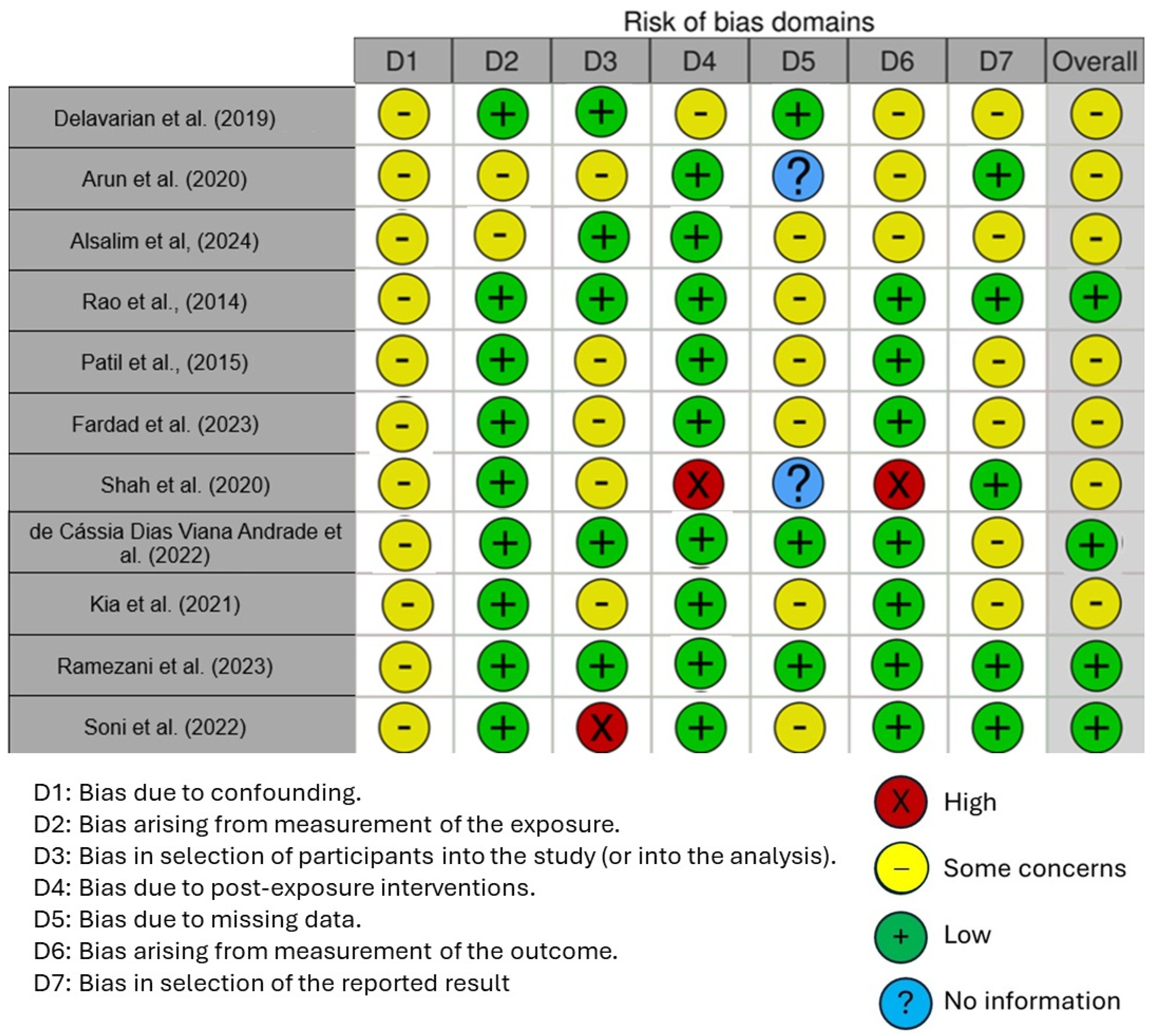
The quality of the included studies was assessed using the Cochrane Risk of Bias Tool (RoB 2) for randomized controlled trials (RCTs), which is designed to evaluate potential biases in five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. Each study was rated as having either low risk, some concerns, or high risk of bias based on these criteria.
Figure 6 displays the risk of bias in the included studies. Most research raises some concerns about bias resulting from confounding. One parameter with a minimal risk of distortion is the measurement’s distortion. Because of participant selection bias, many studies have a low risk of bias. There is minimal risk associated with post-exposure distortion. The bias resulting from missing data is minimal in much research. Measuring outcomes has less bias. Most of the research also shows minimal selection bias in the published results. Five studies had a low risk of bias, according to the results, while seven raised some concerns.
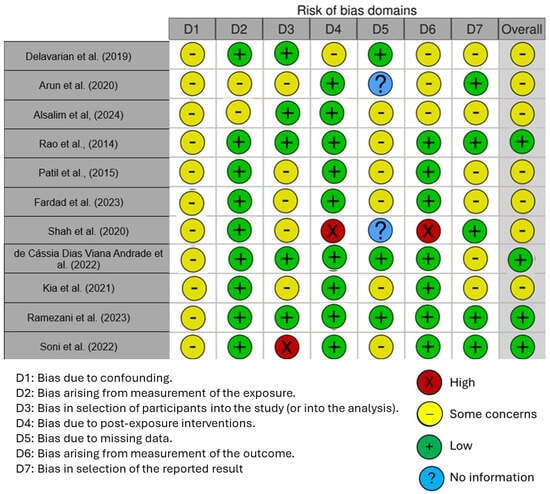
Figure 6.
Risk of bias [7,58,62,63,65,91,93,94,95,96,97].
4. Discussion
Anticancer therapy is widely recognized for its capacity to eliminate cancer cells, and it encompasses many treatments, such as radiotherapy and chemotherapy. An increased synthesis of free radicals, particularly reactive oxygen species (ROS) and reactive nitrogen species (RNS), is a notable adverse impact of these treatments [98]. Indeed, these unstable chemical compounds can harm both malignant and normal cells, since they are produced in high amounts during therapy, and can mediate lipid peroxidation, protein oxidation, and the production of double-strand breaks in DNA. These are some of the mechanisms that lead to toxic consequences in normal tissues, as well as to debilitating disorders, such as oral mucositis (OM) [99]. Since radiotherapy uses ionizing radiation, which interacts with H2O molecules in tissues to cause oxidative stress, an overabundance of ROS/RNS is frequently produced in response to anticancer treatment. On the other hand, certain chemotherapeutic medicaments directly increase the production of free radicals [100]. These processes underscore the need for measures to reduce the oxidative damage caused by cancer therapy, since they not only make treatment more difficult but can also result in therapy interruptions due to severe adverse effects.
Several research studies investigated how defense systems against oxidative stress, especially in the brain, are modulated by the nuclear factor erythroid 2 p45-related factor (Nrf2). Under normal circumstances, the leucine zipper protein (bZIP) Nrf2 is mostly found in the cytoplasm of the cells and, in order to start the transcription of protective genes in response to stress, Nrf2 translocates to the nucleus and attaches to DNA sequences, such as antioxidant response elements (ARE). Although Nrf2 activation is essential for oxidative stress defense (as already stated), overactivation of this protein is linked to a poor prognosis in a number of cancer types. It has been demonstrated that curcumin activates Nrf2, improving the effectiveness of cancer treatments by increasing heme oxygenase-1 (HO-1) and decreasing the Nrf2 inhibitor Keap1.
Additionally, curcumin’s modulatory effects on the Nrf2 signaling pathway improve insulin resistance due to its anti-inflammatory and antioxidant properties, while reducing metabolic reactions that cause inflammation and oxidative stress and promoting glutathione production by activating the Nrf2-HO-1 and Nrf2-Keap1 pathways. Other studies have confirmed the protective role of curcumin through Nrf2 regulation [101].
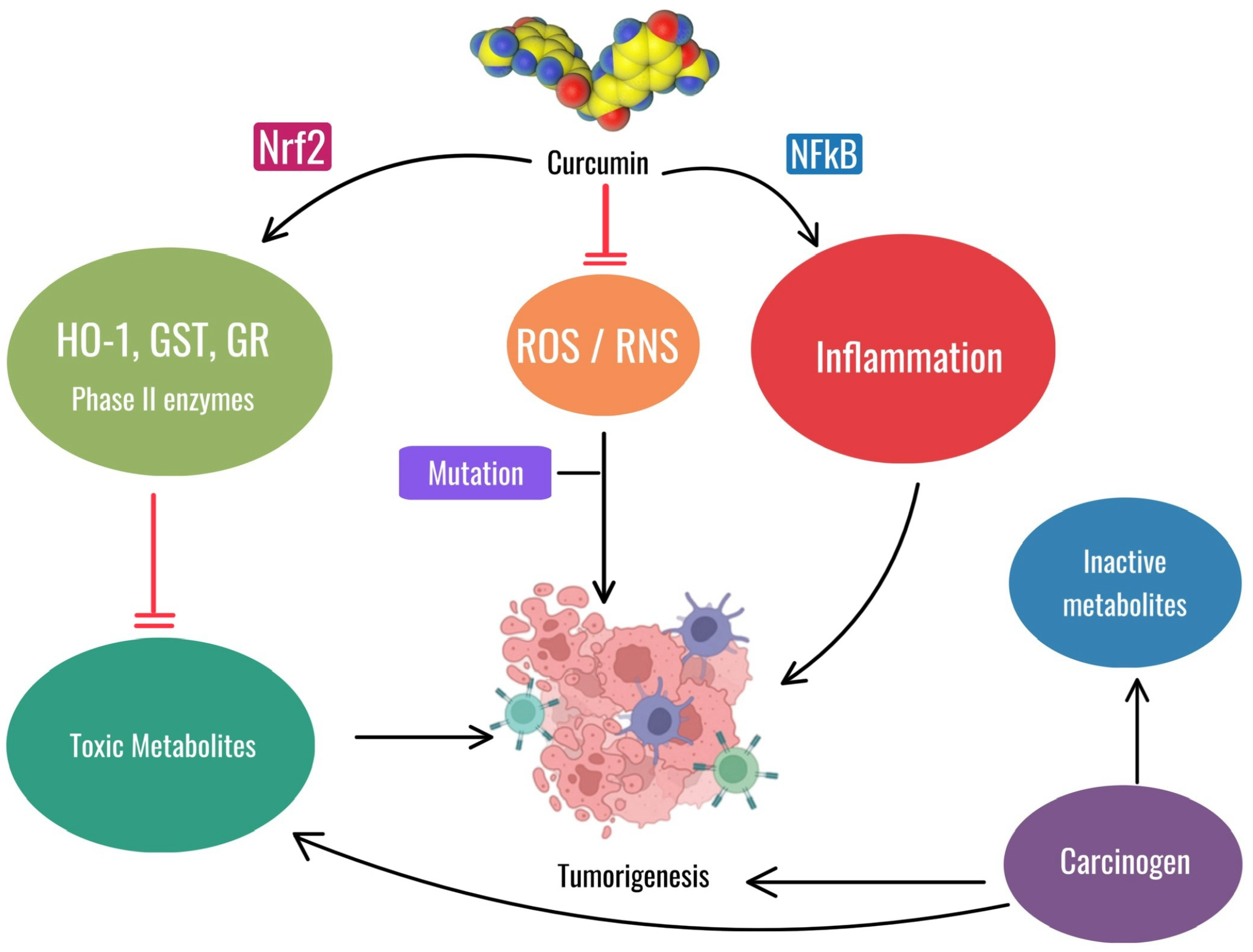
Shahcheraghi SH et al. also described these features with the observation that, similarly to curcumin, the Zinc-curcumin (Zn(II)-curc) compound has been shown to activate Nrf2, leading to anticancer effects through various mechanisms, such as increasing levels of the proteins HO-1, p62/SQSTM1, and Nrf2 itself, while reducing the levels of its inhibitor, Keap1. This interaction between p62/SQSTM1 and Nrf2 could be leveraged to improve cancer therapy outcomes [101]. The antioxidant and anti-inflammatory properties of curcumin may be useful for both oral mucositis and prevention of tumorigenesis, but their applications differ. For OM, curcumin’s role as an antioxidant and anti-inflammatory agent helps reduce the severity of oral mucositis by mitigating oxidative stress and inflammation caused by chemotherapy and radiation therapy. This relieves the painful symptoms of mucositis without interfering with the antitumor efficacy of these treatments. For the prevention of tumorigenesis, activation of Nrf2 and inhibition of NF-kB by curcumin are critical in reducing carcinogenesis by reducing inflammation and oxidative stress, thereby preventing mutations and tumor growth (Figure 7).
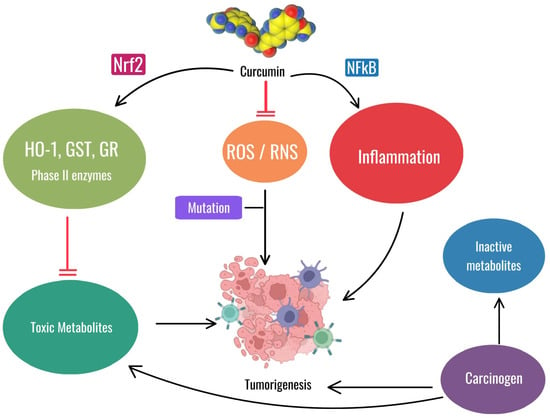
Figure 7.
The image illustrates the dual role of curcumin. In the context of oral mucositis, curcumin reduces oxidative stress (ROS/RNS) and inflammation, aiding in the management of this non-cancerous condition. In a different context, curcumin can prevent tumorigenesis by activating Nrf2, reducing toxic metabolites and inhibiting NF-kB-mediated inflammation, thus counteracting mutations and tumor growth. Chlorhexidine mouthwash is one of the traditional treatments used today, but this agent causes problems by itself while being poorly effective. Newer treatments, such as hyaluronic acid-based formulations (mucosamin) and curcumin extracts of turmeric, are being explored for their potential benefits in treating this condition [17,102].
Inhibiting cancer development may involve reducing oxidative stress by activating antioxidant defenses, restoring tumor suppressor proteins, and modulating inflammation. Curcumin, over time, significantly influences Nrf2-mediated antioxidant enzymes and the tumor suppressor protein p53. On the other hand, inhibiting Nrf2 signaling decreases phase II antioxidant enzymes and p53 levels, while increasing inflammatory signals, such as TGF-β, and altering iNOS and COX2 regulation in lymphoma-bearing mice [101].
Curcumin shows great promise as a treatment therapy for OM and, since OM is a process involving several molecular and cellular events, the therapy needs to be multifactorial. Cancer treatments cause injury to the epithelial cells of the oral mucosa, leading to activation of factors such as TNF-α and IL-6, which are pro-inflammatory. Curcumin could modulate inflammation and tissue damage by inhibiting the transcription factor NF-κB, which regulates the synthesis of inflammatory cytokines. In addition to reducing inflammation, curcumin possesses powerful antioxidant properties that enable it to neutralize ROS, reducing oxidative damage to mucosal cells. This is particularly important, since oxidative stress amplifies inflammation, aggravating mucositis. In addition, curcumin stimulates tissue regeneration by increasing the levels of growth factors such as EGF, which are essential for the repair of damaged mucosa. This accelerates the healing of oral ulcers and reduces the overall duration of OM.
Another significant aspect is curcumin’s ability to modulate the apoptotic pathway, protecting epithelial cells from premature death and maintaining mucosal integrity, thus reducing the severity of ulcers. In addition, curcumin also shows potential as an antimicrobial agent, a relevant aspect in the management of mucositis, where secondary infections may further complicate the clinical picture. By reducing the microbial load in the oral cavity, curcumin helps prevent infectious complications, improving healing. Despite its benefits, curcumin has historically suffered from low bioavailability, limiting its clinical efficacy. However, recent innovations, such as nano-micelles formulations, have significantly improved its ability to reach target tissues, enhancing its therapeutic effects against mucositis and making it a more practical and effective option.
In a study by Delavarian et al., the use of a nanocurcumin oral dosage was evaluated in patients with radiation-induced OM for the treatment of head and neck cancer. RT induces OM as a very common and painful complication in basically all patients with head and neck cancers and is associated with other complications, including mucositis, which results in the inability to eat and an increased risk for infection [56,62,103,104]. A total of 32 patients were involved in the study, and a double-blind, randomized clinical trial was performed to assess the efficacy of SinaCurcumin®, formulated as a nanomicelles to enhance the bioavailability. The results showed that the occurrence of OM in the experimental group was delayed and the intensity was less compared to the control group that received the placebo. There were no significant side effects with nanocurcumin reported in this study’s administration information, and this suggests that it is safe for use and an effective medication for OM. No such reports of the clinical use of orally administered nanocurcumin in the radiation-induced type of OM were previously available. Therefore, this seems to be the first clinical report of the same, which documents the status of nanocurcumin in management and chewing/applicational prospects as a proved analogue for cancer therapy. Further studies with more numbers of cases and favorably designed dosing are warranted to prove this inference and optimize treatment protocols [62].
In the study by Arun et al., SCC of the head and neck is presented as one of the most common cancers in India, accounting for 12% of cases. The conventional methods to treat it include surgery, radiotherapy, and chemotherapy, each of which is often followed complications, including the major one, radiation-induced mucositis, which may cause therapy to be interrupted. Several agents may be used to treat it, but no single agent is approved by the FDA. This study aimed to assess whether turmeric extract, containing curcuminoids, would have the potential to decrease the severity of mucositis [93,105,106]. Curcumin has anti-inflammatory properties, but its oral bioavailability was found to be low. This trial utilized a bioenhanced form of curcumin, BCM-95, combined with essential oils for better absorption. In the randomized, single-blinded clinical trial conducted at R L Jalappa Hospital and Research Centre, Kolar, India, 64 patients with advanced head and neck SCC undergoing post-operative radiotherapy, chemoradiotherapy, or concurrent chemoradiotherapy were included. The subjects were randomized for a test group receiving 1.5 gm/day of turmeric extract capsules against the control group receiving placebo capsules. The results showed that from the third week of the treatment, the severity of mucositis was significantly lower in the turmeric group compared to the control group. Two months after follow-up post-treatment, the majority of the turmeric group had only mild mucositis compared to the control group, which were classified under a higher grade of mucositis [41,107,108]. It was concluded that with no systemic toxicity, the turmeric extract reduced the severity of mucositis and hence was quite effective as a mode of treatment in patients undergoing radiotherapy or chemoradiotherapy. The study emphasizes the possible therapeutic benefits of turmeric extract in the management of radiation-induced mucositis in neck and head cancer patients [93,109].
In 2024, Sarah Adnan Alsalim and others conducted a study to evaluate curcumin oral gel efficacy in relation to RIOM and its connection with salivary EGF. They divided thirty-one patients undergoing radiation in the therapy of neck and head tumors into one of two groups. One received curcumin, while the other was controlled by a magic solution. The second group consisted of a gel formulation which contained 10 mg of Curcuma longa extract, applied three times a day [110]. The difference noted here is that, when compared to the magic solution group, the patients in the curcumin group had a significant difference in WHO and VAS scores regarding the level of mucositis and pain. Moreover, at irradiation and at the end of irradiation, the level of salivary EGF was significantly elevated in the curcumin group. The conclusion of the study was that the curcumin gel appreciably reduced the severity of RIOM and related pain. This can be due to the high levels of EGF, which show a better healing response of the mucosa and control over the injury processes. Further studies involving a larger sample size should be conducted to replicate the findings [63].
In a study, Rao et al. have evaluated the effectiveness of turmeric in the mitigation of RIOM in patients suffering from head and neck malignancies. The common painful side effect of the radiotherapy, oral mucositis, is usually associated with a poor quality of life in ailing patients and reduced adherence to therapy [94,111].
In this study, 80 patients were randomly assigned to use a turmeric gargle or a povidone-iodine gargle during radiation treatment. The results indicated that the turmeric gargle significantly retarded the initiation of OM and reduced the level of its severity compared with the povidone-iodine gargle. Patients who used turmeric had fewer instances of severe mucositis, fewer breaks in treatment, and less weight loss. These results suggest that turmeric can be a very effective and inexpensive solution for the management of radiogen-induced mucositis [94,112,113,114].
In the pilot study conducted in 2015, Patil et al. evaluated the role of curcumin mouthrinse in the management of radio-chemotherapy-induced OM in patients with malignancy. This included 20 patients under treatment using radio-chemotherapy, who were divided randomly into two groups: one using curcumin mouthrinse and another using a standard chlorhexidine mouthwash. The patients’ symptoms of OM were rated at three different time periods: at the beginning, after 10 days, and after 20 days. The evaluation was performed on the WHO scale, OMAS, and NRS [58,115,116]. The results showed that the curcumin mouthrinse fared much better than chlorhexidine in reducing pain, erythema, and ulceration. The scores for pain NRS, erythema levels, and ulceration severity showed a significant improvement with the use of the curcumin mouthrinse, and the differences were statistically significant. Moreover, very minimal adverse effects and excellent compliance were proven in all patients treated with the curcumin mouthrinse in contrast to those who were treated with chlorhexidine. It is therefore clear that curcumin mouthrinse is not only more efficient but also safer in the treatment of OM associated with cancer therapy. The present study thus confirms that curcumin mouthrinse could be a very promising agent for the improvement of quality of life in patients undergoing radio-chemotherapy [58,117,118].
In a randomized, double-blind trial, Fardad et al. evaluated the efficacy of chlorhexidine mouthwash, mucosamin spray, and curcumin gel in treating chemotherapy-induced oral mucositis in patients with malignancy. Their results showed that all three products were effective, but the curcumin gel showed faster and more complete recovery, with fewer side effects. These findings suggest that curcumin might turn out to be an adjunct of value in oral mucositis; however, studies with larger sample sizes are recommended for the confirmation of results [7].
This is a dose-dependent painful syndrome in individuals receiving radiation treatment for cancers of the head and neck. With a view to preventing and managing RIOM, Shah et al. compared the efficacy of a mouthwash containing 0.1% curcumin using nanoparticles with another one containing 0.15% benzydamine. Curcumin showed promise in deferring the onset of RIOM by a fortnight, though both mouthwashes were equally efficacious in reducing the severity of the condition. Although this was a triple-blinded, randomized controlled study, the following were its limitations: small sample size and high loss to follow-up. Curcumin was found to be as safe and efficacious as benzydamine, with an added advantage of delaying the onset of RIOM. Curcumin mouthwash could be a safe and effective option compared to benzydamine in the management of RIOM, delaying its onset. Further studies, with larger sample sizes and at varying doses of curcumin are further indicated to strengthen the findings [95].
The article by Rita de Cássia Dias Viana Andrade et al. describes the comparative study of two kinds of treatment in the management of OM: photobiomodulation and aPDT mediated with curcumin. The results showed that PBM and aPDT decreased the severity of mucositis and the associated pain. Clinical improvement was observed earlier in the aPDT group compared to the other groups: the PBM and control groups. PBM was outstanding, as it improved the tissue healing process and reduced inflammation. On the other hand, aPDT was more effective at reducing the load of Candida yeast. This suggests that both modalities can improve the quality of life of patients with oral mucositis, since it is a non-invasive therapy with antimicrobial, analgesic, and anti-inflammatory effects [91].
According to Kia et al., the text describes how chemotherapy and radiotherapy used during cancer treatment often led to OM. Traditional treatments offer relief but do not prevent OM. Curcumin is a polyphenol with anti-inflammatory properties extracted from turmeric. However, the bioavailability of curcumin is poor, and thus its effectiveness has been improved by the preparation of curcumin nanomicelles. In a clinical trial, it was observed that patients receiving nanomicelle curcumin demonstrated less severe OM conditions and pain as compared to placebo counterparts, which indicated its effectiveness [96].
In a single-blind randomized clinical trial, Ramezani tested the efficacy of oral and topical formulations of curcumin in reducing the severity and pain of radiotherapy-induced ROM. Both formulations of curcumin significantly reduced the symptoms of ROM when compared with the placebo group, though no significant difference was seen between the two groups receiving the curcumin formulations. The anti-inflammatory property of curcumin was brought out as useful in the treatment of ROM and other radiation-related conditions. The study had its limitations, including the small sample size, in part due to the pandemic caused by COVID-19. Future research is needed for replication of the findings with higher levels of curcumin dosages and larger samples [65,119].
In this regard, Soni evaluated the role of a bio-enhanced formulation of turmeric on oral mucositis, which represents one of the common and serious side effects of chemoradiotherapy in head and neck cancer patients. The patients were selected from 60 individuals with cases of oral cancer after radical surgery. These patients were randomized into three groups and received daily either a low dose of 1 g/day or a high dose of 1.5 g/day of BTF or placebo during chemoradiotherapy treatment for six weeks [97,120,121,122]. The study was undertaken to evaluate the role of BTF on a variety of treatment-related toxicities, which includes, among others, dermatitis, oral mucositis, dysphagia or trouble swallowing, mouth discomfort, and weight loss. It was found that, compared with the placebo group, patients who received BTF had lower incidences of severe grade 3 oral mucositis, pain, dysphagia, and dermatitis. The incidence of grade 3 OM was reduced to 25% and 20% for the low and high dose BTF groups, respectively, from 65% in the placebo group. Likewise, grade 3 oral pain, dysphagia, and dermatitis were significantly reduced in the BTF groups. Furthermore, the patients who consumed BTF had lower weight loss compared to their placebo counterparts and also showed greater compliance with treatment. The study concluded that BTF (BCM-95) decreased the severity of oral mucositis, dysphagia, oral pain, and dermatitis associated with chemoradiotherapy in patients suffering from oral malignancy, thus opening up a potential avenue of support for cancer therapy [97,123,124,125,126].
5. Conclusions
Curcumin, a compound derived from turmeric, has shown significant promise in treating RIOM in patients with head and neck cancer, especially in its enhanced forms, such as nanocurcumin and BCM-95. These formulations significantly reduce the severity and pain of mucositis by improving bioavailability and amplifying curcumin’s antioxidant effects. Curcumin’s ability to neutralize ROS plays a key role, reducing oxidative stress, preventing further damage to the oral mucosa, and promoting healing. Studies have shown that curcumin can outperform conventional treatments, such as or chlorhexidine mouthwash, or placebo, improving the quality of life for patients and allowing them to continue cancer treatments without interruptions. Its tolerability is another advantage, as patients experience few side effects, even in its enhanced forms. However, despite these promising results, further research is needed to fully establish curcumin’s role in cancer therapy and RIOM management. This should focus on larger clinical trials, more diverse patient populations, and optimized dosing protocols to ensure consistent and reproducible outcomes. While innovative formulations, such as nanocurcumin, have significantly improved bioavailability, curcumin still faces limitations in terms of its therapeutic potential, particularly when administered in traditional forms. Future studies may explore personalized treatment approaches that incorporate curcumin alongside other complementary therapies, as well as the development of multi-targeted strategies for managing mucositis and other side effects of cancer therapy. The continued investigation into the molecular mechanisms behind curcumin’s anti-inflammatory and antioxidant actions could also uncover new therapeutic pathways, broadening its applications not only in RIOM but also in cancer prevention and adjunctive treatment. In conclusion, curcumin represents a highly promising natural compound with multiple potential applications in cancer therapy, particularly in the management of RIOM. Its continued development and the optimization of delivery methods hold the potential to improve patient outcomes and reduce treatment-related complications, making it an important focus for future research in oncology.
Author Contributions
Conceptualization, G.L., G.M., A.D.I., A.M.I., A.P., I.T., L.F. and P.N.; methodology, A.M.I. and G.M.; software, F.I. and A.D.I.; validation, F.I., G.D. and G.M.; formal analysis, A.P.; investigation, A.P., I.T. and L.F.; resources, G.L., G.M. and A.M.I.; data curation, G.L., A.D.I. and F.I.; writing—original draft preparation, F.I., I.T. and P.N.; writing—review and editing, P.N. and G.D.; visualization, G.M.; supervision, L.F., I.T., P.N. and F.I.; project administration, F.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| aPDT | antimicrobial photodynamic therapy |
| ARE | antioxidant response elements |
| BTF | bio-enhanced turmeric formulation |
| bZIP | zipper protein |
| CT | chemotherapy |
| EGF | epidermal growth factor |
| FDA | food and drug administration |
| GSH | glutathione |
| GSH-px | glutathione peroxidase |
| HO-1 | heme oxygenase-1 |
| HNC | head neck cancer |
| LED | light emitting diodes |
| Nrf2 | nuclear factor erythroid 2 p45-related factor |
| NRS | numerical pain rating scale |
| OM | oral mucositis |
| OMAS | Oral Mucositis Assessment Scale |
| PBM | photobiomodulation |
| RIOM | radiation-induced oral mucositis |
| ROS | reactive oxygen species |
| RT | radiation therapy |
| SCC | squamous cell carcinoma |
| SOD | superoxide dismutase |
| VAS | visual analogue scale |
| WHO | world health organization |
| Zn(II)-curc | zinc-curcumin |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Wojtyłko, M.; Kunstman, P.; Bartylak, H.; Raszewski, Ł.; Osmałek, T.; Froelich, A. A Well-Known Plant and New Therapeutic Strategies: Turmeric and Its Components in Oral Inflammatory Diseases Treatment. Appl. Sci. 2023, 13, 7809. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The Growing Role of Precision and Personalized Medicine for Cancer Treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct Types of Diffuse Large B-Cell Lymphoma Identified by Gene Expression Profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Fardad, F.; Ghasemi, K.; Ansarinejad, N.; Khodakarim, N.; Nasiripour, S.; Farasatinasab, M. A Comparative Study to Assess the Effectiveness of Curcumin, Mucosamin, and Chlorhexidine in Chemotherapy-Induced Oral Mucositis. Explore 2023, 19, 65–70. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; Porta, C.L.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral Mucositis: The Hidden Side of Cancer Therapy. J. Exp. Clin. Cancer Res. CR 2020, 39, 210. [Google Scholar] [CrossRef] [PubMed]
- Al-Rudayni, A.H.M.; Gopinath, D.; Maharajan, M.K.; Menon, R.K. Impact of Oral Mucositis on Quality of Life in Patients Undergoing Oncological Treatment: A Systematic Review. Transl. Cancer Res. 2020, 9, 3126–3134. [Google Scholar] [CrossRef]
- Keefe, D.M.K.; Gibson, R.J.; Hauer-Jensen, M. Gastrointestinal Mucositis. Semin. Oncol. Nurs. 2004, 20, 38–47. [Google Scholar] [CrossRef]
- Oronsky, B.; Goyal, S.; Kim, M.M.; Cabrales, P.; Lybeck, M.; Caroen, S.; Oronsky, N.; Burbano, E.; Carter, C.; Oronsky, A. A Review of Clinical Radioprotection and Chemoprotection for Oral Mucositis. Transl. Oncol. 2018, 11, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. A Hypothesis for the Pathogenesis of Radiation-Induced Oral Mucositis: When Biological Challenges Exceed Physiologic Protective Mechanisms. Implications for Pharmacological Prevention and Treatment. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 4939–4947. [Google Scholar] [CrossRef] [PubMed]
- Kawashita, Y.; Soutome, S.; Umeda, M.; Saito, T. Oral Management Strategies for Radiotherapy of Head and Neck Cancer. Jpn. Dent. Sci. Rev. 2020, 56, 62–67. [Google Scholar] [CrossRef]
- Lalla, R.V.; Saunders, D.P.; Peterson, D.E. Chemotherapy or Radiation-Induced Oral Mucositis. Dent. Clin. N. Am. 2014, 58, 341–349. [Google Scholar] [CrossRef]
- De Sanctis, V.; Bossi, P.; Sanguineti, G.; Trippa, F.; Ferrari, D.; Bacigalupo, A.; Ripamonti, C.I.; Buglione, M.; Pergolizzi, S.; Langendjik, J.A.; et al. Mucositis in Head and Neck Cancer Patients Treated with Radiotherapy and Systemic Therapies: Literature Review and Consensus Statements. Crit. Rev. Oncol. Hematol. 2016, 100, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, É.T.; Silva, M.V.; de Campos, T.A.; de P. Martins, V.; Bezzerra, A.C.B. Antimicrobial Effects of Silver Diamine Fluoride: An In Vivo Study. Am. J. Dent. 2021, 34, 49–53. [Google Scholar]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of Oral Mucositis in Patients Who Have Cancer. Dent. Clin. N. Am. 2008, 52, 61–77,viii. [Google Scholar] [CrossRef]
- Cirillo, N.; Vicidomini, A.; McCullough, M.; Gambardella, A.; Hassona, Y.; Prime, S.S.; Colella, G. A Hyaluronic Acid-Based Compound Inhibits Fibroblast Senescence Induced by Oxidative Stress In Vitro and Prevents Oral Mucositis In Vivo. J. Cell. Physiol. 2015, 230, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; Vincini, M.G.; Zaffaroni, M.; Pepa, M.; Angelicone, I.; Astone, A.; Bergamini, C.; Buonopane, S.; Conte, M.; De Rosa, N.; et al. Management of Radiation-Induced Oral Mucositis in Head and Neck Cancer Patients: A Real-Life Survey among 25 Italian Radiation Oncology Centers. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2023, 32, 38. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A. Potential Prevention: Aloe Vera Mouthwash May Reduce Radiation-Induced Oral Mucositis in Head and Neck Cancer Patients. Chin. J. Integr. Med. 2012, 18, 635–640. [Google Scholar] [CrossRef]
- Chen, L.; Lu, F.; Qian, H.; Wang, H.; Zhang, F. Efficacy of Lvpao Powder on Radiation Therapy-Induced Mucositis: A Retrospective Study of 114 Patients with Head and Neck Carcinoma. Adv. Radiat. Oncol. 2024, 9, 101434. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Macedo, C.; Silva, A.M.; Delerue-Matos, C.; Costa, P.; Rodrigues, F. Natural Products for the Prevention and Treatment of Oral Mucositis-A Review. Int. J. Mol. Sci. 2022, 23, 4385. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Singh, A.K. Oral Mucositis. Natl. J. Maxillofac. Surg. 2020, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Daugėlaitė, G.; Užkuraitytė, K.; Jagelavičienė, E.; Filipauskas, A. Prevention and Treatment of Chemotherapy and Radiotherapy Induced Oral Mucositis. Med. Kaunas Lith. 2019, 55, 25. [Google Scholar] [CrossRef] [PubMed]
- Feller: Inflammation in the Context of Oral Cancer—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=Inflammation+in+the+context+of+oral+cancer&author=Feller,+L.&author=Altini,+M.&author=Lemmer,+J.&publication_year=2013&journal=Oral+Oncol.&volume=49&pages=887%E2%80%93892&doi=10.1016/j.oraloncology.2013.07.003 (accessed on 8 August 2024).
- McGuire, D.B.; Fulton, J.S.; Park, J.; Brown, C.G.; Correa, M.E.P.; Eilers, J.; Elad, S.; Gibson, F.; Oberle-Edwards, L.K.; Bowen, J.; et al. Systematic Review of Basic Oral Care for the Management of Oral Mucositis in Cancer Patients. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2013, 21, 3165–3177. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Ciocia, A.M.; Netti, A.; Viapiano, F.; Palumbo, I.; Trilli, I.; Guglielmo, M.; Inchingolo, A.D.; Dipalma, G.; et al. Benefits of Natural Antioxidants on Oral Health. Antioxidants 2023, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Nashwan, A.J. Use of Chlorhexidine Mouthwash in Children Receiving Chemotherapy: A Review of Literature. J. Pediatr. Oncol. Nurs. Off. J. Assoc. Pediatr. Oncol. Nurses 2011, 28, 295–299. [Google Scholar] [CrossRef]
- Ruggiero, T.; Pol, R.; Camisassa, D.; Simiele, S.; Giaccone, L.; Carossa, S. Treatment of Symptomatic Oral Mucositis with Sodium Hyaluronate and Synthetic Amino Acid Precursors of Collagen in Patients Undergoing Haematopoietic Stem Cell Transplantation. J. Biol. Regul. Homeost. Agents 2018, 32, 737–743. [Google Scholar] [PubMed]
- Ruggiero, T.; Pol, R.; Camisassa, D.; Arata, V.; Martino, I.; Giaccone, L.; Carossa, S. Use of Sodium Hyaluronate and Synthetic Amino Acid Precursors of Collagen for the Symptomatic Treatment of Mucositis in Patients Undergoing Haematopoietic Stem Cell Transplants. J. Biol. Regul. Homeost. Agents 2016, 30, 889–894. [Google Scholar]
- Goodman, A. Neurobiology of Addiction: An Integrative Review. Biochem. Pharmacol. 2008, 75, 266–322. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-Syndromic Multiple Supernumerary Teeth in a Family Unit with a Normal Karyotype: Case Report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current Uses of Chlorhexidine for Management of Oral Disease: A Narrative Review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.I.; Celentano, A.; Paolini, R.; Low, J.T.; Silke, J.; O’ Reilly, L.A.; McCullough, M.; Cirillo, N. High Molecular Weight Hyaluronic Acid Drastically Reduces Chemotherapy-Induced Mucositis and Apoptotic Cell Death. Cell Death Dis. 2023, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Assessing the Topical Application Efficiency of Two Biological Agents in Managing Chemotherapy-Induced Oral Mucositis in Children: A Randomized Clinical Trial—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33996432/ (accessed on 8 August 2024).
- Comparison of Topical Agents That are Effective Against Oral Mucositis Associated with Chemotherapy Using a Rat Antican…. Available online: http://ouci.dntb.gov.ua/en/works/4YxavYP7/ (accessed on 8 August 2024).
- Ozawa, N.; Onda, T.; Hayashi, K.; Honda, H.; Shibahara, T. Effects of Topical Hangeshashinto (TJ-14) on Chemotherapy-Induced Oral Mucositis. Cancer Manag. Res. 2020, 12, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Nakatani, S.; Kobata, K.; Sogawa, N.; Sogawa, C. The Biological Efficacy of Natural Products against Acute and Chronic Inflammatory Diseases in the Oral Region. Medicines 2018, 5, 122. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.D.; Latini, G.; Trilli, I.; Ferrante, L.; Nardelli, P.; Malcangi, G.; Inchingolo, A.M.; Mancini, A.; Palermo, A.; et al. The Role of Curcumin in Oral Health and Diseases: A Systematic Review. Antioxidants 2024, 13, 660. [Google Scholar] [CrossRef]
- Elad, S.; Meidan, I.; Sellam, G.; Simaan, S.; Zeevi, I.; Waldman, E.; Weintraub, M.; Revel-Vilk, S. Topical Curcumin for the Prevention of Oral Mucositis in Pediatric Patients: Case Series. Altern. Ther. Health Med. 2013, 19, 21–24. [Google Scholar] [PubMed]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. Online 2022, 23, 4027. [Google Scholar] [CrossRef]
- Silveira, R.S.; Baldoni, A.O.; Couto, R.O.; Mendonça, T.S.; Domingueti, C.P. In Vivo Efficacy of Turmeric (Curcuma Longa L.) in the Treatment of Peripheral Neuropathy: A Systematic Review of Animal Models. An. Acad. Bras. Cienc. 2023, 95, e20200447. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of Pro-Oxidants and Antioxidants in the Anti-Inflammatory and Apoptotic Effects of Curcumin (Diferuloylmethane). Free Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; Trilli, I.; Del Vecchio, G.; Palmieri, G.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G. Oxidative Stress and Natural Products in Orthodontic Treatment: A Systematic Review. Nutrients 2023, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; Martínez-Aguilar, G.; Sánchez-Meraz, M.A.; Gamboa-Gómez, C.I. Hypoglycemic and Antioxidant Effects of Five Commercial Turmeric (Curcuma Longa) Supplements. J. Food Biochem. 2020, 44, e13389. [Google Scholar] [CrossRef]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in Antidepressant Treatments: An Overview of Potential Mechanisms, Pre-Clinical/Clinical Trials and Ongoing Challenges. Basic Clin. Pharmacol. Toxicol. 2020, 127, 243–253. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.; Yang, T.; Korma, S.; Sitohy, M.; Ali, T.; Selim, S.; AL Jaouni, S.; Salem, H.; Mahmmod, Y.; Soliman, S.; et al. Impacts of Turmeric and Its Principal Bioactive Curcumin on Human Health: Pharmaceutical, Medicinal, and Food Applications: A Comprehensive Review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2012, 15, 195–218. [Google Scholar] [CrossRef]
- Normando, A.G.C.; de Menêses, A.G.; de Toledo, I.P.; Borges, G.Á.; de Lima, C.L.; Dos Reis, P.E.D.; Guerra, E.N.S. Effects of turmeric and curcumin on oral mucositis: A systematic review. Phytother. Res. 2019, 33, 1318–1329. [Google Scholar] [CrossRef]
- Paderni, C.; Compilato, D.; Giannola, L.; Campisi, G. Oral Local Drug Delivery and New Perspectives in Oral Drug Formulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e25–e34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, G.; Wei, Z. Prophylactic and Therapeutic Effects of Curcumin on Treatment-Induced Oral Mucositis in Patients with Head and Neck Cancer: A Meta-Analysis of Randomized Controlled Trials. Nutr. Cancer 2021, 73, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Dharman, S.; Maragathavalli, G.; Shanmugasundaram, K.; Shanmugam, R.K. A Systematic Review and Meta-Analysis on the Efficacy of Curcumin/Turmeric for the Prevention and Amelioration of Radiotherapy/Radiochemotherapy Induced Oral Mucositis in Head and Neck Cancer Patients. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Budi, H.S.; Anitasari, S.; Ulfa, N.M.; Juliastuti, W.S.; Aljunaid, M.; Ramadan, D.E.; Muzari, K.; Shen, Y.-K. Topical Medicine Potency of Musa Paradisiaca Var. Sapientum (L.) Kuntze as Oral Gel for Wound Healing: An In Vitro, In Vivo Study. Eur. J. Dent. 2022, 16, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.; Guledgud, M.V.; Kulkarni, P.K.; Keshari, D.; Tayal, S. Use of Curcumin Mouthrinse in Radio-Chemotherapy Induced Oral Mucositis Patients: A Pilot Study. J. Clin. Diagn. Res. JCDR 2015, 9, ZC59–ZC62. [Google Scholar] [CrossRef]
- Komati, S.; Swain, S.; Rao, M.E.B.; Jena, B.R.; Dasi, V. Mucoadhesive Multiparticulate Drug Delivery Systems: An Extensive Review of Patents. Adv. Pharm. Bull. 2019, 9, 521–538. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; De los Llanos Gandía, M.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Metta, S.; Khan, M.; Amir; Lakshmi, M.; Poojadevi; Shruthi, K. A Review: Pharmaceutical Gels and Its Types with Prominence Role of Its Drug Delivery Systems. IJRAR 2023, 10, 686. [Google Scholar]
- Delavarian, Z.; Pakfetrat, A.; Ghazi, A.; Jaafari, M.R.; Homaei Shandiz, F.; Dalirsani, Z.; Mohammadpour, A.H.; Rahimi, H.R. Oral Administration of Nanomicelle Curcumin in the Prevention of Radiotherapy-Induced Mucositis in Head and Neck Cancers. Spec. Care Dent. Off. Publ. Am. Assoc. Hosp. Dent. Acad. Dent. Handicap. Am. Soc. Geriatr. Dent. 2019, 39, 166–172. [Google Scholar] [CrossRef]
- Alsalim, S.A.; Diajil, A.R. The Effect of Curcumin Oral Gel on Radiation-Induced Oral Mucositis in Relation to Salivary Epidermal Growth Factor. J. Emerg. Med. Trauma Acute Care 2024, 2024, 4. [Google Scholar] [CrossRef]
- Kim, K.H.; Abdi, S. Rediscovery of Nefopam for the Treatment of Neuropathic Pain. Korean J. Pain 2014, 27, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, V.; Ghadirian, S.; Shabani, M.; Boroumand, M.A.; Daneshvar, R.; Saghafi, F. Efficacy of Curcumin for Amelioration of Radiotherapy-Induced Oral Mucositis: A Preliminary Randomized Controlled Clinical Trial. BMC Cancer 2023, 23, 354. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Wu, F.; Gan, X.; Yang, X.; Zhou, L.; Chen, J.; He, Y.; Zhang, R.; Zhu, B.; Liu, L. The Involvement of Descending Pain Inhibitory System in Electroacupuncture-Induced Analgesia. Front. Integr. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef]
- Ospina, J.; Carmona, J.U.; López, C. Short-Term Effects of Two COX-2 Selective Non-Steroidal Anti-Inflammatory Drugs on the Release of Growth Factors and Cytokines from Canine Platelet-Rich Gel Supernatants. Gels 2024, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between Reactive Oxygen Species and Pro-Inflammatory Markers in Developing Various Chronic Diseases: A Review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), Interferons, Transforming Growth Factor β, and TNF-α: Receptors, Functions, and Roles in Diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Nanda, A.; Thangapandi, K.; Banerjee, P.; Dutta, M.; Wangdi, T.; Sharma, P.; Chaudhury, K.; Jana, S.K. Cytokines, Angiogenesis, and Extracellular Matrix Degradation Are Augmented by Oxidative Stress in Endometriosis. Ann. Lab. Med. 2020, 40, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Jacobs, R.; Singh, J. Possible Action Mechanism for Curcumin in Pre-Cancerous Lesions Based on Serum and Salivary Markers of Oxidative Stress. J. Oral Sci. 2010, 52, 251–256. [Google Scholar] [CrossRef]
- Sharma, C.; Kaur, J.; Shishodia, S.; Aggarwal, B.B.; Ralhan, R. Curcumin down Regulates Smokeless Tobacco-Induced NF-kappaB Activation and COX-2 Expression in Human Oral Premalignant and Cancer Cells. Toxicology 2006, 228, 1–15. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, T.J.; Woo, B.H.; Choi, K.U.; Lee, C.H.; Ryu, M.H.; Park, H.R. Curcumin-Induced Autophagy Contributes to the Decreased Survival of Oral Cancer Cells. Arch. Oral Biol. 2012, 57, 1018–1025. [Google Scholar] [CrossRef]
- Okada, N.; Muraoka, E.; Fujisawa, S.; Machino, M. Effects of Curcumin and Capsaicin Irradiated with Visible Light on Murine Oral Mucosa. Vivo Athens Greece 2012, 26, 759–764. [Google Scholar]
- Shehzad, A.; Lee, Y.-S. Curcumin: Multiple Molecular Targets Mediate Multiple Pharmacological Actions: A Review. Drugs Future 2010, 35, 113. [Google Scholar] [CrossRef]
- Rujirachotiwat, A.; Suttamanatwong, S. Curcumin Upregulates Transforming Growth Factor-Β1, Its Receptors, and Vascular Endothelial Growth Factor Expressions in an in Vitro Human Gingival Fibroblast Wound Healing Model. BMC Oral Health 2021, 21, 535. [Google Scholar] [CrossRef]
- Mani, H.; Sidhu, G.S.; Kumari, R.; Gaddipati, J.P.; Seth, P.; Maheshwari, R.K. Curcumin Differentially Regulates TGF-Beta1, Its Receptors and Nitric Oxide Synthase during Impaired Wound Healing. BioFactors Oxf. Engl. 2002, 16, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.; Malcangi, G.; Ferrante, L.; Trilli, I.; Noia, A.; Piras, F.; Mancini, A.; Palermo, A.; Inchingolo, A.; et al. The Interaction of Cytokines in Orthodontics: A Systematic Review. Appl. Sci. 2024, 14, 5133. [Google Scholar] [CrossRef]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting Autophagy Using Natural Compounds for Cancer Prevention and Therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Shamsah, M.S.; Zaidan, T. Curcumin Modulate TGFβii-R to Improve Healing of Oral Ulceration. Int. J. Pharm. Res. 2020, 12, 1288–1294. [Google Scholar] [CrossRef]
- Mehanny, M.; Hathout, R.M.; Geneidi, A.S.; Mansour, S. Exploring the Use of Nanocarrier Systems to Deliver the Magical Molecule; Curcumin and Its Derivatives. J. Control. Release Off. J. Control. Release Soc. 2016, 225, 1–30. [Google Scholar] [CrossRef]
- Rai, A.; Kaur, M.; Gombra, V.; Hasan, S.; Kumar, N. Comparative Evaluation of Curcumin and Antioxidants in the Management of Oral Submucous Fibrosis. J. Investig. Clin. Dent. 2019, 10, e12464. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Dipalma, G.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; D’Oria, M.T.; Isacco, C.G.; Bordea, I.R.; Candrea, S.; Scarano, A.; et al. The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants. Antioxidants 2021, 10, 881. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sharma, D.; Kumar, D.; Singh, G.; Swami, G.; Rathore, M.S. Formulation, Development, and Evaluation of Herbal Effervescent Mouthwash Tablet Containing Azadirachta Indica (Neem) and Curcumin for the Maintenance of Oral Hygiene. Recent Pat. Drug Deliv. Formul. 2020, 14, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the Active Substance of Turmeric: Its Effects on Health and Ways to Improve Its Bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sharma, R.; Sarangal, V.; Kaur, N.; Prashar, P. Evaluation of Anti-Inflammatory Effects of Systemically Administered Curcumin, Lycopene and Piperine as an Adjunct to Scaling and Root Planing: A Clinical Study. Ayu 2017, 38, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.-C.; Chueh, F.-S.; Hsiao, Y.-T.; Cheng, Z.-Y.; Lien, J.-C.; Liu, K.-C.; Peng, S.-F.; Chung, J.-G. Gefitinib and Curcumin-Loaded Nanoparticles Enhance Cell Apoptosis in Human Oral Cancer SAS Cells in Vitro and Inhibit SAS Cell Xenografted Tumor in Vivo. Toxicol. Appl. Pharmacol. 2019, 382, 114734. [Google Scholar] [CrossRef]
- Girisa, S.; Kumar, A.; Rana, V.; Parama, D.; Daimary, U.D.; Warnakulasuriya, S.; Kumar, A.P.; Kunnumakkara, A.B. From Simple Mouth Cavities to Complex Oral Mucosal Disorders-Curcuminoids as a Promising Therapeutic Approach. ACS Pharmacol. Transl. Sci. 2021, 4, 647–665. [Google Scholar] [CrossRef]
- de Cássia Dias Viana Andrade, R.; Azevedo Reis, T.; Rosa, L.P.; de Oliveira Santos, G.P.; da CristinaSilva, F. Comparative Randomized Trial Study about the Efficacy of Photobiomodulation and Curcumin Antimicrobial Photodynamic Therapy as a Coadjuvant Treatment of Oral Mucositis in Oncologic Patients: Antimicrobial, Analgesic, and Degree Alteration Effect. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 30, 7365–7371. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Arun, P.; Sagayaraj, A.; Azeem Mohiyuddin, S.M.; Santosh, D. Role of Turmeric Extract in Minimising Mucositis in Patients Receiving Radiotherapy for Head and Neck Squamous Cell Cancer: A Randomised, Placebo-Controlled Trial. J. Laryngol. Otol. 2020, 134, 159–164. [Google Scholar] [CrossRef]
- Rao, S.; Dinkar, C.; Vaishnav, L.K.; Rao, P.; Rai, M.P.; Fayad, R.; Baliga, M.S. The Indian Spice Turmeric Delays and Mitigates Radiation-Induced Oral Mucositis in Patients Undergoing Treatment for Head and Neck Cancer: An Investigational Study. Integr. Cancer Ther. 2014, 13, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Rath, H.; Sharma, G.; Senapati, S.N.; Mishra, E. Effectiveness of Curcumin Mouthwash on Radiation-Induced Oral Mucositis among Head and Neck Cancer Patients: A Triple-Blind, Pilot Randomised Controlled Trial. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2020, 31, 718–727. [Google Scholar] [CrossRef]
- Kia, S.J.; Basirat, M.; Saedi, H.S.; Arab, S.A. Effects of Nanomicelle Curcumin Capsules on Prevention and Treatment of Oral Mucosits in Patients under Chemotherapy with or without Head and Neck Radiotherapy: A Randomized Clinical Trial. BMC Complement. Med. Ther. 2021, 21, 232. [Google Scholar] [CrossRef]
- Soni, T.P.; Gupta, A.K.; Sharma, L.M.; Singhal, H.; Sharma, S.; Gothwal, R.S. A Randomized, Placebo-Controlled Study to Evaluate the Effect of Bio-Enhanced Turmeric Formulation on Radiation-Induced Oral Mucositis. ORL J. Oto-Rhino-Laryngol. Its. Relat. Spec. 2022, 84, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Oxidative Stress-Inducing Anticancer Therapies: Taking a Closer Look at Their Immunomodulating Effects—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7759788/ (accessed on 17 August 2024).
- DNA Damage by Lipid Peroxidation Products: Implications in Cancer, Inflammation and Autoimmunity—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6690246/ (accessed on 17 August 2024).
- Ionizing Radiation as a Source of Oxidative Stress—The Protective Role of Melatonin and Vitamin D—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7460937/ (accessed on 17 August 2024).
- Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8746993/ (accessed on 17 August 2024).
- Worthington, H.V.; Clarkson, J.E.; Bryan, G.; Furness, S.; Glenny, A.-M.; Littlewood, A.; McCabe, M.G.; Meyer, S.; Khalid, T.; Riley, P. Interventions for Preventing Oral Mucositis for Patients with Cancer Receiving Treatment. Cochrane Database Syst. Rev. 2011, 2011, CD000978. [Google Scholar] [CrossRef]
- Aghamohammadi, A.; Moslemi, D.; Akbari, J.; Ghasemi, A.; Azadbakht, M.; Asgharpour, A.; Hosseinimehr, S.J. The Effectiveness of Zataria Extract Mouthwash for the Management of Radiation-Induced Oral Mucositis in Patients: A Randomized Placebo-Controlled Double-Blind Study. Clin. Oral Investig. 2018, 22, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem Cells: Their Source, Potency and Use in Regenerative Therapies with Focus on Adipose-Derived Stem Cells—A Review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Bassir, S.H.; Wisitrasameewong, W.; Raanan, J.; Ghaffarigarakani, S.; Chung, J.; Freire, M.; Andrada, L.C.; Intini, G. Potential for Stem Cell-Based Periodontal Therapy. J. Cell. Physiol. 2016, 231, 50–61. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Chwatko, G.; Głowacki, R.; Bald, E. Determination of Endogenous Thiols and Thiol Drugs in Urine by HPLC with Ultraviolet Detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3300–3308. [Google Scholar] [CrossRef]
- Ascheim, D.D.; Gelijns, A.C.; Goldstein, D.; Moye, L.A.; Smedira, N.; Lee, S.; Klodell, C.T.; Szady, A.; Parides, M.K.; Jeffries, N.O.; et al. Mesenchymal Precursor Cells as Adjunctive Therapy in Recipients of Contemporary Left Ventricular Assist Devices. Circulation 2014, 129, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.; Piras, F.; Ferrante, L.; Mancini, A.; Palermo, A.; Inchingolo, F.; Dipalma, G. The Interaction between Gut Microbiome and Bone Health. Curr. Opin. Endocrinol. Diabetes Obes. 2024, 31, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Malcangi, G.; Semjonova, A.; Inchingolo, A.M.; Patano, A.; Coloccia, G.; Ceci, S.; Marinelli, G.; Di Pede, C.; Ciocia, A.M.; et al. Oralbiotica/Oralbiotics: The Impact of Oral Microbiota on Dental Health and Demineralization: A Systematic Review of the Literature. Children 2022, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadhi, G.; Al-Rai, S.; Hafed, L. The Therapeutic Potential of Inflamed Gingiva-Derived Mesenchymal Stem Cells in Preclinical Studies: A Scoping Review of a Unique Biomedical Waste. Stem Cells Int. 2021, 2021, 6619170. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Fatone, M.C.; Malcangi, G.; Avantario, P.; Piras, F.; Patano, A.; Di Pede, C.; Netti, A.; Ciocia, A.M.; De Ruvo, E.; et al. Modifiable Risk Factors of Non-Syndromic Orofacial Clefts: A Systematic Review. Children 2022, 9, 1846. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrara, I.; Viapiano, F.; Netti, A.; Buongiorno, S.; Latini, G.; Azzollini, D.; De Leonardis, N.; de Ruvo, E.; et al. Laser Surgical Approach of Upper Labial Frenulum: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 1302. [Google Scholar] [CrossRef]
- Inchingolo, F.; Santacroce, L.; Ballini, A.; Topi, S.; Dipalma, G.; Haxhirexha, K.; Bottalico, L.; Charitos, I.A. Oral Cancer: A Historical Review. Int. J. Environ. Res. Public Health 2020, 17, 3168. [Google Scholar] [CrossRef]
- Ameri, A.; Poshtmahi, S.; Heydarirad, G.; Cramer, H.; Choopani, R.; Hajimehdipoor, H.; Azghandi, S.; Pasalar, M. Effect of Honey-Lemon Spray Versus Benzydamine Hydrochloride Spray on Radiation-Induced Acute Oral Mucositis in Head and Neck Cancer Patients: A Pilot, Randomized, Double-Blind, Active-Controlled Clinical Trial. J. Altern. Complement. Med. N. Y. N. 2021, 27, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Karimyan, N.; Watters, A.; Emperumal, C.P.; Al-Eryani, K.; Enciso, R. Efficacy of Oral and Topical Antioxidants in the Prevention and Management of Oral Mucositis in Head and Neck Cancer Patients: A Systematic Review and Meta-Analyses. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 30, 8689–8703. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.; Latini, G.; Ferrante, L.; Ruvo, E.; Campanelli, M.; Longo, M.; Palermo, A.; Inchingolo, A.; Dipalma, G. Difference in the Intestinal Microbiota between Breastfeed Infants and Infants Fed with Artificial Milk: A Systematic Review. Pathogens 2024, 13, 533. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.M.; Isacco, C.G.; et al. SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs. Microorganisms 2021, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-F.; Wu, H.-J.; Shih, C.-L.; Yeh, T.-P.; Ma, W.-F. Efficacy of Turmeric in the Treatment of Oral Mucositis in Patients with Head and Neck Cancer after Radiotherapy or Chemoradiotherapy: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2024, 15, 1363202. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, E.; Di Prima, G.; Angellotti, G.; Panzarella, V.; De Caro, V. Plant-Derived Polyphenols to Prevent and Treat Oral Mucositis Induced by Chemo- and Radiotherapy in Head and Neck Cancers Management. Cancers 2024, 16, 260. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Scully, C.; Epstein, J.; Sonis, S. Oral Mucositis: A Challenging Complication of Radiotherapy, Chemotherapy, and Radiochemotherapy. Part 2: Diagnosis and Management of Mucositis. Head Neck 2004, 26, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Zhang, Y.; Li, X.; Zhang, Y.; Li, Y.; Zhou, L.; Hu, X. Efficacy of Nonpharmacological Interventions for Severe Radiation-Induced Oral Mucositis among Head and Neck Cancer Patients: A Network Meta-Analysis of Randomised Controlled Trials. J. Clin. Nurs. 2024, 33, 2030–2049. [Google Scholar] [CrossRef]
- Adjunctive Treatments for the Prevention of Chemotherapy- and Radiotherapy-Induced Mucositis—Michael Thomsen, Luis Vitetta, 2018. Available online: https://journals.sagepub.com/doi/10.1177/1534735418794885?icid=int.sj-full-text.similar-articles.2 (accessed on 9 August 2024).
- Lee, S.-Y.; Kim, B.H.; Park, Y.H. Analysis of Dysphagia Patterns Using a Modified Barium Swallowing Test Following Treatment of Head and Neck Cancer. Yonsei Med. J. 2015, 56, 1221–1226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).