Bufotalin Induces Oxidative Stress-Mediated Apoptosis by Blocking the ITGB4/FAK/ERK Pathway in Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability and Cytotoxicity Assays
2.3. Cell Migration and Invasion Assays
2.4. Colony Formation Assay
2.5. Tumor Xenograft Experiments
2.6. Cell Death Manner Assay
2.7. Apoptosis Assay
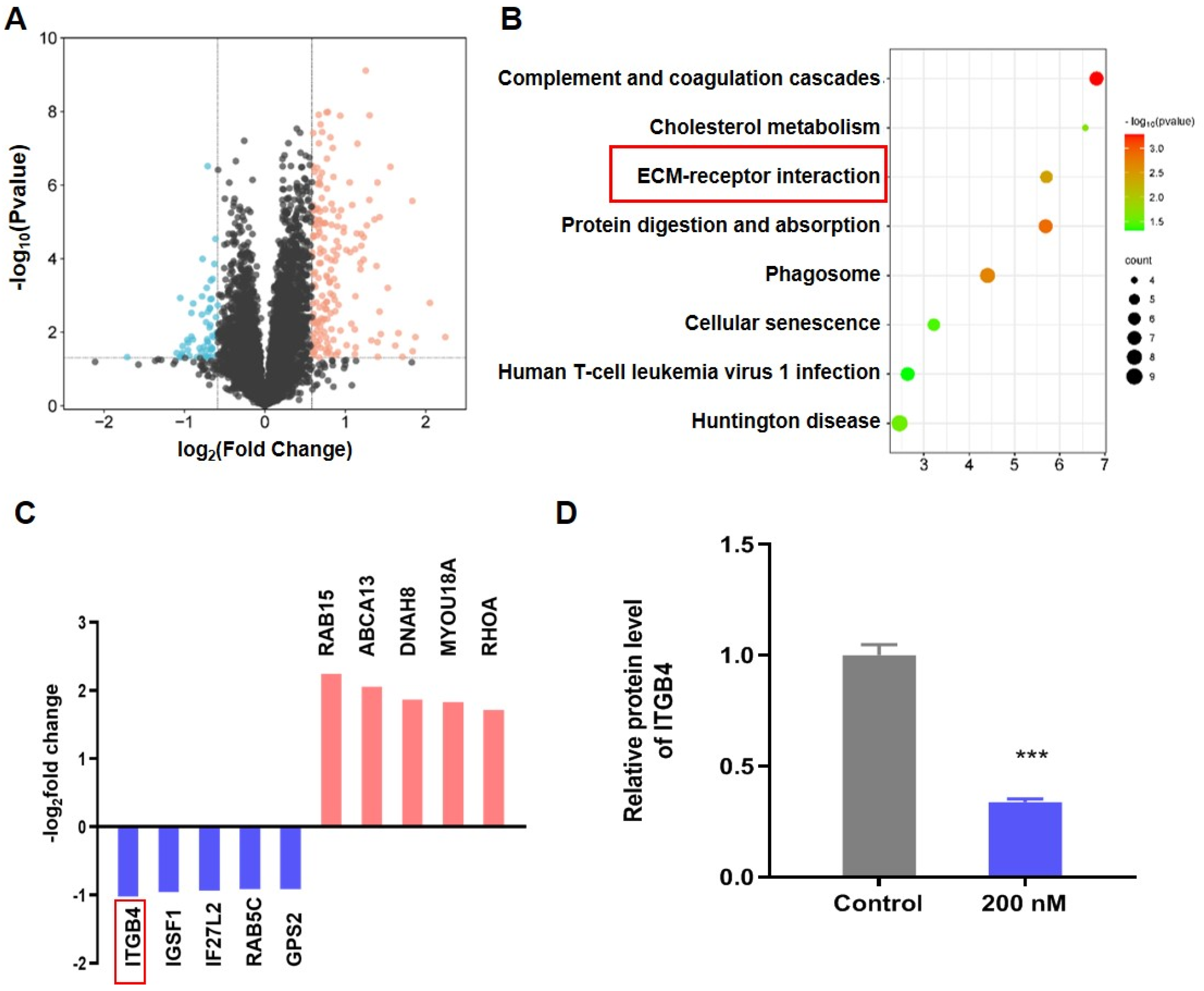
2.8. Quantitative Proteomic Analysis
2.9. Bioinformatic Analysis
2.10. Detection of Adenosine Triphosphate (ATP) and Intracellular Calcium Concentration
2.11. Measurement of Mitochondrial Membrane Potential (MMP) and Mitochondrial Permeability Transition Pore (mPTP)
2.12. Immunofluorescence and IHC Staining
2.13. Detection of the Levels of ROS, Total Thiol, SOD, GPx and MDA
2.14. Western Blot
2.15. Statistical Analysis
3. Results
3.1. BT Alleviates the Malignancy of GBM Cells In Vitro
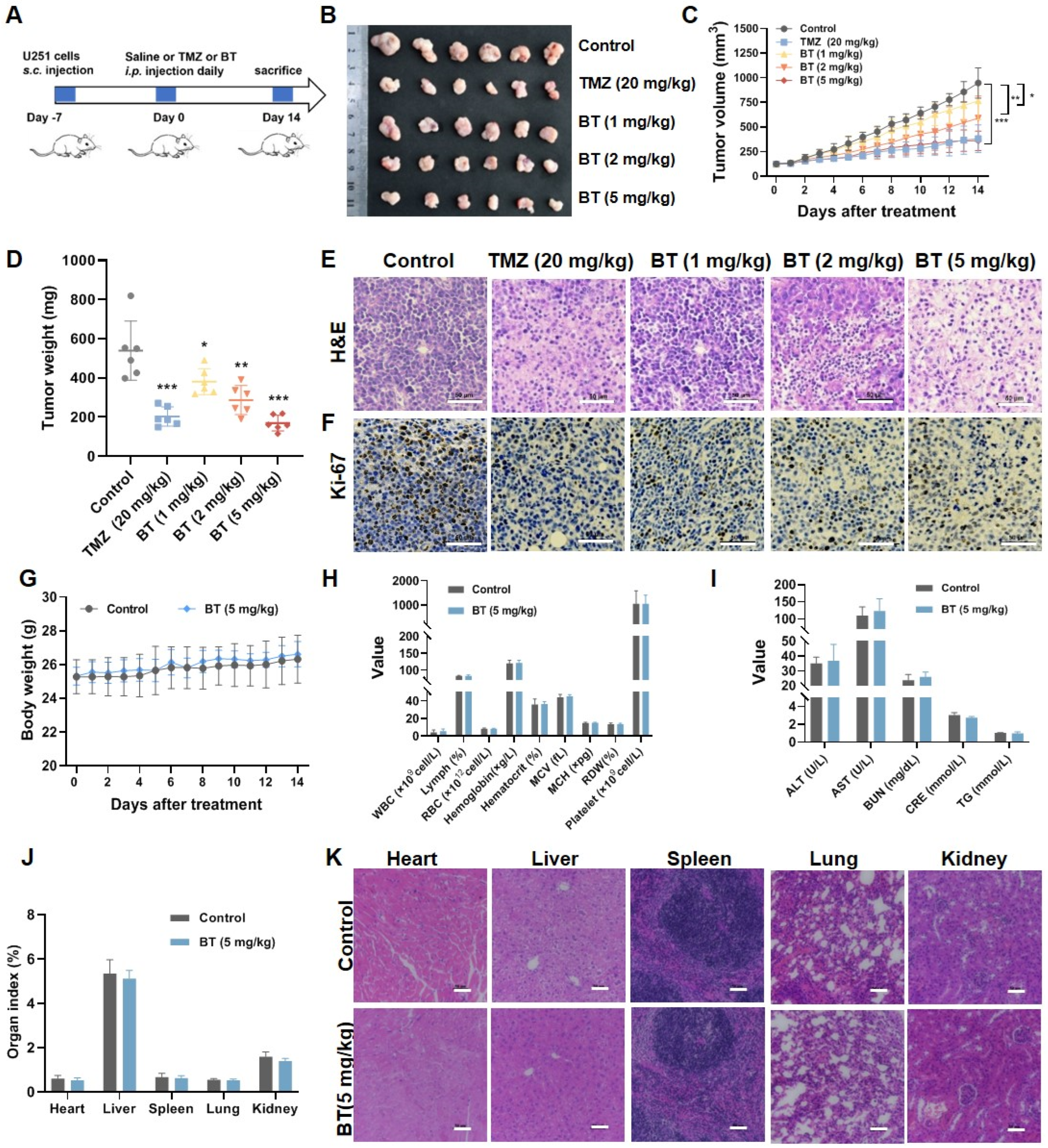
3.2. BT Suppresses GBM Cell Growth In Vivo
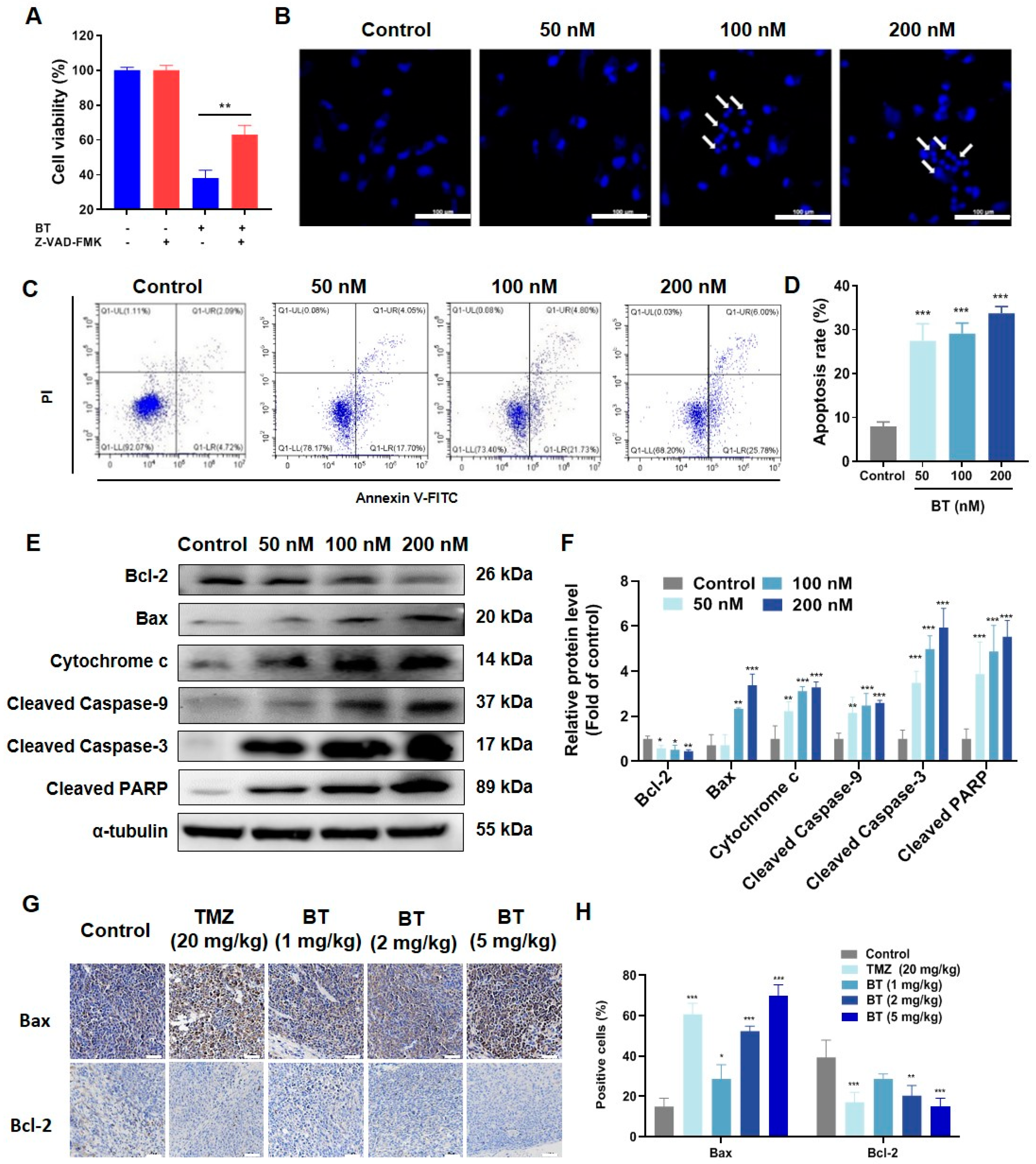
3.3. BT Induces Apoptosis of U251 Cells
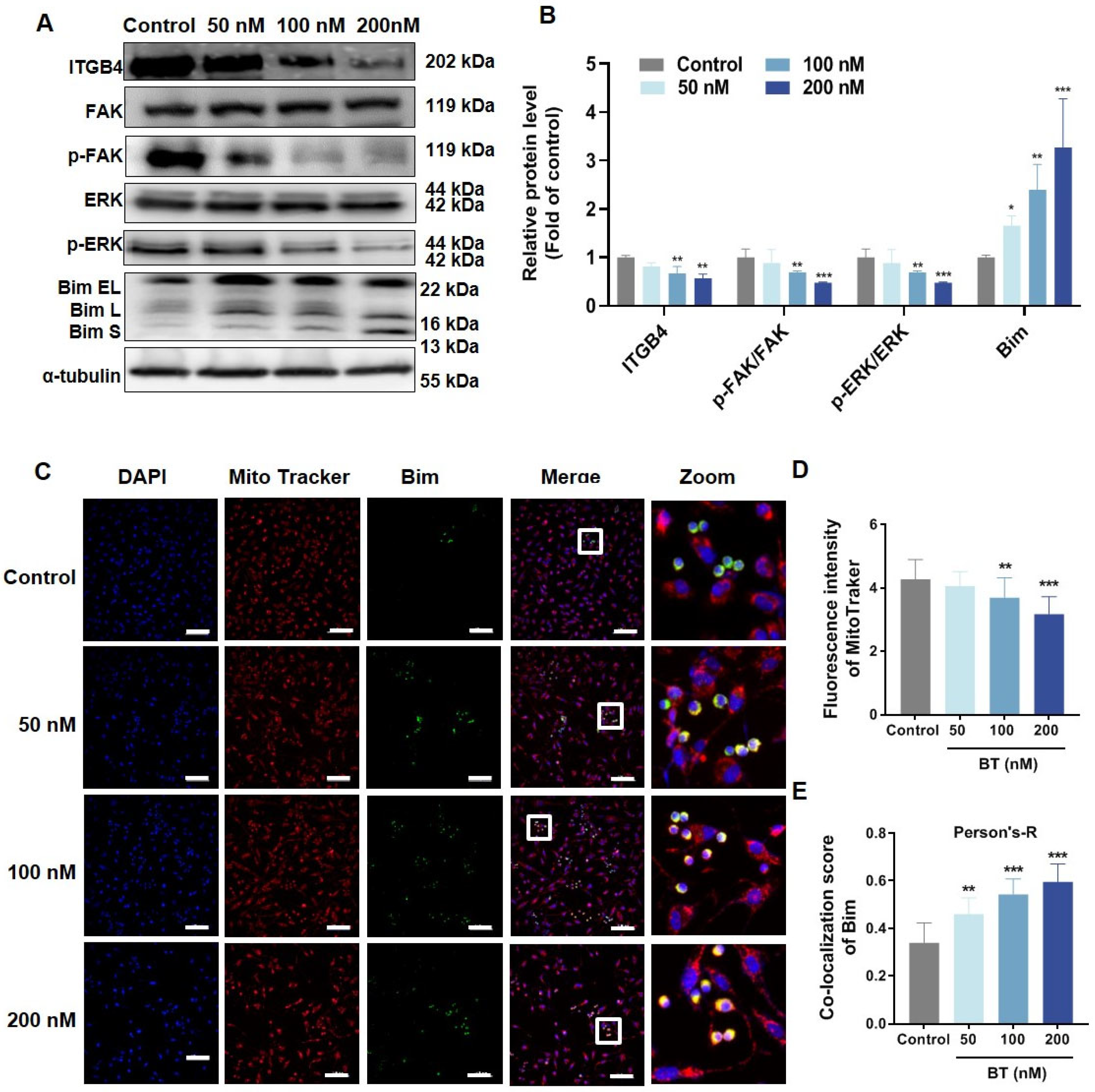
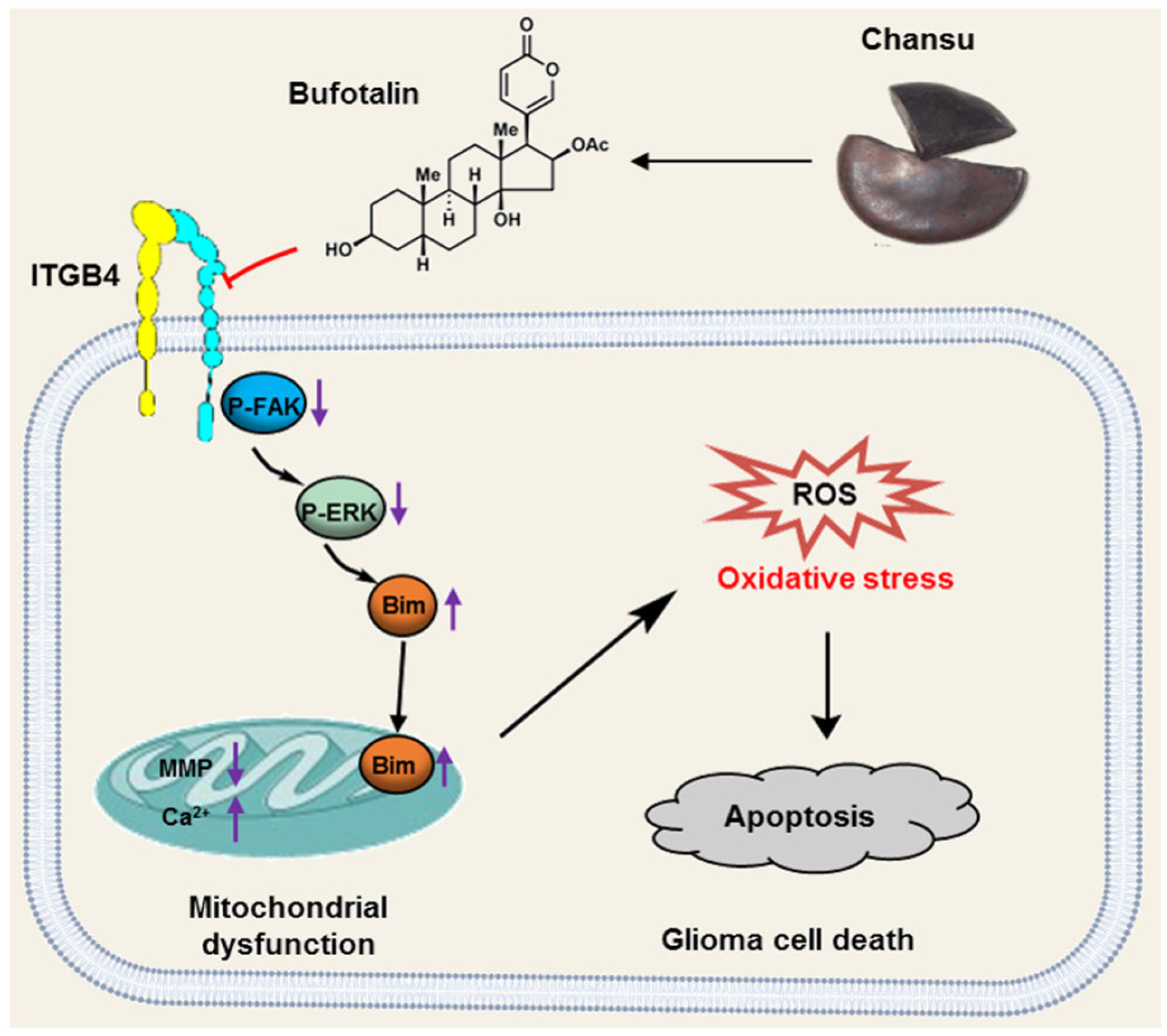
3.4. BT Inhibits ITGB4/FAK/ERK Pathway
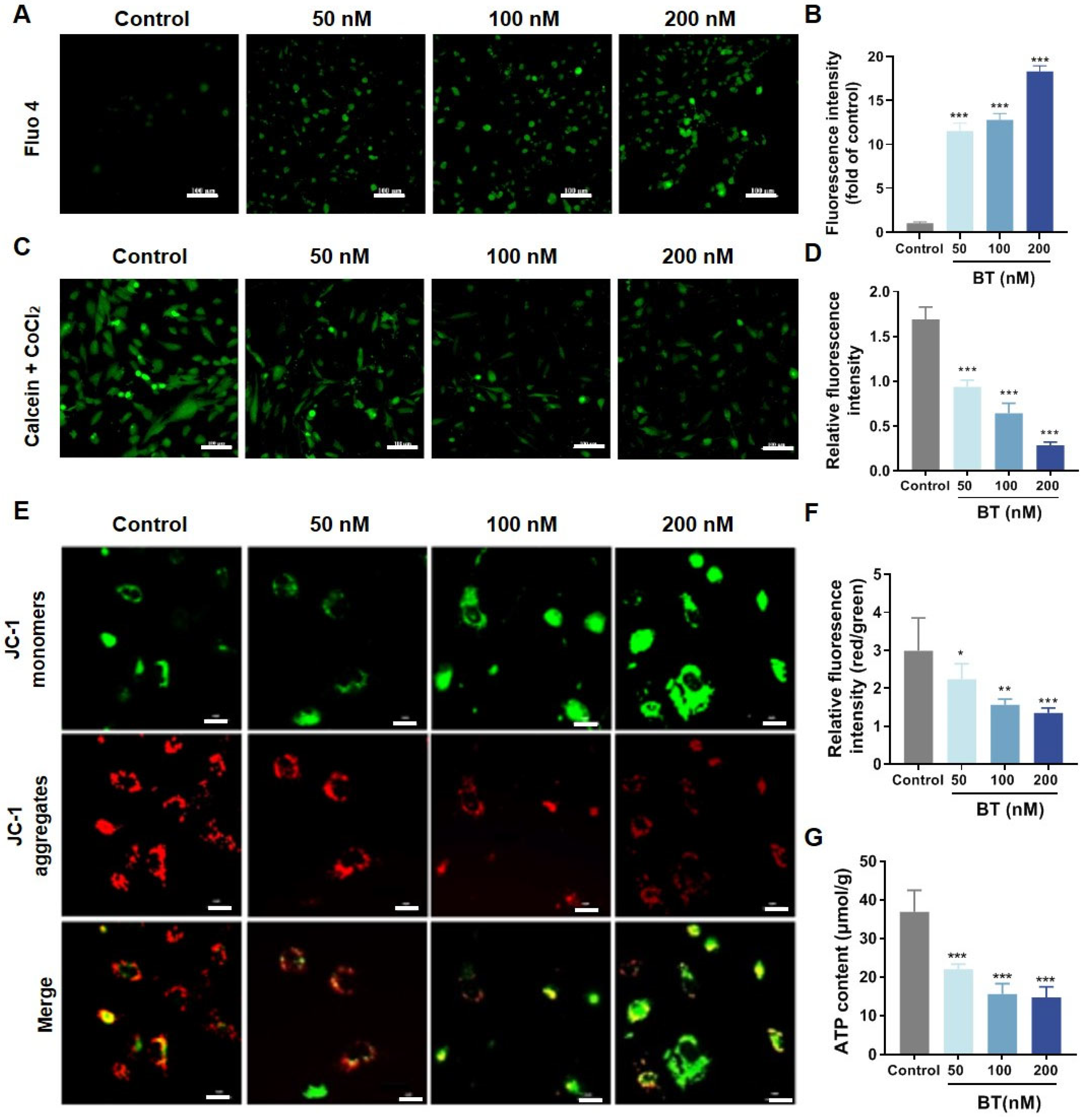
3.5. BT Induces Mitochondrial Dysfunction in U251 Cells
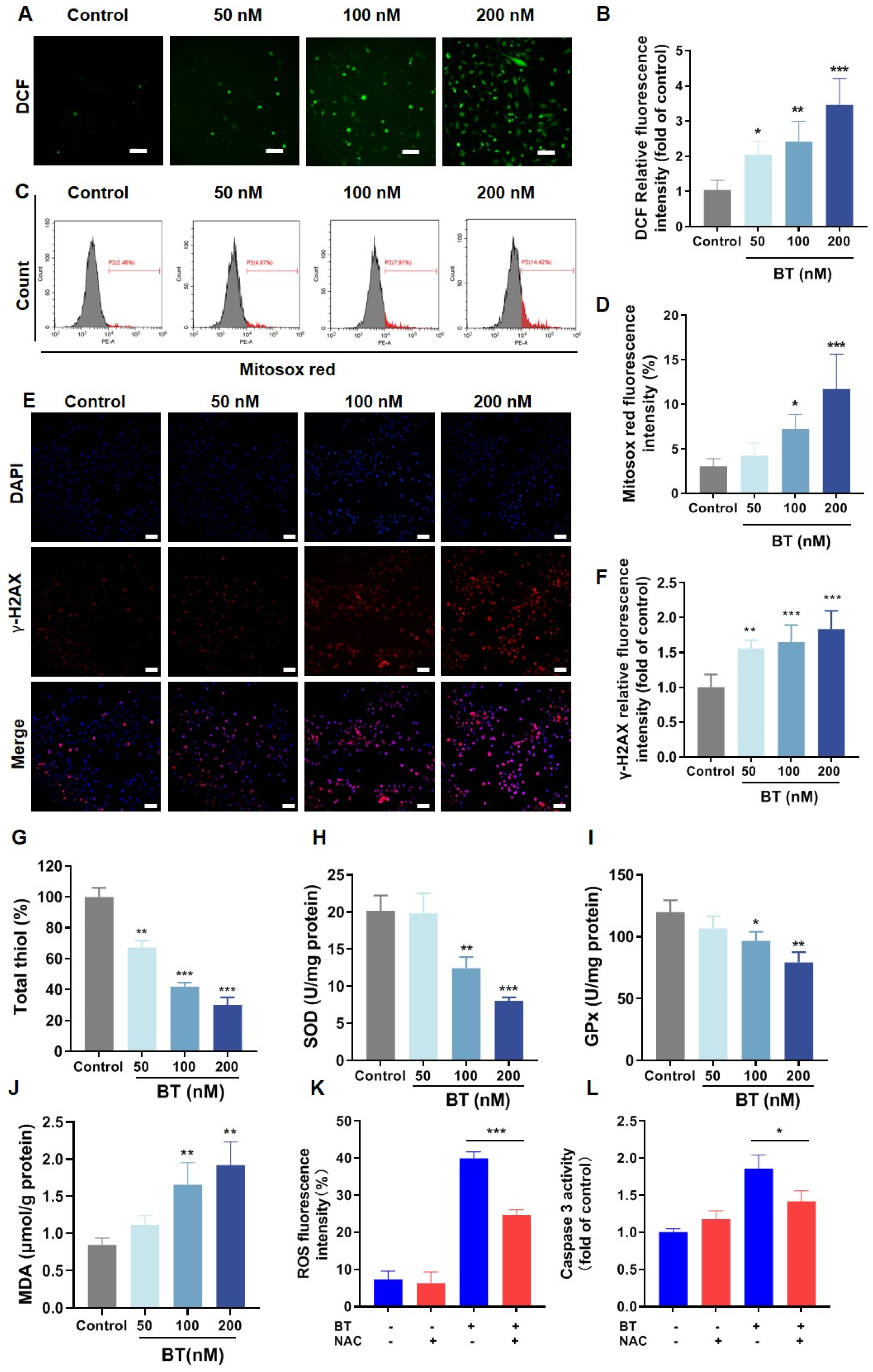
3.6. BT Induces Oxidative Stress in U251 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Smith, H.L.; Wadhwani, N.; Horbinski, C. Major Features of the 2021 WHO Classification of CNS Tumors. Neurotherapeutics 2022, 19, 1691–1704. [Google Scholar] [CrossRef]
- Wang, W.L.; Jiang, Z.R.; Hu, C.; Chen, C.; Hu, Z.Q.; Wang, A.L.; Wang, L.; Liu, J.; Wang, W.C.; Liu, Q.S. Pharmacologically inhibiting phosphoglycerate kinase 1 for glioma with NG52. Acta Pharmacol. Sin. 2021, 42, 633–640. [Google Scholar] [CrossRef]
- Wang, J.; Qi, Q.; Zhou, W.; Feng, Z.; Huang, B.; Chen, A.; Zhang, D.; Li, W.; Zhang, Q.; Jiang, Z.; et al. Inhibition of glioma growth by flavokawain B is mediated through endoplasmic reticulum stress induced autophagy. Autophagy 2018, 14, 2007–2022. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma targeted therapy: Insight into future of molecular approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef]
- Davis, M.E. Epidemiology and Overview of Gliomas. Semin. Oncol. Nurs. 2018, 34, 420–429. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Wang, K.; Lu, J.; Bao, X.; Wang, R.; Qiu, Y.; Wang, T.; Yu, H. Cellular senescence and cancer: Focusing on traditional Chinese medicine and natural products. Cell Prolif. 2020, 53, e12894. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, J.; Zuo, F.; Guo, J.; Sun, X.; Liu, D.; Liu, C. Traditional Chinese Medicine has great potential as candidate drugs for lung cancer: A review. J. Ethnopharmacol. 2023, 300, 115748. [Google Scholar] [CrossRef]
- Zhan, X.; Wu, H.; Wu, H.; Wang, R.; Luo, C.; Gao, B.; Chen, Z.; Li, Q. Metabolites from Bufo gargarizans (Cantor, 1842): A review of traditional uses, pharmacological activity, toxicity and quality control. J. Ethnopharmacol. 2020, 246, 112178. [Google Scholar] [CrossRef]
- Jia, J.; Li, J.; Zheng, Q.; Li, D. A research update on the antitumor effects of active components of Chinese medicine ChanSu. Front. Oncol. 2022, 12, 1014637. [Google Scholar] [CrossRef]
- Zhu, Y.R.; Xu, Y.; Fang, J.F.; Zhou, F.; Deng, X.W.; Zhang, Y.Q. Bufotalin-induced apoptosis in osteoblastoma cells is associated with endoplasmic reticulum stress activation. Biochem. Biophys. Res. Commun. 2014, 451, 112–118. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, S.; Li, J.; Jiang, C.; Ye, M.; Hu, H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis 2011, 16, 394–403. [Google Scholar] [CrossRef]
- Lin, S.; Lv, J.; Peng, P.; Cai, C.; Deng, J.; Deng, H.; Li, X.; Tang, X. Bufadienolides induce p53-mediated apoptosis in esophageal squamous cell carcinoma cells in vitro and in vivo. Oncol. Lett. 2018, 15, 1566–1572. [Google Scholar] [CrossRef]
- Zhang, D.M.; Liu, J.S.; Tang, M.K.; Yiu, A.; Cao, H.H.; Jiang, L.; Chan, J.Y.; Tian, H.Y.; Fung, K.P.; Ye, W.C. Bufotalin from Venenum Bufonis inhibits growth of multidrug resistant HepG2 cells through G2/M cell cycle arrest and apoptosis. Eur. J. Pharmacol. 2012, 692, 19–28. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, B.; Liu, Y.; Xu, L.; Wan, M. Bufotalin induces ferroptosis in non-small cell lung cancer cells by facilitating the ubiquitination and degradation of GPX4. Free Radic. Biol. Med. 2022, 180, 75–84. [Google Scholar] [CrossRef]
- Pan, Z.; Qu, C.; Chen, Y.; Chen, X.; Liu, X.; Hao, W.; Xu, W.; Ye, L.; Lu, P.; Li, D.; et al. Bufotalin induces cell cycle arrest and cell apoptosis in human malignant melanoma A375 cells. Oncol. Rep. 2019, 41, 2409–2417. [Google Scholar] [CrossRef]
- Yu, C.-L.; Hou, H.-M. Plasma pharmacokinetics and tissue distribution of bufotalin in mice following single-bolus injection and constant-rate infusion of bufotalin solution. Eur. J. Drug Metab. Pharmacokinet. 2011, 35, 115–121. [Google Scholar] [CrossRef]
- Liang, S.; Hong, Y.; Qi, Y.; Sun, Q.; Zhu, X.; Wei, Y.; Xu, Y.; Chen, Q. Bufotalin enhances apoptosis and TMZ chemosensitivity of glioblastoma cells by promoting mitochondrial dysfunction via AKT signaling pathway. Aging 2024, 16, 9264–9279. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Ramovs, V.; te Molder, L.; Sonnenberg, A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 2017, 57–58, 213–243. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, L.; Zou, Y.; He, R.; Wu, Q.; Han, C.; Zhang, B. Reciprocal regulation of integrin β4 and KLF4 promotes gliomagenesis through maintaining cancer stem cell traits. J. Exp. Clin. Cancer Res. 2019, 38, 23. [Google Scholar] [CrossRef] [PubMed]
- Davis-Lunn, M.; Goult, B.T.; Andrews, M.R. Clutching at Guidance Cues: The Integrin–FAK Axis Steers Axon Outgrowth. Biology 2023, 12, 954. [Google Scholar] [CrossRef]
- Zhang, X.; Rozengurt, E.; Reed, E.F. HLA Class I Molecules Partner with Integrin β4 to Stimulate Endothelial Cell Proliferation and Migration. Sci. Signal. 2010, 3, ra85. [Google Scholar] [CrossRef] [PubMed]
- Savoia, P.; Fava, P.; Casoni, F.; Cremona, O. Targeting the ERK Signaling Pathway in Melanoma. Int. J. Mol. Sci. 2019, 20, 1483. [Google Scholar] [CrossRef]
- Yuan, M.; Xie, F.; Xia, X.; Zhong, K.; Lian, L.; Zhang, S.; Yuan, L.; Ye, J. UNC5C-knockdown enhances the growth and metastasis of breast cancer cells by potentiating the integrin α6/β4 signaling pathway. Int. J. Oncol. 2020, 56, 139–150. [Google Scholar] [CrossRef]
- Zhu, J.-F.; Liu, Y.; Huang, H.; Shan, L.; Han, Z.-G.; Liu, J.-Y.; Li, Y.-L.; Dong, X.; Zeng, W. MicroRNA-133b/EGFR axis regulates esophageal squamous cell carcinoma metastases by suppressing anoikis resistance and anchorage-independent growth. Cancer Cell Int. 2018, 18, 193. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Tay, E.X.Y.; Ong, D.S.T.; Taneja, R. Mitochondrial Dysfunction at the Center of Cancer Therapy. Antioxid. Redox Signal. 2020, 32, 309–330. [Google Scholar] [CrossRef]
- van Gisbergen, M.W.; Voets, A.M.; Starmans, M.H.W.; de Coo, I.F.M.; Yadak, R.; Hoffmann, R.F.; Boutros, P.C.; Smeets, H.J.M.; Dubois, L.; Lambin, P. How do changes in the mtDNA and mitochondrial dysfunction influence cancer and cancer therapy? Challenges, opportunities and models. Mutat. Res./Rev. Mutat. Res. 2015, 764, 16–30. [Google Scholar] [CrossRef]
- Dewangan, J.; Srivastava, S.; Mishra, S.; Pandey, P.K.; Divakar, A.; Rath, S.K. Chetomin induces apoptosis in human triple-negative breast cancer cells by promoting calcium overload and mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2018, 495, 1915–1921. [Google Scholar] [CrossRef]
- Su, L.; Xu, J.; Lu, C.; Gao, K.; Hu, Y.; Xue, C.; Yan, X. Nano-flow cytometry unveils mitochondrial permeability transition process and multi-pathway cell death induction for cancer therapy. Cell Death Discov. 2024, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Al-Faze, R.; Ahmed, H.A.; El-Atawy, M.A.; Zagloul, H.; Alshammari, E.M.; Jaremko, M.; Emwas, A.-H.; Nabil, G.M.; Hanna, D.H. Mitochondrial dysfunction route as a possible biomarker and therapy target for human cancer. Biomed. J. 2024, 100714. [Google Scholar] [CrossRef] [PubMed]
- Simón, L.; Arazo-Rusindo, M.; Quest, A.F.G.; Mariotti-Celis, M.S. Phlorotannins: Novel Orally Administrated Bioactive Compounds That Induce Mitochondrial Dysfunction and Oxidative Stress in Cancer. Antioxidants 2023, 12, 1734. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, G.; Yang, X.; Wang, M.; Wang, R.; Wan, M.; Wang, J.; Wu, B.; Yan, T.; Jia, Y. A network pharmacology approach and experimental validation to investigate the anticancer mechanism and potential active targets of ethanol extract of Wei-Tong-Xin against colorectal cancer through induction of apoptosis via PI3K/AKT signaling pathway. J. Ethnopharmacol. 2023, 303, 115933. [Google Scholar] [CrossRef]
- Tang, J.-H.; Yang, L.; Chen, J.-X.; Li, Q.-R.; Zhu, L.-R.; Xu, Q.-F.; Huang, G.-H.; Zhang, Z.-X.; Xiang, Y.; Du, L.; et al. Bortezomib inhibits growth and sensitizes glioma to temozolomide (TMZ) via down-regulating the FOXM1–Survivin axis. Cancer Commun. 2019, 39, 81. [Google Scholar] [CrossRef]
- Shukla, S.; Rizvi, F.; Raisuddin, S.; Kakkar, P. FoxO proteins’ nuclear retention and BH3-only protein Bim induction evoke mitochondrial dysfunction-mediated apoptosis in berberine-treated HepG2 cells. Free. Radic. Biol. Med. 2014, 76, 185–199. [Google Scholar] [CrossRef]
- Hwang, K.-E.; Kim, Y.-S.; Hwang, Y.-R.; Kwon, S.-J.; Park, D.-S.; Cha, B.-K.; Kim, B.-R.; Yoon, K.-H.; Jeong, E.-T.; Kim, H.-R. Enhanced apoptosis by pemetrexed and simvastatin in malignant mesothelioma and lung cancer cells by reactive oxygen species-dependent mitochondrial dysfunction and Bim induction. Int. J. Oncol. 2014, 45, 1769–1777. [Google Scholar] [CrossRef]
- Ham, J.; Yun, B.H.; Lim, W.; Song, G. Folpet induces mitochondrial dysfunction and ROS-mediated apoptosis in mouse Sertoli cells. Pestic. Biochem. Physiol. 2021, 177, 104903. [Google Scholar] [CrossRef]
- Taniai, T.; Shirai, Y.; Shimada, Y.; Hamura, R.; Yanagaki, M.; Takada, N.; Horiuchi, T.; Haruki, K.; Furukawa, K.; Uwagawa, T.; et al. Inhibition of acid ceramidase elicits mitochondrial dysfunction and oxidative stress in pancreatic cancer cells. Cancer Sci. 2021, 112, 4570–4579. [Google Scholar] [CrossRef]
- Sledzinska, P.; Bebyn, M.G.; Furtak, J.; Kowalewski, J.; Lewandowska, M.A. Prognostic and Predictive Biomarkers in Gliomas. Int. J. Mol. Sci. 2021, 22, 373. [Google Scholar] [CrossRef]
- Li, F.J.; Hu, J.H.; Ren, X.; Zhou, C.M.; Liu, Q.; Zhang, Y.Q. Toad venom: A comprehensive review of chemical constituents, anticancer activities, and mechanisms. Arch. Pharm. 2021, 354, e2100060. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Ferrer, V.P.; Moura Neto, V.; Mentlein, R. Glioma infiltration and extracellular matrix: Key players and modulators. Glia 2018, 66, 1542–1565. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Giancotti, F.G. Targeting integrin β4 for cancer and anti-angiogenic therapy. Trends Pharmacol. Sci. 2007, 28, 506–511. [Google Scholar] [CrossRef]
- Tai, Y.-L.; Chu, P.-Y.; Lai, I.R.; Wang, M.-Y.; Tseng, H.-Y.; Guan, J.-L.; Liou, J.-Y.; Shen, T.-L. An EGFR/Src-dependent β4 integrin/FAK complex contributes to malignancy of breast cancer. Sci. Rep. 2015, 5, 16408. [Google Scholar] [CrossRef]
- Gupta, N.; Srivastava, S.K. Atovaquone Suppresses the Growth of Metastatic Triple-Negative Breast Tumors in Lungs and Brain by Inhibiting Integrin/FAK Signaling Axis. Pharmaceuticals 2021, 14, 521. [Google Scholar] [CrossRef]
- Ewings, K.E.; Wiggins, C.M.; Cook, S.J. Bim and the Pro-Survival Bcl-2 Proteins: Opposites Attract, ERK Repels. Cell Cycle 2007, 6, 2236–2240. [Google Scholar] [CrossRef]
- Clybouw, C.; Merino, D.; Nebl, T.; Masson, F.; Robati, M.; O’Reilly, L.; Hübner, A.; Davis, R.J.; Strasser, A.; Bouillet, P. Alternative splicing of Bim and Erk-mediated BimEL phosphorylation are dispensable for hematopoietic homeostasis in vivo. Cell Death Differ. 2012, 19, 1060–1068. [Google Scholar] [CrossRef]
- Feng, Y.; Li, T.; Li, Y.; Lin, Z.; Han, X.; Pei, X.; Zhang, Y.; Li, F.; Yang, J.; Shao, D.; et al. Glutaredoxin-1 promotes lymphangioleiomyomatosis progression through inhibiting Bim-mediated apoptosis via COX2/PGE2/ERK pathway. Clin. Transl. Med. 2023, 13, e1333. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Tu, H.-C.; Kim, H.; Wang, G.X.; Bean, G.R.; Takeuchi, O.; Jeffers, J.R.; Zambetti, G.P.; Hsieh, J.J.-D.; Cheng, E.H.-Y. BID, BIM, and PUMA Are Essential for Activation of the BAX- and BAK-Dependent Cell Death Program. Science 2010, 330, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Gogvadze, V.; Norberg, E.; Orrenius, S.; Zhivotovsky, B. Involvement of Ca2+ and ROS in α-tocopheryl succinate-induced mitochondrial permeabilization. Int. J. Cancer 2010, 127, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Andrieux, P.; Chevillard, C.; Cunha-Neto, E.; Nunes, J.P.S. Mitochondria as a Cellular Hub in Infection and Inflammation. Int. J. Mol. Sci. 2021, 22, 11338. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Zanders, E.D.; Svensson, F.; Bailey, D.S. Therapy for glioblastoma: Is it working? Drug Discov. Today 2019, 24, 1193–1201. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Lin, G.; Zhang, R.; Wen, Y.; Luo, C.; Wang, R.; Wang, F.; Peng, S.; Zhang, J. Bufotalin Induces Oxidative Stress-Mediated Apoptosis by Blocking the ITGB4/FAK/ERK Pathway in Glioblastoma. Antioxidants 2024, 13, 1179. https://doi.org/10.3390/antiox13101179
Tan J, Lin G, Zhang R, Wen Y, Luo C, Wang R, Wang F, Peng S, Zhang J. Bufotalin Induces Oxidative Stress-Mediated Apoptosis by Blocking the ITGB4/FAK/ERK Pathway in Glioblastoma. Antioxidants. 2024; 13(10):1179. https://doi.org/10.3390/antiox13101179
Chicago/Turabian StyleTan, Junchao, Guoqiang Lin, Rui Zhang, Yuting Wen, Chunying Luo, Ran Wang, Feiyun Wang, Shoujiao Peng, and Jiange Zhang. 2024. "Bufotalin Induces Oxidative Stress-Mediated Apoptosis by Blocking the ITGB4/FAK/ERK Pathway in Glioblastoma" Antioxidants 13, no. 10: 1179. https://doi.org/10.3390/antiox13101179





