Effects of Astaxanthin on the Physiological State of Porcine Ovarian Granulose Cells Cultured In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Porcine GCs and Porcine GCs Were Treated with AST
2.2. Immunofluorescent Staining
2.3. RNA Isolation, Reverse Transcription, and Quantitative RT-PCR
2.4. Annexin V-FITC/PI Staining and Flow Cytometry
2.5. ROS Assay
2.6. Determination of the Mitochondrial Membrane Potential
2.7. ELISA for Estrogen and Progesterone Levels
2.8. EdU Staining Proliferation
2.9. Statistical Analysis
3. Result
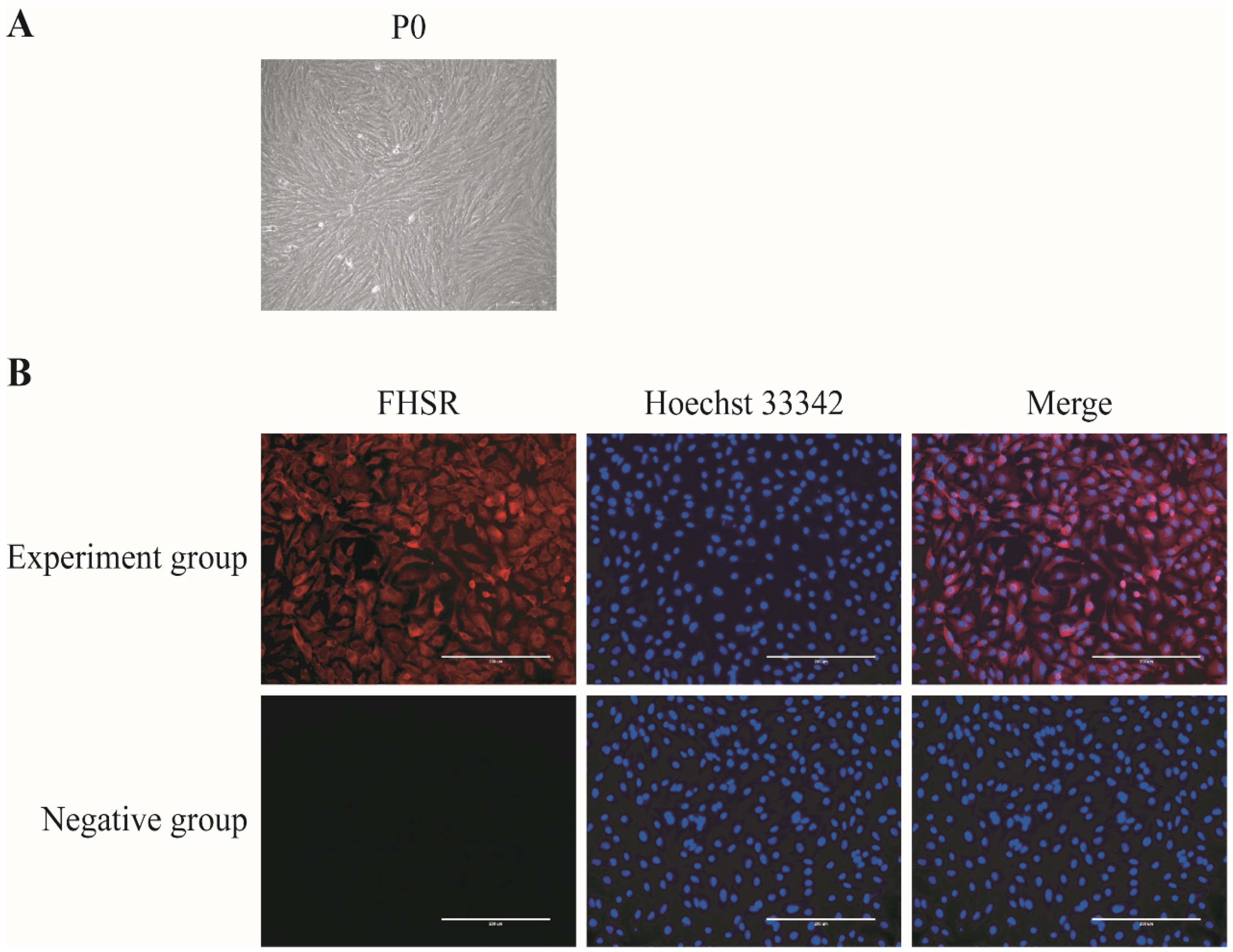
3.1. Isolation, Culture, and Identification of Porcine GCs
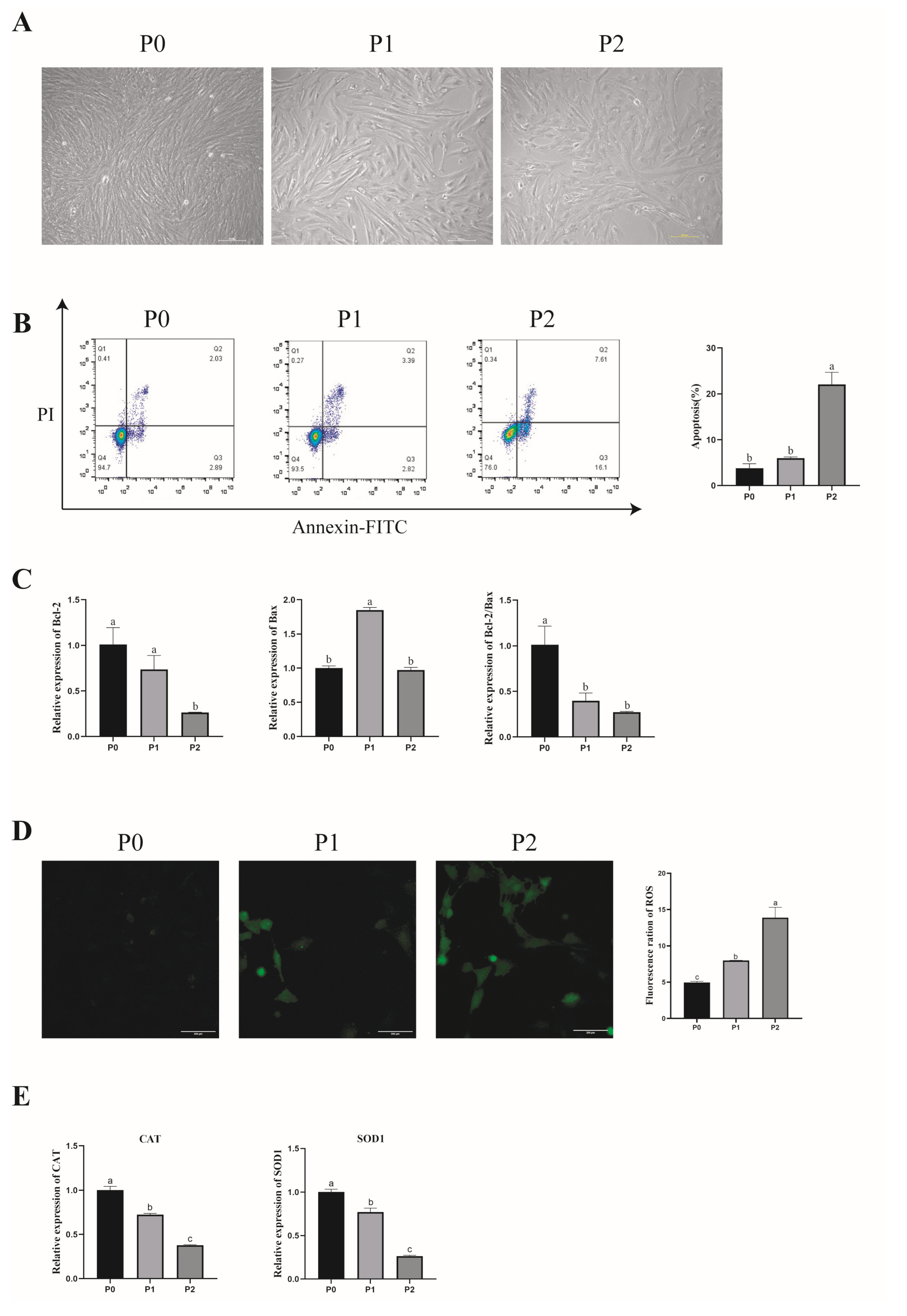
3.2. Effect of In Vitro Subculture of Porcine GCs on Their Morphology and Apoptosis
3.3. Effect of In Vitro Subculture of Porcine GCs on Antioxidant Capacity
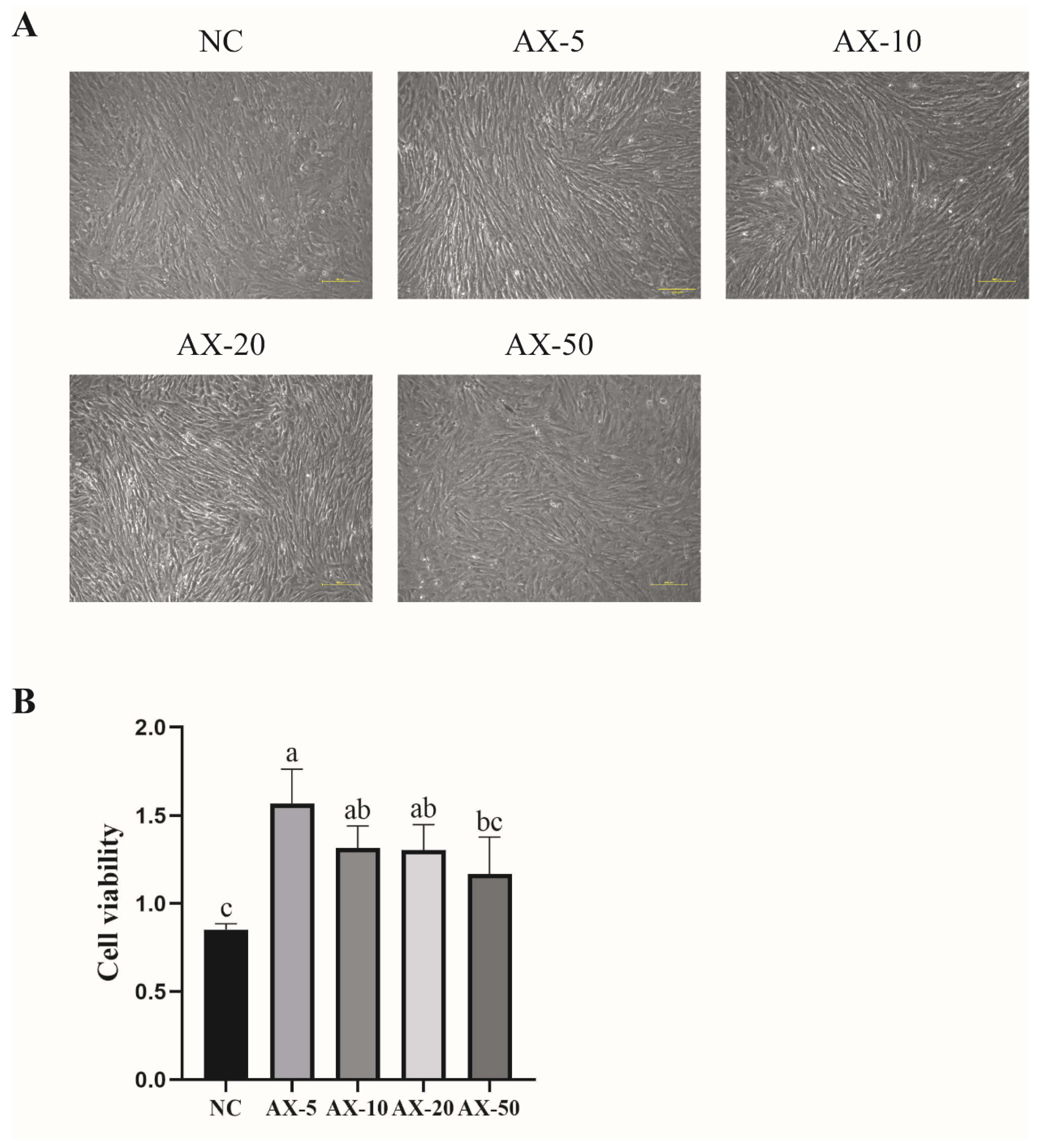
3.4. Effects of Different Concentrations of Astaxanthin on the Morphology and Viability of Porcine GCs
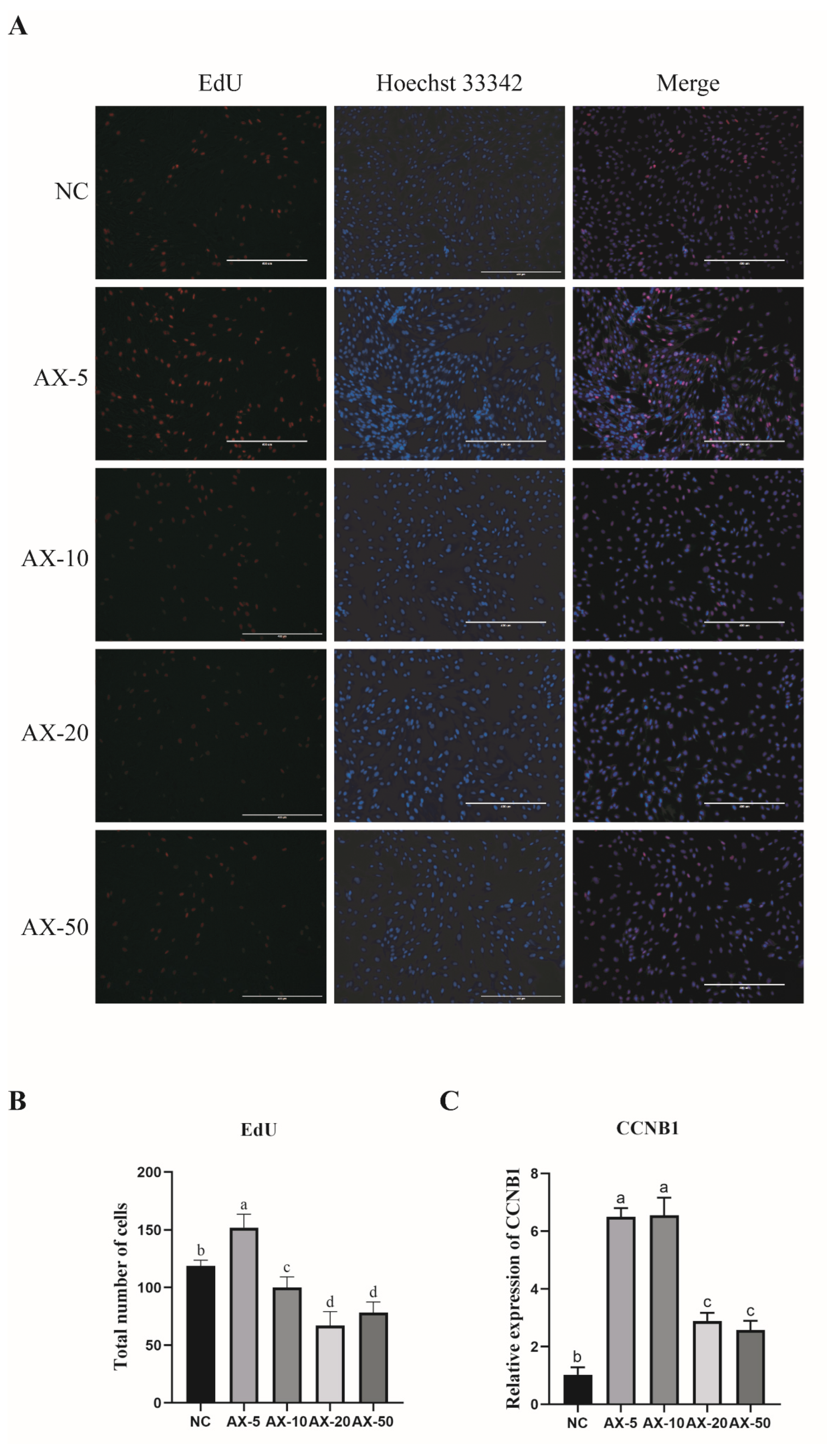
3.5. Effect of Adding Different Concentrations of Astaxanthin on the Proliferation of Porcine GCs
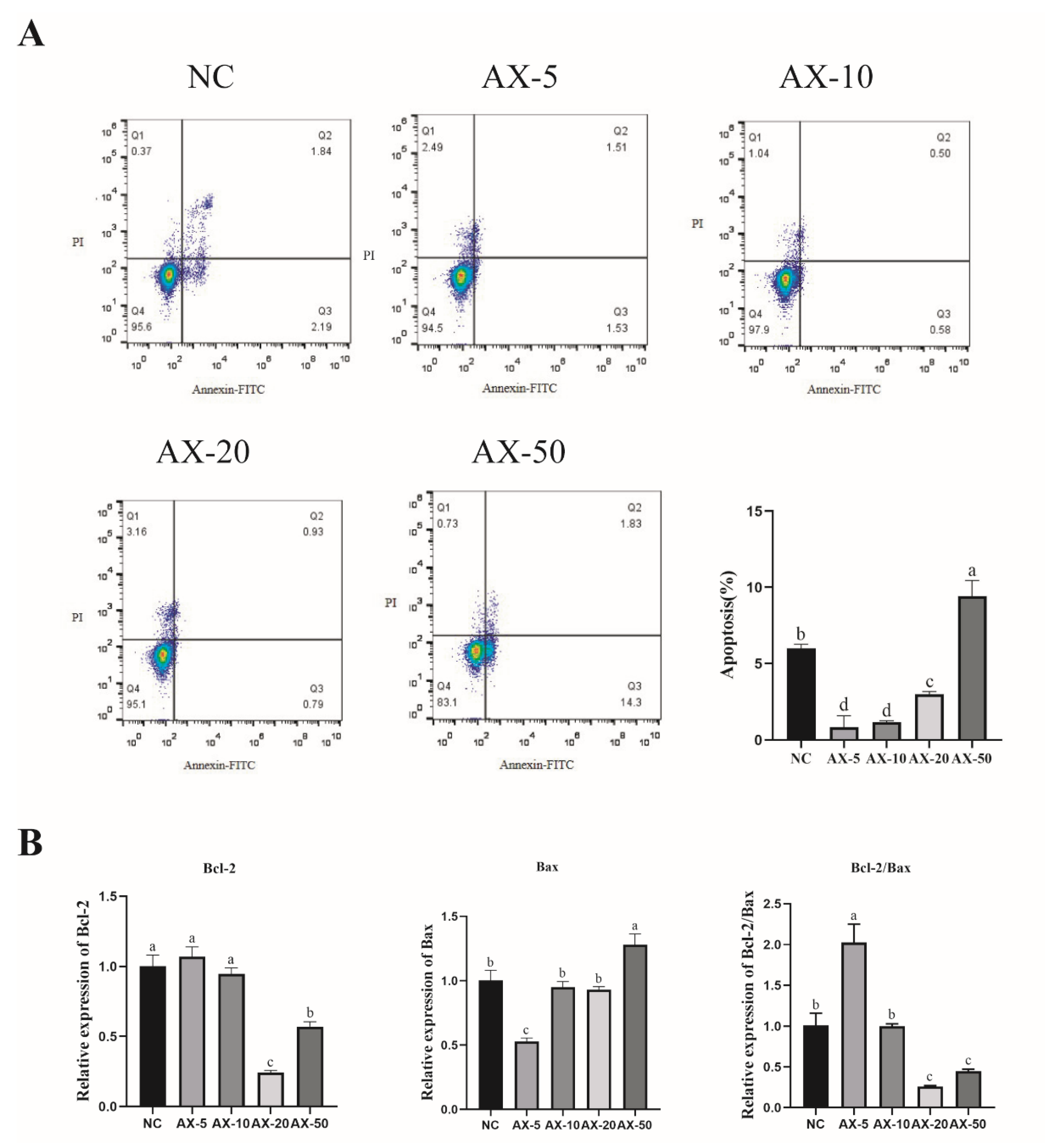
3.6. Effect of Different Concentrations of AST on Apoptosis of Porcine GCs
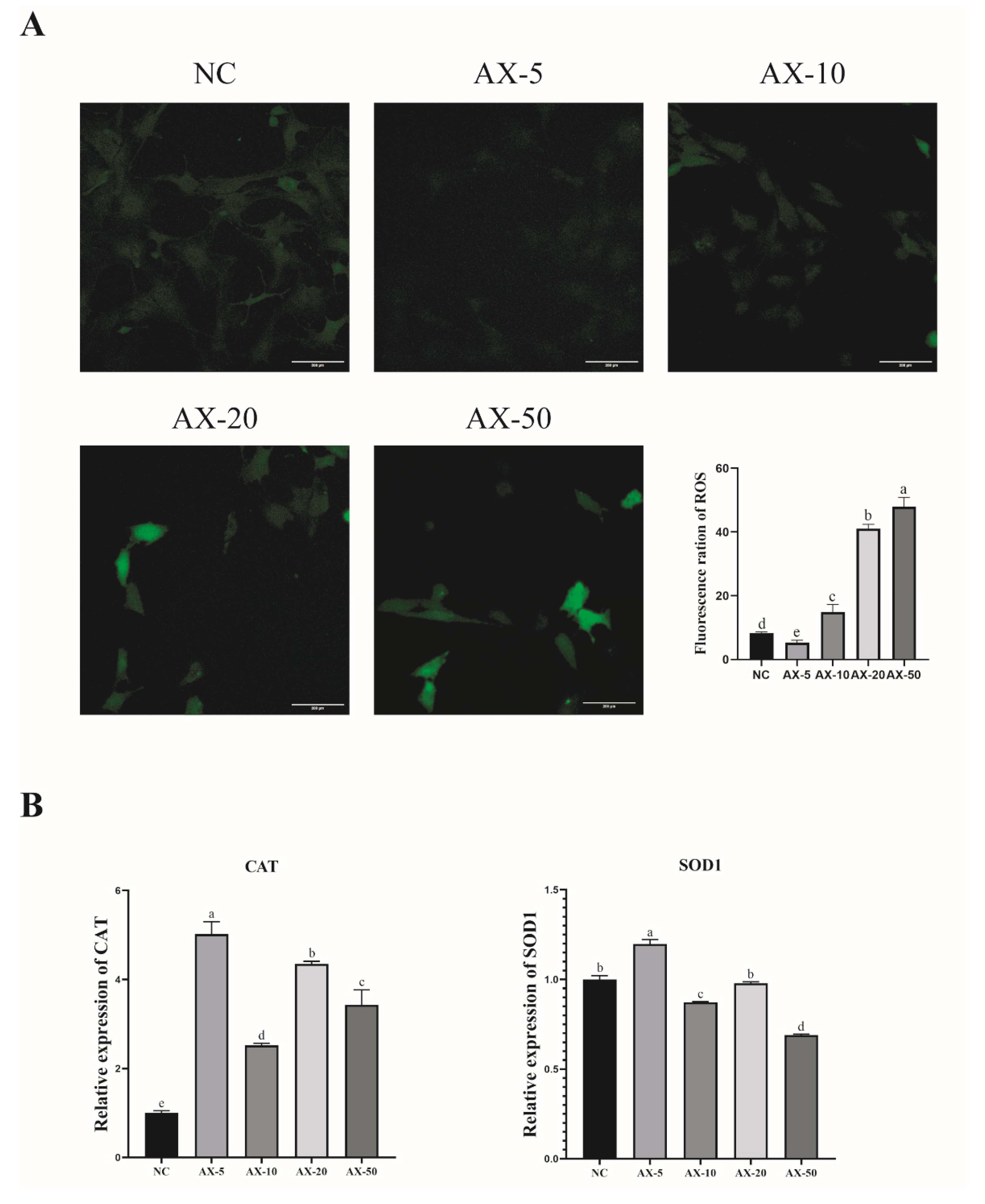
3.7. Effect of Different Concentrations of Astaxanthin on the Antioxidant Properties of Porcine GCs
3.8. Effect of Astaxanthin on the Mitochondrial Function of Porcine GCs
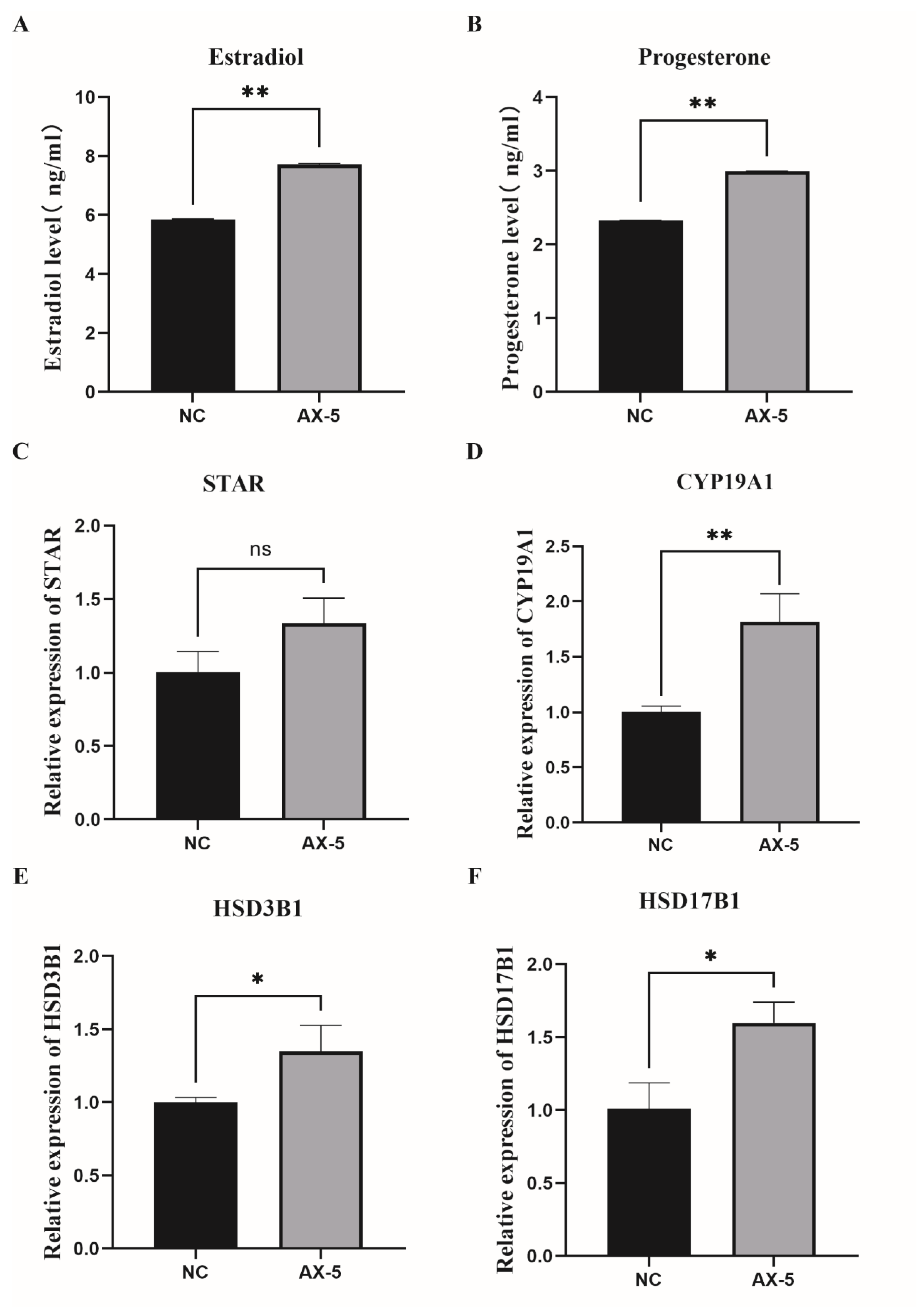
3.9. Hormone Levels in the Astaxanthin of Porcine GCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, X.; Wang, L.; Li, Q.; Wu, W.; Shang, P.; Chamba, Y.; Pan, Z.; Li, Q. miR-130a/TGF-β1 axis is involved in sow fertility by controlling granulosa cell apoptosis. Theriogenology 2020, 157, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Li, M.Q.; Zheng, H.Y.; Yang, C.Y.; Yan, S.F.; Yu, N.Q.; Huang, J.X.; Shang, J.H. Developmental competence of buffalo (Bubalus bubalis) denuded oocytes cocultured with cumulus cells: Protective role of cumulus cells. Theriogenology 2018, 120, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Alemu, T.W.; Pandey, H.O.; Salilew Wondim, D.; Gebremedhn, S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 2018, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Kamada, Y.; Hosoya, T.; Fujita, S.; Nishiyama, Y.; Iwata, N.; Hiramatsu, Y.; Otsuka, F. A regulatory role of androgen in ovarian steroidogenesis by rat granulosa cells. J. Steroid Biochem. Mol. Biol. 2017, 172, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Kulus, M.; Stefańska, K.; Kranc, W.; Chermuła, B.; Bryl, R.; Pieńkowski, W.; Nawrocki, M.J.; Petitte, J.N.; Stelmach, B. Human granulosa cells—Stemness properties, molecular cross-talk and follicular angiogenesis. Cells 2021, 10, 1396. [Google Scholar] [CrossRef]
- Kossowska-Tomaszczuk, K.; De Geyter, C. Cells with stem cell characteristics in somatic compartments of the ovary. BioMed Res. Int. 2013, 2013, 310859. [Google Scholar] [CrossRef]
- Nosratpour, S.; Ndiaye, K. Ankyrin-repeat and SOCS box-containing protein 9 (ASB9) regulates ovarian granulosa cells function and MAPK signaling. Mol. Reprod. Dev. 2021, 88, 830–843. [Google Scholar] [CrossRef]
- Chauvin, S.; Cohen-Tannoudji, J.; Guigon, C.J. Estradiol signaling at the heart of folliculogenesis: Its potential deregulation in human ovarian pathologies. Int. J. Mol. Sci. 2022, 23, 512. [Google Scholar] [CrossRef] [PubMed]
- Sreerangaraja Urs, D.B.; Wu, W.-H.; Komrskova, K.; Postlerova, P.; Lin, Y.-F.; Tzeng, C.-R.; Kao, S.-H. Mitochondrial function in modulating human granulosa cell steroidogenesis and female fertility. Int. J. Mol. Sci. 2020, 21, 3592. [Google Scholar] [CrossRef]
- Taugourdeau, A.; Desquiret-Dumas, V.; Hamel, J.-F.; Chupin, S.; Boucret, L.; Ferré-L’Hotellier, V.; Bouet, P.-E.; Descamps, P.; Procaccio, V.; Reynier, P. The mitochondrial DNA content of cumulus cells may help predict embryo implantation. J. Assist. Reprod. Genet. 2019, 36, 223–228. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, X.; Yuan, Y.; Gao, Y.; Li, X.; Du, R.; Xu, S.; Zhang, R.; Wang, C. Gene expression of granulosa and cumulus cells: The prospect in predicting the quality and developmental competence of oocytes in vitro maturation. Biocell 2020, 44, 487. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010, 139, 685. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Swelum, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Abdo, M. Cellular and functional adaptation to thermal stress in ovarian granulosa cells in mammals. J. Therm. Biol. 2020, 92, 102688. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Zhou, Y.; Lu, Y.; Zhao, C.; Qiu, W.; Chen, J.; Ju, R. Lipotoxicity Impairs Granulosa Cell Function Through Activated Endoplasmic Reticulum Stress Pathway. Reprod. Sci. 2020, 27, 119–131. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.; Luo, M.; Sun, Y.; Wang, G. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulosa cells. Cell Stress Chaperones 2016, 21, 467–475. [Google Scholar] [CrossRef]
- de Barros, F.R.O.; Paula-Lopes, F.F. Cellular and epigenetic changes induced by heat stress in bovine preimplantation embryos. Mol. Reprod. Dev. 2018, 85, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Naqvi, S.R.Z.; Abdelnour, S.A.; Schreurs, N.; Mohammedsaleh, Z.M.; Khan, I.; Shater, A.F.; Abd El-Hack, M.E.; Khafaga, A.F.; Quan, G.; et al. Beneficial effects and health benefits of Astaxanthin molecules on animal production: A review. Res. Vet. Sci. 2021, 138, 69–78. [Google Scholar] [CrossRef]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Rostami, S.; Nekoonam, S.; Rashidi, Z.; Sobhani, A.; Amidi, F. The Effect of Astaxanthin and Metformin on Oxidative Stress in Granulosa Cells of BALB C Mouse Model of Polycystic Ovary Syndrome. Reprod. Sci. 2021, 28, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Dong, F.; Shen, Z.; Wu, H.; Cen, C.; Cui, X.; Bao, S.; Gao, F. PRMT5 regulates ovarian follicle development by facilitating Wt1 translation. Elife 2021, 10, e68930. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Cai, S.; Wang, X.; Li, H.; Zhang, L.; Li, H.; Xiang, W. RNA binding protein IGF2BP1 meditates oxidative stress-induced granulosa cell dysfunction by regulating MDM2 mRNA stability in an m6A-dependent manner. Redox Biol. 2022, 57, 102492. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Hu, Y.; Song, X.; Huang, L.; Zhang, L.; Zhou, X.; Gao, L.; Pang, W.; Yang, G.; Chu, G. Totipotency of miR-184 in porcine granulosa cells. Mol. Cell. Endocrinol. 2022, 558, 111765. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, T.; Guo, Y.; Sun, T.; Li, H.; Zhang, X.; Yin, H.; Cao, G.; Yin, Y.; Wang, H. Oocyte stage-specific effects of MTOR determine granulosa cell fate and oocyte quality in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E5326–E5333. [Google Scholar] [CrossRef]
- Costermans, N.G.; Keijer, J.; van Schothorst, E.M.; Kemp, B.; Keshtkar, S.; Bunschoten, A.; Soede, N.M.; Teerds, K.J. In ovaries with high or low variation in follicle size, granulosa cells of antral follicles exhibit distinct size-related processes. Mol. Hum. Reprod. 2019, 25, 614–624. [Google Scholar] [CrossRef]
- Meng, L.; Teerds, K.; Tao, J.; Wei, H.; Jaklofsky, M.; Zhao, Z.; Liang, Y.; Li, L.; Wang, C.C.; Zhang, S. Characteristics of circular RNA expression profiles of porcine granulosa cells in healthy and atretic antral follicles. Int. J. Mol. Sci. 2020, 21, 5217. [Google Scholar] [CrossRef]
- Son, H.N.; Chi, H.N.Q.; Chung, D.C.; Long, L.T. Morphological changes during replicative senescence in bovine ovarian granulosa cells. Cell Cycle 2019, 18, 1490–1497. [Google Scholar] [CrossRef]
- Regan, S.L.; Knight, P.G.; Yovich, J.L.; Arfuso, F.; Dharmarajan, A. Granulosa cell apoptosis in the ovarian follicle—A changing view. Front. Endocrinol. 2018, 9, 326746. [Google Scholar] [CrossRef]
- Esencan, E.; Beroukhim, G.; Seifer, D.B. Age-related changes in Folliculogenesis and potential modifiers to improve fertility outcomes-A narrative review. Reprod. Biol. Endocrinol. 2022, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, G.S.; Carvalho, K.C.; Ferreira, C.D.S.; Alvarez, P.A.C.; Monteleone, P.A.A.; Baracat, E.C.; Soares, J.M., Jr. Granulosa cells and follicular development: A brief review. Rev. Assoc. Méd. Bras. 2023, 69, e20230175. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jia, Y.; Meng, S.; Luo, Y.; Yang, Q.; Pan, Z. Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review. Int. J. Mol. Sci. 2023, 24, 9205. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.; Esfandyari, S.; Aghahosseini, M.; Rashidi, Z.; Hosseinishental, S.; Brenjian, S.; Sobhani, A.; Amidi, F. Astaxanthin protects human granulosa cells against oxidative stress through activation of NRF2/ARE pathway and its downstream phase II enzymes. Cell J. 2021, 23, 319. [Google Scholar]
- Merhan, O. The biochemistry and antioxidant properties of carotenoids. Carotenoids 2017, 5, 51. [Google Scholar]
- Sathasivam, R.; Ki, J.-S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Naguib, Y.M. A fluorometric method for measurement of peroxyl radical scavenging activities of lipophilic antioxidants. Anal. Biochem. 1998, 265, 290–298. [Google Scholar] [CrossRef]
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38. [Google Scholar]
- Jabarpour, M.; Aleyasin, A.; Nashtaei, M.S.; Lotfi, S.; Amidi, F. Astaxanthin treatment ameliorates ER stress in polycystic ovary syndrome patients: A randomized clinical trial. Sci. Rep. 2023, 13, 3376. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Liu, H.; Pan, Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrinol. 2019, 17, 9. [Google Scholar] [CrossRef]
- Hormozi, M.; Ghoreishi, S.; Baharvand, P. Astaxanthin induces apoptosis and increases activity of antioxidant enzymes in LS-180 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, Z.; Liu, S.; Gao, F.; Zhang, J.; Peng, Z.; Wang, L.; Pan, X. Astaxanthin improves the development of the follicles and oocytes through alleviating oxidative stress induced by BPA in cultured follicles. Sci. Rep. 2022, 12, 7853. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. Mitochondrial Bioenerg. Methods Protoc. 2012, 810, 183–205. [Google Scholar]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Qiang, J.; Tao, Y.-F.; Lu, S.-Q.; Ma, J.-L.; He, J.; Xu, P. Role of Astaxanthin as a Stimulator of Ovarian Development in Nile Tilapia (Oreochromis niloticus) and Its Potential Regulatory Mechanism: Ameliorating Oxidative Stress and Apoptosis. Aquac. Nutr. 2022, 2022, 1245151. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.-Y.; Xiang, D.-C.; Shao, Q.-Y.; Zhang, B.; Liu, S.-N.; Hong, Q.-H.; Quan, G.-B.; Wu, G.-Q. Inhibitory effects of astaxanthin on postovulatory porcine oocyte aging in vitro. Sci. Rep. 2020, 10, 20217. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxidative Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Mathison, B.; Hayek, M.; Zhang, J.; Reinhart, G.; Chew, B. Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dogs. J. Anim. Sci. 2013, 91, 268–275. [Google Scholar] [CrossRef]
- Chou, C.-H.; Chen, M.-J. The effect of steroid hormones on ovarian follicle development. Vitam. Horm. 2018, 107, 155–175. [Google Scholar]
- Li, X.-W.; Yi, B.-J.; Wang, Z.-Y.; Guo, K.; Saleem, M.A.U.; Ma, X.-Y.; Li, X.-N.; Li, J.-L. The ROS/SIRT1/STAR axis as a target for melatonin ameliorating atrazine-induced mitochondrial dysfunction and steroid disorders in granulosa cells. Ecotoxicol. Environ. Saf. 2024, 269, 115780. [Google Scholar] [CrossRef] [PubMed]
- Sechman, A.; Antos, P.; Katarzyńska, D.; Grzegorzewska, A.; Wojtysiak, D.; Hrabia, A. Effects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin on secretion of steroids and STAR, HSD3B and CYP19A1 mRNA expression in chicken ovarian follicles. Toxicol. Lett. 2014, 225, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Guo, Y.; Li, Y.; Jia, Q.; Han, X.; Liu, B.; Chen, J.; Cheng, J.C.; Sun, Y.P. Epigallocatechin-3-gallate stimulates StAR expression and progesterone production in human granulosa cells through the 67-kDa laminin receptor-mediated CREB signaling pathway. J. Cell. Physiol. 2022, 237, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, C.; Oktem, O. P–664 Bone morphogenetic protein–7 (BMP–7) reduces E2 and P4 production of human luteinized granulosa cells by down-regulating the expression of the steroidogenic enzymes StAR and 3B-HSD. Hum. Reprod. 2021, 36, deab130-663. [Google Scholar] [CrossRef]
- He, W.; Wang, H.; Tang, C.; Zhao, Q.; Zhang, J. Dietary supplementation with astaxanthin alleviates ovarian aging in aged laying hens by enhancing antioxidant capacity and increasing reproductive hormones. Poult. Sci. 2023, 102, 102258. [Google Scholar] [CrossRef]

| Genes | Primer Sequences | Length (bp) | Tm (°C) | Accession No. |
|---|---|---|---|---|
| β-actin | F: GATGACGATATTGCTGCGCT R: TTCTCCATGTCGTCCCAGTT | 248 | 60 | XM_021086047.1 |
| Bcl-2 | F: TGAGTTCGGTGGGGTCATGT R: GGCCCATACAGCTCCACAAAG | 159 | 60 | XM_021099593 |
| Bax | F: GGCCCTTTTGCTTCAGGGTTT R: GACACTCGCTCAACTTCTTGG | 119 | 60 | XM_003127290.5 |
| CCNB1 | F: CATCAATTACCTGGACCGCT R: CTGAGGCTTGATGGAGTTGT | 163 | 60 | NM_001170768.1 |
| SOD1 | F: ATTCTGTGATCGCCCTCTCG R: ACTTCCAGCATTTCCCGTCT | 125 | 60 | NM_001190422.1 |
| CAT | F: AGATGAAGCATTGGAAGGAGC R: TCTCAGGAATTCTCTCCCGGT | 162 | 60 | NM_214301.2 |
| STAR | F: AAAGTGATCCCTGACGTGGG R: CGTGAGTGATGACCGTGTCT | 175 | 60 | NM_213755.2 |
| CYP19A1 | F: GGCTATGTGGACGTGTTGACC R: TGAGAAGGAGAGCTTGCCATG | 164 | 60 | NM_214429.1 |
| HSD3B1 | F: CAGCCAGGTATGGCCGAC R: CGGACTACATGTTCCCCCAG | 89 | 60 | NM_001004049.2 |
| HSD17B1 | F: AGTCCTTGGCTTACCAACCG R: TTCTGCATTGGAACCCCTCC | 177 | 60 | NM_001128472.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhou, D.; Gao, L.; Wang, Y.; Wang, Y.; Jia, R.; Bai, Y.; Shi, D.; Lu, F. Effects of Astaxanthin on the Physiological State of Porcine Ovarian Granulose Cells Cultured In Vitro. Antioxidants 2024, 13, 1185. https://doi.org/10.3390/antiox13101185
Yang X, Zhou D, Gao L, Wang Y, Wang Y, Jia R, Bai Y, Shi D, Lu F. Effects of Astaxanthin on the Physiological State of Porcine Ovarian Granulose Cells Cultured In Vitro. Antioxidants. 2024; 13(10):1185. https://doi.org/10.3390/antiox13101185
Chicago/Turabian StyleYang, Xiaofen, Dongping Zhou, Lv Gao, Yanxin Wang, Yun Wang, Ruru Jia, Yuwei Bai, Deshun Shi, and Fenghua Lu. 2024. "Effects of Astaxanthin on the Physiological State of Porcine Ovarian Granulose Cells Cultured In Vitro" Antioxidants 13, no. 10: 1185. https://doi.org/10.3390/antiox13101185






