Lactobacillus Eats Amyloid Plaque and Post-Biotically Attenuates Senescence Due to Repeat Expansion Disorder and Alzheimer’s Disease
Abstract
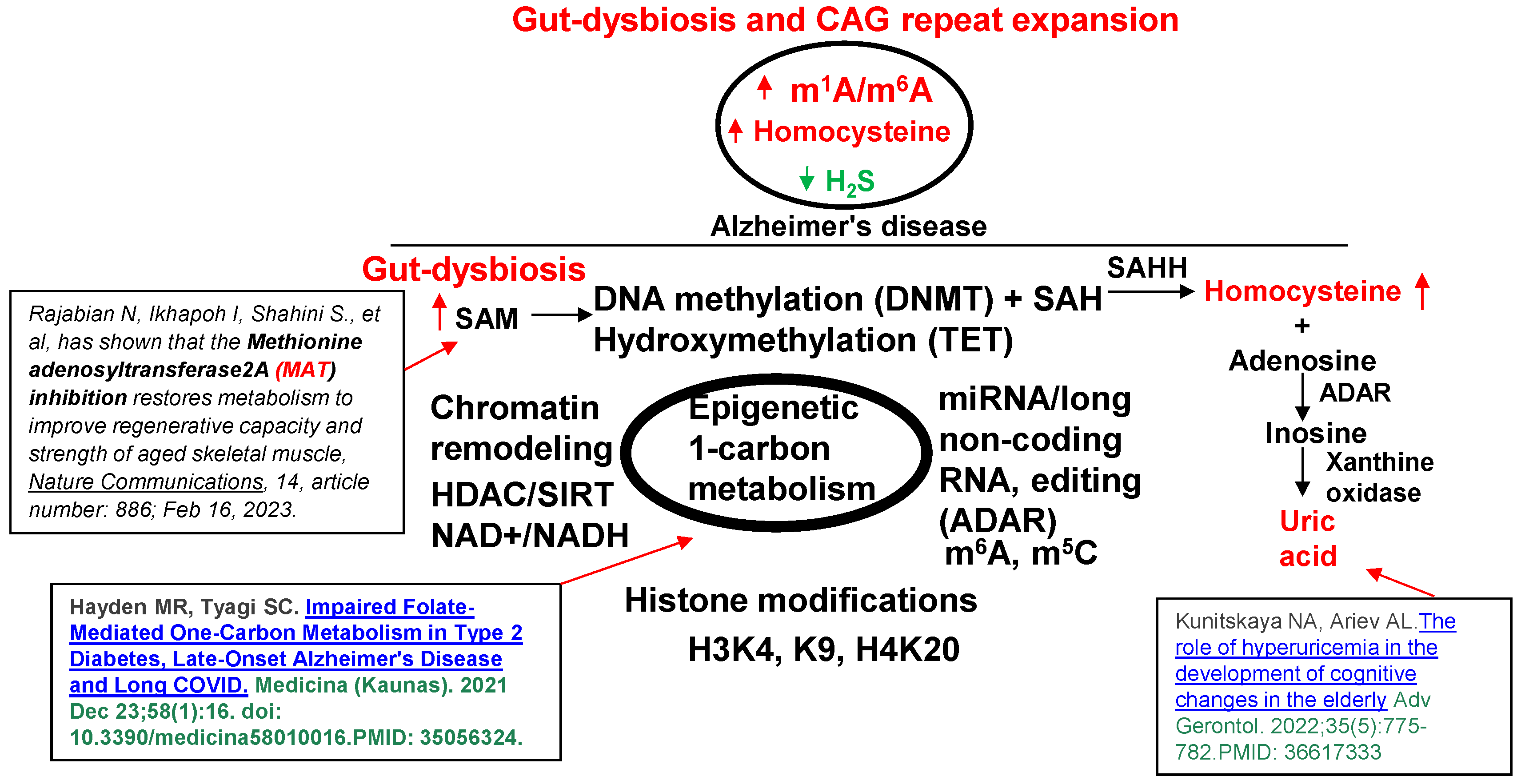
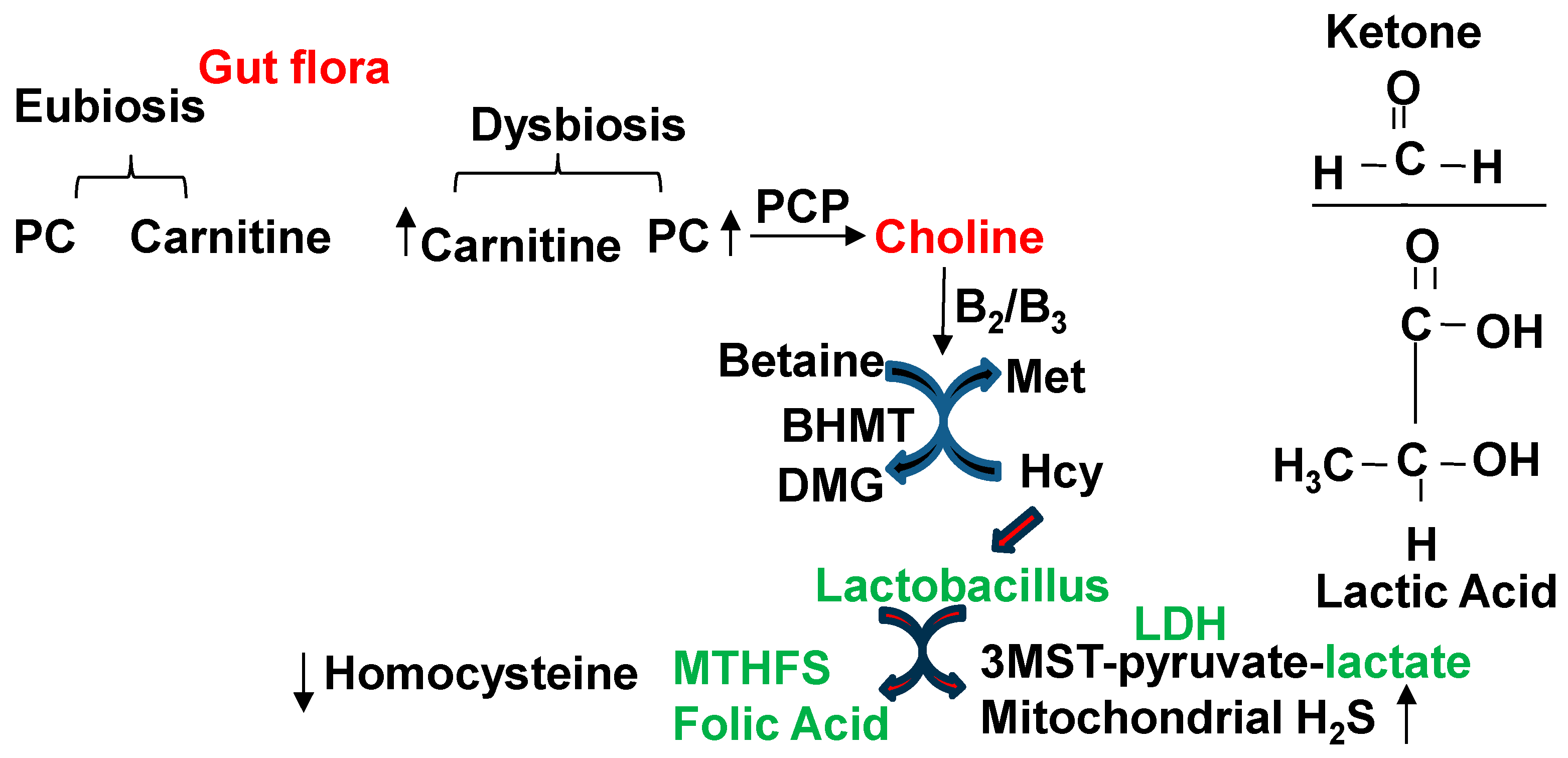
1. Introduction
2. Conclusions and Future Directions
Funding
Conflicts of Interest
Abbreviations
References
- Veeranki, S.; Tyagi, S.C. Dysbiosis and Disease: Many Unknown Ends, Is It Time to Formulate Guidelines for Dysbiosis Research? J. Cell. Physiol. 2016, 232, 2929–2930. [Google Scholar] [CrossRef] [PubMed]
- McCully, H. Pliny’s Pheromonic Abortifacients. Science 1969, 165, 236–237. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am. J. Pathol. 1969, 56, 111–128. [Google Scholar]
- Davidson, Y.S.; Raby, S.; Foulds, P.G.; Robinson, A.; Thompson, J.C.; Sikkink, S.; Yusuf, I.; Amin, H.; DuPlessis, D.; Troakes, C.; et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s Syndrome: Association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011, 122, 703–713. [Google Scholar] [CrossRef]
- Lippa, C.F.; Rosso, A.L.; Stutzbach, L.D.; Neumann, M.; Lee, V.M.-Y.; Trojanowski, J.Q. Transactive Response DNA-Binding Protein 43 Burden in Familial Alzheimer Disease and Down Syndrome. Arch. Neurol. 2009, 66, 1483–1488. [Google Scholar] [CrossRef]
- Ijiri, K.; Zerbini, L.F.; Peng, H.; Correa, R.G.; Lu, B.; Walsh, N.; Zhao, Y.; Taniguchi, N.; Huang, X.-L.; Otu, H.; et al. A Novel Role for GADD45β as a Mediator of MMP-13 Gene Expression during Chondrocyte Terminal Differentiation. J. Biol. Chem. 2005, 280, 38544–38555. [Google Scholar] [CrossRef]
- Bian, Z.; Liu, X.; Feng, T.; Yu, H.; Hu, X.; Hu, X.; Bian, Y.; Sun, H.; Tadokoro, K.; Takemoto, M.; et al. Protective Effect of Rivaroxaban Against Amyloid Pathology and Neuroinflammation Through Inhibiting PAR-1 and PAR-2 in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2022, 86, 111–123. [Google Scholar] [CrossRef]
- Shirafuji, N.; Hamano, T.; Yen, S.-H.; Kanaan, N.M.; Yoshida, H.; Hayashi, K.; Ikawa, M.; Yamamura, O.; Kuriyama, M.; Nakamoto, Y. Homocysteine Increases Tau Phosphorylation, Truncation and Oligomerization. Int. J. Mol. Sci. 2018, 19, 891. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Yu, M.; Zhang, X.; Zhang, M.; Wilson, J.X.; Huang, G. Folic acid administration inhibits amyloid β-peptide accumulation in APP/PS1 transgenic mice. J. Nutr. Biochem. 2015, 26, 883–891. [Google Scholar] [CrossRef]
- Li, W.; Jiang, M.; Zhao, S.; Liu, H.; Zhang, X.; Wilson, J.X.; Huang, G. Folic Acid Inhibits Amyloid β-Peptide Production through Modulating DNA Methyltransferase Activity in N2a-APP Cells. Int. J. Mol. Sci. 2015, 16, 25002–25013. [Google Scholar] [CrossRef]
- Ali, R.; Hameed, R.; Chauhan, D.; Sen, S.; Wahajuddin, M.; Nazir, A.; Verma, S. Multiple Actions of H2S-Releasing Peptides in Human β-Amyloid Expressing C. elegans. ACS Chem. Neurosci. 2022, 13, 3378–3388. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, Y.; Cai, Z.; Heo, G.S.; Yuede, C.M.; Wang, Z.; Lin, K.; Saadi, F.; Trsan, T.; Nguyen, A.T.; et al. Antibody-mediated targeting of human microglial leukocyte Ig-like receptor B4 attenuates amyloid pathology in a mouse model. Sci. Transl. Med. 2024, 16, eadj9052. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Stražar, M.; Mohamed, A.M.; Pacheco, J.A.; Walker, R.L.; Lebar, T.; Zhao, S.; Lockart, J.; Dame, A.; Thurimella, K.; et al. Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria. Cell 2024, 187, 1834–1852.e19. [Google Scholar] [CrossRef] [PubMed]
- Benjannet, S.; Elagoz, A.; Wickham, L.; Mamarbachi, M.; Munzer, J.S.; Basak, A.; Lazure, C.; Cromlish, J.A.; Sisodia, S.; Checler, F.; et al. Post-translational Processing of β-Secretase (β-Amyloid-converting Enzyme) and Its Ectodomain Shedding. J. Biol. Chem. 2001, 276, 10879–10887. [Google Scholar] [CrossRef]
- Creemers, J.W.M.; Dominguez, D.I.; Plets, E.; Serneels, L.; Taylor, N.A.; Multhaup, G.; Craessaerts, K.; Annaert, W.; De Strooper, B. Processing of β-Secretase by Furin and Other Members of the Proprotein Convertase Family. J. Biol. Chem. 2001, 276, 4211–4217. [Google Scholar] [CrossRef]
- Bennett, B.D.; Denis, P.; Haniu, M.; Teplow, D.B.; Kahn, S.; Louis, J.-C.; Citron, M.; Vassar, R. A Furin-like Convertase Mediates Propeptide Cleavage of BACE, the Alzheimer’s β-Secretase. J. Biol. Chem. 2000, 275, 37712–37717. [Google Scholar] [CrossRef]
- Steed, M.M.; Tyagi, N.; Sen, U.; Schuschke, D.A.; Joshua, I.G.; Tyagi, S.C.; Bolduc, V.; Baraghis, E.; Duquette, N.; Thorin-Trescases, N.; et al. Functional consequences of the collagen/elastin switch in vascular remodeling in hyperhomocysteinemic wild-type, eNOS−/−, and iNOS−/− mice. Am. J. Physiol. Cell. Mol. Physiol. 2010, 299, L301–L311. [Google Scholar] [CrossRef]
- Lynch, M.; Pham, W.; Sinclair, B.; O’brien, T.J.; Law, M.; Vivash, L. Perivascular spaces as a potential biomarker of Alzheimer’s disease. Front. Neurosci. 2022, 16, 1021131. [Google Scholar] [CrossRef]
- Jeong, S.H.; Cha, J.; Park, M.; Jung, J.H.; Ye, B.S.; Sohn, Y.H.; Chung, S.J.; Lee, P.H. Association of Enlarged Perivascular Spaces with Amyloid Burden and Cognitive Decline in Alzheimer Disease Continuum. Neurology 2022, 99, E1791–E1802. [Google Scholar] [CrossRef]
- Bown, C.W.; Khan, O.A.; Liu, D.; Remedios, S.W.; Pechman, K.R.; Terry, J.G.; Nair, S.; Davis, L.T.; Landman, B.A.; Gifford, K.A.; et al. Enlarged perivascular space burden associations with arterial stiffness and cognition. Neurobiol. Aging 2023, 124, 85–97. [Google Scholar] [CrossRef]
- Hayden, M.R.; Tyagi, S.C. Impaired Folate-Mediated One-Carbon Metabolism in Type 2 Diabetes, Late-Onset Alzheimer’s Disease and Long COVID. Medicina 2021, 58, 16. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, R.A.; Bosetti, F.; Emr, M.; Gladman, J.T.; Koenig, J.I.; Moy, C.S.; Pahigiannis, K.; Waddy, S.P.; Koroshetz, W. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell. Mol. Neurobiol. 2016, 36, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, D.M.; Zhao, Q.; Morgan, D.; Gordon, M.N.; Everhart, A.; Wilson, J.G.; Lee, J.E.; Colton, C.A. Diverse Inflammatory Responses in Transgenic Mouse Models of Alzheimer’s Disease and the Effect of Immunotherapy on These Responses. ASN Neuro 2011, 3, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Lominadze, D.; Tyagi, N.; Sen, U.; Ovechkin, A.; Tyagi, S.C. Homocysteine alters cerebral microvascular integrity and causes remodeling by antagonizing GABA-A receptor. Mol. Cell. Biochem. 2012, 371, 89–96. [Google Scholar] [CrossRef][Green Version]
- Lominadze, D.; Roberts, A.M.; Tyagi, N.; Moshal, K.S.; Tyagi, S.C. Homocysteine causes cerebrovascular leakage in mice. Am. J. Physiol. Circ. Physiol. 2006, 290, H1206–H1213. [Google Scholar] [CrossRef]
- Sudduth, T.L.; Weekman, E.M.; Price, B.R.; Gooch, J.L.; Woolums, A.; Norris, C.M.; Wilcock, D.M. Time-course of glial changes in the hyperhomocysteinemia model of vascular cognitive impairment and dementia (VCID). Neuroscience 2017, 341, 42–51. [Google Scholar] [CrossRef]
- Cheng, M.; Xue, H.; Li, X.; Yan, Q.; Zhu, D.; Wang, Y.; Shi, Y.; Fu, C. Prevalence of hyperhomocysteinemia (HHcy) and its major determinants among hypertensive patients over 35 years of age. Eur. J. Clin. Nutr. 2021, 76, 616–623. [Google Scholar] [CrossRef]
- Gallistl, S.; Sudi, K.; Mangge, H.; Erwa, W.; Borkenstein, M. Insulin is an independent correlate of plasma homocysteine levels in obese children and adolescents. Diabetes Care 2000, 23, 1348–1352. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, J.; Cheng, L.; Fan, K.; Xu, M.; Ren, W.; Chen, Y.; Geng, D.; Cheng, H.; Luan, X.; et al. Corrigendum: Age-Dependent Association Between Elevated Homocysteine and Cognitive Impairment in a Post-stroke Population: A Prospective Study. Front. Nutr. 2021, 8, 736283. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Wang, L.; Jia, J.; Hong, Z.; Zhang, L.; Zhang, J. Effects of chemerin and homocysteine levels and their associations with oc-currence and development of ischemic cerebrovascular disease. Lipids Health Dis. 2021, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Bin Bae, J.; Han, J.W.; Song, J.; Lee, K.; Kim, T.H.; Kwak, K.P.; Kim, B.J.; Kim, S.G.; Kim, J.L.; Moon, S.W.; et al. Hypohomocysteinemia may increases the risk of dementia and Alzheimer’s disease: A nationwide population-based prospective cohort study. Clin. Nutr. 2021, 40, 4579–4584. [Google Scholar] [CrossRef]
- Loscalzo, J. Homocysteine and Dementias. N. Engl. J. Med. 2002, 346, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Fridman, O. Hyperhomocysteinemia: Atherothrombosis and neurotoxicity. Acta Physiol. Pharmacol. Ther. Latinoam. 1999, 49, 21–30. [Google Scholar]
- Hackam, D.G.; Peterson, J.C.; Spence, J. What level of plasma homocyst(e)ine should be treated? Effects of vitamin therapy on progression of carotid atherosclerosis in patients with homocyst(e)ine levels above and below 14 μmol/L. Am. J. Hypertens. 2000, 13, 105–110. [Google Scholar] [CrossRef]
- Schnyder, G.; Roffi, M.; Pin, R.; Flammer, Y.; Lange, H.; Eberli, F.R.; Meier, B.; Turi, Z.G.; Hess, O.M. Decreased Rate of Coronary Restenosis after Lowering of Plasma Homocysteine Levels. N. Engl. J. Med. 2001, 345, 1593–1600. [Google Scholar] [CrossRef]
- Folbergrová, J. NMDA and Not Non-NMDA Receptor Antagonists Are Protective against Seizures Induced by Homocysteine in Neonatal Rats. Exp. Neurol. 1994, 130, 344–350. [Google Scholar] [CrossRef]
- Sieklucka, M.; Bortolotto, Z.; Heim, C.; Block, F.; Sontag, K.H. Decreased susceptibility to seizures induced by bicuculline after transient bilateral clamping of the carotid arteries in rats. J. Neural Transm. 1991, 83, 127–137. [Google Scholar] [CrossRef]
- Hoek, J.; Schoenmakers, S.; Ringelberg, B.; Reijnders, I.F.; Willemsen, S.P.; De Rijke, Y.B.; Mulders, A.G.; Steegers-Theunissen, R.P. Periconceptional maternal and paternal homocysteine levels and early utero-placental (vascular) growth trajectories: The Rotterdam periconception cohort. Placenta 2021, 115, 45–52. [Google Scholar] [CrossRef]
- Schwinger, C.; Sharma, S.; Chandyo, R.K.; Hysing, M.; Kvestad, I.; Ulak, M.; Ranjitkar, S.; Shrestha, M.; Shrestha, L.P.; McCann, A.; et al. Cobalamin and folate status in women during early pregnancy in Bhaktapur, Nepal. J. Nutr. Sci. 2021, 10, e57. [Google Scholar] [CrossRef]
- Wang, B.; Jian, L.; Li, H.; Li, Z.; Luo, H.; Gao, Y. Folic acid supplementation during pregnancy modulates hepatic methyl metabolism and genes expression profile of neonatal lambs of different litter sizes. Br. J. Nutr. 2021, 128, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, T.; Tong, Y.; Le, J.; Yao, Q.; Tao, J.; Liu, H.; Jiao, W.; Mei, Y.; Chen, J.; et al. Gut-microbiome-expressed 3β-hydroxysteroid dehydrogenase degrades estradiol and is linked to depression in premenopausal females. Cell Metab. 2023, 35, 685–694.e5. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, B.; Vasanthakumar, A.; Vasanthakumar, A.; Li, Q.S.; Li, Q.S.; Nudelman, K.N.; Nudelman, K.N.; Risacher, S.L.; Risacher, S.L.; et al. Integrative analysis of DNA methylation and gene expression identifies genes associated with biological aging in Alzheimer’s disease. Alzheimer’s Dementia Diagn. Assess. Dis. Monit. 2022, 14, e12354. [Google Scholar] [CrossRef]
- Zhao, J.; Huai, J. Role of primary aging hallmarks in Alzheimer’s disease. Theranostics 2023, 13, 197–230. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kim, J.K.; Shin, Y.J.; Son, Y.H.; Lee, D.Y.; Park, H.S.; Kim, D.H. Alleviation of Cognitive Impairment-like Behaviors, Neuroinflammation, Colitis, and Gut Dysbiosis in 5xFAD Transgenic and Aged Mice by Lactobacillus mucosae and Bifidobacterium longum. Nutrients 2023, 15, 3381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, S.; Zhang, H.; Zhao, W.; Ding, J.; Dai, R.; Xu, K.; He, C.; Liu, J.; Yang, L.; et al. Dynamic distribution of gut microbiota during Alzheimer’s disease progression in a mice model. APMIS 2023, 131, 480–490. [Google Scholar] [CrossRef]
- López-Villodres, J.A.; Escamilla, A.; Mercado-Sáenz, S.; Alba-Tercedor, C.; Rodriguez-Perez, L.M.; Arranz-Salas, I.; Sanchez-Varo, R.; Bermúdez, D. Microbiome Alterations and Alzheimer’s Disease: Modeling Strategies with Transgenic Mice. Biomedicines 2023, 11, 1846. [Google Scholar] [CrossRef] [PubMed]
- Navalón-Monllor, V.; Soriano-Romaní, L.; Silva, M.; Hazas, M.-C.L.d.L.; Hernando-Quintana, N.; Diéguez, T.S.; Esteve, P.M.; Nieto, J.A. Microbiota dysbiosis caused by dietetic patterns as a promoter of Alzheimer’s disease through metabolic syndrome mechanisms. Food Funct. 2023, 14, 7317–7334. [Google Scholar] [CrossRef]
- Wu, G.; Xu, J.; Wang, Q.; Fang, Z.; Fang, Y.; Jiang, Y.; Zhang, X.; Cheng, X.; Sun, J.; Le, G. Methionine-Restricted Diet: A Feasible Strategy Against Chronic or Aging-Related Diseases. J. Agric. Food Chem. 2022, 71, 5–19. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Li, B.; Xie, Y.; Shi, Y.; Le, G. Dietary methionine restriction improves gut microbiota composition and prevents cognitive impairment ind-galactose-induced aging mice. Food Funct. 2022, 13, 12896–12914. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, Y.; Zhou, Y.; Liu, Q.; Chen, X.; Liu, X.; Grune, T.; Shi, L.; Hou, M.; Liu, Z. Effects of methionine intake on cognitive function in mild cognitive impairment patients and APP/PS1 Alzheimer’s Disease model mice: Role of the cystathionine-β-synthase/H2S pathway. Redox Biol. 2023, 59, 102595. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, M.; Xu, Y.; Qian, J.; Le, G.; Xie, Y. Dietary Methionine via Dose-Dependent Inhibition of Short-Chain Fatty Acid Production Capacity Contributed to a Potential Risk of Cognitive Dysfunction in Mice. J. Agric. Food Chem. 2022, 70, 15225–15243. [Google Scholar] [CrossRef] [PubMed]
- George, A.K.; Master, K.; Majumder, A.; Homme, R.P.; Laha, A.; Sandhu, H.S.; Tyagi, S.C.; Singh, M. Circular RNAs constitute an inherent gene regulatory axis in the mammalian eye and brain. Can. J. Physiol. Pharmacol. 2019, 97, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; George, A.K.; Homme, R.P.; Majumder, A.; Laha, A.; Sandhu, H.S.; Tyagi, S.C. Circular RNAs profiling in the cystathionine-β-synthase mutant mouse reveals novel gene targets for hyperhomocysteinemia induced ocular disorders. Exp. Eye Res. 2018, 174, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Eyob, W.; George, A.K.; Homme, R.P.; Stanisic, D.; Sandhu, H.; Tyagi, S.C.; Singh, M. Regulation of the parental gene GRM4 by circGrm4 RNA transcript and glutamate-mediated neurovascular toxicity in eyes. Mol. Cell. Biochem. 2021, 476, 663–673. [Google Scholar] [CrossRef]
- Scalise, M.; Galluccio, M.; Console, L.; Pochini, L.; Indiveri, C. The Human SLC7A5 (LAT1): The Intriguing Histidine/Large Neutral Amino Acid Transporter and Its Relevance to Human Health. Front. Chem. 2018, 6, 243. [Google Scholar] [CrossRef]
- Tyagi, S.C. Homocyst(E)Ine and Heart Disease: Pathophysiology of Extracellular Matrix. Clin. Exp. Hypertens. 1999, 21, 181–198. [Google Scholar] [CrossRef]
- Schaevitz, L.; Berger-Sweeney, J.; Ricceri, L. One-carbon metabolism in neurodevelopmental disorders: Using broad-based nutraceutics to treat cognitive deficits in complex spectrum disorders. Neurosci. Biobehav. Rev. 2014, 46, 270–284. [Google Scholar] [CrossRef]
- George, A.K.; Singh, M.; Pushpakumar, S.; Homme, R.P.; Hardin, S.J.; Tyagi, S.C. Dysbiotic 1-carbon metabolism in cardiac muscle remodeling. J. Cell. Physiol. 2019, 235, 2590–2598. [Google Scholar] [CrossRef]
- Rajabian, N.; Ikhapoh, I.; Shahini, S.; Choudhury, D.; Thiyagarajan, R.; Shahini, A.; Kulczyk, J.; Breed, K.; Saha, S.; Alaa Mohamed, M.; et al. Methionine adenosyltransferase2A inhibition restores metabolism to improve re-generative capacity and strength of aged skeletal muscle. Nat. Commun. 2023, 14, 886. [Google Scholar] [CrossRef]
- Kunitskaya, N.A.; Ariev, A.L. The role of hyperuricemia in the development of cognitive changes in the elderly. Adv. Gerontol. 2022, 35, 775–782. [Google Scholar] [PubMed]
- Sendžikaitė, G.; Hanna, C.W.; Stewart-Morgan, K.R.; Ivanova, E.; Kelsey, G. A DNMT3A PWWP mutation leads to methylation of bivalent chromatin and growth retardation in mice. Nat. Commun. 2019, 10, 1884. [Google Scholar] [CrossRef]
- Mathiyalagan, P.; Adamiak, M.; Mayourian, J.; Sassi, Y.; Liang, Y.; Agarwal, N.; Jha, D.; Zhang, S.; Kohlbrenner, E.; Chepurko, E.; et al. FTO-Dependent N6 -Methyladenosine Regulates Cardiac Function During Remodeling and Repair. Circulation 2019, 139, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Malinow, M.; Levenson, J.; Giral, P.; Nieto, F.; Razavian, M.; Segond, P.; Simon, A. Role of blood pressure, uric acid, and hemorheological parameters on plasma homocyst(e)ine concentration. Atherosclerosis 1995, 114, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Sutton-Tyrrell, K.; Bostom, A.; Selhub, J.; Zeigler-Johnson, C. High Homocysteine Levels Are Independently Related to Isolated Systolic Hypertension in Older Adults. Circulation 1997, 96, 1745–1749. [Google Scholar] [CrossRef]
- Unger, T.; Becker, H.; Dietz, R.; Ganten, D.; Lang, R.E.; Rettig, R.; Schömig, A.; Schwab, N.A. Antihypertensive effect of the GABA receptor agonist muscimol in spontaneously hypertensive rats. Role of the sympathoadrenal axis. Circ. Res. 1984, 54, 30–37. [Google Scholar] [CrossRef]
- Kishi, T.; Hirooka, Y.; Sakai, K.; Shigematsu, H.; Shimokawa, H.; Takeshita, A. Overexpression of eNOS in the RVLM Causes Hypotension and Bradycardia Via GABA Release. Hypertension 2001, 38, 896–901. [Google Scholar] [CrossRef]
- Tyagi, S.C.; Smiley, L.M.; Mujumdar, V.S. Homocyst(e)ine impairs endocardial endothelial function. Can. J. Physiol. Pharmacol. 1999, 77, 950–957. [Google Scholar] [CrossRef]
- Tyagi, S.C.; Lominadze, D.; Roberts, A.M. Homocysteine in Microvascular Endothelial Cell Barrier Permeability. Cell Biochem. Biophys. 2005, 43, 37–44. [Google Scholar] [CrossRef]
- Kim, H.; Noh, M.; Zhang, H.; Kim, Y.; Park, S.; Park, J.; Kwon, Y.-G. Long-term administration of CU06-1004 ameliorates cerebrovascular aging and BBB injury in aging mouse model. Fluids Barriers CNS 2023, 20, 9. [Google Scholar] [CrossRef]
- Cheng, Z.; Dai, L.; Wu, Y.; Cao, Y.; Chai, X.; Wang, P.; Liu, C.; Ni, M.; Gao, F.; Wang, Q.; et al. Correlation of blood–brain barrier leakage with cerebral small vessel disease including cerebral microbleeds in Alzheimer’s disease. Front. Neurol. 2023, 14, 1077860. [Google Scholar] [CrossRef] [PubMed]
- Shibly, A.Z.; Sheikh, A.M.; Michikawa, M.; Tabassum, S.; Azad, A.K.; Zhou, X.; Zhang, Y.; Yano, S.; Nagai, A. Analysis of Cerebral Small Vessel Changes in AD Model Mice. Biomedicines 2022, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Valencia, M.M.; Yu, Y.; Ouchi, Y.; Takahashi, K.; Shokhirev, M.N.; Lande, K.; Williams, A.E.; Fresia, C.; Kurita, M.; et al. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice. Cell 2023, 186, 715–731.e19. [Google Scholar] [CrossRef]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of White Fat: Novel Insight Into Factors, Mechanisms, and Therapeutics. J. Cell. Physiol. 2016, 232, 61–68. [Google Scholar] [CrossRef]
- Paul, B.D.; Pieper, A.A. Protective Roles of Hydrogen Sulfide in Alzheimer’s Disease and Traumatic Brain Injury. Antioxidants 2023, 12, 1095. [Google Scholar] [CrossRef]
- Gu, Y.; Xiao, X.; Pan, R.; Zhang, J.; Zhao, Y.; Dong, Y.; Cui, H. Lactobacillus plantarum dy-1 fermented barley extraction activates white adipocyte browning in high-fat diet-induced obese rats. J. Food Biochem. 2021, 45, e13680. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a myokine and exerkine: Drivers and signals of physiology and metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef]
- Guerrero-Encinas, I.; González-González, J.N.; Santiago-López, L.; Muhlia-Almazán, A.; Garcia, H.S.; Mazorra-Manzano, M.A.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Protective Effect of Lacticaseibacillus casei CRL 431 Postbiotics on Mitochondrial Function and Oxidative Status in Rats with Aflatoxin B1–Induced Oxidative Stress. Probiotics Antimicrob. Proteins 2021, 13, 1033–1043. [Google Scholar] [CrossRef]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ. Res. 2023, 132, 882–898. [Google Scholar] [CrossRef]
- Tomita, I.; Tsuruta, H.; Yasuda-Yamahara, M.; Yamahara, K.; Kuwagata, S.; Tanaka-Sasaki, Y.; Chin-Kanasaki, M.; Fujita, Y.; Nishi, E.; Katagiri, H.; et al. Ketone bodies: A double-edged sword for mammalian life span. Aging Cell 2023, 22, e13833. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Albano, C.; Silvetti, T.; Brasca, M. Screening of lactic acid bacteria producing folate and their potential use as adjunct cultures for cheese bio-enrichment. FEMS Microbiol. Lett. 2020, 367, fnaa059. [Google Scholar] [CrossRef]
- Panigrahi, P.; Parida, S.; Nanda, N.C.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, G.; Kreutz, M.R. Neuronal DNA Methyltransferases: Epigenetic Mediators between Synaptic Activity and Gene Expression? Neuroscientist 2017, 24, 171–185. [Google Scholar] [CrossRef]
- Bayraktar, G.; Kreutz, M.R. The Role of Activity-Dependent DNA Demethylation in the Adult Brain and in Neurological Disorders. Front. Mol. Neurosci. 2018, 11, 169. [Google Scholar] [CrossRef]
- Gavin, D.P.; Chase, K.A.; Sharma, R.P. Active DNA demethylation in post-mitotic neurons: A reason for optimism. Neuropharmacology 2013, 75, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-C.; Lang, L.-Q.; Hu, J.; Zhang, X.-L.; Zhong, M.-K.; Ma, C.-L. A novel epigenetic marker, Ten-eleven translocation family member 2 (TET2), is identified in the intractable epileptic brain and regulates ATP binding cassette subfamily B member 1 (ABCB1) in the blood–brain barrier. Bioengineered 2022, 13, 6638–6649. [Google Scholar] [CrossRef]
- Johnson, E.C.B.; Carter, E.K.; Dammer, E.B.; Duong, D.M.; Gerasimov, E.S.; Liu, Y.; Liu, J.; Betarbet, R.; Ping, L.; Yin, L.; et al. Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat. Neurosci. 2022, 25, 213–225. [Google Scholar] [CrossRef]
- Dey, K.K.; Sun, H.; Wang, Z.; Niu, M.; Wang, H.; Jiao, Y.; Sun, X.; Li, Y.; Peng, J. Proteomic Profiling of Cerebrospinal Fluid by 16-Plex TMT-Based Mass Spectrometry. Methods Mol. Biol. 2022, 2420, 21–37. [Google Scholar] [CrossRef]
- Erickson, A.; Zhou, S.; Luo, J.; Li, L.; Huang, X.; Even, Z.; Huang, H.; Xu, H.-M.; Peng, J.; Lu, L.; et al. Genetic architecture of protein expression and its regulation in the mouse brain. BMC Genom. 2021, 22, 875. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Manczak, M.; Pradeepkiran, J.A.; Morton, H.; Reddy, P.H. A partial reduction of Drp1 improves cognitive behavior and enhances mitophagy, autophagy and dendritic spines in a transgenic Tau mouse model of Alzheimer disease. Hum. Mol. Genet. 2021, 31, 1788–1805. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.Y.; Cardiff, K.; Yang, X.; Wilfred, B.S.; Gilsdorf, J.; Shear, D. Selective Brain Cooling Reduces Motor Deficits Induced by Combined Traumatic Brain Injury, Hypoxemia and Hemorrhagic Shock. Front. Neurol. 2018, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Desplats, P.A. Perinatal programming of neurodevelopment: Epigenetic mechanisms and the prenatal shaping of the brain. Adv. Neurobiol. 2015, 10, 335–361. [Google Scholar] [CrossRef]
- Williams, B.J.; Bimonte-Nelson, H.A.; Granholm-Bentley, A.-C. ERK-mediated NGF signaling in the rat septo-hippocampal pathway diminishes with age. Psychopharmacology 2006, 188, 605–618. [Google Scholar] [CrossRef]
- Finneran, D.J.; Nash, K.R. Neuroinflammation and fractalkine signaling in Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 30. [Google Scholar] [CrossRef]
- Pandey, U.B.; Ward, C. Matrin-3 dysfunction in myopathy and motor neuron degeneration. Neural Regen. Res. 2022, 17, 575–576. [Google Scholar] [CrossRef]
- Fortuna, T.R.; Kour, S.; Anderson, E.N.; Ward, C.; Rajasundaram, D.; Donnelly, C.J.; Hermann, A.; Wyne, H.; Shewmaker, F.; Pandey, U.B. DDX17 is involved in DNA damage repair and modifies FUS toxicity in an RGG-domain dependent manner. Acta Neuropathol. 2021, 142, 515–536. [Google Scholar] [CrossRef]
- Anderson, E.N.; Morera, A.A.; Kour, S.; Cherry, J.D.; Ramesh, N.; Gleixner, A.; Schwartz, J.C.; Ebmeier, C.; Old, W.; Donnelly, C.J.; et al. Traumatic injury compromises nucleocytoplasmic transport and leads to TDP-43 pathology. eLife 2021, 10, e67587. [Google Scholar] [CrossRef]
- Winslow, W.; McDonough, I.; Tallino, S.; Decker, A.; Vural, A.S.; Velazquez, R. IntelliCage Automated Behavioral Phenotyping Reveals Behavior Deficits in the 3xTg-AD Mouse Model of Alzheimer’s Disease Associated With Brain Weight. Front. Aging Neurosci. 2021, 13, 720214. [Google Scholar] [CrossRef]
- Robison, L.S.; Gannon, O.J.; Thomas, M.A.; Salinero, A.E.; Abi-Ghanem, C.; Poitelon, Y.; Belin, S.; Zuloaga, K.L. Role of sex and high-fat diet in metabolic and hypothalamic disturbances in the 3xTg-AD mouse model of Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 285. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dai, H.; Dai, X.; Yin, J.; Cui, Y.; Liu, X.; Gonzalez, G.; Yuan, J.; Tang, F.; Wang, N.; et al. m1A in CAG repeat RNA binds to TDP-43 and induces neurodegeneration. Nature 2023, 623, 580–587. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Miramontes, R.; Chillon-Marinas, C.; Maimon, R.; Vazquez-Sanchez, S.; Lau, A.L.; McClure, N.R.; England, W.E.; Singha, M.; Stocksdale, J.T.; et al. Aberrant splicing in Huntington’s disease via disrupted TDP-43 activity accompanied by altered m6A RNA modification. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-P.; Hua, K.-F.; Tsai, F.-T.; Lin, T.-Y.; Cheng, C.-Y.; Yang, D.-I.; Hsu, H.-T.; Ju, T.-C. A selective inhibitor of the NLRP3 inflammasome as a potential therapeutic approach for neuroprotection in a transgenic mouse model of Huntington’s disease. J. Neuroinflamm. 2022, 19, 56. [Google Scholar] [CrossRef]
- Menéndez-González, M.; Clarimón, J.; Rosas-Allende, I.; Blázquez, M.; Martín, E.S.S.; García-Fernández, C.; Lleó, A.; Dols-Icardo, O.; Illán-Gala, I.; Morís, G.; et al. HTT gene intermediate alleles in neurodegeneration: Evidence for association with Alzheimer’s disease. Neurobiol. Aging 2018, 76, 215.e9–215.e14. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.Y.; Keene, C.D.; Jayadev, S.; Bird, T. The Co-Occurrence of Alzheimer’s Disease and Huntington’s Disease: A Neuropathological Study of 15 Elderly Huntington’s Disease Subjects. J. Huntington’s Dis. 2014, 3, 209–217. [Google Scholar] [CrossRef]
- Moschini, V.; Mazzeo, S.; Bagnoli, S.; Padiglioni, S.; Emiliani, F.; Giacomucci, G.; Morinelli, C.; Ingannato, A.; Freni, T.; Belloni, L.; et al. CAG Repeats within the Non-pathological Range in the HTT Gene Influence Personality Traits in Patients with Subjective Cognitive Decline: A 13-Year Follow-Up Study. Front. Psychiatry 2022, 13, 826135. [Google Scholar] [CrossRef]
- Rao, K.S. Mechanisms of Disease: DNA repair defects and neurological disease. Nat. Clin. Pract. Neurol. 2007, 3, 162–172. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyagi, S.C. Lactobacillus Eats Amyloid Plaque and Post-Biotically Attenuates Senescence Due to Repeat Expansion Disorder and Alzheimer’s Disease. Antioxidants 2024, 13, 1225. https://doi.org/10.3390/antiox13101225
Tyagi SC. Lactobacillus Eats Amyloid Plaque and Post-Biotically Attenuates Senescence Due to Repeat Expansion Disorder and Alzheimer’s Disease. Antioxidants. 2024; 13(10):1225. https://doi.org/10.3390/antiox13101225
Chicago/Turabian StyleTyagi, Suresh C. 2024. "Lactobacillus Eats Amyloid Plaque and Post-Biotically Attenuates Senescence Due to Repeat Expansion Disorder and Alzheimer’s Disease" Antioxidants 13, no. 10: 1225. https://doi.org/10.3390/antiox13101225
APA StyleTyagi, S. C. (2024). Lactobacillus Eats Amyloid Plaque and Post-Biotically Attenuates Senescence Due to Repeat Expansion Disorder and Alzheimer’s Disease. Antioxidants, 13(10), 1225. https://doi.org/10.3390/antiox13101225




