Discovery of Effective Inhibitors Against Phosphodiesterase 9, a Potential Therapeutic Target of Alzheimer’s Disease with Antioxidant Capacities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Remarks
2.1.2. General Procedure for the Preparation of the Intermediates and Pyrazolopyrimidinone Derivatives
2.2. In Vitro Assay for Potential PDE9 Inhibitors
2.2.1. Expression and Purification of PDE9 Protein
2.2.2. Enzymatic Assays Against PDE9
2.3. Antioxidant Activity Assay
2.4. Molecular Docking
2.5. Molecular Dynamics Simulations
2.6. Prediction of Pharmacokinetic Properties
3. Results and Discussion
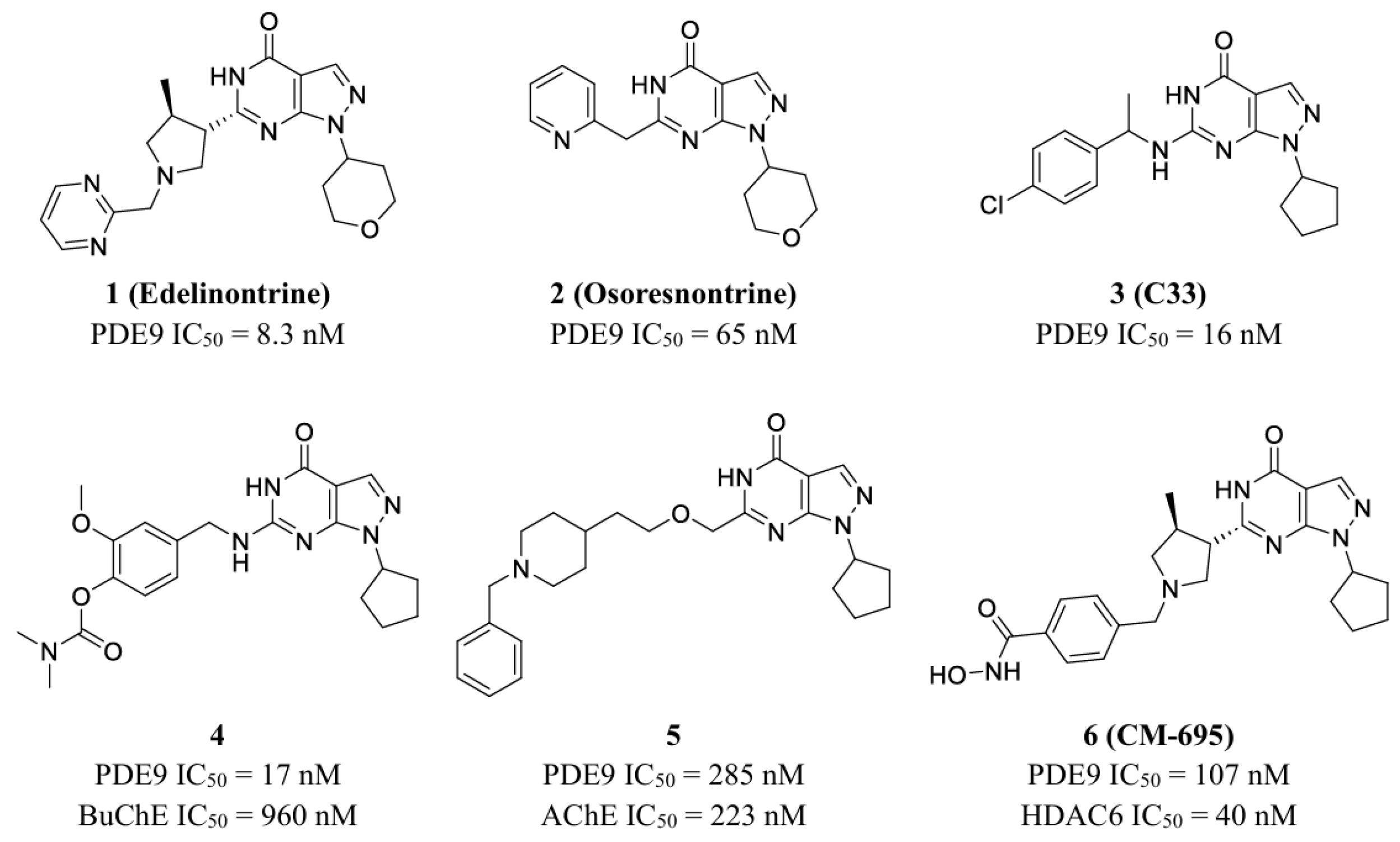
3.1. Multitarget-Directed Ligand Design Strategy
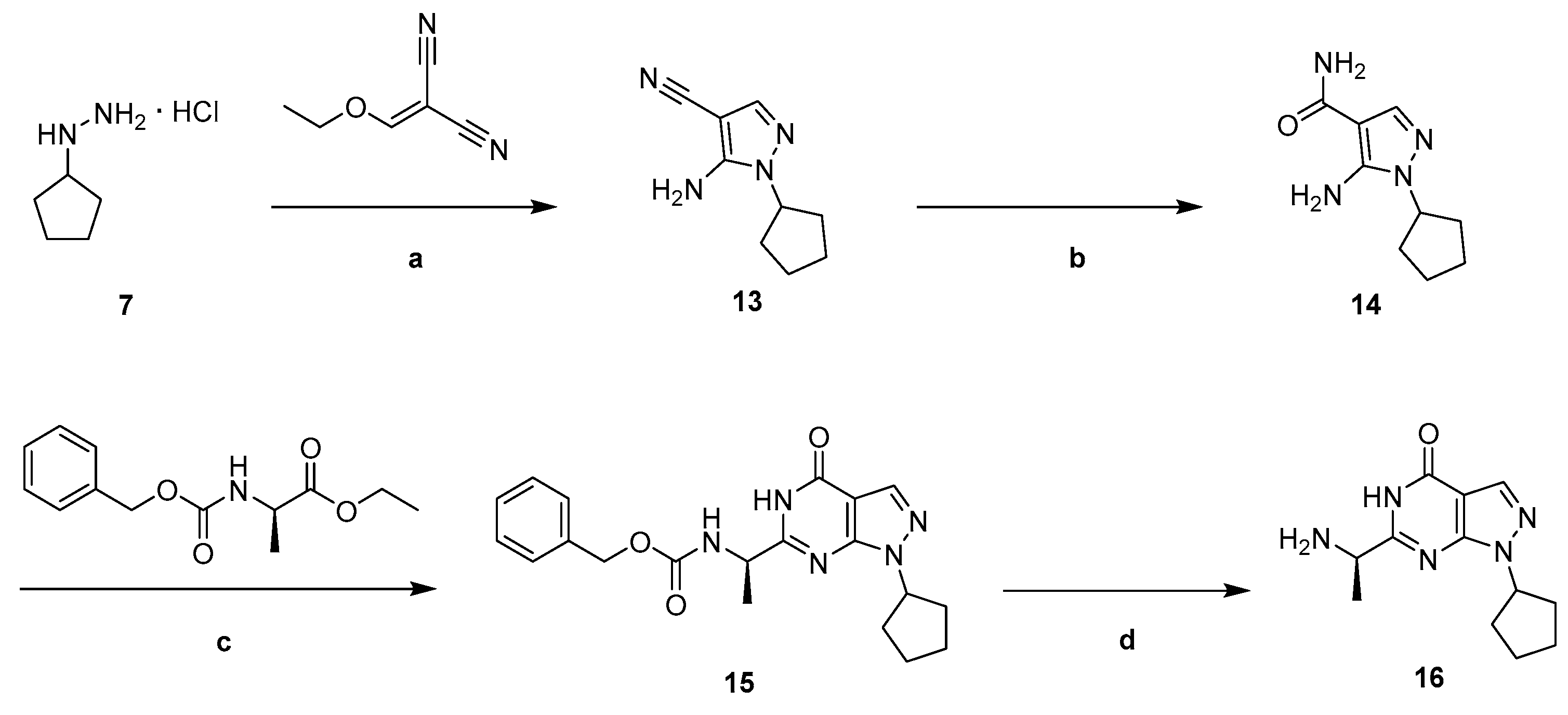
3.2. Chemistry
3.3. Biological Evaluation of Designed Compounds
3.3.1. Inhibition of Designed Compounds Against PDE9
3.3.2. Antioxidant Activity of Designed Compounds by the ORAC Method
3.4. Pharmacokinetic Properties
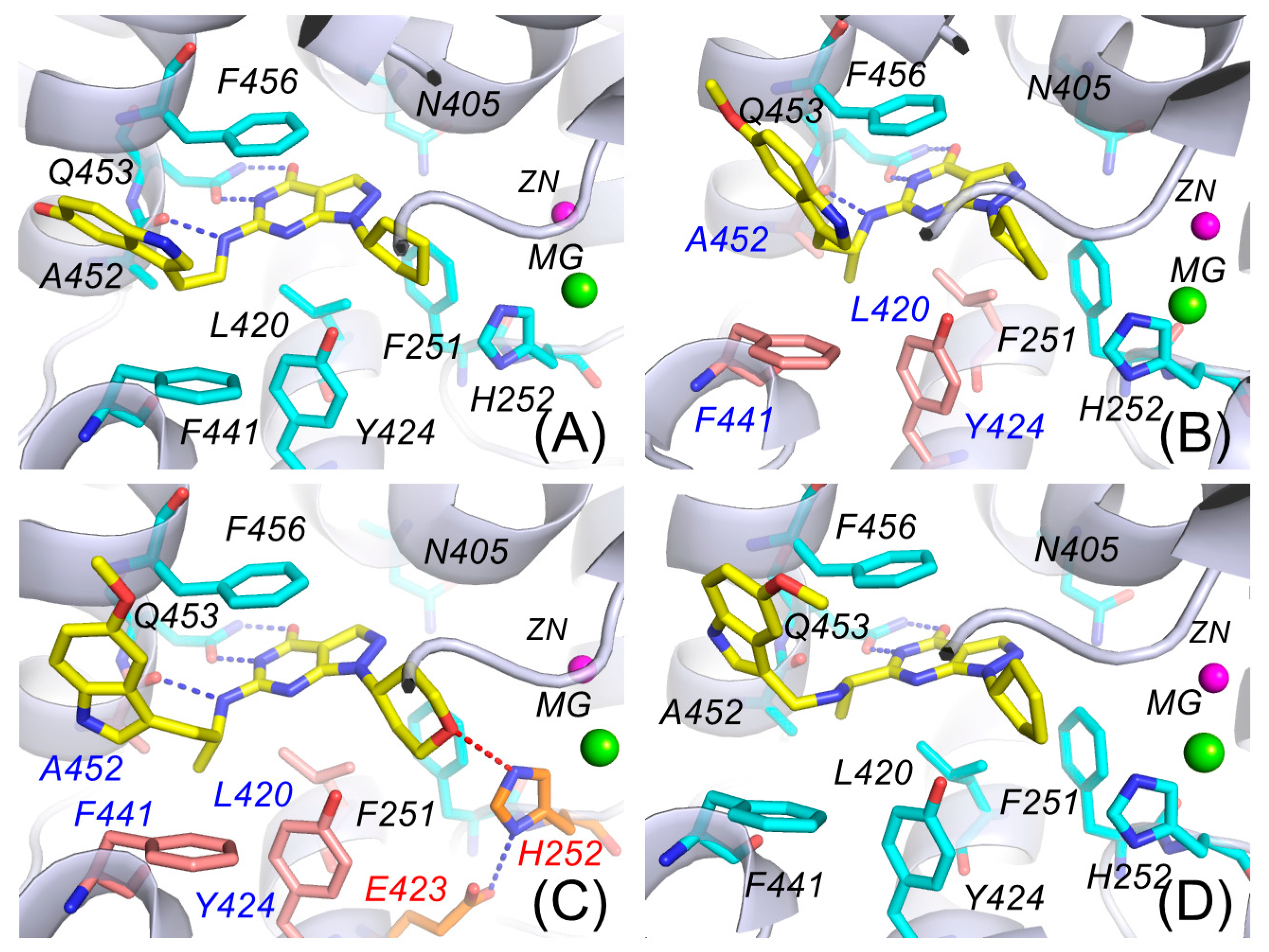
3.5. Structure-Activity Relationship Analysis Based on Molecular Docking
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, andcare: 2020 report of theLancet Commission. Lancet 2020, 396, 413–446, Erratum in Lancet 2023, 402, 1132. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s Disease: The Challenge of the Second Century. Sci. Transl. Med. 2011, 3, 77sr1. [Google Scholar] [CrossRef]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Chau, S.A.; Kircanski, I.; Lanctôt, K.L. Current and Emerging Drug Treatment Options for Alzheimer’s Disease A Systematic Review. Drugs 2011, 71, 2031–2065. [Google Scholar] [CrossRef]
- Reardon, S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature 2023, 613, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Möller, C.; Lannfelt, L. Lecanemab, Aducanumab, and Gantenerumab—Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics 2023, 20, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Turgutalp, B.; Kizil, C. Multi-target drugs for Alzheimer’s disease. Trends Pharmacol. Sci. 2024, 45, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Dai, C.L.; Liu, F.; Iqbal, K. Multi-Targets: An Unconventional Drug Development Strategy for Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 837649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Zhang, Y.L.; Wang, J.X.; Xia, Y.L.; Zhang, J.X.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative Stress in Alzheimer’s Disease: Are We Connecting the Dots? J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin: Clinical Perspectives in Neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Wong, K.Y.; Aquili, L.; Uddin, M.S.; Heng, B.C.; Tipoe, G.L.; Wong, K.H.; Fung, M.L.; Lim, L.W. Role of melatonin in Alzheimer’s disease: From preclinical studies to novel melatonin-based therapies. Front. Neuroendocrinol. 2022, 65, 100986. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Beavo, J.A.; Brunton, L.L. Cyclic nucleotide research—Still expanding after half a century. Nat. Rev. Mol. Cell Biol. 2002, 3, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Lakics, V.; Karran, E.H.; Boess, F.G. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 2010, 59, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Guipponi, M.; Scott, H.S.; Kudoh, J.; Kawasaki, K.; Shibuya, K.; Shintani, A.; Asakawa, S.; Chen, H.M.; Lalioti, M.D.; Rossier, C.; et al. Identification and characterization of a novel cyclic nucleotide phosphodiesterase gene that maps to 21q22.3: Alternative splicing of mRNA transcripts, genomic structure and sequence. Hum. Genet. 1998, 103, 386–392. [Google Scholar] [CrossRef]
- Patel, N.S.; Klett, J.; Pilarzyk, K.; Lee, D.I.; Kass, D.; Menniti, F.S.; Kelly, M.P. Identification of new PDE9A isoforms and how their expression and subcellular compartmentalization in the brain change across the life span. Neurobiol. Aging 2018, 65, 217–234. [Google Scholar] [CrossRef]
- Wang, P.; Wu, P.; Egan, R.W.; Billah, M.M. Identification and characterization of a new human type 9 eGMP-specific phosphodiesterase splice variant (PDE9A5)—Differential tissue distribution and subcellular localization of PDE9A variants. Gene 2003, 314, 15–27. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Harms, J.F.; Tingley, F.D.; Schmidt, K.; Adamowicz, W.O.; Romegialli, A.; Kleiman, R.J.; Barry, C.J.; Coskran, T.M.; O’Neill, S.M.; et al. PDE9A-mediated regulation of cGMP: Developing a biomarker for a novel therapy for Alzheimer’s disease. Alzheimers Dement. 2009, 5, 331. [Google Scholar] [CrossRef]
- Harms, J.F.; Menniti, F.S.; Schmidt, C.J. Phosphodiesterase 9A in Brain Regulates cGMP Signaling Independent of Nitric-Oxide. Front. Neurosci. 2019, 13, 837. [Google Scholar] [CrossRef] [PubMed]
- García-Osta, A.; Cuadrado-Tejedor, M.; García-Barroso, C.; Oyarzábal, J.; Franco, R. Phosphodiesterases as Therapeutic Targets for Alzheimer’s Disease. ACS Chem. Neurosci. 2012, 3, 832–844. [Google Scholar] [CrossRef]
- Hutson, P.H.; Finger, E.N.; Magliaro, B.C.; Smith, S.M.; Converso, A.; Sanderson, P.E.; Mullins, D.; Hyde, L.A.; Eschle, B.K.; Turnbull, Z.; et al. The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 (6-[(3,4)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydro-2-pyran-4-yl)-1,5-dihydro-4-pyrazolo[3,4-]pyrimidin-4-one) enhances synaptic plasticity and cognitive function in rodents. Neuropharmacology 2011, 61, 665–676. [Google Scholar] [PubMed]
- Rosenbrock, H.; Giovannini, R.; Schänzle, G.; Koros, E.; Runge, F.; Fuchs, H.; Marti, A.; Reymann, K.G.; Schröder, U.H.; Fedele, E.; et al. The Novel Phosphodiesterase 9A Inhibitor BI 409306 Increases Cyclic Guanosine Monophosphate Levels in the Brain, Promotes Synaptic Plasticity, and Enhances Memory Function in Rodents. J. Pharmacol. Exp. Ther. 2019, 371, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Kroker, K.S.; Rast, G.; Giovannini, R.; Marti, A.; Dorner-Ciossek, C.; Rosenbrock, H. Inhibition of acetylcholinesterase and phosphodiesterase-9A has differential effects on hippocampal early and late LTP. Neuropharmacology 2012, 62, 1964–1974. [Google Scholar] [CrossRef]
- Jankowska, A.; Wesolowska, A.; Pawlowski, M.; Chlon-Rzepa, G. Multifunctional Ligands Targeting Phosphodiesterase as the Future Strategy for the Symptomatic and Disease-Modifying Treatment of Alzheimer’s Disease. Curr. Med. Chem. 2020, 27, 5351–5373. [Google Scholar] [CrossRef]
- Rabal, O.; Sánchez-Arias, J.A.; Cuadrado-Tejedor, M.; de Miguel, I.; Pérez-González, M.; García-Barroso, C.; Ugarte, A.; de Mendoza, A.E.H.; Sáez, E.; Espelosin, M.; et al. Multitarget Approach for the Treatment of Alzheimer’s Disease: Inhibition of Phosphodiesterase 9 (PDE9) and Histone Deacetylases (HDACs) Covering Diverse Selectivity Profiles. ACS Chem. Neurosci. 2019, 10, 4076–4101. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zhang, T.H.; Xie, S.S.; Yan, J.; Wu, Y.N.; Li, X.S.; Huang, L.; Luo, H.B. Discovery of novel PDE9 inhibitors capable of inhibiting Aβ aggregation as potential candidates for the treatment of Alzheimer’s disease. Sci. Rep. 2016, 6, 21826. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Han, C.A.; Zhang, C.; Zhou, Q.; Zhang, B.; Le, M.L.; Huang, M.X.; Wu, Y.N.; Luo, H.B. Discovery of effective phosphodiesterase 2 inhibitors with antioxidant activities for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2021, 41, 128016. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.N.; Shao, Y.X.; Hou, J.Y.; Cui, W.J.; Liang, B.B.; Huang, Y.C.; Li, Z.; Wu, Y.N.; Zhu, X.H.; Liu, P.Q.; et al. Structural Asymmetry of Phosphodiesterase-9A and a Unique Pocket for Selective Binding of a Potent Enantiomeric Inhibitor. Mol. Pharmacol. 2015, 88, 836–845. [Google Scholar] [CrossRef]
- Wu, Y.N.; Zhou, Q.; Zhang, T.H.; Li, Z.; Chen, Y.P.; Zhang, P.; Yu, Y.F.; Geng, H.J.; Tian, Y.J.; Zhang, C.; et al. Discovery of Potent, Selective, and Orally Bioavailable Inhibitors against Phosphodiesterase-9, a Novel Target for the Treatment of Vascular Dementia. J. Med. Chem. 2019, 62, 4218–4224. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Jain, A.N. Surflex: Fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.J.; Wang, J.M.; Li, Y.Y.; Wang, W. Assessing the Performance of the MM/PBSA and MM/GBSA Methods. 1. The Accuracy of Binding Free Energy Calculations Based on Molecular Dynamics Simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Greene, D.; Homeyer, N.; et al. Amber16; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Pittsburgh, PA, USA, 2004. [Google Scholar]
- Stote, R.H.; Karplus, M. Zinc binding in proteins and solution: A simple but accurate nonbonded representation. Proteins 1995, 23, 12–31. [Google Scholar] [CrossRef]
- Essmann, U.P.L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Forester, T.R.; Smith, W. SHAKE, rattle, and roll: Efficient constraint algorithms for linked rigid bodies. J. Comput. Chem. 2000, 21, 157. [Google Scholar] [CrossRef]



 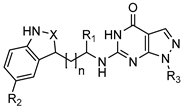 17a–e, 17g–h 17f | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comp. | n | X | R1 | R2 | R3 | Binding Free Energy (kcal/mol) 1 | PDE9 IC50 (nM) | ORAC 4 |
| 17a | 1 | CH | -CH3 | -H | 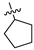 | −31.42 ± 2.48 | 78% 2 68% 3 | 0.37 ± 0.04 |
| 17b | 1 | CH | -H | -OH | 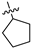 | −31.87 ± 3.14 | 91 ± 4 | 2.00 ± 0.27 |
| 17c | 1 | CH | -CH3 | -OCH3 | 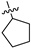 | −39.59 ± 2.50 | 1.8 | 0.32 ± 0.06 |
| 17d | 1 | CH | -CH3 | -OCH3 | 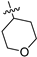 | −37.89 ± 2.59 | 89 ± 4 | 2.60 ± 0.05 |
| 17e | 2 | CH | -CH3 | -H | 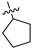 | −35.24 ± 2.50 | 83% 2 67% 3 | 0.33 ± 0.002 |
| 17f | 1 | CH2 | -CH3 | -H | 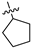 | −32.67 ± 3.21 | 67% 2 59% 3 | 0.66 ± 0.04 |
| 17g | 1 | N | -CH3 | -H | 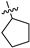 | −37.37 ± 2.68 | 82% 2 70% 3 | 0.17 ± 0.004 |
| 17h | 1 | N | -CH3 | -OCH3 | 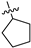 | −38.67 ± 2.73 | 69% 2 65% 3 | 0.22 ± 0.12 |
 | ||||||
|---|---|---|---|---|---|---|
| Comp. | X | R1 | R2 | Binding Free Energy (kcal/mol) 1 | PDE9 IC50 (nM) | ORAC 4 |
| 18a | CH | -H | -H | −32.78 ± 2.27 | 56% 2 46% 3 | 0.60 ± 0.06 |
| 18b | CH | -H | -F | −36.12 ± 2.74 | 76% 2 61% 3 | 0.88 ± 0.08 |
| 18c | CH | -H | -OCH3 | −37.38 ± 3.19 | 194 ± 26 | 1.61 ± 0.11 |
| 18d | CH | -CH3 | -OCH3 | −36.09 ± 2.85 | 214 ± 20 | 1.09 ± 0.02 |
| 18e | N | -H | -H | −34.68 ± 2.48 | 47% 2 | 0.15 ± 0.03 |
| 18f | N | -H | -OCH3 | −32.70 ± 2.81 | 28% 2 | 0.24 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Wu, X.-N.; Luo, W.-H.; Huang, Q.-H.; Feng, L.-L.; Wu, Y.; Zhang, C. Discovery of Effective Inhibitors Against Phosphodiesterase 9, a Potential Therapeutic Target of Alzheimer’s Disease with Antioxidant Capacities. Antioxidants 2025, 14, 123. https://doi.org/10.3390/antiox14020123
Zhou Q, Wu X-N, Luo W-H, Huang Q-H, Feng L-L, Wu Y, Zhang C. Discovery of Effective Inhibitors Against Phosphodiesterase 9, a Potential Therapeutic Target of Alzheimer’s Disease with Antioxidant Capacities. Antioxidants. 2025; 14(2):123. https://doi.org/10.3390/antiox14020123
Chicago/Turabian StyleZhou, Qian, Xu-Nian Wu, Wei-Hao Luo, Qing-Hua Huang, Ling-Ling Feng, Yinuo Wu, and Chen Zhang. 2025. "Discovery of Effective Inhibitors Against Phosphodiesterase 9, a Potential Therapeutic Target of Alzheimer’s Disease with Antioxidant Capacities" Antioxidants 14, no. 2: 123. https://doi.org/10.3390/antiox14020123
APA StyleZhou, Q., Wu, X.-N., Luo, W.-H., Huang, Q.-H., Feng, L.-L., Wu, Y., & Zhang, C. (2025). Discovery of Effective Inhibitors Against Phosphodiesterase 9, a Potential Therapeutic Target of Alzheimer’s Disease with Antioxidant Capacities. Antioxidants, 14(2), 123. https://doi.org/10.3390/antiox14020123








