Exploring the Therapeutic Potential of Theobroma cacao L.: Insights from In Vitro, In Vivo, and Nanoparticle Studies on Anti-Inflammatory and Anticancer Effects
Abstract
:1. Introduction
2. Experimental Paper Selection Criteria
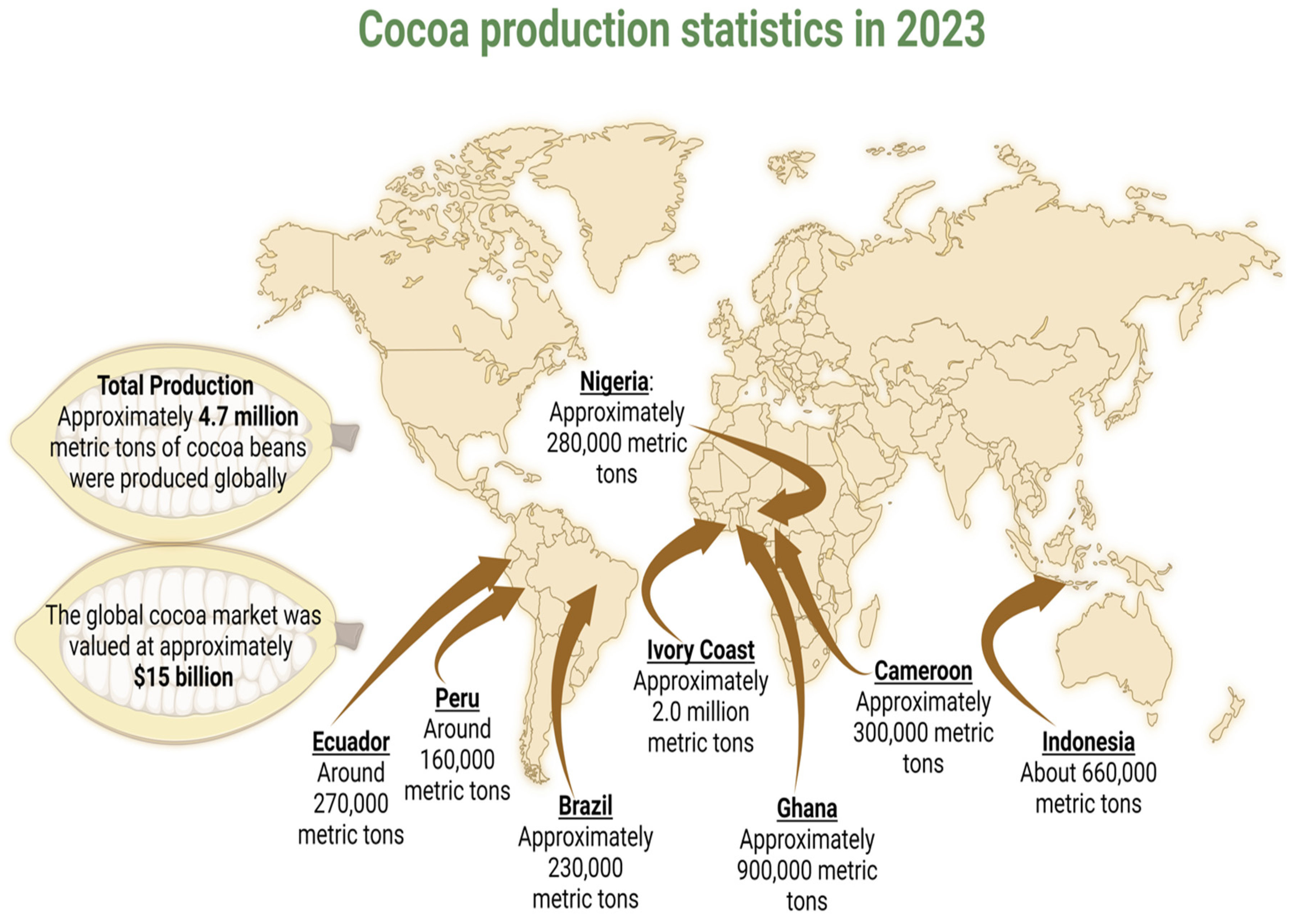
3. Agricultural Livelihoods and Socio-Economic Development
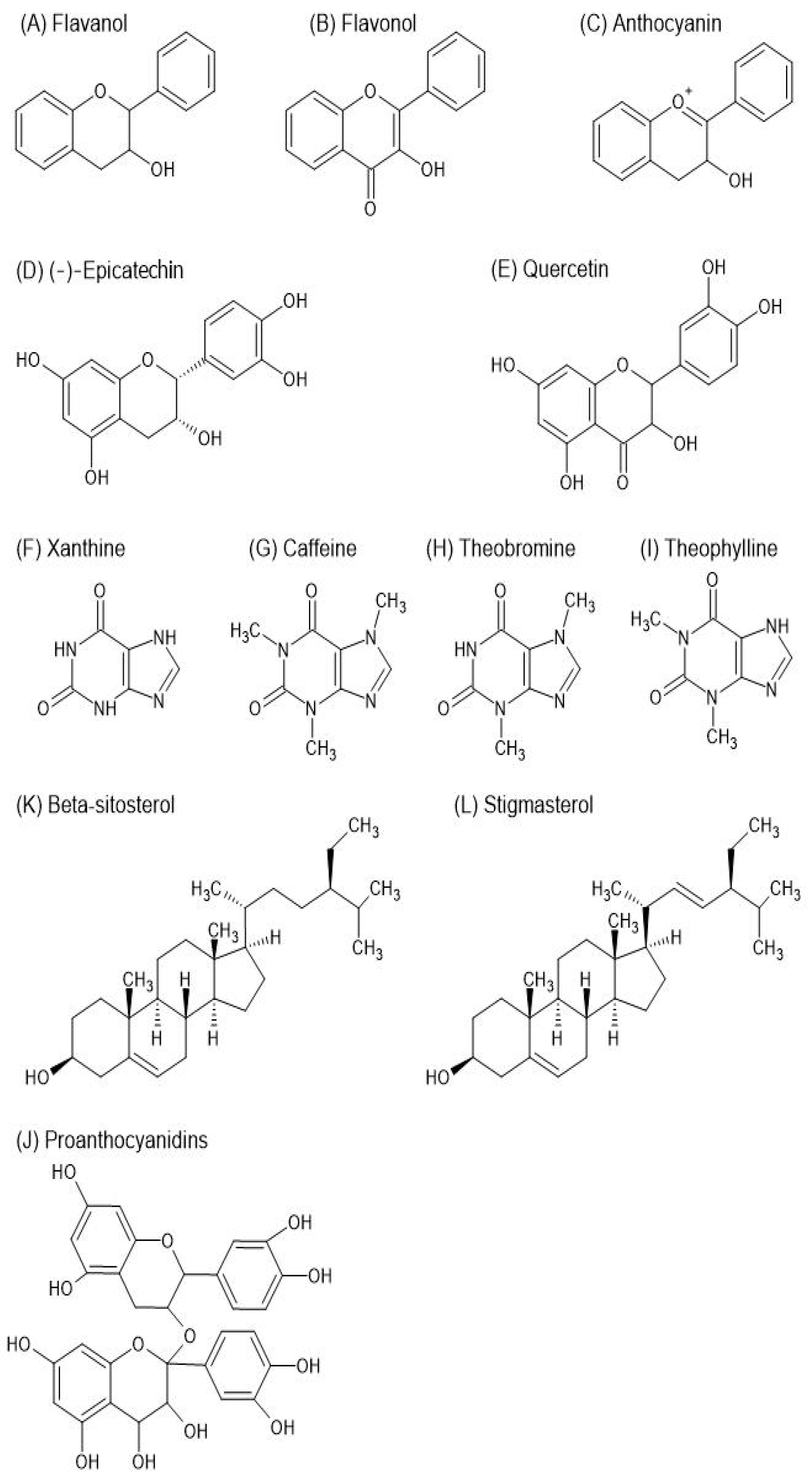
4. Theobroma cacao L.—Characteristics, Secondary Metabolites, and Health Benefits
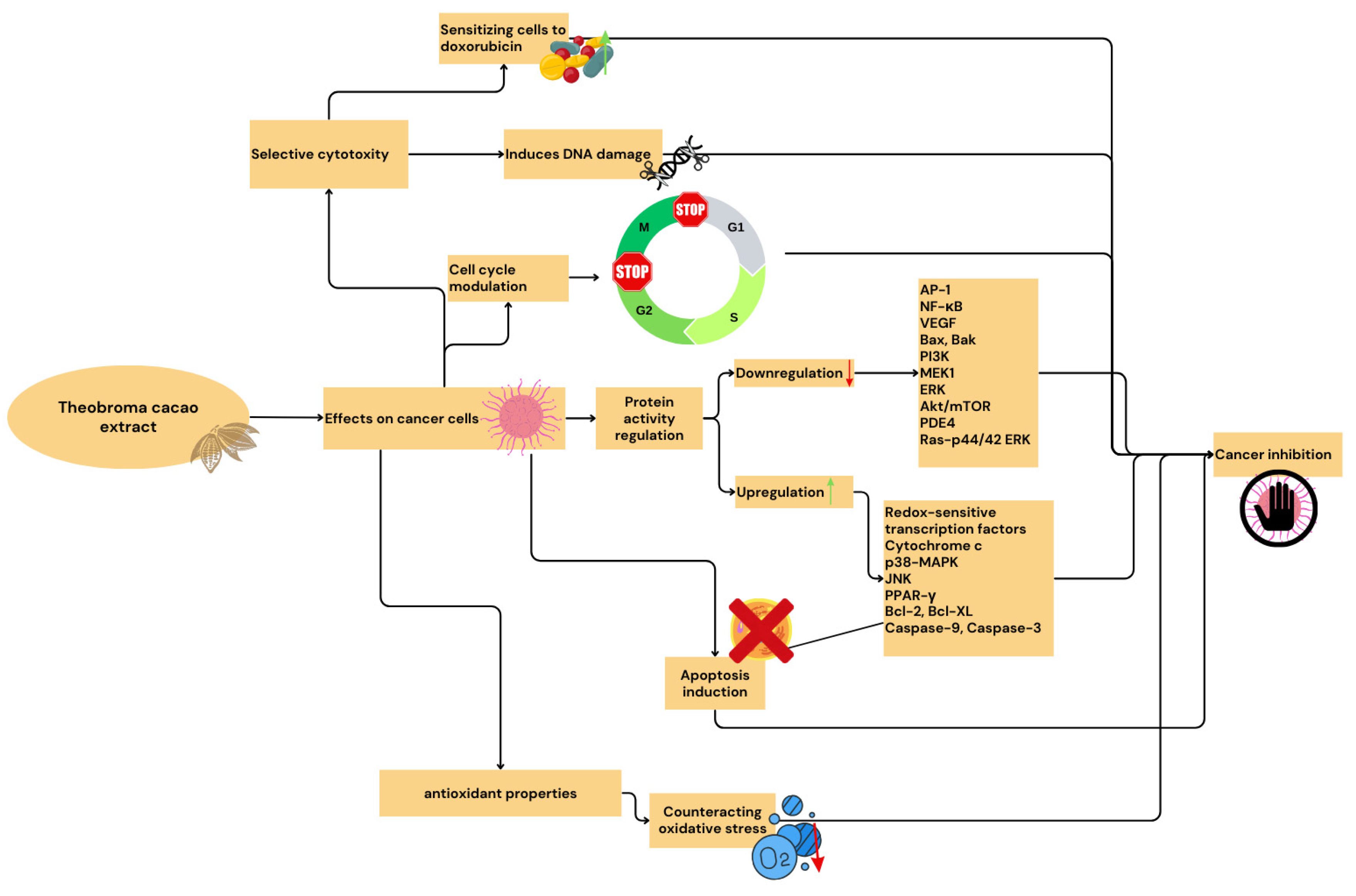
5. Anticancer and Anti-Inflammatory Effects of Theobroma cacao Extract In Vitro and In Vivo
| Part of the Plant | Class of Compounds | Cell Line | Ic50 | Activity/Mechanism/Effects | Ref. |
|---|---|---|---|---|---|
| Cocoa beans | phenylpropenyl-amino acids, hydroxycinnamic-amino acid conjugates, procyanidin compounds, fatty acids and lysophospholipids | MCF-7, Hep-G2, OE19, Caco-2 | Indonesian cocoa beans: MCF-7—254.20 µg/mL Hep-G2—122.00 µg/mL OE19—903.30 µg/mL Caco-2—104.90 µg/mL Peruvian cocoa beans: MCF-7—708.30 µg/mL Hep-G2—199.70 µg/mL OE19—>1000 µg/mL Caco-2—133.90 µg/mL | The extract did not significantly inhibit proliferation of all cancer cell lines. | [48] |
| Cocoa leaf extract | - | MCF-7 | 41.43 µg/mL | The extract did not significantly inhibit proliferation of all cancer cell lines. | [34] |
| Cacao fruit powder | polyphenols flavonoids | HeLa, CaCo2 | HeLa: 1810 µg/mL, 2170 µg/mL CaCo2: 2650 µg/mL | The extract inhibited proliferation of both cancer cell lines. | [54] |
| Theobromine extract | theobromine | LoVo, LoVo/Dx | - | Theobromine highly enhanced cytotoxic and resistance reversal potency of MAE-TPR. | [55] |
| Cocoa bean phenolic extract | protocatechuic acid, p-hydroxybenzoic acid, ideain, catechin, chlorogenic acid, caffeic acid, epicatechin, cyanidin, quercetin, kaempferol, procyanidin B1, procyanidin B2, clovamide | AML12, MLP29 | - | The cocoa extract inhibited drug-triggered cytotoxicity in liver, possibly by activating autophagy. The phenolic compounds protected the cells from celecoxib-induced viability inhibition. Apoptotic pathways (e.g., Bax) are the main target for cocoa extract. | [50] |
| Edel cocoa bean extract from fermented and unfermented cocoa beans | polyphenols | Human gingival fibroblast cells | 1552.877 µg/mL (from fermented cocoa extract) 32.1282 µg/mL (from unfermented cocoa extract) | The extract from fermented cocoa beans did not show a cytotoxic effect, but the extract from unfermented beans showed a cytotoxic effect on fibroblast cells. | [49] |
| Cocoa leaf extract | methanolic extract | MCF-7 | 6.4 µg/mL (concentration after 48 h) | The compounds present in the methanolic leaf extract induced apoptosis in breast cancer cells by inducing cell shrinkage and membrane blebbing. The bioactive fraction upregulated pro-apoptotic genes (DDIT3, HRK, GADD45G) and increased the activity of caspase 3, caspase 8, and caspase 9. | [56] |
| Cocoa powder | caffeine, theobromine, flavonols, procyanidins | Caco-2 | - | The cocoa extract had an anti-proliferative effect by blocking cells at the G2/M phase. Cocoa extract causes polyamine biosynthesis inhibition. | [52] |
| Cocoa leaf, bark, husk, unfermented cocoa shell, fermented cocoa shell, root, cherelle, pith extracts | methanolic extract | MCF-7, A549, HeLa, HepG2, HT-29, MDA-MB-231, WRL-68 | For MCF-7 cell line: Leaf—41.43 µg/mL Bark—71.97 µg/mL Husk—62.23 µg/mL Unfermented shell—65.03 µg/mL Fermented shell—242.33 µg/mL Pith—329.67 µg/mL Root—76.40 µg/mL Cherelle—68.90 µg/mL | The cocoa leaf extract had strong cytotoxic activity against the MCF-7 cell line. The hexane-partitioned fraction had the highest cytotoxic effect. | [57] |
| Theobromine extract | theobromine | U87-MG | - | Theobromine showed antiproliferative properties on the U87-MG cell line. This activity was mediated by the modulation of proteins associated with proliferative and anti-apoptotic pathways (e.g., PDE4, ERK, NF-κB, Akt/mTOR) and the activation of the pro-apoptotic pathway by JNK, p38-MAPK. | [58] |
| Phenolic cocoa powder extract | theobromine, procyanidin B1, procyanidin B2, catechin, epicatechin | HepG2 | - | The cocoa extract protected cells from oxidative stress. | [59] |
| Roasted cocoa, unroasted cocoa, roasted fermented cocoa, unroasted fermented cocoa, | phenols | A549 | - | Cocoa bean extracts inhibited cell proliferation, stopped the cell cycle in different phases, and increased apoptosis process in the A549 cell line. | [53] |
| Cocoa seeds | albumin, globulin, prolamin, glutelin | L5178Y | Unfermented cocoa: Albumin—3140 (µg protein/mL) Globulin—2890 (µg protein/mL) Glutelin—580 (µg protein/mL) Semi-fermented cocoa: Albumin—1510 (µg protein/mL) Globulin—2210 (µg protein/mL) Glutelin—220 (µg protein/mL) | Antitumor activity was observed only in the albumin fraction that inhibited the growth of lymphoma cells. It may be associated with sulfur and hydrophobic amino acids. Antioxidant activity was observed in the glutelin and albumin fractions. No correlation was found between antitumor and antioxidant activity. | [51] |
| Cocoa leaf, bark, husk, fermented and unfermented shell, pith, root, cherelle | methanolic extract | MCF-7, MDA-MB-231, HepG2, HT-29, A549, HeLa, WRL-68 | 41.4 μg/mL–857.04 μg/mL | The root extract had the highest antioxidant activity, but only the cherelle extract inhibited lipid peroxidation. The leaf extract had highest antiproliferative potential. A negative correlation was found between antioxidant activity, total phenolic content, and anticancer effect. | [60] |
| Procyanidin rich cocoa powder extract | procyanidins, flavan-3-ol, catechin | OAW42, OVCAR3 | - | The procyanidin-rich extract increased the intracellular level of ROS. The treatment induced caspase-3-dependent death and the downregulation of MMP2 (a matrix metalloprotease associated with metastasis). | [61] |
| Cocoa bean husk | polyphenol, flavonoids | PC3, DU145 | - | Bean husk includes large amounts of phenolic compounds, which demonstrated antioxidant and anticancer activity on prostate cancer cell lines. | [62] |
| Cocoa pod husk | methanolic extract, lupeol, syringaresinol, catechol, squalene | MCF-7, HeLa | MCF—7: 161.53 μg/mL, 45.36 μg/mL, 53.91 μg/mL HeLa: 272.58 μg/mL, 82.44 μg/mL, 120.71 μg/mL | The ethyl acetate partition derived from cocoa pod husk had moderate activity against MCF-7 cells and low activity against HeLa. The extract demonstrated high levels of lupeol, syringaresinol, catechol and squalene, which showed anticancer activity. | [63] |
| Part of the Plant | Class of Compounds | Cell Line | Dose | Activity/Mechanism/Effects | Ref. |
|---|---|---|---|---|---|
| Cocoa pod husk | Pectin | Mice peritoneal macrophages | 25, 50, 100, 200, 400 μg·mL−1 | Optimized pectin, partially deacetylated pectin, de-esterified pectin, and homogalacturonan pectin obtained from cocoa pod husk are able to modulate some macrophage functions, e.g., the secretion of pro-inflammatory factors (NO, TNF-α, IL-12) and anti-inflammatory IL-10. The optimized pectin fraction showed anti-inflammatory activity, while homogalacturonan pectin increased the number of activated macrophages. | [74] |
| Theobromine extract | Theobromine | CaCo-2 | 10–30 μM | Theobromine protected membrane structure integrity by decreasing the level of specific inflammatory factors such as cytokines and matrix metalloproteinases. | [73] |
| Cocoa powder extract | Flavonoids Polyphenols | NR8383, RAW 264.7 | 5–100 μg/mL | Cocoa extract lowered the level of TNF-α, IL-1α, IL-6, NO and MCP-1. | [64] |
| Aqueous cocoa extract | Polyphenols | J744A.1 | 0.25%, 0.05% | Cocoa extract contains compounds that suppress NO production in macrophages activated by LPS and IFN-γ. | [72] |
| Dried cocoa beans | Polyphenols | HUVEC | 25, 50, 100 ppm | Cocoa extract prevents increases in IL-6 and sVCAM-1 levels in human endothelial cells following induction by plasma from preeclamptic patients. | [65] |
| “Guiana” cocoa pods | Theobromine, Caffeine, Epicatechin, Procyanidin A1, Procyanidin A2, Procyanidin B2, Procyanidin C1 | J774-A1 | 100 μL | Guiana cocoa shows better inhibition of IL-6 production and stimulation of TNF-α secretion. | [66] |
| Cocoa beans | Phenolic extract | THP-1 | 0.1–100 μM | Cocoa extract reduced the inflammatory response in M1 macrophage by increasing the secretion of anti-inflammatory cytokines. It also caused a metabolic switch from the M1 pro-inflammatory to the M2 anti-inflammatory type. | [69] |
| Cocoa bean shell | Theobromine, Caffeine, Protocatechuic acid, Catechin, Epicatechin, Procyanidin B2, Procyanidin B, Procyanidin A, Quercitin-3-O-glycosides | CaCo-2 | 10, 25, 50 μg/mL | The extracts inhibited IL-8 and TLR2, and TLR4, indicating that cocoa could interfere with oxysterol-mediated inflammation. | [67] |
| Theobromine extract | Theobromine | RAW 264.7 | 1–500 μg/mL | Theobromine activated MAPK and NF-κB signaling pathways, which enhance immune effects. Theobromine increases production of inflammatory factors by p38, JNK, and NF-κB pathways. It also upregulates the expression of iNOS. Higher expression of COX-2 causes increased PGE2 production. | [68] |
| Procyanidin-rich cocoa extract | Flavanols, Catechin, Epicatechin | CaCo-2, HT-29 | Max concetration—100 μg/mL or 10–25 μg/mL | Cocoa extract prevents loss of gut barrier function and epithelial inflammation. | [70] |
| Cocoa beans | Polyphenols | GMSM-K | 31.25, 62.5, 125, 250, 500 μg/mL | Cocoa extract inhibited F. nucleatum-induced inflammatory response in monocytes and oral epithelial cells. The extract improves the barrier function of oral epithelial cells. | [71] |
| Cocoa powder | Aqueous and ethanolic extract | PBMC, THP-1 | 0.5–10 μg/mL | Cocoa extracts suppressed mitogen-induced degradation of tryptophan. IFN-γ and neopterin production were strongly inhibited by extracts. | [75] |
| Cocoa extract | - | HUVEC | 6.25, 12.5, 25, 50, 100 μg/mL | The cocoa extract inhibited angiotensin-covering enzyme activity and increased the NO level. | [76] |
6. Antitumor and Anti-Inflammatory Effects of Theobroma cacao Extract in an In Vitro and In Vivo Model in Combination with Nanoparticles
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDP | Gross domestic product |
| ALT | Alanine transaminase |
| BDNF | Brain-derived neurotrophic factor |
| HRS | Hodgkin and Reed-Sternberg |
| THP-1 | Human acute monocytic leukemia |
| TAMs | Tumor-associated macrophages |
| ROS | Reactive oxygen species |
| MMP2 | Matrix mettaloproteinase-2 |
| MAPK | Mitogen activated protein kinases |
| NO | Nitric oxide |
| TNF | Tumor necrosis factor |
| COX-2 | Cyclo-oxygenase-2 |
| PTP1B | Protein tyrosine phosphatase 1B |
| PGE2 | Prostaglandin E2 |
| BMP-2 | Bone morphogenetic protein-2 |
| EPR | Permeability and retention effect |
References
- Díaz-Valderrama, J.R.; Leiva-Espinoza, S.T.; Aime, M.C. The history of cacao and its diseases in the Americas. Phytopathology 2020, 110, 1604–1619. [Google Scholar] [CrossRef] [PubMed]
- McNeil, C. Chocolate in Mesoamerica: A Cultural History of Cacao; University Press of Florida: Gainesville, FL, USA, 2006. [Google Scholar] [CrossRef]
- Richardson, J.E.; Whitlock, B.A.; Meerow, A.W.; Madriñán, S. The age of chocolate: A diversification history of Theobroma and Malvaceae. Front. Ecol. Evol. 2015, 3, 120. [Google Scholar] [CrossRef]
- Cuatrecasas, J. Cacao and its allies: A taxonomic revision of the genus Theobroma, Contrib. U.S. Natl. Herb. 1964, 35, 379–614. [Google Scholar]
- Motamayor, J.C.; Lachenaud, P.; Da Silva e Mota, J.W.; Loor, R.; Kuhn, D.N.; Brown, J.S.; Schnell, R.J. Geographic and genetic population differentiation of the Amazonian chocolate tree (Theobroma cacao L.). PLoS ONE 2008, 3, e3311. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, T.; Justeson, J. The history of the word for cacao in ancient Mesoamerica. Anc. Mesoam. 2007, 18, 193–237. [Google Scholar] [CrossRef]
- Zeng, H.; Locatelli, M.; Bardelli, C.; Amoruso, A.; Coisson, J.D.; Travaglia, F.; Arlorio, M.; Brunelleschi, S. Anti-inflammatory properties of clovamide and Theobroma cacao phenolic extracts in human monocytes: Evaluation of respiratory burst, cytokine release, NF-κB activation, and PPARγ modulation. J. Agric. Food Chem. 2011, 59, 5342–5350. [Google Scholar] [CrossRef]
- Verna, R. The history and science of chocolate. Malays. J. Pathol. 2013, 35, 111. [Google Scholar]
- Ebuehi, O.A.; Anams, C.; Gbenle, O.D.; Ajagun-Ogunleye, M.O. Hydro-ethanol seed extract of Theobroma cacao exhibits antioxidant activities and potential anticancer property. J. Food Biochem. 2019, 43, e12767. [Google Scholar] [CrossRef]
- Baharum, Z.; Akim, A.M.; Hin, T.Y.Y.; Hamid, R.A.; Kasran, R. Theobroma cacao: Review of the extraction, isolation, and bioassay of its potential anti-cancer compounds. Trop. Life Sci. Res. 2016, 27, 21–42. [Google Scholar]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479–3485. [Google Scholar] [CrossRef]
- Jalil, A.; Ismail, A. Polyphenols in cocoa and cocoa products: Is there a link between antioxidant properties and health? Molecules 2008, 13, 2190–2219. [Google Scholar] [CrossRef] [PubMed]
- Corti, R.; Flammer, A.J.; Hollenberg, N.K.; Lüscher, T.F. Cocoa and cardiovascular health. Contemp. Rev. Cardiovasc. Med. Cocoa 2009, 119, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Ellam, S.; Williamson, G. Cocoa and human health. Annu. Rev. Nutr. 2013, 33, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Salvatori, T.; Villarini, M.; Moretti, M. Can chocolate consumption reduce cardio-cerebrovascular risk? A systematic review and meta-analysis. Nutrition 2018, 46, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Cocchi, C.A.; Lanfredini, M.; Keen, C.L.; Gershwin, M.E. Chocolate at heart: The anti-inflammatory impact of cocoa flavanols. Mol. Nutr. Food Res. 2008, 52, 1340–1348. [Google Scholar] [CrossRef]
- Patil, P.P.; Khanal, P.; Patil, V.S.; Charla, R.; Harish, D.R.; Patil, B.M.; Roy, S. Effect of Theobroma cacao L. on the efficacy and toxicity of doxorubicin in mice bearing Ehrlich ascites carcinoma. Antioxidants 2022, 11, 1094. [Google Scholar] [CrossRef]
- Ruzaidi, A.; Abbe Maleyki, M.; Amin, I.; Nawalyah, A.; Muhajir, H. Protective effect of polyphenol-rich extract prepared from Malaysian cocoa (Theobroma cacao) on glucose levels and lipid profiles in streptozotocin-induced diabetic rats. J. Sci. Food Agric. 2008, 88, 1442–1447. [Google Scholar] [CrossRef]
- Kosoko, A.M.; Olurinde, O.J.; Akinloye, O.A. Doxorubicin induced neuro-and cardiotoxicities in experimental rats: Protection against oxidative damage by Theobroma cacao stem bark. Biochem. Biophys. Rep. 2017, 10, 303–317. [Google Scholar] [CrossRef]
- Zhang, D. Theobroma. In Wild Crop Relatives: Genomic and Breeding Resources: Plantation and Ornamental Crops; Springer: Berlin/Heidelberg, Germany, 2011; pp. 277–296. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2024, 20, 101–124. [Google Scholar] [CrossRef]
- Farley, R. CHOCOLATE CITY, VANILLA SUBURBS REVISITED: The Racial Integration of Detroit’s Suburbs. Du Bois Rev. Soc. Sci. Res. Race 2022, 19, 1–29. [Google Scholar] [CrossRef]
- Brown, A.L.; Bakke, A.J.; Hopfer, H. Understanding American premium chocolate consumer perception of craft chocolate and desirable product attributes using focus groups and projective mapping. PLoS ONE 2020, 15, e0240177. [Google Scholar] [CrossRef] [PubMed]
- Boysen, O.; Ferrari, E.; Nechifor, V.; Tillie, P. Impacts of the Cocoa Living Income Differential Policy in Ghana and Côte D’Ivoire; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar] [CrossRef]
- De Souza, P.A.; Moreira, L.F.; Sarmento, D.H.A.; da Costa, F.B. Cacao—Theobroma cacao. Exot. Fruits 2018, 69–76. [Google Scholar] [CrossRef]
- Bekele, F.; Phillips-Mora, W. Cacao (Theobroma cacao L.) breeding, Adv. Plant Breed. Strateg. Ind. Food Crops 2019, 6, 409–487. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J.L. Cocoa polyphenols and their potential benefits for human health. Oxid. Med. Cell Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef] [PubMed]
- Smit, H.J. Theobromine and the Pharmacology of Cocoa. In Methylxanthines. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Jalal, M.A.F.; Collin, H.A. Polyphenols of mature plant, seedling, and tissue cultures of Theobroma cacao. Phytochemistry 1977, 16, 1377–1380. [Google Scholar] [CrossRef]
- Tauchen, J.; Bortl, L.; Huml, L.; Miksatkova, P.; Doskocil, I.; Marsik, P.; Villegas, P.P.P.; Flores, Y.B.; Van Damme, P.V.; Lojka, B.; et al. Phenolic composition, antioxidant and anti-proliferative activities of edible and medicinal plants from the Peruvian Amazon. Rev. Bras. Farmacogn. 2016, 26, 728–737. [Google Scholar] [CrossRef]
- Tokede, O.A.; Gaziano, J.M.; Djoussé, L. Effects of cocoa products/dark chocolate on serum lipids: A meta-analysis. Eur. J. Clin. Nutr. 2011, 65, 879–886. [Google Scholar] [CrossRef]
- Montagna, M.T.; Diella, G.; Triggiano, F.; Caponio, G.R.; De Giglio, O.; Caggiano, G.; Di Ciaula, A.; Portincasa, P. Chocolate, “Food of the Gods”: History, science, and human health. Int. J. Environ. Res. Public Health 2019, 16, 4960. [Google Scholar] [CrossRef]
- Zainal, B.; Abdah, M.; Taufiq-Yap, Y.; Roslida, A.; Rosmin, K. Anticancer agents from non-edible parts of Theobroma cacao. Nat. Prod. Chem. Res. 2014, 2. [Google Scholar] [CrossRef]
- Weikart, D.K.; Indukuri, V.V.; Racine, K.C.; Coleman, K.M.; Kovac, J.; Cockburn, D.W.; Hopfer, H.; Neilson, A.P.; Lambert, J.D. Effect of processing on the anti-inflammatory efficacy of cocoa in a high fat diet-induced mouse model of obesity. J. Nutr. Biochem. 2022, 109, 109117. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Lambert, J.D. Modulation of metabolic syndrome-related inflammation by cocoa. Mol. Nutr. Food Res. 2013, 57, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and dark chocolate polyphenols: From biology to clinical applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Badrie, N.; Bekele, F.; Sikora, E.; Sikora, M. Cocoa agronomy, quality, nutritional, and health aspects. Crit. Rev. Food Sci. Nutr. 2015, 55, 620–659. [Google Scholar] [CrossRef] [PubMed]
- Cerri, M.; Reale, L.; Zadra, C. Metabolite Storage in Theobroma cacao L. Seed: Cyto-Histological and Phytochemical Analyses. Front. Plant Sci. 2019, 10, 1599. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations, and cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Sohrab, S.S.; Raj, R.; Nagar, A.; Hawthorne, S.; Paiva-Santos, A.C.; Kamal, M.A.; El-Daly, M.M.; Azhar, E.I.; Sharma, A. Chronic inflammation’s transformation to cancer: A nanotherapeutic paradigm. Molecules 2023, 28, 4413. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lu, Y.; Zheng, J.; Jiang, X.; Shen, H.; Shang, X.; Lu, Y.; Fu, P. Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: New insights and therapeutic implications. Cell Death Dis. 2023, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, M.; Pacifico, S.; Nugnes, R.; Russo, C.; Orlo, E.; Piccolella, S.; Isidori, M. Theobroma cacao Criollo var. beans: Biological properties and chemical profile. Foods 2021, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Fitriyani, N.; Sutjiati, R.; Arina, Y.M.D.A. Cytotoxicity test of cocoa bean extract (Theobroma cacao L.) fermented and unfermented Edel varieties against human gingival fibroblast cells. Health Not. 2021, 5, 363–367. [Google Scholar]
- Arlorio, M.; Bottini, C.; Travaglia, F.; Locatelli, M.; Bordiga, M.; Coïsson, J.D.; Martelli, A.; Tessitore, L. Protective activity of Theobroma cacao L. phenolic extract on AML12 and MLP29 liver cells by preventing apoptosis and inducing autophagy. J. Agric. Food Chem. 2009, 57, 10612–10618. [Google Scholar] [CrossRef]
- Preza, A.M.; Jaramillo, M.E.; Puebla, A.M.; Mateos, J.C.; Hernández, R.; Lugo, E. Antitumor activity against murine lymphoma L5178Y model of proteins from cacao (Theobroma cacao L.) seeds in relation with in vitro antioxidant activity. BMC Complement. Altern. Med. 2010, 10, 61. [Google Scholar] [CrossRef]
- Carnésecchi, S.; Schneider, Y.; Lazarus, S.A.; Coehlo, D.; Gossé, F.; Raul, F. Flavanols and procyanidins of cocoa and chocolate inhibit growth and polyamine biosynthesis of human colonic cancer cells. Cancer Lett. 2002, 175, 147–155. [Google Scholar] [CrossRef]
- Bauer, D.; de Abreu, J.P.; Oliveira, H.S.; Goes-Neto, A.; Koblitz, M.G.; Teodoro, A.J. Antioxidant activity and cytotoxicity effect of cocoa beans subjected to different processing conditions in human lung carcinoma cells. Oxid. Med. Cell. Longev. 2016, 2016, 7428515. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Cock, I.E. Acai, cacao and maca extracts: Anticancer activity and growth inhibition of microbial triggers of selected autoimmune inflammatory diseases. Pharmacogn. Commun. 2016, 6, 204–214. [Google Scholar] [CrossRef]
- Środa-Pomianek, K.; Michalak, K.; Palko-Łabuz, A.; Poła, A.; Dzięgiel, P.; Puła, B.; Świątek, P.; Wesołowska, O. Cytotoxic and multidrug resistance reversal activity of phenothiazine derivative is strongly enhanced by theobromine, a phytochemical from cocoa. Eur. J. Pharmacol. 2019, 849, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Abu Bakar, M.F.; Md Akim, A.; Bin Baharum, Z.; Ellulu, M.S.; Fadel, A. Induction of apoptosis and modulation of caspase activity on MCF-7 human breast cancer cells by bioactive fractionated cocoa leaf extract. Asian Pac. J. Cancer Prev. 2023, 24, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Zainal, B.; Abdah, M.; Taufiq Yap, Y.; Roslida, A.; Mohd Redzuan, S.; Kasran, R. Bioactivity-guided fractionation of potent anti-cancer properties from non-edible tissues of Theobroma cacao. Malays. Cocoa J. 2016, 9, 170–181. [Google Scholar]
- Sugimoto, N.; Miwa, S.; Hitomi, Y.; Nakamura, H.; Tsuchiya, H.; Yachie, A. Theobromine, the primary methylxanthine found in Theobroma cacao, prevents malignant glioblastoma proliferation by negatively regulating phosphodiesterase-4, extracellular signal-regulated kinase, Akt/mammalian target of rapamycin kinase, and nuclear factor-kappa B. Nutr. Cancer 2014, 66, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Ramos, S.; Mateos, R.; Granado Serrano, A.B.; Izquierdo-Pulido, M.; Bravo, L.; Goya, L. Protection of human HepG2 cells against oxidative stress by cocoa phenolic extract. J. Agric. Food Chem. 2008, 56, 7765–7772. [Google Scholar] [CrossRef]
- Baharum, Z.; Akim, A.M.; Taufiq-Yap, Y.H.; Hamid, R.A.; Kasran, R. In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of Theobroma cacao. Molecules 2014, 19, 18317–18331. [Google Scholar] [CrossRef]
- Taparia, S.S.; Khanna, A. Procyanidin-rich extract of natural cocoa powder causes ROS-mediated caspase-3 dependent apoptosis and reduction of pro-MMP-2 in epithelial ovarian carcinoma cell lines. Biomed. Pharmacother. 2016, 83, 130–140. [Google Scholar] [CrossRef]
- Choi, J.; Yang, C.; Lim, W.; Song, G.; Choi, H. Antioxidant and apoptotic activity of cocoa bean husk extract on prostate cancer cells. Mol. Cell. Toxicol. 2022, 18, 1–11. [Google Scholar] [CrossRef]
- Yahya, M.; Ginting, B.; Saidi, N. Anticancer and antiretroviral activities of methanolic extract from Theobroma cacao L pod husk: Focusing on the ethyl acetate partition. F1000Research 2022, 11, 1395. [Google Scholar] [CrossRef]
- Ramiro, E.; Franch, A.; Castellote, C.; Pérez-Cano, F.; Permanyer, J.; Izquierdo-Pulido, M.; Castell, M. Flavonoids from Theobroma cacao down-regulate inflammatory mediators. J. Agric. Food Chem. 2005, 53, 8506–8511. [Google Scholar] [CrossRef]
- Rahayu, B.; Baktiyani, S.C.; Nurdiana, N. Theobroma cacao increases cells viability and reduces IL-6 and sVCAM-1 level in endothelial cells induced by plasma from preeclamptic patients. Pregnancy Hypertens. 2016, 6, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Jean-Marie, E.; Bereau, D.; Poucheret, P.; Guzman, C.; Boudard, F.; Robinson, J.C. Antioxidative and immunomodulatory potential of the endemic French Guiana wild cocoa “Guiana”. Foods 2021, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Testa, G.; Sottero, B.; Poli, G.; Zeppa, G.; Biasi, F. A dietary mixture of oxysterols induces in vitro intestinal inflammation through TLR2/4 activation: The protective effect of cocoa bean shells. Antioxidants 2019, 8, 151. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, I.W.; Ha, S.K. Immunostimulatory activities of theobromine on macrophages via the activation of MAPK and NF-κB signaling pathways. Curr. Issues Mol. Biol. 2022, 44, 4216–4228. [Google Scholar] [CrossRef] [PubMed]
- Dugo, L.; Belluomo, M.G.; Fanali, C.; Russo, M.; Cacciola, F.; Maccarrone, M.; Sardanelli, A.M. Effect of cocoa polyphenolic extract on macrophage polarization from proinflammatory M1 to anti-inflammatory M2 state. Oxid. Med. Cell. Longev. 2017, 2017, 6293740. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Glisan, S.L.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Lambert, J.D.; Neilson, A.P. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J. Nutr. Biochem. 2015, 26, 827–831. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Huacho, P.M.; Grenier, D. A cocoa (Theobroma cacao L.) extract impairs the growth, virulence properties, and inflammatory potential of Fusobacterium nucleatum and improves oral epithelial barrier function. PLoS ONE 2021, 16, e0252029. [Google Scholar] [CrossRef]
- Ono, K.; Takahashi, T.; Kamei, M.; Mato, T.; Hashizume, S.; Kamiya, S.; Tsutsumi, H. Effects of an aqueous extract of cocoa on nitric oxide production of macrophages activated by lipopolysaccharide and interferon-gamma. Nutrition 2003, 19, 681–685. [Google Scholar] [CrossRef]
- Iaia, N.; Rossin, D.; Sottero, B.; Venezia, I.; Poli, G.; Biasi, F. Efficacy of theobromine in preventing intestinal CaCo-2 cell damage induced by oxysterols. Arch. Biochem. Biophys. 2020, 694, 108591. [Google Scholar] [CrossRef]
- Amorim, J.C.; Vriesmann, L.C.; Petkowicz, C.L.; Martinez, G.R.; Noleto, G.R. Modified pectin from Theobroma cacao induces potent pro-inflammatory activity in murine peritoneal macrophage. Int. J. Biol. Macromol. 2016, 92, 1040–1048. [Google Scholar] [CrossRef]
- Jenny, M.; Santer, E.; Klein, A.; Ledochowski, M.; Schennach, H.; Ueberall, F.; Fuchs, D. Cacao extracts suppress tryptophan degradation of mitogen-stimulated peripheral blood mononuclear cells. J. Ethnopharmacol. 2009, 122, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.A.; Persson, K.; Hägg, S.; Andersson, R.G. Effects of cocoa extract and dark chocolate on angiotensin-converting enzyme and nitric oxide in human endothelial cells and healthy volunteers—A nutrigenomics perspective. J. Cardiovasc. Pharmacol. 2011, 57, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Boriollo, M.F.G.; Alves, V.E.; Silva, T.A.; Silva, J.J.; Barros, G.B.S.; Dias, C.T.S.; Höfling, J.F.; Oliveira, N.M.S. Decrease of the DXR-induced genotoxicity and nongenotoxic effects of Theobroma cacao revealed by micronucleus assay. Braz. J. Biol. 2021, 81, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Muhvić, D.; Pavletić, A.; Ðudarić, L. Cocoa polyphenols exhibit antioxidant, anti-inflammatory, anticancerogenic, and anti-necrotic activity in carbon tetrachloride-intoxicated mice. J. Funct. Foods 2016, 23, 177–187. [Google Scholar] [CrossRef]
- Patil, P.P.; Kumar, P.; Khanal, P.; Patil, V.S.; Darasaguppe, H.R.; Bhandare, V.V.; Bhatkande, A.; Shukla, S.; Joshi, R.K.; Patil, B.M.; et al. Computational and experimental pharmacology to decode the efficacy of Theobroma cacao L. against doxorubicin-induced organ toxicity in EAC-mediated solid tumor-induced mice. Front. Pharmacol. 2023, 14, 1174867. [Google Scholar] [CrossRef]
- Bisson, J.F.; Guardia-Llorens, M.A.; Hidalgo, S.; Rozan, P.; Messaoudi, M. Protective effect of Acticoa powder, a cocoa polyphenolic extract, on prostate carcinogenesis in Wistar-Unilever rats. Eur. J. Cancer Prev. 2008, 17, 54–61. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, S.; Park, J.Y.; Harvatine, K.; Lambert, J.D. Dietary cocoa reduces metabolic endotoxemia and adipose tissue inflammation in high-fat fed mice. J. Nutr. Biochem. 2014, 25, 439–445. [Google Scholar] [CrossRef]
- Izzuddin, A.F.A.; Nurkesuma, A.; No, J.K.; Timur, J.J. The potential of cocoa (Theobroma cacao L.) pods extract in periodontal dressing to rabbit gingival wound healing. Sci. Coop. Med. Work 2015, 1–16. Available online: http://med-scoop.org/medPapers/2015/IWDN-2015/2.Izzuddin.pdf (accessed on 7 November 2024).
- Dare, C.A.; Onwumelu, R.N.; Oyedapo, O.O. Biochemical studies on the effects of polyphenols from fermented and unfermented acetone extracts of cocoa (Theobroma cacao L.) seeds on antioxidant enzymes of streptozotocin-induced diabetic rats. Niger. J. Biochem. Mol. Biol. 2013, 28, 45–59. [Google Scholar]
- Rahayu, Y.C.; Setiawatie, E.M.; Rahayu, R.P.; Sakinah, N.N.; Kusumawardani, B.; Gunadi, A.; Ryandhana, P.H. Effects of cocoa pod husk extract (Theobroma cacao L.) on alveolar bone in experimental periodontitis rats. Trends Sci. 2023, 20, 6535. [Google Scholar] [CrossRef]
- Oyeleke, S.A.; Ajayi, A.M.; Umukoro, S.; Aderibigbe, A.O.; Ademowo, O.G. Anti-inflammatory activity of Theobroma cacao L. stem bark ethanol extract and its fractions in experimental models. J. Ethnopharmacol. 2018, 222, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Irondi, E.A.; Olanrewaju, S.; Oboh, G.; Olasupo, F.; Boligon, A.A. Inhibitory potential of cocoa leaves polyphenolics-rich extract on xanthine oxidase and angiotensin 1-converting enzyme. J. Biol. Act. Prod. Nat. 2017, 7, 39–51. [Google Scholar] [CrossRef]
- Pérez-Berezo, T.; Ramírez-Santana, C.; Franch, A.; Ramos-Romero, S.; Castellote, C.; Pérez-Cano, F.J.; Castell, M. Effects of a cocoa diet on an intestinal inflammation model in rats. Exp. Biol. Med. 2012, 237, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kundu, J.K.; Kim, S.O.; Chun, K.S.; Lee, H.J.; Surh, Y.J. Cocoa polyphenols inhibit phorbol ester-induced superoxide anion formation in cultured HL-60 cells and expression of cyclooxygenase-2 and activation of NF-kappaB and MAPKs in mouse skin in vivo. J. Nutr. 2006, 136, 1150–1155. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Zakłos-Szyda, M.; Juśkiewicz, J.; Bojczuk, M.; Oracz, J.; Budryn, G.; Miśkiewicz, K.; Krysiak, W.; Zduńczyk, Z.; Jurgoński, A. Cocoa bean (Theobroma cacao L.) phenolic extracts as PTP1B inhibitors, hepatic HepG2 and pancreatic β-TC3 cell cytoprotective agents and their influence on oxidative stress in rats. Food Res. Int. 2016, 89, 946–957. [Google Scholar] [CrossRef]
- Osukoya, O.; Fadaka, A.; Adewale, O.; Oluloye, O.; Ojo, O.; Ajiboye, B.; Adewumi, D.; Kuku, A. In vitro anthelmintic and antioxidant activities of the leaf extracts of Theobroma cacao L. AIMS Agric. Food 2019, 4, 568–577. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Oehler, J.B.; Rajapaksha, W.; Albrecht, H. Emerging applications of nanoparticles in the diagnosis and treatment of breast cancer. J. Pers. Med. 2024, 14, 723. [Google Scholar] [CrossRef]
- Garg, P.; Pareek, S.; Kulkarni, P.; Salgia, R.; Singhal, S.S. Nanoengineering solutions for cancer therapy: Bridging the gap between clinical practice and translational research. J. Clin. Med. 2024, 13, 3466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.D.; Hu, Y.J.; Yu, L.; Zhou, X.G.; Wu, J.M.; Tang, Y.; Qin, D.L.; Fan, Q.Z.; Wu, A.G. Nanoparticles: A hope for the treatment of inflammation in CNS. Front. Pharmacol. 2021, 12, 683935. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s frontier in combatting infectious and inflammatory diseases: Prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Yang, Y.P.; Nicol, C.J.B.; Wang, C.J. Gold nanoparticles in neurological diseases: A review of neuroprotection. Int. J. Mol. Sci. 2024, 25, 2360. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, B.; Morshed, R.; Tobias, A.; Cheng, Y.; Ahmed, A.U.; Lesniak, M.S. Drug-loaded nanoparticle systems and adult stem cells: A potential marriage for the treatment of malignant glioma? Oncotarget 2013, 4, 378–396. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Chowdhury, N.R.; MacGregor-Ramiasa, M.; Zilm, M.; Majewski, P.; Vasilev, K. ‘Chocolate’ silver nanoparticles: Synthesis, antibacterial activity and cytotoxicity. J. Colloid Interface Sci. 2016, 482, 151–158. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Rostami-Vartooni, A.; Bagherzadeh, M. Green synthesis of Pd/CuO nanoparticles by Theobroma cacao L. seeds extract and their catalytic performance for the reduction of 4-nitrophenol and phosphine-free Heck coupling reaction under aerobic conditions. J. Colloid Interface Sci. 2015, 448, 106–113. [Google Scholar] [CrossRef]
- Roy Chowdhury, N.; Cowin, A.J.; Zilm, P.; Vasilev, K. “Chocolate” gold nanoparticles—One pot synthesis and biocompatibility. Nanomaterials 2018, 8, 496. [Google Scholar] [CrossRef]
- Fazal, S.; Jayasree, A.; Sasidharan, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Green synthesis of anisotropic gold nanoparticles for photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2014, 6, 8080–8089. [Google Scholar] [CrossRef]
- Anooj, E.S.; Praseetha, P.K. Cocoa bean-extract mediated graphene quantum dots as antimicrobial, anticancer and plant growth regulators. Int. J. Recent Technol. Eng. 2019, 8, 269–273. [Google Scholar] [CrossRef]

| Part of the Plant | Class of Compounds | Organism | Dose | N | Exposure Time | Activity/Mechanism/Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Cocoa seeds glycolic extract | polyphenols | Mice bone marrow | Genotoxicity test: 0.5, 1.0, 1.5, and 2.0 g/kg of theobroma extract Antigenotoxicity test: 2.0 g/kg of theobroma extract and 5.0 mg/kg of doxorubicin | 5 male mice and 5 female mice | 24 h 48 h | The combination of cocoa extract (2 g/kg) with doxorubicin (5 mg/kg) partially increases systemic toxicity. The extract enhances the toxic effects of doxorubicin. | [77] |
| Cocoa cake extract | polyphenols, flavonoids, gallic acid, procyanidin B1, epigallocatechin, catechin, procyanidin B2, epicatechin, epigallocatechin gallate, vanillin, p-coumaric acid, m-coumaric acid, quercetin | BALB/cN mice liver and blood samples | Cocoa extract—34.5 mg/kg Epicatechin extract—2.24 mg/kg | 4 groups of 8 female BALB/cN mice | 2 weeks | Cocoa polyphenols improved cellular redox state and molecular signaling pathways associated with oxidative stress. They also decreased hepatic cell necrosis and suppressed Pkm2. Hepatotoxicity was decreased by lowering the activity of serum transaminases and phosphatic phosphatase activity. The extract acted as a carcinogenic inhibitor by down-regulating Hsp 90 and returning the expression of Hsp70 to normal levels. | [78] |
| Cocoa nibs powder | polyphenols, alkaloids, flavonoids | Female BALB/c mice vital organs (heart, liver, kidney) and blood samples | Doxorubicin-treated mice—4.91 mg/kg Cocoa extract-treated mice—200 mg/kg Doxorubicin- and cocoa-treated mice—4.91 mg/kg of doxorubicin and 200 mg/kg of cocoa extract | 5 groups of 16 mice | 21 days | Cocoa extract protects organs against doxorubicin-induced intoxication (heart, liver, kidney). It also has a synergistic anticancer effect, enhancing doxorubicin activity. | [79] |
| Acticoa powder | flavonoids, flavonol, epicatechin, procyanidins | Wistar–Unilever rat prostate | Acticoa powder 24 mg/kg, Acticoa powder 48 mg/kg | 4 groups of 15 rats | 9 months | Daily consumption of cocoa products influence plasma flavonol concentration, enhancing the antioxidant potential of plasma. Treatment with Acticoa powder protects rats from CI prostate tumor. | [80] |
| Cocoa pods powder | polyphenols, flavonoids, alkaloids | BALB/c mice Ehrlich ascites carcinoma tumor cells | Doxorubicin-treated mice—4.91 mg/kg Cocoa extract-treated mice—200 mg/kg Doxorubicin and cocoa-treated mice—4.91 mg/kg of doxorubicin and 200 mg/kg of cocoa extract Pretreated mice—200 mg/kg of cocoa extract for 21 days before cancer induction. After cancer induction, the mice received 200 mg/kg of cocoa extract and 4.91 mg/kg of doxorubicin | 6 groups of 10 mice | 21 days | Cocoa powder protects against vital organ damage induced by doxorubicin without compromising chemotherapeutic effects. Moreover, it can neutralize free radicals. | [17] |
| Part of the Plant | Class of Compounds | Organism | Dose | N | Exposure Time | Activity/Mechanism/Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Cocoa polyphenols from cocoa powder | Polyphenols, Gallic acid, Epicatechin. | Skin from ears of ICR mice | With 12-O-tetradecanoylphorbol-13-acetate: 4 mg/kg 20 mg/kg 40 mg/kg 200 mg/kg Without 12-O-tetradecanoylphorbol-13-acetate: 200 mg/kg | Groups of 6 female mice | 5 h | Cocoa polyphenols lower the activity of COX-2 expression, inhibit the activation of MAPK and NF-κB pathways. | [84] |
| Unsweetened cocoa powder | Polyphenols. | Male C57BL/6 mice | 80 mg/g of unsweetened cocoa powder | Low-fat diet 23 mice High-fat diet 21 mice High-fat diet cocoa treated 24 mice | 18 weeks | Cocoa supplementation decreases adipose tissue inflammation by downregulating genes associated with NF-κB. | [82] |
| Cocoa pod extract | Polyphenols, Flavonoids, Tannins. | Rabbit | Concentration of cocoa: 0%, 5%, 10%, 15% | 36 male rabbits | 3 days 5 days 7 days | Cocoa pod extract can accelerate the speed of wound healing. | [83] |
| Cocoa bean phenolic extract | Phenols | Wistar rats | Raw cocoa bean: 2.25% of diet; Roasted cocoa bean: 2.45% of diet; Flavan-3-ol: 0.114% of diet | 5 groups of 8 male rats | 4 weeks | A diet enriched with cocoa bean extract causes fat tissue reduction, PTP1B inhibition, hepatic steatosis attenuation, ROS protection, and improved serum lipid profile, and increases serum ACl. It shows anti-obesity properties. | [89] |
| Leaf extracts | Tannins, Phenols, Saponins, Terpenoids, Flavonoids, Glycosides. | African earthworms (Pheretima posthuma) | 10, 25, 50 mg/mL | - | - | Cocoa leaf extracts have antioxidant and anthelmintic activity. | [90] |
| Cocoa pods powder | Flavonoids, Alkaloids, Tannins, Saponins. | Wistar rats | Fermented cocoa: 150 mg/kg 300 mg/kg | 35 rats | 21 days | Anti-hyperglycemia compounds were discovered in fermented cocoa polyphenol extracts. | [85] |
| Cocoa pod husk extract | Polyphenols | Wistar rats | Cocoa pod husk extract 100 mg/mL | 24 male rats | 7 days 14 days | Cocoa pod husk extract increases alveolar bone regeneration in periodontitis by increasing osteoblast numbers and BMP-2 expression. | [86] |
| Stem bark extract | - | Wistar rats | Cocoa stem bark: Extract 250 mg/kg Ethylacetate fraction 65, 125 and 250 mg/kg | 7 groups of 10 rats | 72 h | Ethanol extracts of cocoa stem bark reduce inflammation by decreasing inflammatory mediator production (TNF-α). | [81] |
| Cocoa leaves | Polyphenols | Male Wistar rats | 10, 20, 30, 40 μg/mL | 3 male rats | 7 days acclimatization | Polyphenol-enriched cocoa leaf extract inhibits xanthine oxidase and angiotensin 1-converting enzyme. Leaf extract may be useful in preventing oxidative stress. | [87] |
| Cocoa | Procyanidin B2 Catechin, Epicatechin, Isoquercetin, Quercetin | Female Wistar rats | 5% cocoa diet | 4 groups of 12 rats | 3 weeks | Cocoa-containing diet shows anti-inflammatory potential. In addition, lower colon cell infiltration was observed. | [88] |
| Part of the Plant | Nanoparticles | Dose | Activity/Mechanism/Effects | Ref. |
|---|---|---|---|---|
| Cocoa powder | Silver nanoparticles | 25, 50 mg/mL | An insignificant cytotoxic effect on human dermal fibroblast cells has been demonstrated. | [101] |
| Cocoa seeds | Palladium/Copper (II) oxide nanoparticles | 15 mL of cocoa seed extract | The chemical components of cocoa (e.g., catechin, phenolic acids) were identified as stabilizing, reducing and capping agents. This method of obtaining nanoparticles shows lower toxicity and is environmentally friendly. | [102] |
| Cocoa powder | Gold nanoparticles | 0.1, 1, 2.5, 10, 50 mg/mL | Gold nanoparticles are not toxic for human dermal fibroblast. | [103] |
| Cocoa seed extract | Gold nanoparticles | 200, 250, 270, 300 μL | Anisotropic b-AuNPs (derived from T. cacao [cocoa] seed extract) demonstrated excellent photothermal properties in A431 epidermal cancer cells using a laser power density of 6 W/cm2. The b-AuNP nanoparticles exhibited near-infrared absorbance at 700–1000 nm, facilitating an effective photothermal therapy against A431 cancer cells. | [104] |
| Cocoa bean extract | Graphene nanoparticles | IC50—31.2 µg/mL | The cytotoxicity studies showed that the synthesized CSE-GQDs exhibited dose-dependent toxicity on human breast cancer (MCF-7) cell lines. | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitarek, P.; Merecz-Sadowska, A.; Sikora, J.; Osicka, W.; Śpiewak, I.; Picot, L.; Kowalczyk, T. Exploring the Therapeutic Potential of Theobroma cacao L.: Insights from In Vitro, In Vivo, and Nanoparticle Studies on Anti-Inflammatory and Anticancer Effects. Antioxidants 2024, 13, 1376. https://doi.org/10.3390/antiox13111376
Sitarek P, Merecz-Sadowska A, Sikora J, Osicka W, Śpiewak I, Picot L, Kowalczyk T. Exploring the Therapeutic Potential of Theobroma cacao L.: Insights from In Vitro, In Vivo, and Nanoparticle Studies on Anti-Inflammatory and Anticancer Effects. Antioxidants. 2024; 13(11):1376. https://doi.org/10.3390/antiox13111376
Chicago/Turabian StyleSitarek, Przemysław, Anna Merecz-Sadowska, Joanna Sikora, Weronika Osicka, Igor Śpiewak, Laurent Picot, and Tomasz Kowalczyk. 2024. "Exploring the Therapeutic Potential of Theobroma cacao L.: Insights from In Vitro, In Vivo, and Nanoparticle Studies on Anti-Inflammatory and Anticancer Effects" Antioxidants 13, no. 11: 1376. https://doi.org/10.3390/antiox13111376
APA StyleSitarek, P., Merecz-Sadowska, A., Sikora, J., Osicka, W., Śpiewak, I., Picot, L., & Kowalczyk, T. (2024). Exploring the Therapeutic Potential of Theobroma cacao L.: Insights from In Vitro, In Vivo, and Nanoparticle Studies on Anti-Inflammatory and Anticancer Effects. Antioxidants, 13(11), 1376. https://doi.org/10.3390/antiox13111376










