Effects of Caffeine, Zinc, and Their Combined Treatments on the Growth, Yield, Mineral Elements, and Polyphenols of Solanum lycopersicum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Yield Measurements and Sampling
2.3. Ethylene Measurements
2.4. Zinc and Mineral Element Analyses
2.5. Caffeine-13C Extraction and Polyphenol Extraction
2.6. Caffeine and Selected Polyphenols Analyses
2.7. HPLC-MS/MS Method for Polyphenols
2.8. HPLC-MS/MS for Caffeine
2.9. DPPH Assay
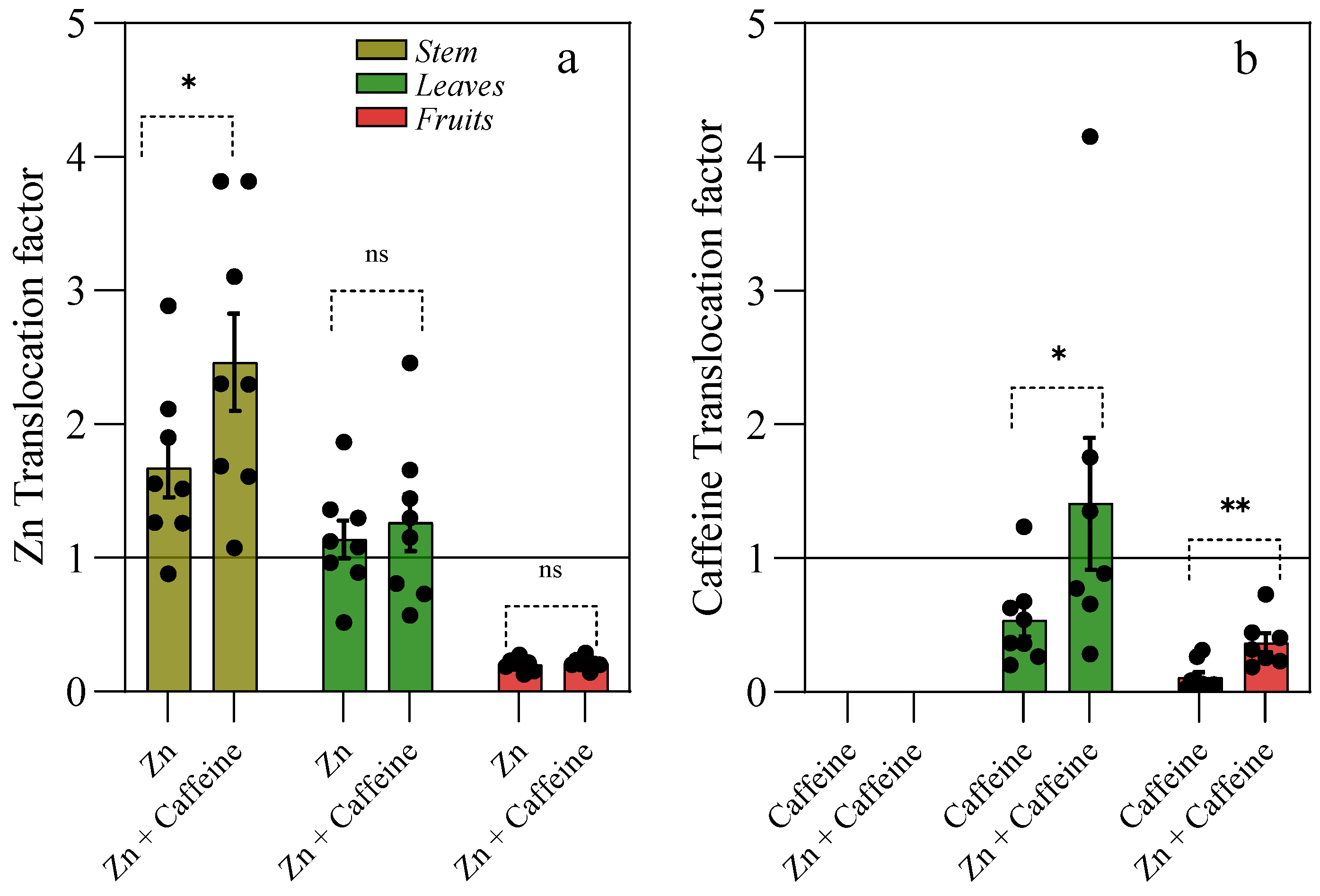
2.10. Translocation Factor
2.11. Risk Assessment
2.12. Statistical Analysis
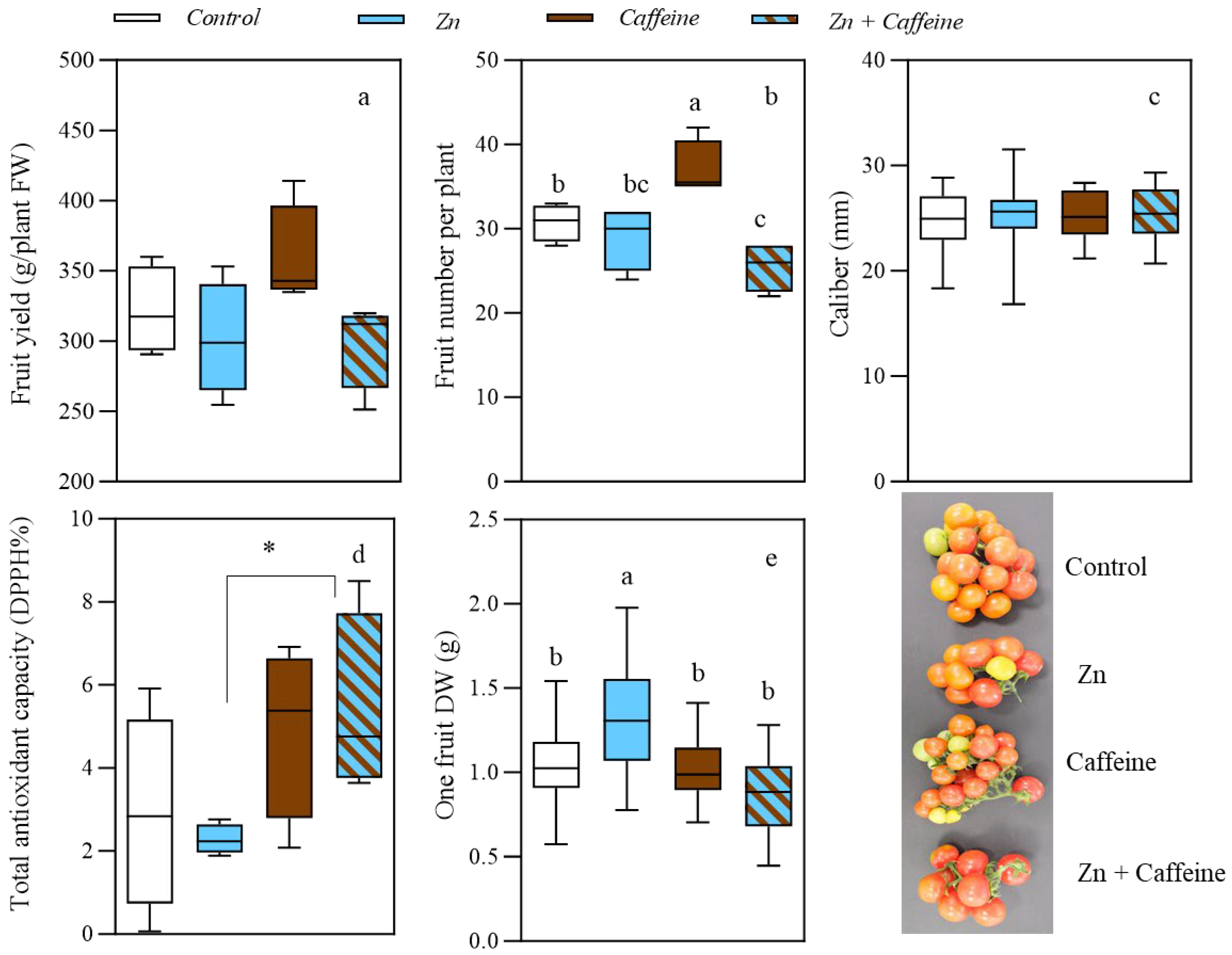
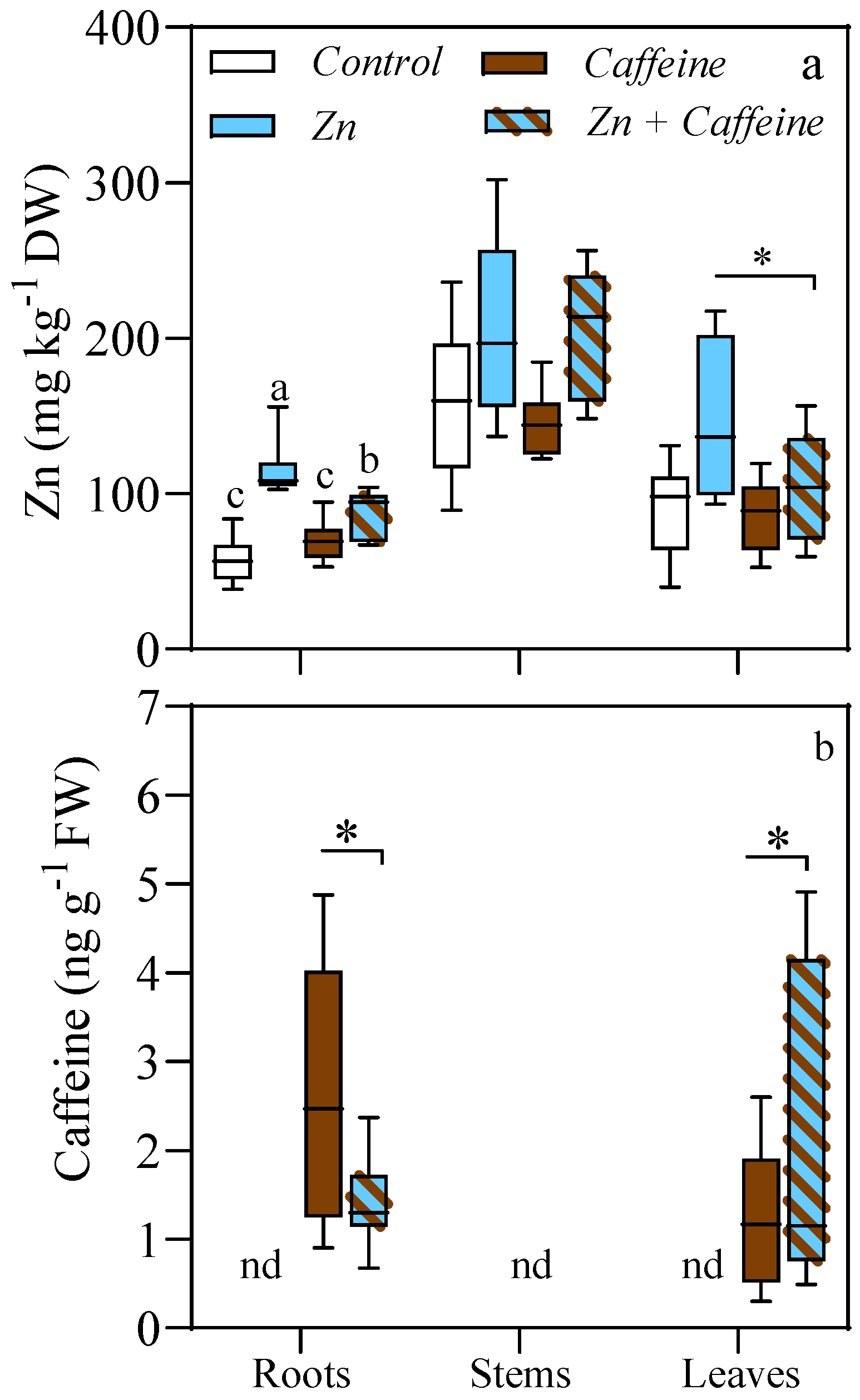
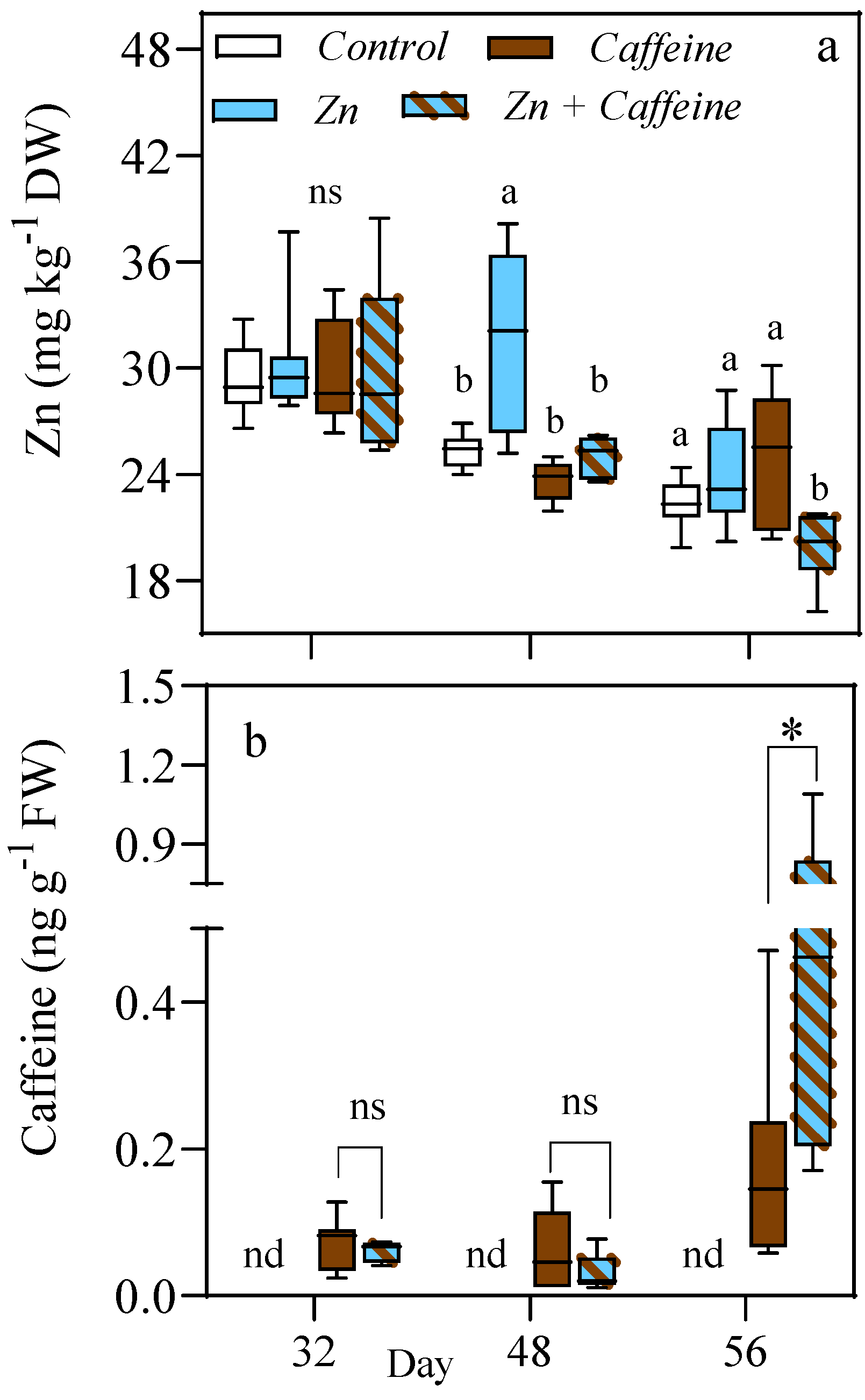
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kavian, S.; Safarzadeh, S.; Yasrebi, J. Zinc improves growth and antioxidant enzyme activity in Aloe vera plant under salt stress. South Afr. J. Bot. 2022, 147, 1221–1229. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Rhizosphere biology. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 369–388. [Google Scholar]
- Prasad, P.N.S.; Subbarayappa, C.T.; Sathish, A.; Ramamurthy, V. Impact of Zinc Fertilization on Tomato (Solanum lycopersicum L.) Yield, Zinc use Efficiency, Growth and Quality Parameters in Eastern Dry Zone (EDZ) Soils of Karnataka, India. Int. J. Plant Soil Sci. 2021, 33, 20–38. [Google Scholar] [CrossRef]
- Júnior, C.A.M.; Luchiari, N.D.C.; Gomes, P.C.F.L. Occurrence of caffeine in wastewater and sewage and applied techniques for analysis: A review. Eclet. Quim. 2019, 44, 11–26. [Google Scholar] [CrossRef]
- Korekar, G.; Kumar, A.; Ugale, C. Occurrence, fate, persistence and remediation of caffeine: A review. Environ. Sci. Pollut. Res. 2020, 27, 34715–34733. [Google Scholar] [CrossRef] [PubMed]
- Edwards, Q.A.; Sultana, T.; Kulikov, S.M.; Garner-O’neale, L.D.; Yargeau, V.; Metcalfe, C.D. Contaminants of Emerging Concern in Wastewaters in Barbados, West Indies. Bull. Environ. Contam. Toxicol. 2018, 101, 1–6. [Google Scholar] [CrossRef]
- Vieira, L.; Soares, A.; Freitas, R. Caffeine as a contaminant of concern: A review on concentrations and impacts in marine coastal systems. Chemosphere 2022, 286, 131675. [Google Scholar] [CrossRef]
- Pierattini, E.C.; Francini, A.; Raffaelli, A.; Sebastiani, L. Degradation of exogenous caffeine by Populus alba and its effects on endogenous caffeine metabolism. Environ. Sci. Pollut. Res. 2016, 23, 7298–7307. [Google Scholar] [CrossRef]
- Vitória, A.; Mazzafera, P. Cytokinin-like effects of caffeine in bioassays. Biol. Plant. 1997, 39, 329–335. [Google Scholar] [CrossRef]
- Emanuil, N.; Akram, M.S.; Ali, S.; Majrashi, A.; Iqbal, M.; El-Esawi, M.A.; Ditta, A.; Alharby, H.F. Exogenous Caffeine (1,3,7-Trimethylxanthine) Application Diminishes Cadmium Toxicity by Modulating Physio-Biochemical Attributes and Improving the Growth of Spinach (Spinacia oleracea L.). Sustainability 2022, 14, 2806. [Google Scholar] [CrossRef]
- Jené, L.; Munné-Bosch, S. Hormonal involvement in boosting the vegetative vigour underlying caffeine-related improvements in lentil production. Plant Sci. 2023, 336, 111856. [Google Scholar] [CrossRef] [PubMed]
- Truta, E.; Zamfirache, M.M.; Olteanu, Z. Caffeine induced genotoxic effects in Phaseolus vulgaris and Raphanus sativus. Bot. Serbica 2011, 35, 49–54. [Google Scholar]
- Kumar, G.; Tripathi, A. Mutagenic response of caffeine in Capsicum annuum. J. Indian Bot. Soc. 2004, 83, 136–140. [Google Scholar]
- Ghiyasi, M.; Danesh, Y.R.; Amirnia, R.; Najafi, S.; Mulet, J.M.; Porcel, R. Foliar Applications of ZnO and Its Nanoparticles Increase Safflower (Carthamus tinctorius L.) Growth and Yield under Water Stress. Agronomy 2023, 13, 192. [Google Scholar] [CrossRef]
- Minnocci, A.; Francini, A.; Romeo, S.; Sgrignuoli, A.D.; Povero, G.; Sebastiani, L. Zn-localization and anatomical changes in leaf tissues of green beans (Phaseolus vulgaris L.) following foliar application of Zn-lignosulfonate and ZnEDTA. Sci. Hortic. 2018, 231, 15–21. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- FAOSTATS. 2022. Available online: https://www.fao.org/faostat/en/#search/tomato (accessed on 23 March 2024).
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef]
- Sbartai, H.; Djebar, M.R.; Rouabhi, R.; Sbartai, I.; Berrebbah, H. Antioxidative response in tomato plants Lycopersicon esculentum L. roots and leaves to zinc. Am. Eurasian J. Toxicol. Sci. 2011, 3, 41–46. [Google Scholar]
- Romeo, S.; Francini, A.; Ariani, A.; Sebastiani, L. Phytoremediation of Zn: Identify the diverging resistance, uptake and biomass production behaviours of poplar clones under high zinc stress. Water Air Soil. Pollut. 2014, 225, 1–12. [Google Scholar] [CrossRef]
- Pavithra, G.J.; Mahesh, S.; Parvathi, M.S.; Basavarajeshwari, R.M.; Nataraja, K.N.; Shankar, A.G. Comparative growth responses and transcript profiling of zinc transporters in two tomato varieties under different zinc treatments. Indian J. Plant Physiol. 2016, 21, 208–212. [Google Scholar] [CrossRef]
- Carbonare, L.D.; White, M.D.; Shukla, V.; Francini, A.; Perata, P.; Flashman, E.; Sebastiani, L.; Licausi, F. Zinc Excess Induces a Hypoxia-Like Response by Inhibiting Cysteine Oxidases in Poplar Roots. Plant Physiol. 2019, 180, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Smyth, D.A. Effect of methylxanthine treatment on rice seedling growth. J. Plant Growth Regul. 1992, 11, 125–128. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Sudesh, K.Y. Retardation in seedling growth and induction of early senescence in plants upon caffeine exposure is related to its negative effect on Rubisco. Photosynthetica 2009, 47, 293–297. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- European Food Safety Authority. EFSA Explains Risk Assessment—Caffeine; European Food Safety Authority: Parma, Italy, 2015; Available online: https://data.europa.eu/doi/10.2805/618813 (accessed on 3 August 2023).
- Antoine, J.M.; Fung, L.A.H.; Grant, C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017, 4, 181–187. [Google Scholar] [CrossRef]
- Jené, L.; Mirabent, C.; Campillos, S.; Munné-Bosch, S. Impact of environmental conditions, stress severity and dose application on caffeine-related improved lentil productivity. Environ. Exp. Bot. 2022, 203, 105064. [Google Scholar] [CrossRef]
- Ansari, A.M. The effect of caffeine and nicotine on different plant growth. Int. J. Adv. Res. Innov. Ideas Educ. 2020, 6, 846–858. Available online: www.IJARIIT.com (accessed on 3 August 2023).
- Sledz, W.; Motyka, A.; Zoledowska, S.; Paczek, A.; Los, E.; Rischka, J. Influence of exogenously supplemented caffeine on cell division, germination, and growth of economically important plants. In The Question of Caffeine; IntechOpen: London, UK, 2017; pp. 127–142. [Google Scholar] [CrossRef]
- Vannucchi, F.; Traversari, S.; Raffaelli, A.; Francini, A.; Sebastiani, L. Populus alba tolerates and efficiently removes caffeine and zinc excesses using an organ allocation strategy. Plant Growth Regul. 2020, 92, 597–606. [Google Scholar] [CrossRef]
- Trebolazabala, J.; Maguregui, M.; Morillas, H.; García-Fernandez, Z.; de Diego, A.; Madariaga, J.M. Uptake of metals by tomato plants (Solanum lycopersicum) and distribution inside the plant: Field experiments in Biscay (Basque Country). J. Food Compos. Anal. 2017, 59, 161–169. [Google Scholar] [CrossRef]
- Rajam, K.; Rajendran, S.; Banu, N.N. Effect of Caffeine-Zn2+ System in Preventing Corrosion of Carbon Steel in Well Water. J. Chem. 2013, 2013, 521951. [Google Scholar] [CrossRef]
- Castillo-Gonzalez, J.; Ojeda-Barrios, D.; Hernandez-Rodríguez, A.; Gonzalez-Franco, A.C.; Robles-Hernandez, L.; Lopez-Ochoa, G.R. Zinc metalloenzymes in plants. Interciencia 2018, 43, 242–248. [Google Scholar]
- Ali, M.Y.; Ibn Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2021, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Ciudad-Mulero, M.; Pinela, J.; Carvalho, A.M.; Barros, L.; Fernández-Ruiz, V.; Ferreira, I.C.F.R.; Sánchez-Mata, M.d.C.; Morales, P. Bioaccessibility of Macrominerals and Trace Elements from Tomato (Solanum lycopersicum L.) Farmers’ Varieties. Foods 2022, 11, 1968. [Google Scholar] [CrossRef]
- Roohanitaziani, R.; de Maagd, R.A.; Lammers, M.; Molthoff, J.; Meijer-Dekens, F.; van Kaauwen, M.P.W.; Finkers, R.; Tikunov, Y.; Visser, R.G.F.; Bovy, A.G. Exploration of a Resequenced Tomato Core Collection for Phenotypic and Genotypic Variation in Plant Growth and Fruit Quality Traits. Genes 2020, 11, 1278. [Google Scholar] [CrossRef]
- Bénard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Génard, M. Effects of Low Nitrogen Supply on Tomato (Solanum lycopersicum) Fruit Yield and Quality with Special Emphasis on Sugars, Acids, Ascorbate, Carotenoids, and Phenolic Compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef]
- Georgé, S.; Tourniaire, F.; Gautier, H.; Goupy, P.; Rock, E.; Caris-Veyrat, C. Changes in the contents of carotenoids, phenolic compounds and vitamin C during technical processing and lyophilisation of red and yellow tomatoes. Food Chem. 2011, 124, 1603–1611. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Lombardi, S.; Gaspari, A.; Grosso, M.; Ritieni, A. Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Various Typologies of Canned Tomatoes. Front. Nutr. 2022, 9, 849163. [Google Scholar] [CrossRef]
- Francini, A.; Giro, A.; Ferrante, A. Biochemical and molecular regulation of phenylpropanoids pathway under abiotic stresses. In Plant Signaling Molecules—Role and Regulation under Stressful Environments; Elsevier: Amsterdam, The Netherlands, 2019; pp. 183–192. [Google Scholar]
- Özcan, F.Ö.; Aldemir, O.; Karademir, B. Flavones (Apigenin, Luteolin, Crhysin) and Their Importance for Health. Mellifera 2020, 20, 16–27. [Google Scholar]
- Bounar, A.; Boukaka, K.; Leghouchi, E. Determination of heavy metals in tomatoes cultivated under green houses and human health risk assessment. Qual. Assur. Saf. Crop. Foods 2020, 12, 76–86. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Krishnan, S.; Kumar, S.; Nejad, Z.D.; Khan, M.M.; Alam, J. Evaluating heavy metals contamination in soil and vegetables in the region of North India: Levels, transfer and potential human health risk analysis. Environ. Toxicol. Pharmacol. 2021, 82, 103563. [Google Scholar] [CrossRef] [PubMed]
- Kloke, A.; Sauerbeck, D.R.; Vetter, H. The Contamination of Plants and Soils with Heavy Metals and the Transport of Metals in Terrestrial Food Chains. In Changing Metal Cycles and Human Health; Nriagu, J.O., Ed.; Springer: Berlin, Germany, 1984; pp. 113–141. [Google Scholar] [CrossRef]
- Khan, S.; Farooq, R.; Shahbaz, S.; Khan, M.A.; Sadique, M. Health risk assessment of heavy metals for population via con-sumption of vegetables. World Appl. Sci. J. 2009, 6, 1602–1606. [Google Scholar]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total. Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.M.; Choi, J.Y.; Nho, E.Y.; Dang, Y.M.; Jamila, N.; Khan, N.; Seo, H.-Y.; Kim, K.S. Determination of Essential and Toxic Elements in Vegetables from South Korea. Anal. Lett. 2017, 50, 663–681. [Google Scholar] [CrossRef]

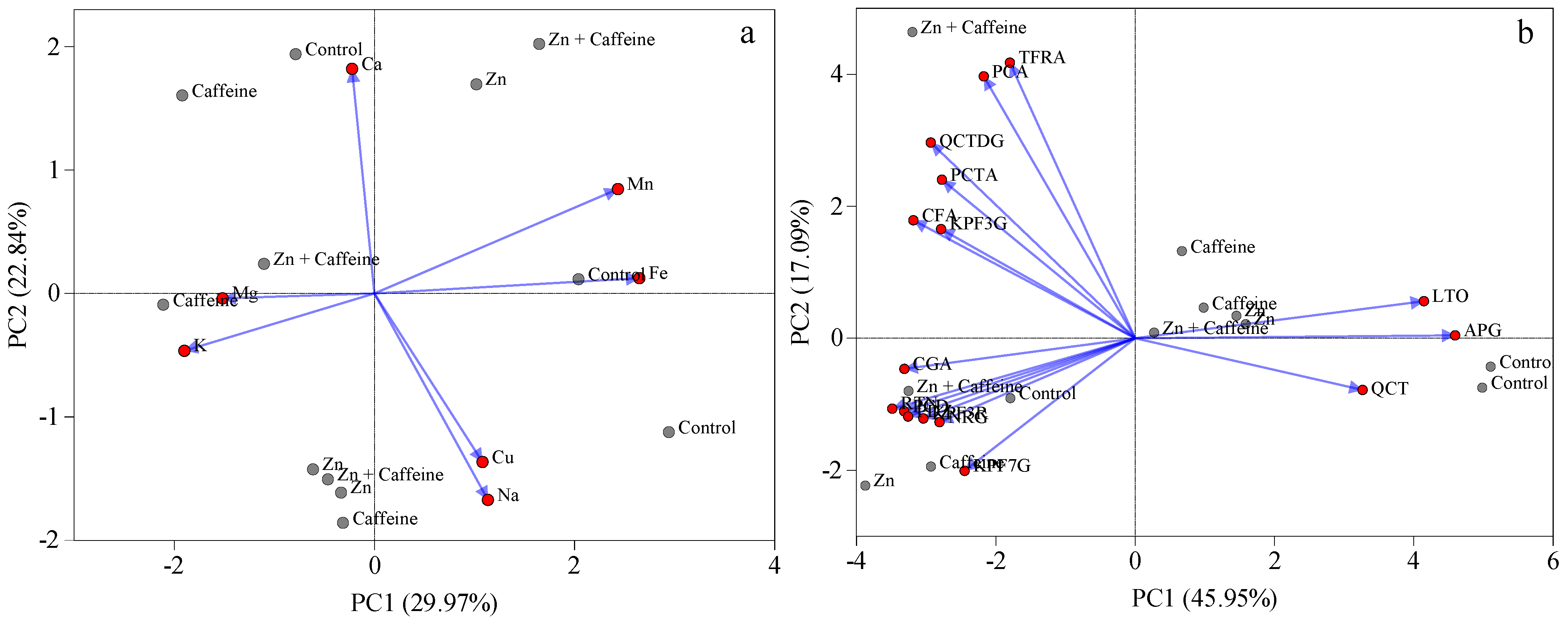
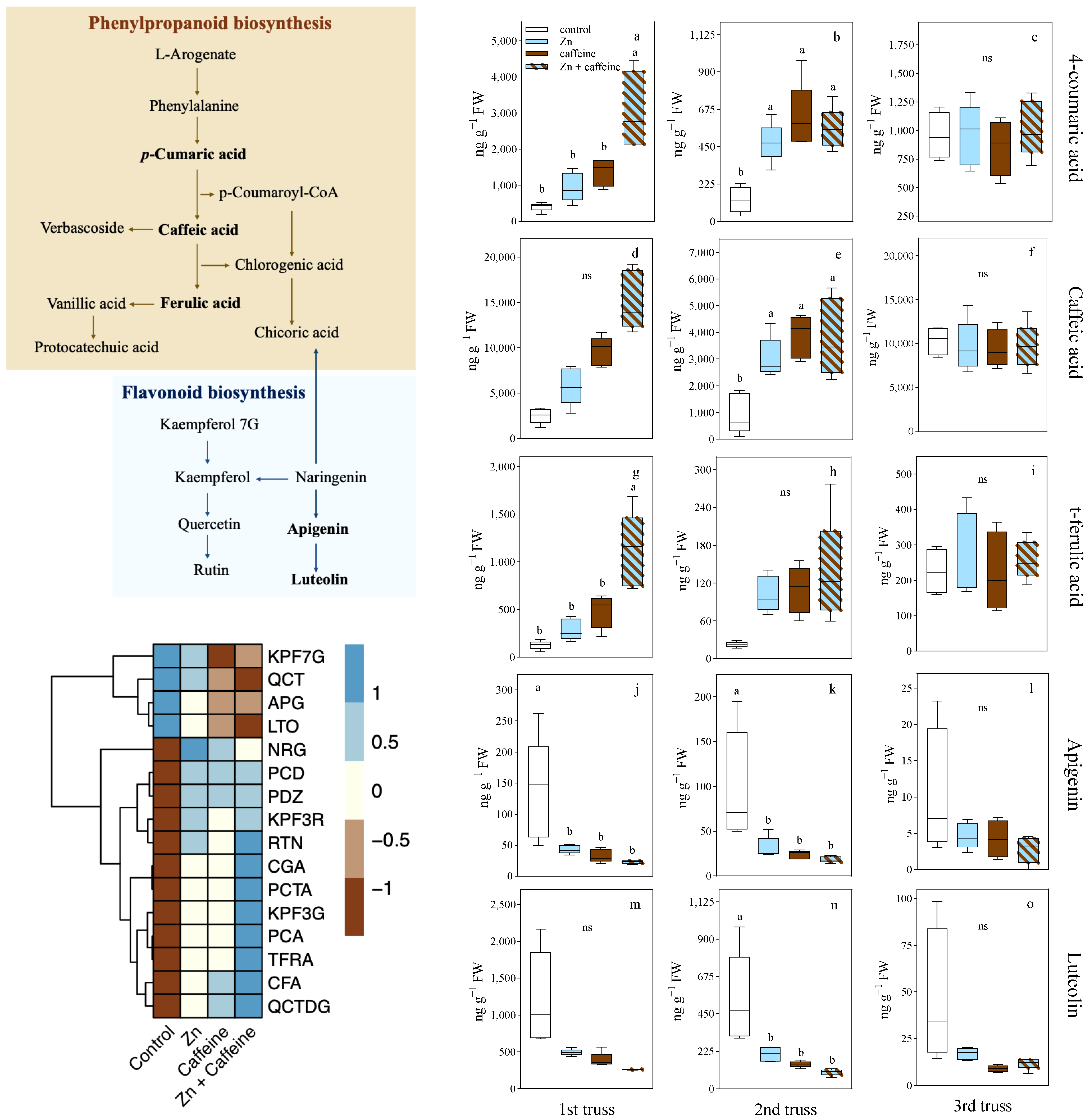
| Treatments | |||||
|---|---|---|---|---|---|
| Day of Treatment | Control | Zn | Caffeine | Zn + Caffeine | |
| Total soluble solids content (°Brix) | 32 | 8.9 ± 0.9 | 9.4 ± 1.4 | 8.8 ± 0.8 | 8.4 ± 1.2 |
| 48 | 9.0 ± 1.0 | 10.0 ± 1.4 | 9.0 ± 0.8 | 9.0 ± 1.1 | |
| 56 | 10.4 ± 2.2 | 8.9 ± 0.8 | 9.0 ± 0.7 | 9.0 ± 1.4 | |
| Ethylene (nl g−1 h−1) | 32 | 0.37 ± 0.3 | 0.40 ± 0.2 | 0.18 ± 0.1 | 0.52 ± 0.5 |
| 48 | 0.16 ± 0.1 | 0.45 ± 0.5 | 0.13 ± 0.1 | 0.14 ± 0.1 | |
| 56 | 0.32 ± 0.3 | 0.48 ± 0.6 | 0.25 ± 0.2 | 0.44 ± 0.3 | |
| Treatments | |||||
|---|---|---|---|---|---|
| Compound | Control | Zn | Caffeine | Zn + Caffeine | |
| third Truss | PCTA | 921 ± 220 | 722 ± 258 | 542 ± 294 | 863 ± 385 |
| NRG | 747 ± 551 | 1935 ± 1312 | 1816 ± 2150 | 641 ± 212 | |
| QCT | 221 ± 139 | 259 ± 79 | 197 ± 61 | 204 ± 92 | |
| CGA | 125,798 ± 41,569 | 141,023 ± 42,128 | 131,831 ± 59,148 | 187,224 ± 62,235 | |
| PCD | 84 ± 25 | 111 ± 33 | 108 ± 26 | 98 ± 27 | |
| PDZ | 1584 ± 1146 | 2878 ± 1693 | 1939 ± 710 | 1878 ± 1201 | |
| KPF7G | 320 ± 29 | 374 ± 133 | 334 ± 209 | 303 ± 103 | |
| KPF3G | 65 ± 31 | 102 ± 44 | 94 ± 38 | 119 ± 64 | |
| KPF3R | 15,648 ± 5099 | 22,661 ± 4649 | 19,293 ± 10,572 | 20,822 ± 8325 | |
| RTN | 309,569 ± 97,652 | 375,194 ± 74,526 | 353,134 ± 72,683 | 365,085 ± 10,102 | |
| QCTDG | 916 ± 396 | 1263 ± 229 | 1304 ± 742 | 1283 ± 427 | |
| second Truss | PCTA | 173 ± 117 | 833 ± 586 | 595 ± 153 | 709 ± 234 |
| NRG | 76 ± 41 | 544 ± 818 | 299 ± 63 | 428 ± 421 | |
| QCT | 444 ± 245 | 204 ± 116 | 131 ± 27 | 94 ± 15 | |
| CGA | 8637 ± 5989 | 48,542 ± 23,516 | 47,921 ± 15,864 | 74,721 ± 45,677 | |
| PCD | 14 ± 10 | 67 ± 29 | 70 ± 26 | 80 ± 27 | |
| PDZ | 289 ± 238 | 719 ± 230 | 1304 ± 793 | 1466 ± 607 | |
| KPF7G | 81 ± 58 | 43 ± 23 | 41 ± 18 | 70 ± 56 | |
| KPF3G | 65 ± 14 | 85 ± 22 | 88 ± 15 | 88 ± 17 | |
| KPF3R | 120 ± 10 | 44 ± 5 | 142 ± 75 | 149 ± 93 | |
| RTN | 30,355 ± 20,331 | 153,394 ± 5505 | 161,355 ± 91,172 | 224,005 ± 94,321 | |
| QCTDG | 183 ± 143 | 799 ± 60 | 1309 ± 965 | 1127 ± 594 | |
| first Truss | PCTA | 402 ± 170 | 522 ± 117 | 675 ± 152 | 1110 ± 422 |
| NRG | 273 ± 117 | 405 ± 231 | 460 ± 112 | 757 ± 374 | |
| QCT | 863 ± 681 | 239 ± 81 | 166 ± 19 | 156 ± 50 | |
| CGA | 57,333 ± 29,331 | 92,037 ± 41,689 | 70,646 ± 22,055 | 123,978 ± 25,866 | |
| PCD | 43 ± 20 | 58 ± 18 | 58 ± 18 | 66 ± 25 | |
| PDZ | 457 ± 198 | 1049 ± 547 | 1123 ± 466 | 1206 ± 471 | |
| KPF7G | 124 ± 65 | 64 ± 13 | 74 ± 25 | 68 ± 41 | |
| KPF3G | 34 ± 14 | 36 ± 14 | 57 ± 19 | 140 ± 134 | |
| KPF3R | 3499 ± 1613 | 6151 ± 2594 | 6108 ± 2879 | 9657 ± 6951 | |
| RTN | 72,725 ± 32,123 | 151,346 ± 59,515 | 122,042 ± 32,231 | 222,966 ± 119,537 | |
| QCTDG | 367 ± 194 | 851 ± 756 | 1004 ± 513 | 2398 ± 3216 |
| Truss Clusters | Zn | Zn + Caffeine | |
|---|---|---|---|
| Znaverage (mg kg−1 FW) | first | 3.79 | 2.29 |
| second | 3.71 | 1.99 | |
| third | 2.85 | 1.52 | |
| THQ of Zn | first | 0.009 | 0.005 |
| second | 0.008 | 0.004 | |
| third | 0.006 | 0.003 | |
| Caffeine | Zn + Caffeine | ||
| Caffeineaverage (ng g−1 FW) | first | 0.072 | 0.059 |
| second | 0.060 | 0.034 | |
| third | 0.181 | 0.460 | |
| THQ of caffeine | first | 1.73 × 10−5 | 1.42 × 10−5 |
| second | 1.44 × 10−5 | 8.16 × 10−6 | |
| third | 4.33 × 10−5 | 1.10 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vichi, E.; Francini, A.; Raffaelli, A.; Sebastiani, L. Effects of Caffeine, Zinc, and Their Combined Treatments on the Growth, Yield, Mineral Elements, and Polyphenols of Solanum lycopersicum L. Antioxidants 2024, 13, 1100. https://doi.org/10.3390/antiox13091100
Vichi E, Francini A, Raffaelli A, Sebastiani L. Effects of Caffeine, Zinc, and Their Combined Treatments on the Growth, Yield, Mineral Elements, and Polyphenols of Solanum lycopersicum L. Antioxidants. 2024; 13(9):1100. https://doi.org/10.3390/antiox13091100
Chicago/Turabian StyleVichi, Elena, Alessandra Francini, Andrea Raffaelli, and Luca Sebastiani. 2024. "Effects of Caffeine, Zinc, and Their Combined Treatments on the Growth, Yield, Mineral Elements, and Polyphenols of Solanum lycopersicum L." Antioxidants 13, no. 9: 1100. https://doi.org/10.3390/antiox13091100
APA StyleVichi, E., Francini, A., Raffaelli, A., & Sebastiani, L. (2024). Effects of Caffeine, Zinc, and Their Combined Treatments on the Growth, Yield, Mineral Elements, and Polyphenols of Solanum lycopersicum L. Antioxidants, 13(9), 1100. https://doi.org/10.3390/antiox13091100











