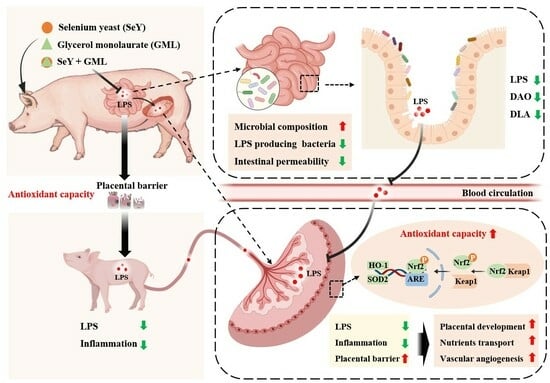
Enhanced Antioxidative Capacity Transfer between Sow and Fetus via the Gut–Placenta Axis with Dietary Selenium Yeast and Glycerol Monolaurate Supplementation during Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Management
2.2. Sample Collection
2.3. Se Content Measurement
2.4. Chemical Analyses
2.5. Total RNA Extraction and Real-Time Quantitative PCR
2.6. Western Blotting Analysis
2.7. Bacterial Data Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of Dietary Addition of SeY and GML on Placental Weight and LPS Levels in Sow, Placenta, and Piglet
3.2. Effect of Dietary Addition of SeY and GML on Se Status along the Maternal–Placental–Fetal Axis
3.3. Effect of Dietary Addition of SeY and GML on Oxidative Stress in the Maternal–Placental–Fetal Axis
3.4. Effect of Dietary Addition of SeY and GML on Inflammation along the Placenta–Piglets Axis
3.5. Effect of Dietary Addition of SeY and GML on the Intestinal and Placental Barriers in Sows
3.6. Effect of Dietary Addition of SeY and GML on Maternal Placental Development
3.7. Effect of Dietary Addition of SeY or GML on Sow Placental Nutrient Transport
3.8. Effect of Dietary Addition of SeY and GML on the Gut Microbiota of Sows
4. Discussion
4.1. Intestine-Derived LPS Participates in Mediating the Effects of Selenium and GML
4.2. Selenium and GML Reshape Sow Gut Microbiota Composition to Mitigate Systemic LPS Translocation from Mother to Fetus
4.3. Selenium and GML Enhance the Antioxidative Status of the Placenta and Fetus during Pregnancy
4.4. Intestine-Derived LPS Modulates the Antioxidative Transmission between the Mother and Fetus
4.5. Placenta Is a Key Functional Site for Antioxidative Transmission between the Mother and Fetus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Hu, C.; Yan, Y.; Ji, F.; Zhou, H. Maternal Obesity Increases Oxidative Stress in Placenta and It Is Associated with Intestinal Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 671347. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.; Lakic, N.; Simin, N.; Nikolic, A.; Sudji, J.; Bozin, B. Biomarkers of oxidative stress in amniotic fluid and complications in pregnancy. J. Matern. Neonatal Med. 2011, 25, 104–108. [Google Scholar] [CrossRef]
- Marseglia, L.; D’angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxid. Med. Cell. Longev. 2014, 2014, 358375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Flowers, W.L.; Saraiva, A.; Yeum, K.-J.; Kim, S.W. Effect of social ranks and gestation housing systems on oxidative stress status, reproductive performance, and immune status of sows. J. Anim. Sci. 2013, 91, 5848–5858. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, R.; Shi, M.; Wang, L.; Yan, J.; Gong, J.; Zhang, Q.; He, J.; Wu, S. Placental Malfunction, Fetal Survival and Development Caused by Sow Metabolic Disorder: The Impact of Maternal Oxidative Stress. Antioxidants 2023, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Burton, G. The role of oxidative stress in placental-related diseases of pregnancy. J. Gynecol. Obstet. Biol. Reprod. 2016, 45, 775–785. [Google Scholar] [CrossRef]
- James, J.; Carter, A.; Chamley, L. Human placentation from nidation to 5 weeks of gestation. Part II: Tools to model the crucial first days. Placenta 2012, 33, 335–342. [Google Scholar] [CrossRef]
- Krishnan, L.; Nguyen, T.; McComb, S. From mice to women: The conundrum of immunity to infection during pregnancy. J. Reprod. Immunol. 2013, 97, 62–73. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, T.B.; Lippi, L.L.; Bevilacqua, E.; Bernardi, M.M. LPS exposure increases maternal corticosterone levels, causes placental injury and increases IL-1β levels in adult rat offspring: Relevance to autism. PLoS ONE 2013, 8, e82244. [Google Scholar] [CrossRef]
- Tran, H.T.; Liong, S.; Lim, R.; Barker, G.; Lappas, M. Resveratrol ameliorates the chemical and microbial induction of inflammation and insulin resistance in human placenta, adipose tissue and skeletal muscle. PLoS ONE 2017, 12, e0173373. [Google Scholar] [CrossRef] [PubMed]
- Kamal, D.A.M.; Salamt, N.; Yusuf, A.N.M.; Kashim, M.I.A.M.; Mokhtar, M.H. Potential Health Benefits of Curcumin on Female Reproductive Disorders: A Review. Nutrients 2021, 13, 3126. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.; Fisinin, V. Selenium in sow nutrition. Anim. Feed Sci. Technol. 2016, 211, 18–30. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Peterson, M.L. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Dong, S.; Ma, Y. Supplementation with alpha-glycerol monolaurate during late gestation and lactation enhances sow performance, ameliorates milk composition, and improves growth of suckling piglets. J. Anim. Sci. Biotechnol. 2023, 14, 47. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, M.; Xiong, L.; Lin, T.; Zhang, S.; Yue, X.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal supplementation with glycerol monolaurate improves the intestinal health of suckling piglets by inhibiting the NF-κB/MAPK pathways and improving oxidative stability. Food Funct. 2023, 14, 3290–3303. [Google Scholar] [CrossRef]
- Chen, J.; Han, J.H.; Guan, W.T.; Chen, F.; Wang, C.X.; Zhang, Y.Z.; Lv, Y.T.; Lin, G. Selenium and vitamin E in sow diets: I. Effect on antioxidant status and reproductive performance in multiparous sows. Anim. Feed Sci. Technol. 2016, 221, 111–123. [Google Scholar] [CrossRef]
- Chen, J.; Han, J.; Guan, W.; Chen, F.; Wang, C.; Zhang, Y.; Lv, Y.; Lin, G. Selenium and vitamin E in sow diets: II. Effect on selenium status and antioxidant status of the progeny. Anim. Feed. Sci. Technol. 2016, 221, 101–110. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Jiang, Z.; Cai, H.; Li, Y.; Mo, Q.; Deng, L.; Zhong, H.; Liu, T.; Zhang, H.; Kang, J.X.; et al. Modulation of the gut microbiota during high-dose glycerol monolaurate-mediated amelioration of obesity in mice fed a high-fat diet. mBio 2020, 11, e00190-20. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-L.; Zhu, Y.-H.; Shi, M.; Li, T.-T.; Li, N.; Wu, G.-Y.; Bazer, F.W.; Zang, J.-J.; Wang, F.-L.; Wang, J.-J. Within-litter variation in birth weight: Impact of nutritional status in the sow. J. Zhejiang Univ. B 2015, 16, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, H.; Guo, L.; Gou, X.; Chen, G.; Lin, D.; Fan, D.; Guo, X.; Liu, Z. Association between gut microbiota and preeclampsia-eclampsia: A two-sample Mendelian randomization study. BMC Med. 2022, 20, 443. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, P.; Liu, M.; Zheng, H.; He, Y.; Chen, M.-X.; Tang, W.; Yue, X.; Huang, Y.; Zhuang, L.; et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 2020, 69, 513–522. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, Y.; Huang, S.; Zhang, L.; Cao, C.; Baker, P.N.; Tong, C.; Zheng, P.; Qi, H. Altered gut bacterial and metabolic signatures and their interaction in gestational diabetes mellitus. Gut Microbes 2020, 12, 1840765. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Tao, C.; Guo, H.; Xue, Y.; Zhao, R.; Yao, W. Heat stress during late gestation disrupts maternal microbial transmission with altered offspring’s gut microbial colonization and serum metabolites in a pig model. Environ. Pollut. 2020, 266, 115111. [Google Scholar] [CrossRef]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-gut microbiota interactions and gestational diabetes mellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2017, 67, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.; Alim, A.; Ren, D.; Zhao, Y.; Yang, X. Regulatory Effects of Stachyose on Colonic and Hepatic Inflammation, Gut Microbiota Dysbiosis, and Peripheral CD4+ T Cell Distribution Abnormality in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2019, 67, 11665–11674. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Tan, J.; Li, Z.; Wang, L.; Shi, M.; Li, B.; Liu, M.; Yuan, X.; He, J.; Wu, X. Effect of dietary resveratrol on placental function and reproductive performance of late pregnancy sows. Front. Nutr. 2022, 9, 1001031. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shen, Q.; Lyu, W.; Lv, L.; Wang, W.; Yu, M.; Yang, H.; Tao, S.; Xiao, Y. Clostridium butyricum and Its Derived Extracellular Vesicles Modulate Gut Homeostasis and Ameliorate Acute Experimental Colitis. Microbiol. Spectr. 2022, 10, e0136822. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, B.; Ji, L.; Lv, X.; Feng, Y.; Li, L.; Wu, X. Dietary lysozyme improves growth performance and intestinal barrier function of weaned piglets. Anim. Nutr. 2023, 14, 249–258. [Google Scholar] [CrossRef]
- Oliphant, K.; Ali, M.; D’souza, M.; Hughes, P.D.; Sulakhe, D.; Wang, A.Z.; Xie, B.; Yeasin, R.; Msall, M.E.; Andrews, B.; et al. Bacteroidota and Lachnospiraceae integration into the gut microbiome at key time points in early life are linked to infant neurodevelopment. Gut Microbes 2021, 13, 1997560. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Zhang, S.; Yang, F.; Zhou, H.; Song, Y.; Wang, B.; Li, H. Sodium alginate and galactooligosaccharides ameliorate metabolic disorders and alter the composition of the gut microbiota in mice with high-fat diet-induced obesity. Int. J. Biol. Macromol. 2022, 215, 113–122. [Google Scholar] [CrossRef]
- Kim, H.S.; Cho, J.H.; Park, H.W.; Yoon, H.; Kim, M.S.; Kim, S.C. Endotoxin-neutralizing antimicrobial proteins of the human placenta. J. Immunol. 2002, 168, 2356–2364. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Liu, J.; Zhong, F.; Zhang, C.; Chen, Y.; Sun, T.; Ji, C.; Ma, D. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat. Commun. 2022, 13, 2522. [Google Scholar] [CrossRef]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Charron, M.J.; Williams, L.; Seki, Y.; Du, X.Q.; Chaurasia, B.; Saghatelian, A.; Summers, S.A.; Katz, E.B.; Vuguin, P.M.; Reznik, S.E. Antioxidant Effects of N-Acetylcysteine Prevent Programmed Metabolic Disease in Mice. Diabetes 2020, 69, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The antioxidant properties of selenium and vitamin E; their role in periparturient dairy cattle health regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Wassmann, K.; Nickenig, G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 2004, 44, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; DeNicola, G.M. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Galasso, M.; Gambino, S.; Romanelli, M.G.; Donadelli, M.; Scupoli, M.T. Browsing the oldest antioxidant enzyme: Catalase and its multiple regulation in cancer. Free. Radic. Biol. Med. 2021, 172, 264–272. [Google Scholar] [CrossRef]
- Mou, D.; Ding, D.; Yan, H.; Qin, B.; Dong, Y.; Li, Z.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; et al. Maternal supplementation of organic selenium during gestation improves sows and offspring antioxidant capacity and inflammatory status and promotes embryo survival. Food Funct. 2020, 11, 7748–7761. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Gallagher, K.; Beck, C.; Kumar, R.; Gernand, A.D. Maternal-fetal inflammation in the placenta and the developmental origins of health and disease. Front. Immunol. 2020, 11, 531543. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obstet. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [PubMed]
- Jena, M.K.; Sharma, N.R.; Petitt, M.; Maulik, D.; Nayak, N.R. Pathogenesis of Preeclampsia and Therapeutic Approaches Targeting the Placenta. Biomolecules 2020, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Muruganandan, S.; Shallie, P.D.; Dhal, S.; Petitt, M.; Nayak, N.R. VEGF Maintains Maternal Vascular Space Homeostasis in the Mouse Placenta through Modulation of Trophoblast Giant Cell Functions. Biomolecules 2021, 11, 1062. [Google Scholar] [CrossRef]
- Hu, C.; Wu, Z.; Huang, Z.; Hao, X.; Wang, S.; Deng, J.; Yin, Y.; Tan, C. Nox2 impairs VEGF-A-induced angiogenesis in placenta via mitochondrial ROS-STAT3 pathway. Redox Biol. 2021, 45, 102051. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Sferruzzi-Perri, A.N. Human placental development and function. Semin. Cell Dev. Biol. 2022, 131, 66–77. [Google Scholar] [CrossRef]
- Kyllo, H.M.; Wang, D.; Lorca, R.A.; Julian, C.G.; Moore, L.G.; Wilkening, R.B.; Rozance, P.J.; Brown, L.D.; Wesolowski, S.R. Adaptive responses in uteroplacental metabolism and fetoplacental nutrient shuttling and sensing during placental insufficiency. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E556–E568. [Google Scholar] [CrossRef]
- Ericsson, A.; Säljö, K.; Sjöstrand, E.; Jansson, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport. J. Physiol. 2007, 581, 1323–1332. [Google Scholar] [CrossRef]
- Cuffe, J.S.; Walton, S.L.; Singh, R.R.; Spiers, J.G.; Bielefeldt-Ohmann, H.; Wilkinson, L.; Little, M.H.; Moritz, K.M. Mid-to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J. Physiol. 2014, 592, 3127–3141. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, J.; Ma, Z.; Fu, Z.; Zhao, Y.; Zeng, X.; Lin, G.; Zhang, S.; Guan, W.; Chen, F. Enhanced Antioxidative Capacity Transfer between Sow and Fetus via the Gut–Placenta Axis with Dietary Selenium Yeast and Glycerol Monolaurate Supplementation during Pregnancy. Antioxidants 2024, 13, 141. https://doi.org/10.3390/antiox13020141
Zhang J, Wang J, Ma Z, Fu Z, Zhao Y, Zeng X, Lin G, Zhang S, Guan W, Chen F. Enhanced Antioxidative Capacity Transfer between Sow and Fetus via the Gut–Placenta Axis with Dietary Selenium Yeast and Glycerol Monolaurate Supplementation during Pregnancy. Antioxidants. 2024; 13(2):141. https://doi.org/10.3390/antiox13020141
Chicago/Turabian StyleZhang, Jiawen, Jun Wang, Ziwei Ma, Zhichao Fu, Yueqi Zhao, Xiangfang Zeng, Gang Lin, Shihai Zhang, Wutai Guan, and Fang Chen. 2024. "Enhanced Antioxidative Capacity Transfer between Sow and Fetus via the Gut–Placenta Axis with Dietary Selenium Yeast and Glycerol Monolaurate Supplementation during Pregnancy" Antioxidants 13, no. 2: 141. https://doi.org/10.3390/antiox13020141