Investigating the Impact of Disrupting the Glutamine Metabolism Pathway on Ammonia Excretion in Crucian Carp (Carassius auratus) under Carbonate Alkaline Stress Using Metabolomics Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Experimental Design and Feeding Management
2.3. UPLC-Q-TOF/MS Analysis
2.3.1. Sample Preparation
2.3.2. Detection Using UPLC-Q-TOF/MS
2.4. Data Processing
3. Results
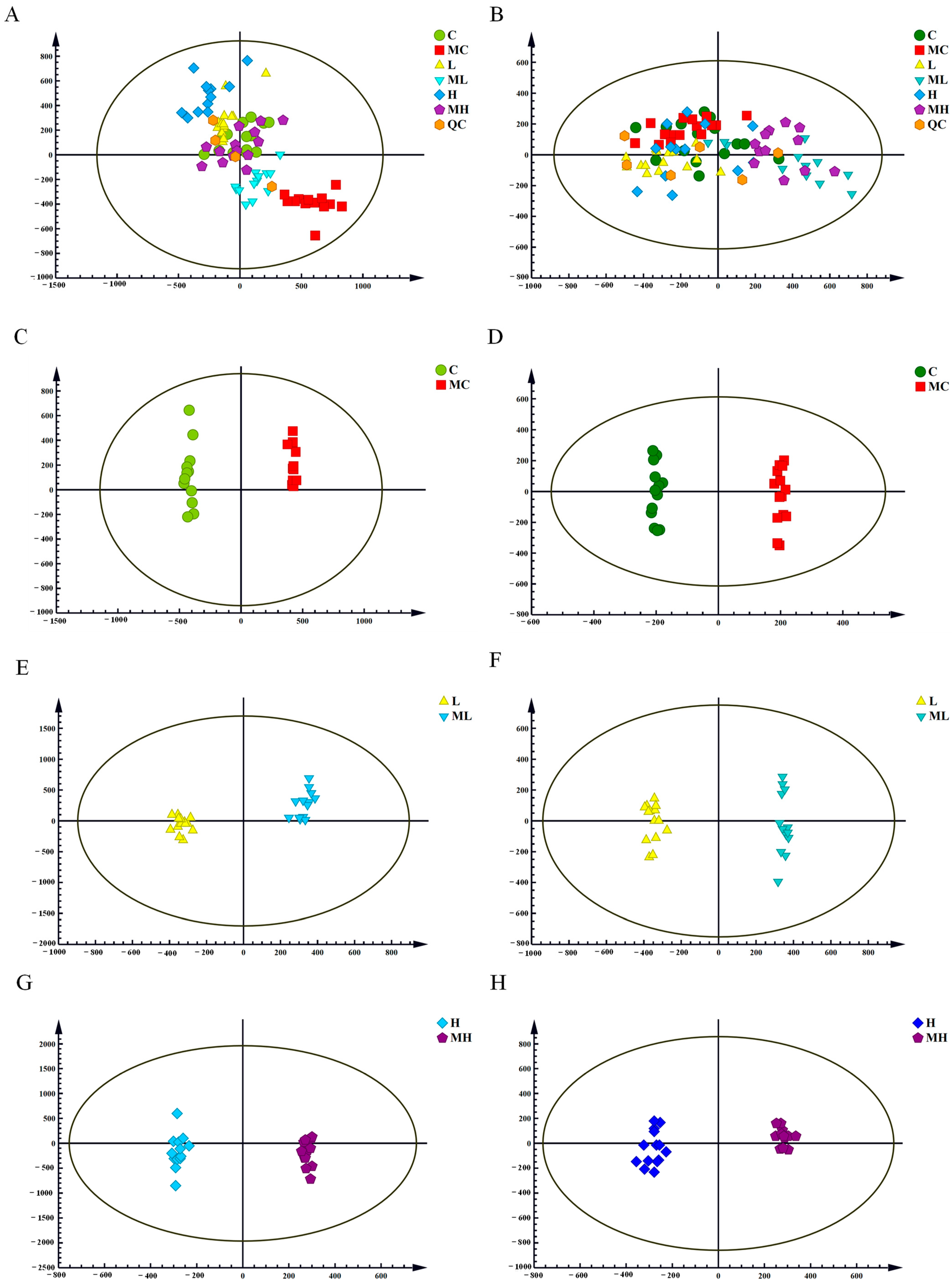
3.1. Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
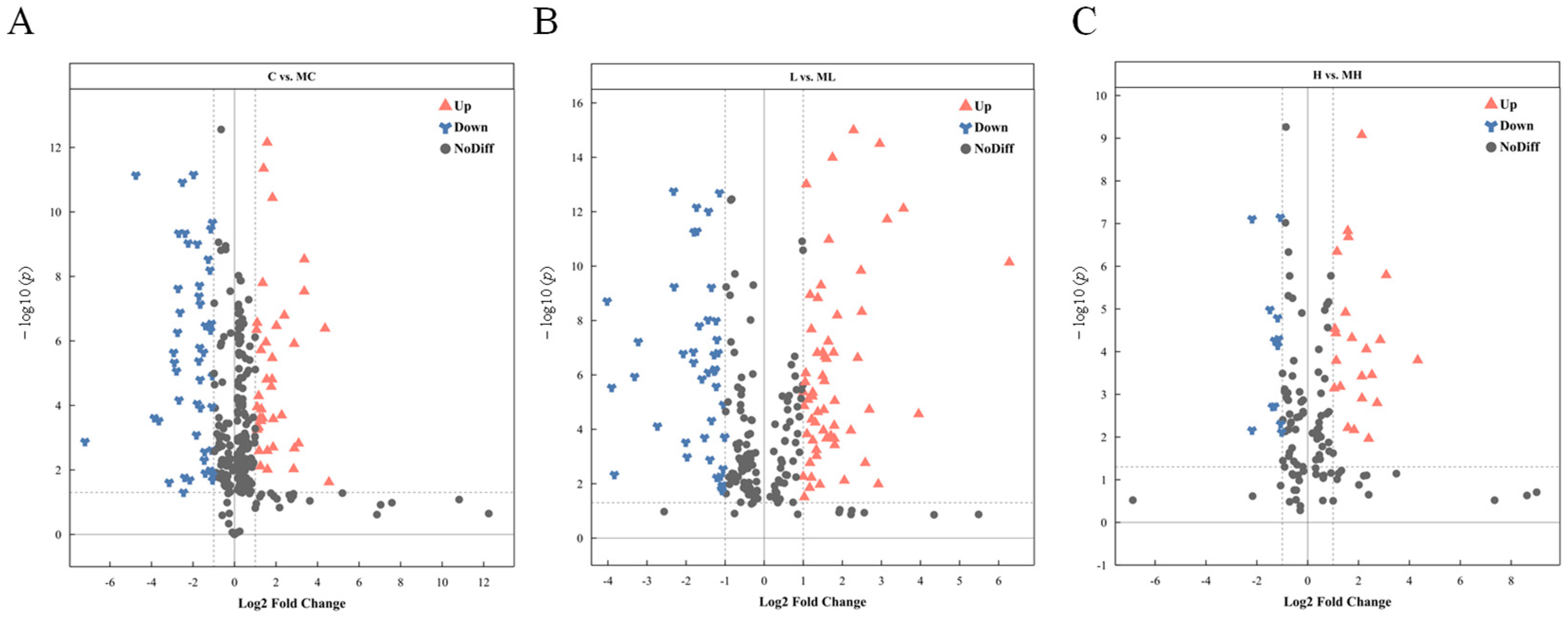
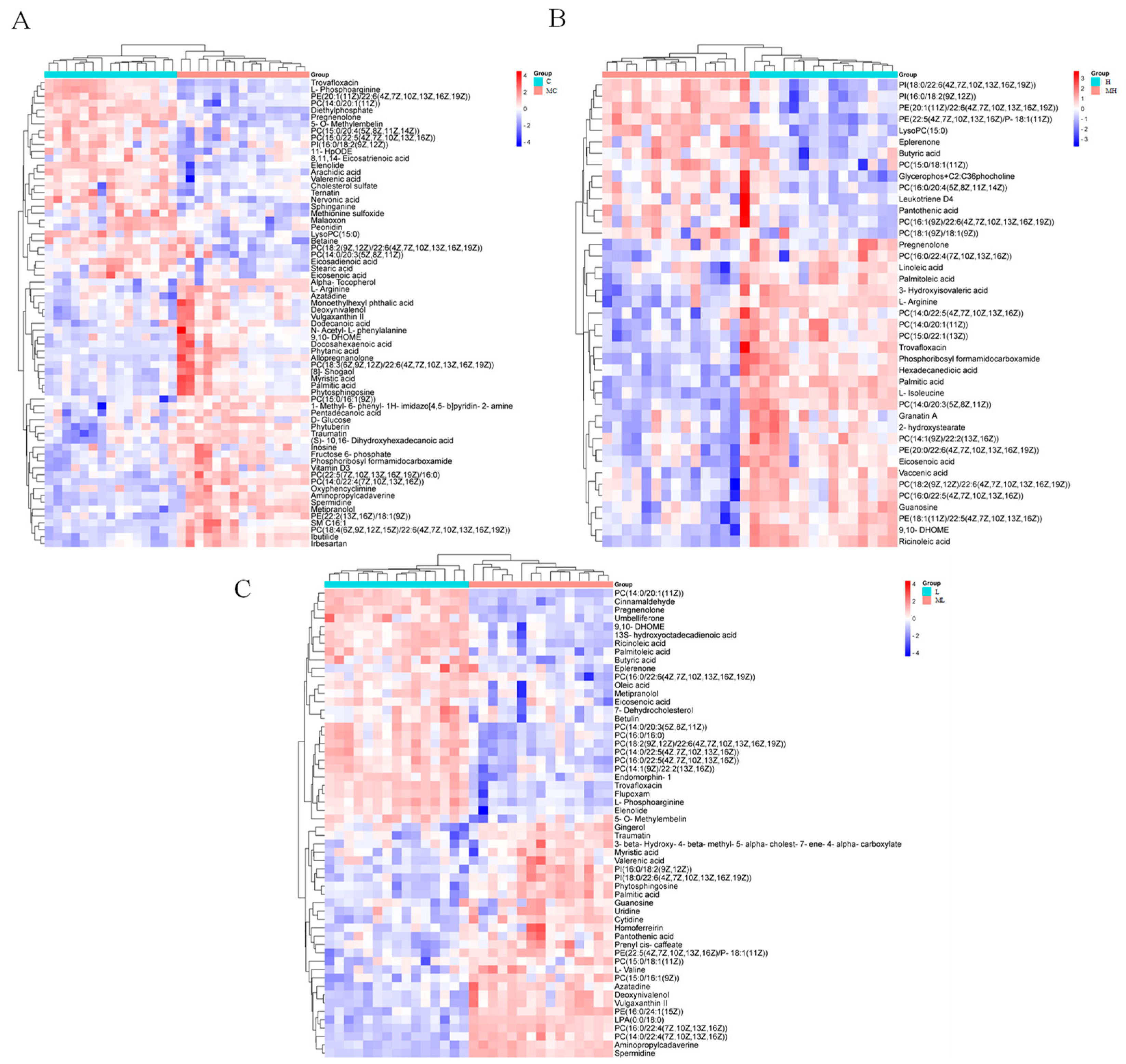
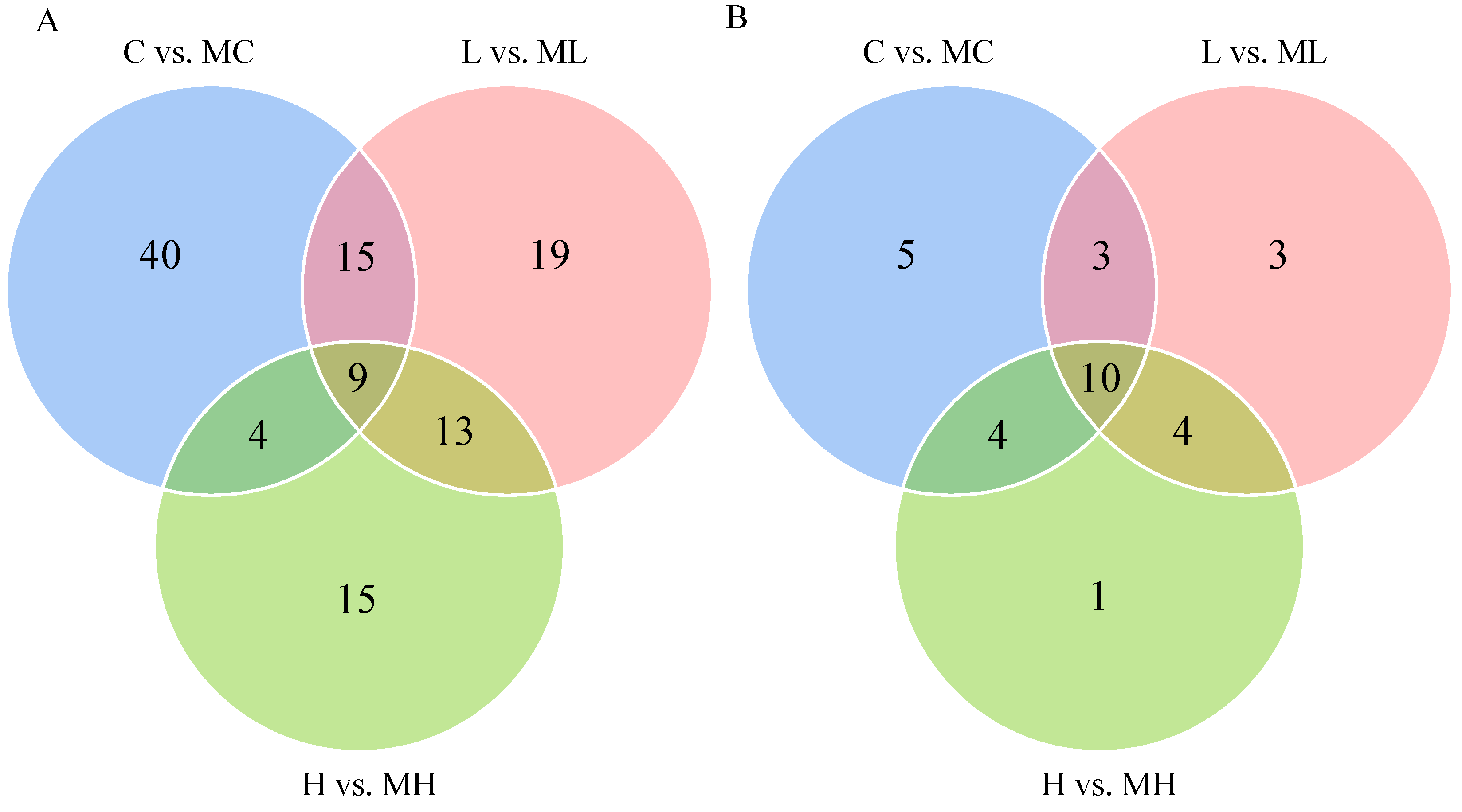
3.2. Identification of Differentially Expressed Metabolites
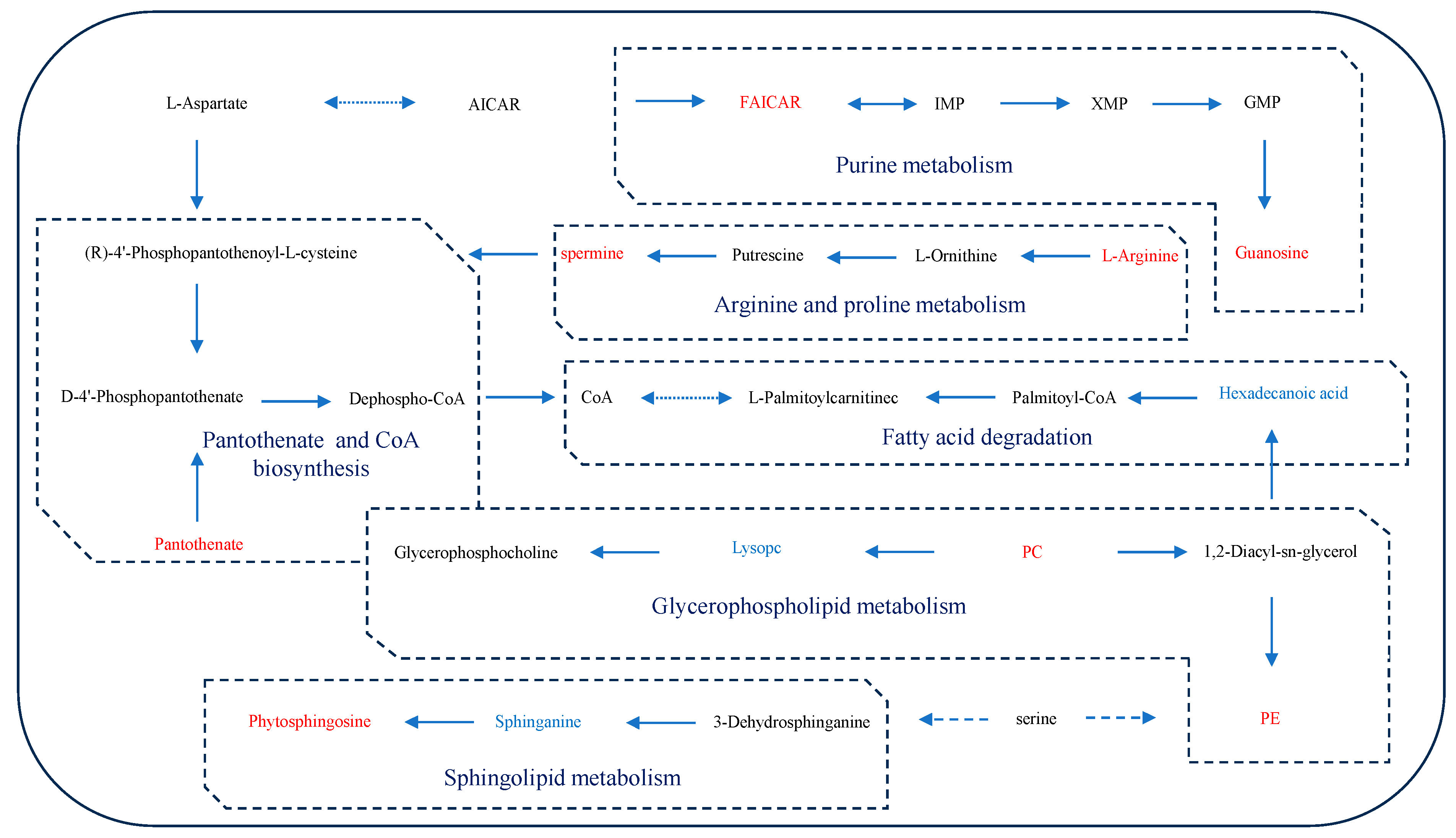
3.3. Enrichment Analysis of Metabolic Pathways
4. Discussions
4.1. Oxidative Stress
4.2. Obstruction of Ammonia Excretion
4.3. Energy Metabolism Disorder
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Z.; Lai, Q.; Zhou, K.; Rizalita, R.E.; Wang, H. Developmental biology of medaka fish (Oryzias latipes) exposed to alkalinity stress. J. Appl. Ichthyol. 2010, 26, 397–402. [Google Scholar] [CrossRef]
- Sherif, A.H.; Okasha, L.A.; Kassab, A.S.; Abass, M.E.; Kasem, E.A. Long-term exposure to lead nitrate and zinc sulfate Nile tilapia impact the Aeromonas hydrophila treatment. Mol. Biol. Rep. 2024, 51, 71. [Google Scholar] [CrossRef] [PubMed]
- Okomoda, V.T.; Isah, S.; Solomon, S.G.; Ikhwanuddin, M. Salinity tolerance in Clarias gariepinus (Burchell, 1822): Insight on blood parameter variations and gill histological changes. Fish Physiol. Biochem. 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Linlin, A.; Zhang, Y.; Xu, B.; Zhang, H.; Li, Y.; Wang, L.; Liang, J.; Zhou, W.; Feng, Z.; Zhang, H. Comprehensive analyses of annexins in naked carp (Gymnocypris przewalskii) unveil their roles in saline-alkaline stress. Aquaculture 2023, 579, 740175. [Google Scholar]
- Tao, S.; Li, X.; Wang, J.; Bai, Y.; Wang, J.; Yang, Y.; Zhao, Z. Examination of the relationship of carbonate alkalinity stress and ammonia metabolism disorder-mediated apoptosis in the Chinese mitten crab, Eriocheir sinensis: Potential involvement of the ROS/MAPK signaling pathway. Aquaculture 2023, 579, 740179. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, T.; Zhang, T.; Sun, M.; Ning, Z.; Chen, Y.; Mu, W. Effects of chronic saline-alkaline stress on gill, liver and intestinal histology, biochemical, and immune indexes in Amur minnow (Phoxinus lagowskii). Aquaculture 2024, 579, 740153. [Google Scholar] [CrossRef]
- Wen, J.; Chen, S.L.; Xu, W.Y.; Zheng, G.D.; Zou, S.M. Effects of high NaHCO3 alkalinity on growth, tissue structure, digestive enzyme activity, and gut microflora of grass carp juvenile. Environ. Sci. Pollut. Res. 2023, 30, 85223–85236. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Zhang, Q.; Ma, Y.; Hou, M.; Sa, R.; Ma, J.; Lü, X. Improving biological traits by soda alkali-saline land diking for fish. Trans. Chin. Soc. Agric. Eng. 2018, 34, 142–146. [Google Scholar]
- Fan, Z.; Wu, D.; Li, J.; Li, C.; Zheng, X.; Wang, L. Phosphorus Nutrition in Songpu Mirror Carp (Cyprinus carpio Songpu) during Chronic Carbonate Alkalinity Stress: Effects on Growth, Intestinal Immunity, Physical Barrier Function, and Intestinal Microflora. Front. Immunol. 2022, 13, 900793. [Google Scholar] [CrossRef]
- Li, H.; Lai, Q.; Yao, Z.; Liu, Y.; Gao, P.; Zhou, K.; Sun, Z. Ammonia excretion and blood gas variation in naked carp (Gymnocypris przewalskii) exposed to acute hypoxia and high alkalinity. Fish Physiol. Biochem. 2020, 46, 1981–1990. [Google Scholar] [CrossRef]
- Song, L.; Zhao, Y.; Song, Y.; Zhao, L.; Ma, C.; Zhao, J. Effects of saline-alkaline water on growth performance, nutritional processing, and immunity in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 544, 737036. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Liu, Z.; Sun, J.; Sun, T.; Lei, M. Histological, Physiological and Transcriptomic Analysis Reveal the Acute Alkalinity Stress of the Gill and Hepatopancreas of Litopenaeus vannamei. Mar. Biotechnol. 2023, 25, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Liang, L.Q. Advances of research of physiological and molecular mechanisms related to alkali-saline adaptation for fish species inhabiting alkali-saline water. J. Fish. China 2021, 45, 798–812. [Google Scholar]
- Zhou, C.; Xu, L.; Song, H.; Feng, J.; Hu, Z.; Yang, M.J.; Shi, P.; Li, Y.R.; Guo, Y.J.; Li, H.Z. Examination of the regulation of energy metabolism, antioxidant response, and ammonia detoxification in hard clam, Mercenaria mercenaria, under hypersalinity stress. Aquaculture 2023, 563, 738916. [Google Scholar] [CrossRef]
- Lv, L.; Ren, J.; Zhang, H.; Sun, C.; Dong, Y.; Lin, Z. Transcriptomic analysis of gill and hepatopancreas in razor clam (Sinonovacula constricta) exposed to acute ammonia. Front. Mar. Sci. 2022, 9, 832494. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, M.; Li, S.; Wei, X.; Ding, L.; Han, S.; Wang, P.; Lv, B.; Chen, Z.; Sun, Y. Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to saline-alkaline stress in the gills of crucian carp (Carassius auratus). Sci. Total Environ. 2022, 822, 153622. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.Q.; Zhao, J.L.; Zhao, Y. Study on the timing sequence of two pathway of Oreochromis niloticus ammonia metabolism under the stress of carbonate alkalinity. Freshw. Fish. 2018, 48, 25–32. [Google Scholar]
- Sun, Y.C.; Wu, S.; Du, N.N.; Song, Y.; Xu, W. High-throughput metabolomics enables metabolite biomarkers and metabolic mechanism discovery of fish in response to alkalinity stress. RSC Adv. 2018, 8, 14983–14990. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.J.; Wei, X.F.; Geng, C.Y.; Liu, W.Z.; Han, L.; Yuan, F.Y.; Wang, P.; Sun, Y.C. Effects of α-ketoglutarate supplementation on serum metabolism of crucian carp under carbonate alkaline stress based on UPLC-Q-TOF/MS metabolomics. J. Fish. Sci. China 2023, 30, 138–149. [Google Scholar]
- Li, S.W.; Han, S.C.; Liu, Y.J.; Ding, L.; Wei, X.F.; Wang, P.; Sun, Y.C. Metabolomics of rainbow trout liver under heat stress. J. Fish. Sci. China 2022, 29, 1168–1178. [Google Scholar]
- Su, H.; Li, Y.; Ma, D.; Fan, J.; Zhong, Z.; Zhu, H. Metabolism responses in the intestine of Oreochromis mossambicus exposed to salinity, alkalinity and salt-alkalinity stress using LC-MS/MS-based metabolomics. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 45, 101044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Metabolomics analysis reveals the response mechanism to carbonate alkalinity toxicity in the gills of Eriocheir sinensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rong, H.N.; Liu, Z.Y.; Chen, H.C.; Wang, Z.Z. Differential analysis on the effect of biological phenotypes on body weight of Carassius auratus gibelio in two pond aquaculture modes. Oceanol. Limnol. Sin. 2022, 53, 1161–1169. [Google Scholar]
- Choi, J.H.; Lee, J.H.; Jo, A.H.; Choi, Y.J.; Choi, C.Y.; Kang, J.C.; Kim, J.H. Microplastic polyamide toxicity: Neurotoxicity, stress indicators and immune responses in crucian carp, Carassius carassius. Ecotoxicol. Environ. Saf. 2023, 265, 115469. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, M.J.; Park, Y.S.; Kang, C.K.; Kim, J.H.; Choi, C.Y. Effects of microfiber and bead microplastic exposure in the goldfish Carassius auratus: Bioaccumulation, antioxidant responses, and cell damage. Aquat. Toxicol. 2023, 263, 106684. [Google Scholar] [CrossRef]
- Lacy, B.; Rahman, M.S. Interactive effects of high temperature and pesticide exposure on oxidative status, apoptosis, and renin expression in kidney of goldfish: Molecular and cellular mechanisms of widespread kidney damage and renin attenuation. J. Appl. Toxicol. 2022, 42, 1787–1806. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Wang, C.; Ye, B.; Lv, L.; Ye, Q.; Xie, S.; Hu, G.; Zou, J. Dietary Antimicrobial Peptides Improve Intestinal Function, Microbial Composition and Oxidative Stress Induced by Aeromonas hydrophila in Pengze Crucian Carp (Carassius auratus var. Pengze). Antioxidants 2022, 11, 1756. [Google Scholar] [CrossRef]
- Wilkie, M.P.; Stecyk, J.A.; Couturier, C.S.; Sidhu, S.; Sandvik, G.K.; Nilsson, G.E. Reversible brain swelling in crucian carp (Carassius carassius) and goldfish (Carassius auratus) in response to high external ammonia and anoxia. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 184, 65–75. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yao, M.Z.; Li, S.W.; Chen, Z.X.; Wang, P.; Sun, Y.C. Research on Metabolomics Analysis Method for Fish Gills Target Organs Based on UPLC-QTOFMS. J. Instrum. Anal. 2022, 41, 1193–1199. [Google Scholar]
- Zhang, H.; Zhang, X.; Zhao, X.; Xu, J.; Lin, C.; Jing, P.; Hu, L.; Zhao, S.; Wang, X.; Li, B. Discrimination of dried sea cucumber (Apostichopus japonicus) products from different geographical origins by sequential windowed acquisition of all theoretical fragment ion mass spectra (SWATH-MS)-based proteomic analysis and chemometrics. Food Chem. 2019, 274, 592–602. [Google Scholar] [CrossRef]
- Ouyang, H.; Deng, N.; Xu, J.; Huang, J.; Han, C.; Liu, D.; Liu, S.; Yan, B.; Han, L.; Li, S. Effects of hyperosmotic stress on the intestinal microbiota, transcriptome, and immune function of mandarin fish (Siniperca chuatsi). Aquaculture 2023, 563, 738901. [Google Scholar] [CrossRef]
- Yun, X.; Zhou, J.; Wang, J.; Li, Q.; Wang, Y.; Zhang, W.; Fan, Z. Biological toxicity effects of florfenicol on antioxidant, immunity and intestinal flora of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2023, 265, 115520. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Deng, Q.; Qiu, M.; Deng, Y.; Sun, L.; Fang, Z.; Liao, J.; Zhao, J.; Gooneratne, R. Iturin A Strongly Inhibits the Growth and T-2 Toxin Synthesis of Fusarium oxysporum: A Morphological, Cellular, and Transcriptomics Study. Foods 2023, 12, 1278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Z.; Huang, J.; Yang, H.; Wang, Q.; Zhang, Y. Analyzing the impact and mechanism of bisphenol A on testicular lipid metabolism in Gobiocypris rarus through integrated lipidomics and transcriptomics. Ecotoxicol. Environ. Saf. 2023, 265, 115498. [Google Scholar] [CrossRef]
- Zhu, S.; Ye, M.; Xu, J.; Guo, C.; Zheng, H.; Hu, J.; Chen, J.; Wang, Y.; Xu, S.; Yan, X. Lipid profile in different parts of edible jellyfish Rhopilema esculentum. J. Agric. Food Chem. 2015, 63, 8283–8291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ma, A.; Yang, S.; Huang, Z. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 102937. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cai, B.; Tian, C.; Jiang, D.; Shi, H.; Huang, Y.; Zhu, C.; Li, G.; Deng, S. RNA Sequencing (RNA-Seq) Analysis Reveals Liver Lipid Metabolism Divergent Adaptive Response to Low-and High-Salinity Stress in Spotted Scat (Scatophagus argus). Animals 2023, 13, 1503. [Google Scholar] [CrossRef]
- Li, X.; Shen, Y.; Bao, Y.; Wu, Z.; Yang, B.; Jiao, L.; Zhang, C.; Tocher, D.R.; Zhou, Q.; Jin, M. Physiological responses and adaptive strategies to acute low-salinity environmental stress of the euryhaline marine fish black seabream (Acanthopagrus schlegelii). Aquaculture 2022, 554, 738117. [Google Scholar] [CrossRef]
- Fang, M.; Lei, Z.; Ruilin, M.; Jing, W.; Leqiang, D. High temperature stress induced oxidative stress, gut inflammation and disordered metabolome and microbiome in tsinling lenok trout. Ecotoxicol. Environ. Saf. 2023, 266, 115607. [Google Scholar] [CrossRef]
- Ferreira, I.A.; Peixoto, D.; Losada, A.P.; Quiroga, M.I.; do Vale, A.; Costas, B. Early innate immune responses in European sea bass (Dicentrarchus labrax L.) following Tenacibaculum maritimum infection. Front. Immunol. 2023, 14, 1254677. [Google Scholar] [CrossRef]
- Yao, M.X.; Yu, H.X.; Mo, H.L.; Zhang, Z.H.; Song, Q.C.; Liu, Q.; Yang, Q.Y.; Wang, L.X.; Li, Y. Structural and pharmacological characterization of a medium-chain fatty acid receptor GPR84 in common carp (Cyprinus carpio). Dev. Comp. Immunol. 2023, 153, 105126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Liang, L.Q.; Liew, H.J.; Chang, Y.M.; Sun, B.; Wang, S.Y.; Mi, B.H.; Zhang, L.M. Identification and analysis of long non-coding RNAs in Leuciscus waleckii adapted to highly alkaline conditions. Front. Physiol. 2021, 12, 665268. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.F.; Liu, Y.J.; Li, S.W.; Ding, L.; Han, S.C.; Chen, Z.X.; Lu, H.; Wang, P.; Sun, Y.C. Stress response and tolerance mechanisms of NaHCO3 exposure based on biochemical assays and multi-omics approach in the liver of crucian carp (Carassius auratus). Ecotoxicol. Environ. Saf. 2023, 253, 114633. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.O.; Ratko, J.; Côrrea, A.P.N.; da Silva, N.G.; Pereira, D.M.C.; Schleger, I.C.; Neundorf, A.K.A.; de Souza, M.R.D.P.; Herrerias, T.; Donatti, L. Assessing physiological responses and oxidative stress effects in Rhamdia voulezi exposed to high temperatures. Fish Physiol. Biochem. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Ding, J.; Zhang, Y.; Hou, C.; Shen, W.; Wu, X.; Zhu, J. Effects of hypoxia stress on oxidative stress, apoptosis and microorganisms in the intestine of large yellow croaker (Larimichthys crocea). Aquaculture 2024, 581, 740444. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Quan, J.; Lu, J.; Zhao, G.; Sun, J. Metabonomics analysis reveals the protective effect of nano-selenium against heat stress of rainbow trout (Oncorhynchus mykiss). J. Proteom. 2022, 259, 104545. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Zhao, X.; Wu, F.; Wang, C.; Wang, C.; White, J.C.; Zhao, W.; Zhou, L.; Yan, S.; Tian, S. Charge-specific adverse effects of polystyrene nanoplastics on zebrafish (Danio rerio) development and behavior. Environ. Int. 2022, 163, 107154. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.F.; Cheng, C.H.; Chen, T.; Zhang, X.; Wu, X.F.; Jiang, X.; Ren, C.H.; Hu, C.Q. Study on Sex Differential Metabolites and Metabolic Pathway of Parental Tropical Sea Cucumbers Holothuria scabra. Prog. Fish. Sci. 2021, 42, 55–67. [Google Scholar]
- Li, Q.Q.; Xiang, Q.Q.; Lian, L.H.; Chen, Z.Y.; Luo, X.; Ding, C.Z.; Chen, L.Q. Metabolic profiling of nanosilver toxicity in the gills of common carp. Ecotoxicol. Environ. Saf. 2021, 222, 112548. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.; Wei, X.; Geng, C.; Liu, W.; Han, L.; Yuan, F.; Wang, P.; Sun, Y. Effects of Saline-Alkaline Stress on Metabolome, Biochemical Parameters, and Histopathology in the Kidney of Crucian Carp (Carassius auratus). Metabolites 2023, 13, 159. [Google Scholar] [CrossRef]
- Zhang, J.M.; Fu, B.; Li, Y.c.; Sun, J.H.; Xie, J.; Wang, G.J.; Tian, J.J.; Kaneko, G.; Yu, E.M. The effect of nitrite and nitrate treatment on growth performance, nutritional composition and flavor-associated metabolites of grass carp (Ctenopharyngodon idella). Aquaculture 2023, 562, 738784. [Google Scholar] [CrossRef]
- Fauzi, I.A.; Haga, Y.; Kondo, H.; Hirono, I.; Satoh, S. Dietary citrulline improves survival of rainbow trout Oncorhynchus mykiss juveniles challenged with Vibrio anguillarum. Aquaculture 2020, 528, 735491. [Google Scholar] [CrossRef]
- Gao, J.; Xu, G.; Xu, P. Gills full-length transcriptomic analysis of osmoregulatory adaptive responses to salinity stress in Coilia nasus. Ecotoxicol. Environ. Saf. 2021, 226, 112848. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, Q.; Kan, D.; Zhao, W.; Guo, H.; Lv, L. Effects of ammonia-N exposure on the growth, metabolizing enzymes, and metabolome of Macrobrachium rosenbergii. Ecotoxicol. Environ. Saf. 2020, 189, 110046. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Deng, F.; Yuan, M.; Chen, M.; Zeng, L.; Ouyang, Y.; Chen, X.; Zhao, B.; Yang, Z.; Tian, Z. Metabolomics reveals the defense mechanism of histidine supplementation on high-salt exposure-induced hepatic oxidative stress. Life Sci. 2023, 314, 121355. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
- Duan, Y.; Nan, Y.; Zhu, X.; Yang, Y.; Xing, Y. The adverse impacts of ammonia stress on the homeostasis of intestinal health in Pacific white shrimp (Litopenaeus vannamei). Environ. Pollut. 2023, 340, 122762. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Li, P.; Liu, Y.; Song, M.; Li, F.; Ou, J.; Lai, J. Integrated metabolomic and transcriptomic responses to heat stress in a high-altitude fish, Triplophysa siluroides. Fish Shellfish. Immunol. 2023, 142, 109118. [Google Scholar] [CrossRef]
- Lei, Y.; Yuan, Z.; Zeng, Q.; Wan, B.; Liu, J.; Wang, W. Dynamic N6-methyladenosine RNA methylation landscapes reveal epi-transcriptomic modulation induced by ammonia nitrogen exposure in the Pacific whiteleg shrimp Litopenaeus vannamei. J. Hazard. Mater. 2023, 458, 131996. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, Y.; Guo, Z.; Xu, G.; Xu, P. Transcriptomic analysis reveals different responses to ammonia stress and subsequent recovery between Coilia nasus larvae and juveniles. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 230, 108710. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Guo, Z.; Zhong, H.; Luo, Y.; Wang, J.; Tang, Z.; Huang, T.; Li, M.; Zhu, J. Transcriptomics integrated with metabolomics reveals the effect of Lycium barbarum polysaccharide on apoptosis in Nile tilapia (Oreochromis niloticus). Genomics 2022, 114, 229–240. [Google Scholar] [CrossRef]
- Yuan, L.; Fan, L.; Dai, H.; He, G.; Zheng, X.; Rao, S.; Yang, Z.; Jiao, X.A. Multi-omics reveals the increased biofilm formation of Salmonella typhimurium M3 by the induction of tetracycline at sub-inhibitory concentrations. Sci. Total Environ. 2023, 899, 165695. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, Z.; Qian, J.; Tu, Z.; Yang, C.; Deng, Y.; Xue, Y.; Shang, Y.; Hu, M.; Wang, Y. Effects of diel-cycling hypoxia and salinity on lipid metabolism and fatty acid composition of the oyster Crassostrea hongkongensis. Mar. Environ. Res. 2023, 191, 106124. [Google Scholar] [CrossRef]
- Hou, L.; Wang, M.; Li, H.; Zhu, L.; Kong, X.; Gu, W.; Bi, K.; Du, J.; Meng, Q. Integrated pathological, proteomic and metabolomic analyses reveal significant changes of Eriocheir sinensis hepatopancreatic in response to the microsporidian Hepatospora eriocheir infection. Aquaculture 2023, 577, 739994. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, J.Y.; Lai, X.H.; Liu, X.X.; Zhang, H.; Wang, X.F.; Tang, B.G. Metabolomic analysis of Trachinotus ovatus under flow velocity stress. Haiyang Xuebao 2023, 45, 53–63. [Google Scholar]
- Yu, Y.; Zhao, P.; Zhai, S. Dietary bile acids supplementation mainly regulates the amino acid metabolic pathways without decreasing bile acids levels in the liver of farmed European eel (Anguilla anguilla) juveniles. Aquac. Rep. 2022, 26, 101283. [Google Scholar] [CrossRef]
- Deng, W.; Sun, J.; Chang, Z.G.; Gou, N.N.; Wu, W.Y.; Luo, X.L.; Zhou, J.S.; Yu, H.B.; Ji, H. Energy response and fatty acid metabolism in Onychostoma macrolepis exposed to low-temperature stress. J. Therm. Biol. 2020, 94, 102725. [Google Scholar] [CrossRef]
- Liu, S.; Tian, F.; Qi, D.; Qi, H.; Wang, Y.; Xu, S.; Zhao, K. Physiological, metabolomic, and transcriptomic reveal metabolic pathway alterations in Gymnocypris przewalskii due to cold exposure. BMC Genom. 2023, 24, 545. [Google Scholar] [CrossRef]
- Bi, B.; Yuan, Y.; Zhao, Y.; He, M.; Song, H.; Kong, L.; Gao, Y. Effect of crowding stress on growth performance, the antioxidant system and humoral immunity in hybrid sturgeon. Aquac. Rep. 2023, 28, 101468. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Geng, C.; Liu, W.; Liu, Y.; Ding, L.; Wang, P. Investigating the Impact of Disrupting the Glutamine Metabolism Pathway on Ammonia Excretion in Crucian Carp (Carassius auratus) under Carbonate Alkaline Stress Using Metabolomics Techniques. Antioxidants 2024, 13, 170. https://doi.org/10.3390/antiox13020170
Sun Y, Geng C, Liu W, Liu Y, Ding L, Wang P. Investigating the Impact of Disrupting the Glutamine Metabolism Pathway on Ammonia Excretion in Crucian Carp (Carassius auratus) under Carbonate Alkaline Stress Using Metabolomics Techniques. Antioxidants. 2024; 13(2):170. https://doi.org/10.3390/antiox13020170
Chicago/Turabian StyleSun, Yanchun, Chuanye Geng, Wenzhi Liu, Yingjie Liu, Lu Ding, and Peng Wang. 2024. "Investigating the Impact of Disrupting the Glutamine Metabolism Pathway on Ammonia Excretion in Crucian Carp (Carassius auratus) under Carbonate Alkaline Stress Using Metabolomics Techniques" Antioxidants 13, no. 2: 170. https://doi.org/10.3390/antiox13020170
APA StyleSun, Y., Geng, C., Liu, W., Liu, Y., Ding, L., & Wang, P. (2024). Investigating the Impact of Disrupting the Glutamine Metabolism Pathway on Ammonia Excretion in Crucian Carp (Carassius auratus) under Carbonate Alkaline Stress Using Metabolomics Techniques. Antioxidants, 13(2), 170. https://doi.org/10.3390/antiox13020170






