Effects of Ferulic Acid on Respiratory Metabolism, Oxidative Lesions, and Apoptotic Parameters in Gills and Red Blood Cells of Carp (Cyprinus carpio Var. Jian) Response to Copper
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Experiment
2.2.1. Experiment Diets
2.2.2. Feeding Trial
2.2.3. CuSO4 Exposure
2.2.4. Metabolic Experiments
2.3. Cell Experiment
2.3.1. Apoptosis Induction
2.3.2. Cytoprotection Assays
2.3.3. Hemolysis and Apoptosis Measurement
2.3.4. Cytochrome c Measurement
2.4. Biochemical Analysis
2.5. Caspase Measurement
2.6. Statistical Analysis
3. Results
3.1. Influences of Dietary FA on Growth Performance and Respiratory Metabolism in Cu-Treated Carp
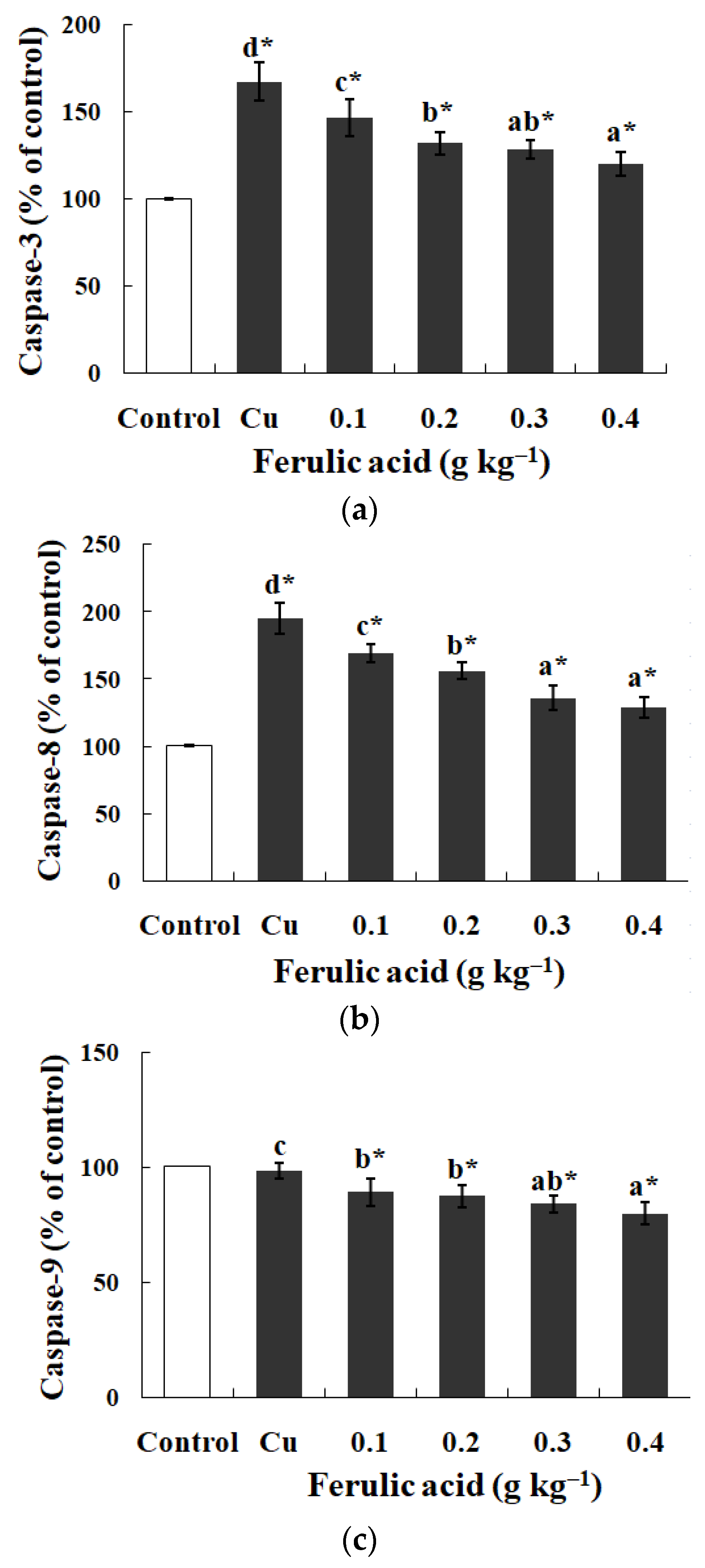
3.2. Influences of Dietary FA on Biochemical Parameters in Gills of Cu-Treated Carp
3.3. Influences of Dietary FA on Hematology and Biochemical Index in Red Blood Cells of Cu-Treated Carp
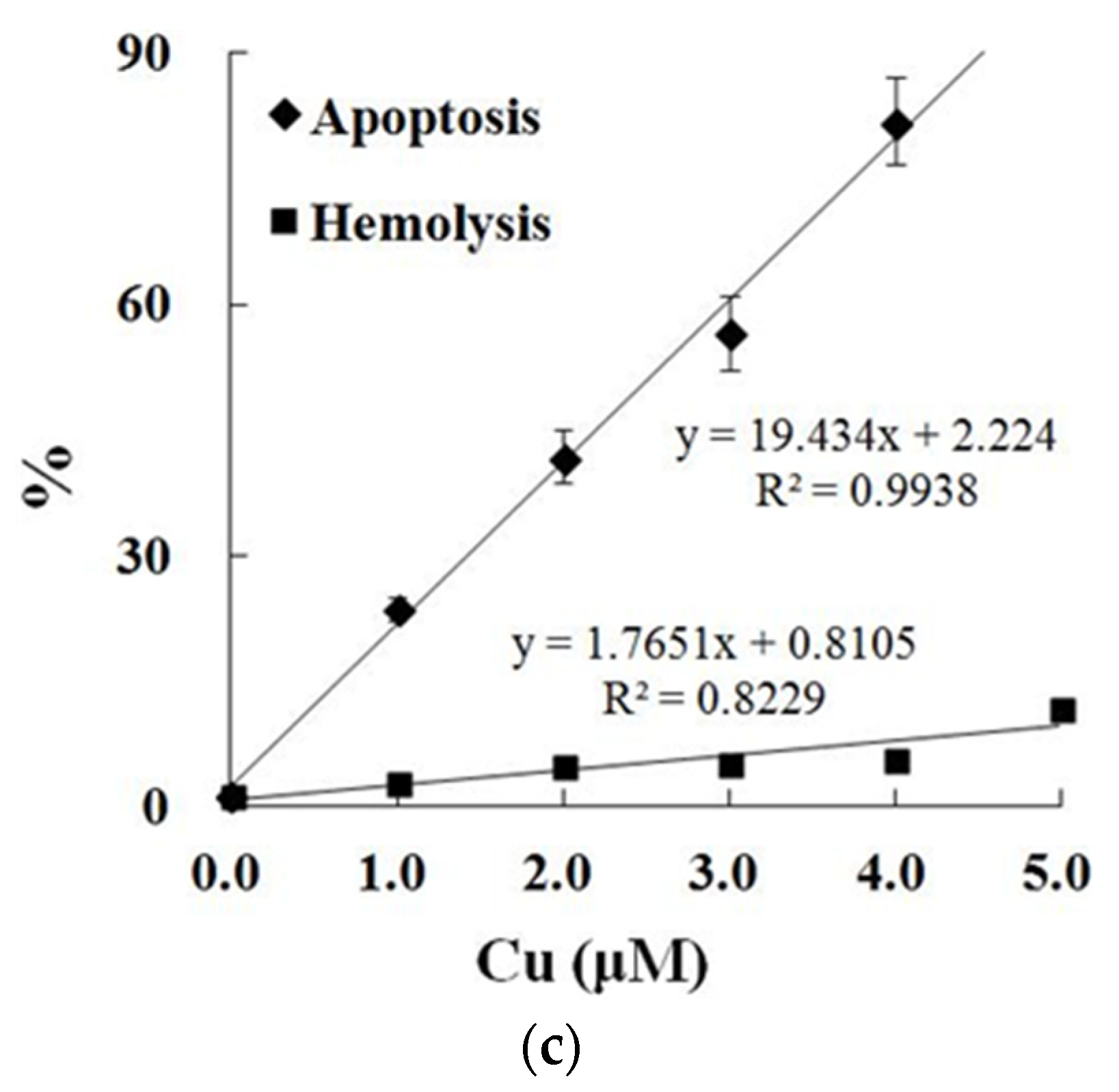
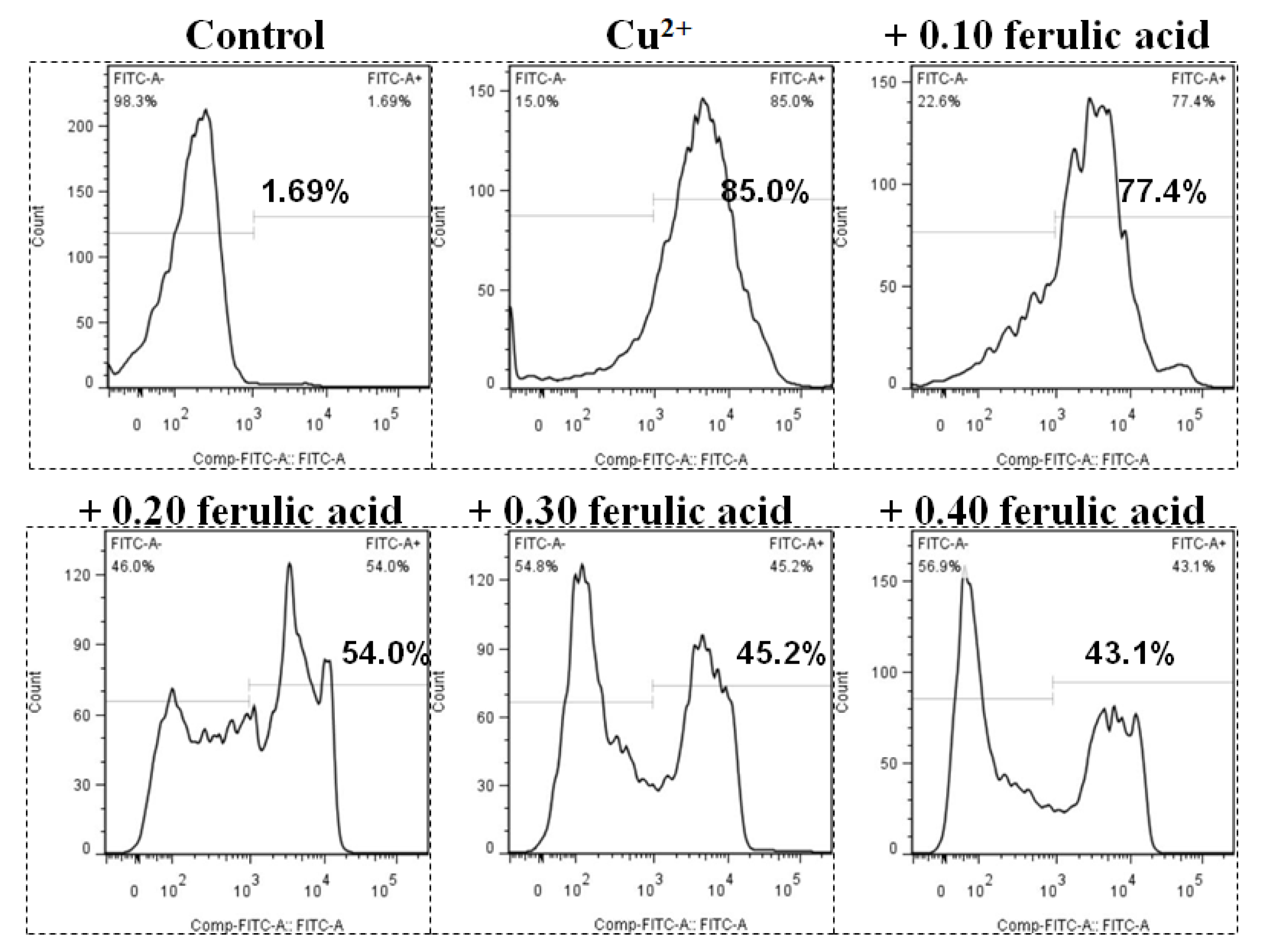
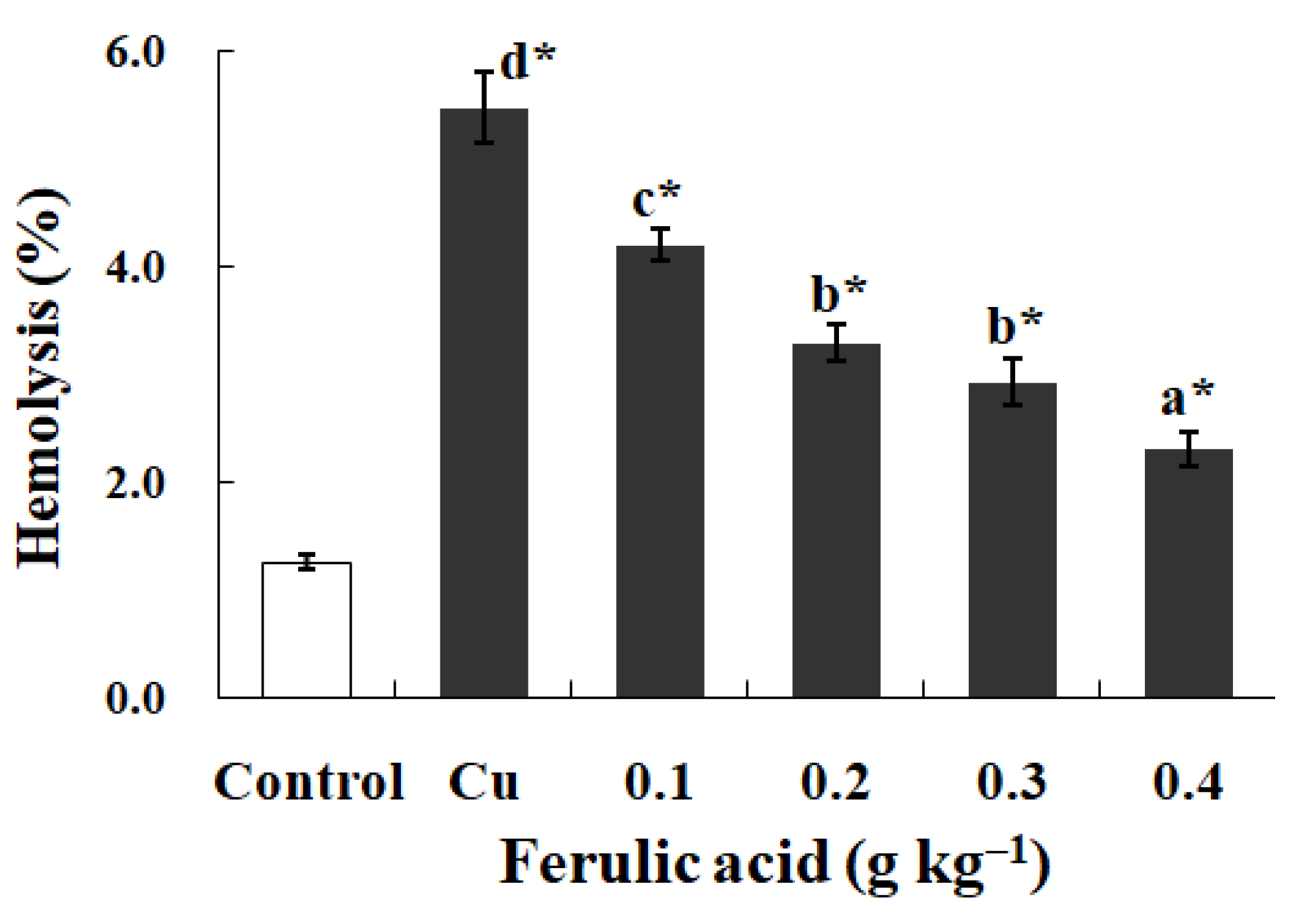
3.4. PS Exposure (a Biomarker of Apoptosis) and Hemolysis Caused by Cu in Carp Red Blood Cells
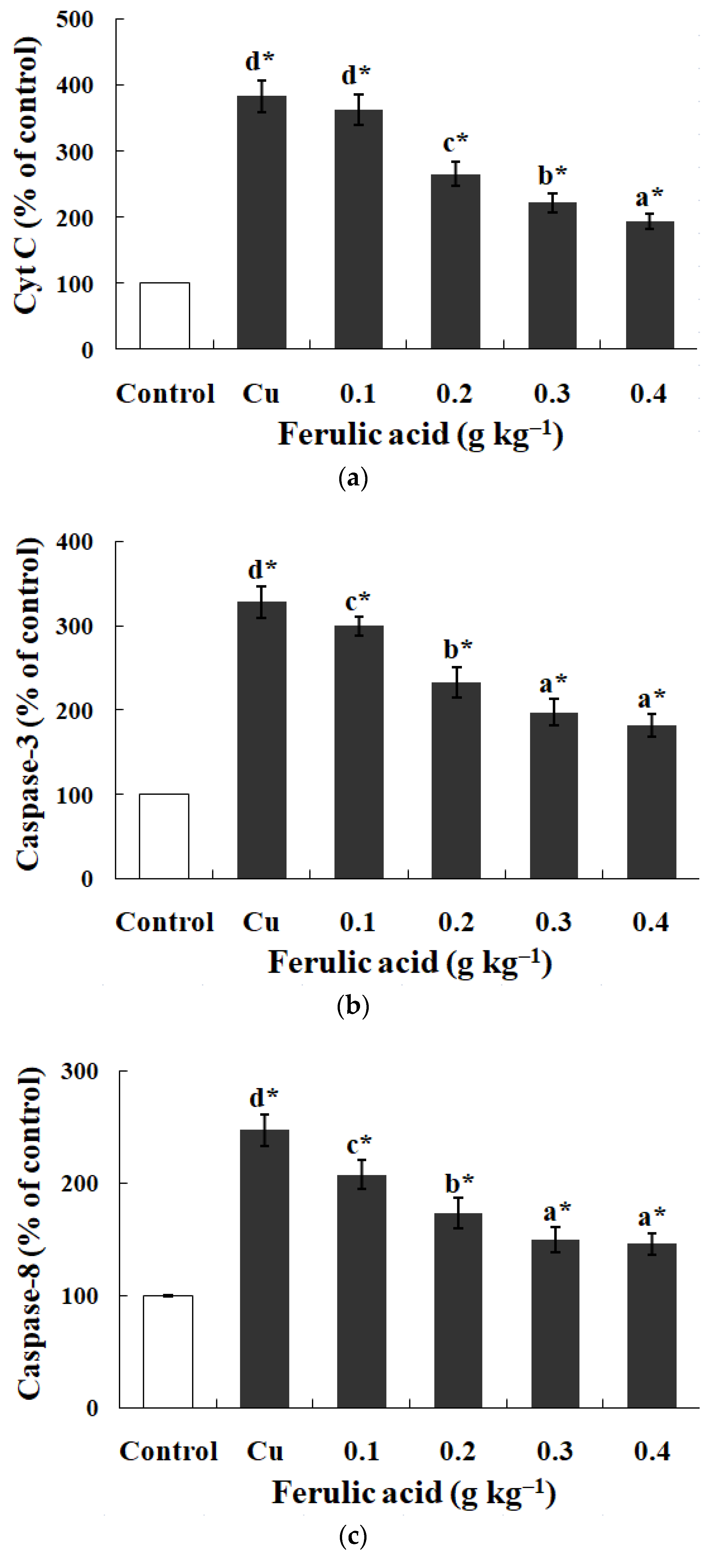
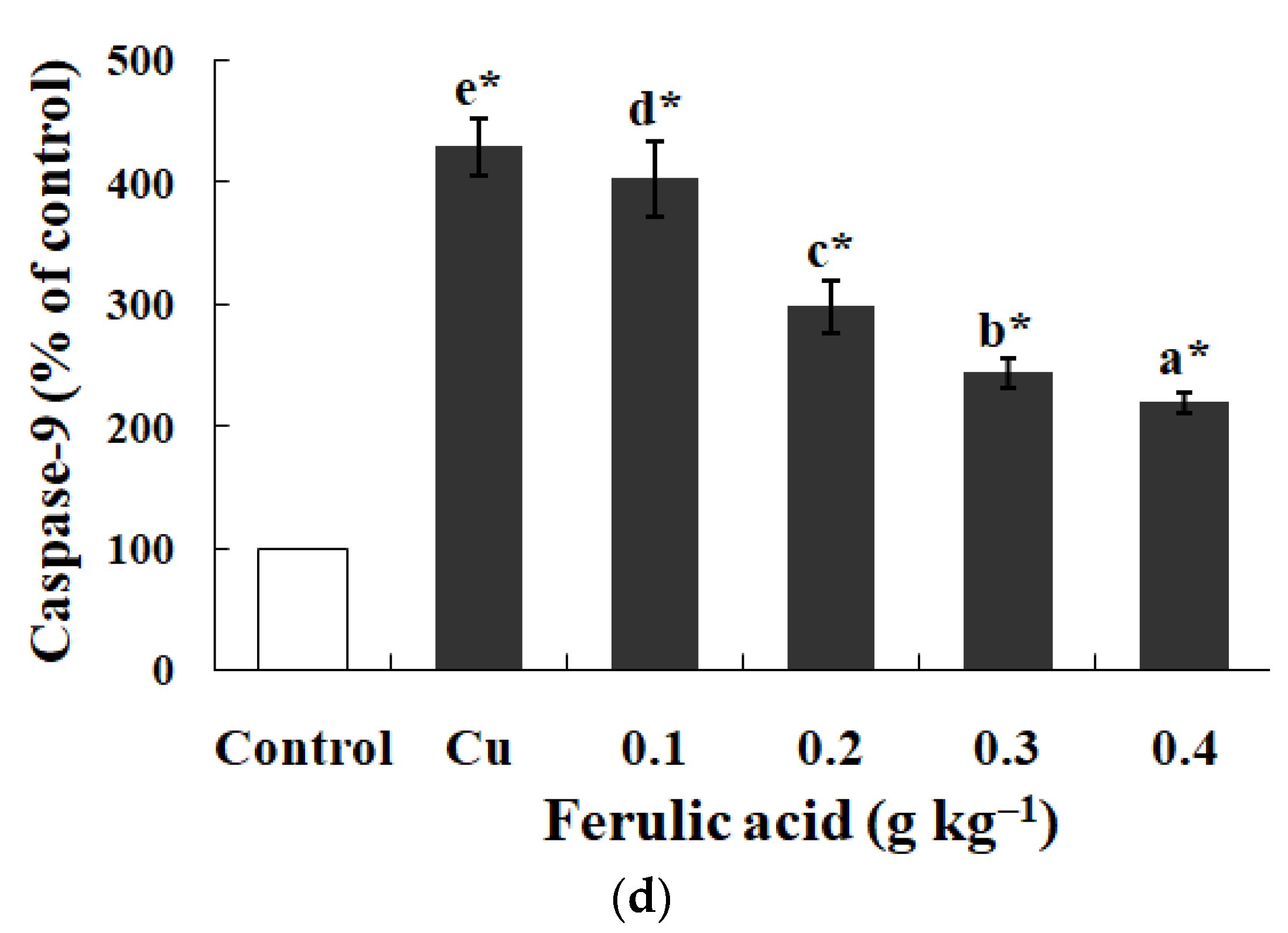
3.5. Effects of Dietary FA on Apoptosis Parameters in Carp Red Blood Cells Treated with Cu
3.6. Effects of FA on Oxidative Lesion Parameters in Carp Red Blood Cells Treated with Cu
4. Discussion
4.1. Dietary FA Improved Respiratory Metabolism in Cu-Treated Fish
4.2. Dietary FA Suppressed Oxidative Lesions and Apoptosis in Gills of Cu-Treated Fish
4.3. Cu Caused Apoptosis and Hemolysis in Red Blood Cells of Fish
4.4. Dietary FA Suppressed Oxidative Lesions and Apoptosis in Fish Red Blood Cells Treated with Cu
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riggio, M.; Filosa, S.; Parisi, E.; Scudiero, R. Changes in zinc, copper and metallothionein contents during oocyte growth and early development of the teleost Danio rerio (zebrafish). Comp. Biochem. Physiol. Part C 2003, 135, 191–196. [Google Scholar] [CrossRef]
- Mazon, A.F.; Fernandes, M.N. Toxicity and differential tissue accumulation of copper in the tropical freshwater fish, Prochilodus scrofa (Prochilodontidae). Bull. Environ. Contam. Toxicol. 1999, 63, 797–804. [Google Scholar] [CrossRef]
- Scudiero, R.; Cretì, P.; Trinchella, F. Evaluation of cadmium, lead and metallothionein contents in the tissues of mussels (Mytilus galloprovincialis) from the Campania coast (Italy): Levels and seasonal trends. Comptes Rendus Biol. 2014, 337, 451–458. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.C.; Paez-Osuna, F.; Hillaire-Marcel, C.; Soto-Jiménez, M.; Ghaleb, B. Principal component analysis applied to the assessment of metal pollution from urban wastes in the Culiacán River Estuary. Bull. Environ. Contam. Toxicol. 2001, 67, 0741–0748. [Google Scholar] [CrossRef]
- Sampaio, F.G.; Boijink, C.L.; Oba, E.T.; Santos, L.R.B.; Kalinin, A.L.; Rantin, F.T. Antioxidant defenses and biochemical changes in pacu (Piaractus mesopotamicus) in response to single and combined copper and hypoxia exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 43–51. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, K.; Yang, X.L. The Efficacy of copper sulfate in controlling infection of Saprolegnia parasitica. J. World Aquac. Soc. 2014, 45, 220–225. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Abicht, H.K.; Knauer, K. Copper-induced oxidative stress in rainbow trout gill cells. Aquat. Toxicol. 2008, 86, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, M.; Modra, H.; Slaninova, A.; Svobodova, Z. Metals as a cause of oxidative stress in fish: A review. Vet. Med. 2011, 56, 537–546. [Google Scholar] [CrossRef]
- Mazon, A.F.; Cerqueira, C.C.C.; Fernandes, M.N. Gill cellular changes induced by copper exposure in the South American tropical freshwater fish Prochilodus scrofa. Environ. Res. 2002, 88, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Parvez, S.; Ansari, R.A.; Ali, M.; Kaur, M.; Hayat, F.; Ahmad, F.; Raisuddin, S. Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish, Channa punctata Bloch. Chem.-Biol. Interact. 2008, 174, 183–192. [Google Scholar] [CrossRef]
- Luzio, A.; Monteiro, S.M.; Fontainhas-Fernandes, A.A.; Pinto-Carnide, O.; Matos, M.; Coimbra, A.M. Copper induced upregulation of apoptosis related genes in zebrafish (Danio rerio) gill. Aquat. Toxicol. 2013, 128–129, 183–189. [Google Scholar] [CrossRef]
- Scudiero, R.; Trinchella, F.; Riggio, M.; Parisi, E. Structure and expression of genes involved in transport and storage of iron in red-blooded and hemoglobin-less antarctic notothenioids. Gene 2007, 397, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kamunde, C.; Clayton, C.; Wood, C.M. Waterborne vs. dietary copper uptake in rainbow trout and the effects of previous waterborne copper exposure. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 283, R69–R78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoyle, I.; Shaw, B.J.; Handy, R.D. Dietary copper exposure in the African walking catfish, Clarias gariepinus: Transient osmoregulatory disturbances and oxidative stress. Aquat. Toxicol. 2007, 83, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, K.X.; Wang, W.; Wang, C.G.; Shen, Y.C. Effects of copper on hemocyte apoptosis, ROS production, and gene expression in White shrimp Litopenaeus vannamei. Biol. Trace Elem. Res. 2017, 179, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Witeska, M.; Kondera, E.; Lipionoga, J.; Jastrzębska, A. Changes in oxygen consumption rate and red blood parameters in common carp Cyprinus carpio L. after acute copper and cadmium exposures. Fresenius Environ. Bull. 2010, 19, 115–122. [Google Scholar]
- Campa-Córdova, A.I.; Núñez-Vázquez, E.J.; Luna-González, A.; Romero-Geraldo, M.J.; Ascencio, F. Superoxide dismutase activity in juvenile Litopenaeus vannamei and Nodipecten subnodosus exposed to the toxic dinoflagellate Prorocentrum lima. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Wu, P.; Kuang, S.Y.; Liu, Y.; Jiang, J.; Hu, K.; Li, S.H.; Tang, L.; Feng, L.; Zhou, X.Q. Myo-inositol prevents copper-induced oxidative damage and changes in antioxidant capacity in various organs and the enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian). Aquat. Toxicol. 2011, 105, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Lu, L.; Zhang, R.M.; Luo, L.; Yuan, Z.; Zhang, S.F.; Jiang, J.; Liu, S.M.; Dong, T.T.; Liang, Q.; et al. The extracts of Angelica sinensis inhibit lipid oxidation in fish erythrocytes and improve growth, digestive, absorptive and antioxidant capacity in juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2019, 25, 119–133. [Google Scholar]
- Baker, R.T.M.; Handy, R.D.; Davies, S.J.; Snook, J.C. Chronic dietary exposure to copper affects growth, tissue lipid peroxidation, and metal composition of the grey mullet, Chelon labrosus. Mar. Environ. Res. 1998, 45, 357–365. [Google Scholar] [CrossRef]
- Dautremepuits, C.; Betoulle, S.; Paris-Palacios, S.; Vernet, G. Humoral immune factors modulated by copper and chitosan in healthy or parasitised carp (Cyprinus carpio L.) by Ptychobothrium sp. (Cestoda). Aquat. Toxicol. 2004, 68, 325–338. [Google Scholar] [CrossRef]
- Khangarot, B.S.; Tripathi, D.M. Changes in humoral and cell-mediated immune responses and in skin and respiratory surfaces of catfish, Saccobranchus fossilis, following copper exposure. Ecotoxicol. Environ. Saf. 1991, 22, 291–308. [Google Scholar] [CrossRef]
- Rougier, F.; Troutaud, D.; Ndoye, A.; Deschaux, P. Non-specific immune response of Zebrafish, Brachydanio rerio (Hamilton-Buchanan) following copper and zinc exposure. Fish Shellfish. Immunol. 1994, 4, 115–127. [Google Scholar] [CrossRef]
- Subathra, S.; Karuppasamy, R. Toxic effects of copper on bioenergetics and growth rates in fingerlings and adult age of the fish, Mystus vittatus (Bloch, 1794). J. Fish. Aquat. Sci. 2007, 2, 285–293. [Google Scholar] [CrossRef]
- Tenorio-Rodriguez, P.A.; Murillo-Álvarez, J.I.; Campa-Cordova, A.I.; Angulo, C. Antioxidant screening and phenolic content of ethanol extracts of selected Baja California Peninsula macroalgae. J. Food Sci. Technol. 2017, 54, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.J.; Wu, F.; Jiang, M.; Yang, C.G.; Liu, W.; Tian, J.; Lu, X.; Wen, H. Ferulic acid: A natural compound as an efficient feed additive for GIFT (Oreochromis niloticus). Aquac. Nutr. 2018, 24, 27–35. [Google Scholar] [CrossRef]
- Yu, L.J.; Wen, H.; Jiang, M.; Wu, F.; Tian, J.; Lu, X.; Xiao, J.R.; Liu, W. Effects of ferulic acid on intestinal enzyme activities, morphology, microbiome composition of genetically improved farmed tilapia (Oreochromis niloticus) fed oxidized fish oil. Aquaculture 2020, 528, 735543. [Google Scholar] [CrossRef]
- Adam, A.; Crespy, V.; Levrat-Verny, M.A.; Leenhardt, F.; Leuillet, M.; Demigne, C.; Remesy, C. The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J. Nutr. 2002, 132, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Anson, N.M.; Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, M.; Jokura, H.; Fujii, A.; Tokimitsu, I.; Hase, T.; Saito, I. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. Am. J. Hypertens. 2007, 20, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Yang, D.D.; Li, Z.H.; He, M.Q.; Li, F.Y.; Jiang, J.; Tang, S.Y.; Peng, P.Y.; Du, W.H.; Ma, Y.T.; et al. Effects of Angelica sinensis extracts on lipid oxidation in fish feeds and growth performance of juvenile Jian carp (Cyprinus carpio var. Jian). Anim. Nutr. 2019, 5, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Zhao, C.R.; Jiang, J.; Feng, L.; Liu, Y. Dietary lysine requirement of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2008, 14, 381–386. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Su, S.L. Juvenile tilapia (Oreochromis niloticus×Oreochromis aureus) requires dietary myo-inositol for maximal growth. Aquaculture 2005, 243, 273–277. [Google Scholar] [CrossRef]
- Li, H.T.; Lu, L.; Wu, M.; Xiong, X.Q.; Luo, L.; Ma, Y.T.; Liu, Y. The effects of dietary extract of mulberry leaf on growth performance, hypoxia-reoxygenation stress and biochemical parameters in various organs of fish. Aquac. Rep. 2020, 18, 100494. [Google Scholar] [CrossRef]
- Ai, Q.H.; Mai, K.S.; Tan, B.P.; Xu, W.; Zhang, W.B.; Ma, H.M.; Liufu, Z.G. Effects of dietary vitamin C on survival, growth, and immunity of large yellow croaker, Pseudosciaena crocea. Aquaculture 2006, 261, 327–336. [Google Scholar] [CrossRef]
- Ai, Q.H.; Mai, K.S.; Zhang, W.B.; Xu, W.; Tan, B.P.; Zhang, C.X.; Li, H.T. Effects of exogenous enzymes (phytase, non-starch polysaccharide enzyme) in diets on growth, feed utilization, nitrogen and phosphorus excretion of Japanese seabass, Lateolabrax japonicus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 502–508. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Ma, Q.Q.; Limbu, S.M.; Du, Z.Y.; Zhang, N.N.; Li, E.C.; Chen, L.Q. Effects of dietary protein to energy ratios on growth, body composition and digestive enzyme activities in Chinese mitten-handed crab, Eriocheir sinensis. Aquac. Res. 2017, 48, 2243–2252. [Google Scholar] [CrossRef]
- Lin, Y.H.; Shiau, S.Y. Mutual sparing of dietary requirements for alpha-tocopherol and selenium in grouper, Epinephelus malabaricus. Aquaculture 2009, 294, 242–245. [Google Scholar] [CrossRef]
- Li, H.T.; Ma, Y.T.; Liu, Y.; Wu, M.; Long, J.; Jing, X.Q.; Zhou, S.S.; Yuan, P.; Jiang, J. Integrated biomarker parameters response to the toxic effects of high stocking density, CuSO4, and trichlorfon on fish and protective role mediated by Angelica sinensis extract. Fish Physiol. Biochem. 2020, 46, 1679–1698. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Wu, M.; Wang, J.; Qin, C.J.; Long, J.; Zhou, S.S.; Yuan, P.; Jing, X.Q. Protective role of Angelica sinensis extract on trichlorfon-induced oxidative damage and apoptosis in gills and erythrocytes of fish. Aquaculture 2020, 519, 734895. [Google Scholar] [CrossRef]
- Campa-Córdova, A.I.; Hernandez-Saavedra, N.Y.; Philippis, R.D.; Ascencio, F. Generation of superoxide anion and SOD activity in haemocytes and muscle of American white shrimp (Litopenaeus vannamei) as a response to β-glucan and sulphated polysaccharide. Fish Shellfish. Immunol. 2002, 12, 353–366. [Google Scholar] [CrossRef]
- Li, H.T.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Li, S.H.; Zhou, X.Q. Oxidative stress parameters and anti-apoptotic response to hydroxyl radicals in fish erythrocytes: Protective effects of glutamine, alanine, citrulline and proline. Aquat. Toxicol. 2013, 126, 169–179. [Google Scholar] [CrossRef]
- Li, H.T.; Jiang, W.D.; Liu, Y.; Jiang, J.; Zhang, Y.A.; Wu, P.; Zeng, Y.Y.; Zhou, X.Q.; Feng, L. Dietary glutamine improves the function of erythrocytes through its metabolites in juvenile carp (Cyprinus carpio var. Jian). Aquaculture 2017, 474, 86–94. [Google Scholar] [CrossRef]
- Li, H.T.; Jiang, W.D.; Liu, Y.; Jiang, J.; Zhang, Y.A.; Wu, P.; Zhao, J.; Duan, X.D.; Zhou, X.Q.; Feng, L. The metabolites of glutamine prevent hydroxyl radical-induced apoptosis through inhibiting mitochondria and calcium ion involved pathways in fish erythrocytes. Free Radic. Biol. Med. 2016, 92, 126–140. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chen, P.; Liang, X.F.; Wu, X.F.; Wang, C.P.; Gu, X.; Xue, M. Dietary N-Carbamylglutamate (NCG) alleviates liver metabolic disease and hepatocyte apoptosis by suppressing ERK1/2-mTOR-S6K1 signal pathway via promoting endogenous arginine synthesis in Japanese seabass (Lateolabrax japonicus). Fish Shellfish. Immunol. 2019, 90, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, H.Y.; Shiau, S.Y. Dietary folic acid requirement of grouper, Epinephelus malabaricus, and its effects on non-specific immune responses. Aquaculture 2011, 317, 133–137. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.Q.; Feng, L.; Liu, Y.; Jiang, J. Effects of glutamine on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells of Jian carp (Cyprinus carpio var. Jian). Aquaculture 2009, 288, 285–289. [Google Scholar] [CrossRef]
- Jiang, W.D.; Feng, L.; Liu, Y.; Jiang, J.; Zhou, X.Q. Myo-inositol prevents oxidative damage, inhibits oxygen radical generation and increases antioxidant enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Res. 2009, 40, 1770–1776. [Google Scholar] [CrossRef]
- Li, H.T.; Zhou, X.Q.; Wu, M.; Deng, M.L.; Wang, C.; Hou, J.J.; Mou, P.J. The cytotoxicity and protective effects of Astragalus membranaceus extracts and butylated hydroxyanisole on hydroxyl radical-induced apoptosis in fish erythrocytes. Anim. Nutr. 2016, 2, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Wu, M.; Jiang, J.; Sun, X.M.; Chen, L.J.; Feng, M.; Yuan, D.Y.; Wen, Z.Y.; Qin, C.J. The extracts of Angelica sinensis restore the digestive and absorptive capacity through improving antioxidant status in digestive organs of fish treated with trichlorfon. Aquac. Res. 2019, 50, 490–504. [Google Scholar] [CrossRef]
- Darbkin, D.L. Spectrophotometric studies. XIV the crystallographic and optical properties of the hemoglobin of man in comparison with these of other species. J. Biol. Chem. 1946, 164, 703–772. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Li, E.C.; Qin, J.G.; Du, Z.Y.; Yu, N.; Kong, Y.Q.; Feng, D.X.; Chen, L.Q. Effect of oxidized fish oil and α -tocopherol on growth, antioxidation status, serum immune enzyme activity and resistance to Aeromonas hydrophila challenge of Chinese mitten crab Eriocheir sinensis. Aquac. Nutr. 2015, 21, 414–424. [Google Scholar] [CrossRef]
- Boyd, C.E.; Massaut, L. Risks associated with the use of chemicals in pond aquaculture. Aquac. Eng. 1999, 20, 113–132. [Google Scholar] [CrossRef]
- Fang, L.; Ling, F.; XIAO, X.; LIU, C.; Xin, H.E. Recent advance in studies on Angelica sinensis. Chin. Herb. Med. 2012, 4, 12–25. [Google Scholar]
- Yu, L.; Wu, F.; Liu, W.; Tian, J.; Lu, X.; Wen, H. Semisynthetic ferulic acid derivative: An efficient feed additive for Genetically Improved Farmed Tilapia (Oreochromis niloticus). Aquac. Res. 2017, 48, 5017–5028. [Google Scholar] [CrossRef]
- Bao, J.; Xing, Y.; Feng, C.; Kou, S.; Jiang, H.; Li, X. Acute and sub-chronic effects of copper on survival, respiratory metabolism, and metal accumulation in Cambaroides dauricus. Sci. Rep. 2020, 10, 16700. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Harauz, G.; Ballantyne, J.S. Regulation of fish gill Na(+)-K(+)-ATPase by selective sulfatide-enriched raft partitioning during seawater adaptation. J. Biol. Chem. 2005, 280, 36545–36550. [Google Scholar] [CrossRef]
- Kondera, E.; Witeska, M. Cadmium and copper reduce hematopoietic potential in common carp (Cyprinus carpio L.) head kidney. Fish Physiol. Biochem. 2013, 39, 755–764. [Google Scholar] [CrossRef]
- Yang, F.; Pei, R.; Zhang, Z.; Liao, J.; Yu, W.; Qiao, N.; Han, Q.; Li, Y.; Hu, L.; Guo, J.; et al. Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol. Vitr. 2019, 54, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Su, S.Y.; Tang, N.Y.; Ho, T.Y.; Lo, W.Y.; Hsieh, C.L. Ferulic acid inhibits nitric oxide-induced apoptosis by enhancing GABA(B1) receptor expression in transient focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2010, 31, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Tang, S.Y.; Du, W.H.; Jiang, J.; Peng, P.Y.; Yuan, P.; Liao, Y.H.; Long, J.; Zhou, S.S. The effects of ethoxyquin and Angelica sinensis extracts on lipid oxidation in fish feeds and growth, digestive and absorptive capacities and antioxidant status in juvenile red carp (Cyprinus carpio var. xingguonensis): A comparative study. Fish Physiol. Biochem. 2019, 45, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Benedetto, R.D.; Varı, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.R.; Gravato, C.; Soares, A.M.V.M.; Morgado, F.; Guilhermino, L. Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: Linking biomarkers to behaviour. Chemosphere 2009, 76, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.S.; Wu, L.Y.; Yang, S.E.; Cheng, H.Y.; Tsai, C.C.; Wu, C.R.; Lin, L.W. Ferulic acid reverses the cognitive dysfunction caused by amyloid β peptide 1-40 through anti-oxidant activity and cholinergic activation in rats. Am. J. Chin. Med. 2015, 43, 319–335. [Google Scholar] [CrossRef]
- Zhai, Q.; Ji, H.; Zheng, Z.; Yu, X.; Sun, L.; Liu, X. Copper induces apoptosis in BA/F3beta cells: Bax, reactive oxygen species, and NFkappaB are involved. J. Cell. Physiol. 2000, 184, 161–170. [Google Scholar] [CrossRef]
- Feng, Q.; Boone, A.N.; Vijayan, M.M. Copper impact on heat shock protein 70 expression and apoptosis in rainbow trout hepatocytes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 345–355. [Google Scholar] [CrossRef]
- Chan, P.C.; Peller, O.G.; Kesner, L. Copper(II)-catalyzed lipid peroxidation in liposomes and erythrocyte membranes. Lipids 1982, 17, 331–337. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Filipe, P.M.; Manso, C.F. Protective effects of a 21-aminosteroid against copper-induced erythrocyte and plasma lipid peroxidation. Eur. J. Pharmacol. 1992, 220, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, J.; Kaji, H.; Ichiyama, K.; Makino, K.; Gohda, E.; Yamamoto, I.; Tai, A. Inhibition of free radical-induced erythrocyte hemolysis by 2-O-substituted ascorbic acid derivatives. Free Radic. Biol. Med. 2007, 43, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, D.; Carloni, M.; Falcioni, G. Oxidative damage in trout erythrocyte in response to “in vitro” copper exposure. Mar. Environ. Res. 2010, 69, 172–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cimen, M.Y. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ates, B.; Orun, I.; Talas, Z.S.; Durmaz, G.; Yilmaz, I. Effects of sodium selenite on some biochemical and hematological parameters of rainbow trout (Oncorhynchus mykiss Walbaum, 1792) exposed to Pb2+ and Cu2+. Fish Physiol. Biochem. 2008, 34, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hai-Yan, Y.E.; Chen, Y.Q.; Si-Ming, Y.U. Ferulic acid protects against apoptosis of PC12 cells induced by kainic acid. Chin. J. Pathophysiol. 2013, 29, 1175–1180. [Google Scholar]
- Mandal, D.; Moitra, P.K.; Saha, S.; Basu, J. Caspase 3 regulates phosphatidylserine externalization and phagocytosis of oxidatively stressed erythrocytes. FEBS Lett. 2002, 513, 184–188. [Google Scholar] [CrossRef]
- Mandal, D.; Mazumder, A.; Das, P.; Kundu, M.; Basu, J. Fas-, caspase 8-, and caspase 3-dependent signaling regulates the activity of the aminophospholipid translocase and phosphatidylserine externalization in human erythrocytes. J. Biol. Chem. 2005, 280, 39460–39467. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Moyes, C.D.; Tufts, B.L. The effects of cell ageing on metabolism in Rainbow trout (Oncorhynchus mykiss) red blood cells. J. Exp. Biol. 2000, 203, 1039–1045. [Google Scholar] [CrossRef]
- Tiihonenk, K.; Nikinmaa, M. Substrate utilization by carp (Cyprinus carpio) erythrocytes. J. Exp. Biol. 1991, 161, 509–514. [Google Scholar] [CrossRef]
- Boyko, M.; Stepensky, D.; Gruenbaum, B.F.; Gruenbaum, S.E.; Melamed, I.; Ohayon, S.; Glazer, M.; Shapira, Y.; Zlotnik, A. Pharmacokinetics of glutamate–oxaloacetate transaminase and glutamate–pyruvate transaminase and their blood glutamate lowering activity in naïve rats. Neurochem. Res. 2012, 37, 2198–2205. [Google Scholar] [CrossRef]
- Gottlieb, M.; Wang, Y.; Teichberg, V.I. Blood-mediated scavenging of cerebrospinal fluid glutamate. J. Neurochem. 2003, 87, 119–126. [Google Scholar] [CrossRef]
- Johnson, R.M.; Goyette, G.; Ravindranath, Y.; Ho, Y.S. Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free Radic. Biol. Med. 2005, 39, 1407–1417. [Google Scholar] [CrossRef]
- Rola, R.C.; Marins, L.F.; Nery, L.E.M.; Rosa, C.E.; Sandrini, J.Z. Responses to ROS inducer agents in zebrafish cell line: Differences between copper and UV-B radiation. Fish Physiol. Biochem. 2014, 40, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.J.; Kim, K.B.; Bae, S.; Choi, B.G.; An, S.; Ahn, K.J.; Kim, S.Y. Pretreatment of ferulic acid protects human dermal fibroblasts against ultraviolet a irradiation. Ann. Dermatol. 2016, 28, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qu, Z.; Zhang, J.; Huo, L.; Gao, J.; Gao, W.Y. Ferulic acid ameliorates memory impairment in d-galactose-induced aging mouse model. Int. J. Food Sci. Nutr. 2016, 67, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Winston, G.W.; Giulioz, R.T.D. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Kochhann, D.; Pavanato, M.A.; Llesuy, S.F.; Correa, L.M.; Riffel, A.P.K.; Loro, V.L.; Mesko, M.F.; Flores, É.M.M.; Dressler, V.L.; Baldisserotto, B. Bioaccumulation and oxidative stress parameters in silver catfish (Rhamdia quelen) exposed to different thorium concentrations. Chemosphere 2009, 77, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Jannuzzi, A.T.; Kara, M.; Alpertunga, B. Celastrol ameliorates acetaminophen-induced oxidative stress and cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2018, 37, 742–751. [Google Scholar] [CrossRef]

| Ingredients (%) | 0.00 g kg−1 | 0.10 g kg−1 | 0.20 g kg−1 | 0.30 g kg−1 | 0.40 g kg−1 |
|---|---|---|---|---|---|
| Fish meal | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Soybean meal | 30.55 | 30.55 | 30.55 | 30.55 | 30.55 |
| Wheat flour | 36.17 | 36.17 | 36.17 | 36.17 | 36.17 |
| DL-methionine | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 |
| Threonine | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Fish oil | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 |
| Soybean oil | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 |
| Ca(H2PO4)2·H2O | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Vitamin mixture 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral mixture 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Microcrystalline cellulose | 1.00 | 0.99 | 0.98 | 0.97 | 0.96 |
| FA | 0.00 | 0.01 | 0.02 | 0.03 | 0.04 |
| Proximate analysis (%) | |||||
| Dry matter | 93.78 | 93.67 | 93.70 | 94.10 | 93.84 |
| Crude protein | 33.90 | 33.82 | 34.06 | 34.03 | 33.91 |
| Crude lipid | 5.55 | 5.48 | 5.54 | 5.61 | 5.53 |
| Crude ash | 7.56 | 7.72 | 7.69 | 7.58 | 7.75 |
| Item | 0.00 FA | 0.10 FA | 0.20 FA | 0.30 FA | 0.40 FA | Regression Equation | R2 |
|---|---|---|---|---|---|---|---|
| IBW (g fish−1) | 9.65 ± 0.31 a | 9.71 ± 0.36 a | 9.71 ± 0.33 a | 9.61 ± 0.30 a | 9.69 ± 0.32 a | - | - |
| FBW (g fish−1) | 19.48 ± 0.62 a | 20.45 ± 1.05 ab | 21.26 ± 0.71 bc | 22.49 ± 1.19 cd | 23.11 ± 1.08 d | Y = 9.3X + 19.49 | 0.992 |
| WG (g fish−1) | 9.83 ± 0.59 a | 10.74 ± 0.73 ab | 11.55 ± 0.94 b | 12.88 ± 1.01 c | 13.43 ± 0.98 c | Y = 9.34X + 9.82 | 0.988 |
| SGR (% day−1) | 2.34 ± 0.12 a | 2.48 ± 0.08 ab | 2.61 ± 0.20 b | 2.83 ± 0.13 c | 2.90 ± 0.15 c | Y = 1.47X + 2.34 | 0.982 |
| FI (g fish−1) | 16.71 ± 0.37 a | 17.11 ± 0.46 a | 18.50 ± 0.28 b | 20.25 ± 0.26 c | 21.35 ± 0.43 d | Y = 12.42X + 16.30 | 0.969 |
| FE (%) | 58.77 ± 2.70 a | 62.72 ± 3.00 a | 62.44 ± 5.00 a | 63.53 ± 4.37 a | 62.84 ± 3.73 a | Y = −56.50X2 + 31.55X + 59.14 | 0.880 |
| Item | 0.00 FA | 0.00 FA+Cu | 0.10 FA+Cu | 0.20 FA+Cu | 0.30 FA+Cu | 0.40 FA+Cu | Regression Equation | R2 |
|---|---|---|---|---|---|---|---|---|
| W (g fish−1) | 21.30 ± 0.26 | 21.20 ± 0.25 a | 21.43 ± 0.38 a | 21.33 ± 0.35 a | 21.50 ± 0.30 a | 21.57 ± 0.29 a | - | - |
| V (L bottle−1) | 4.26 ± 0.05 | 4.24 ± 0.05 a | 4.29 ± 0.08 a | 4.27 ± 0.07 a | 4.30 ± 0.06 a | 4.31 ± 0.06 a | - | - |
| FDO (mg L−1) | 5.33 ± 0.09 | 6.39 ± 0.09 d* | 6.25 ± 0.13 cd* | 6.12 ± 0.10 c* | 5.89 ± 0.10 b* | 5.65 ± 0.13 a* | Y = −2.14X2 − 0.98X + 6.39 | 0.998 |
| FAC (μmol L−1) | 71.51 ± 1.63 | 61.70 ± 0.96 a* | 62.96 ± 1.16 ab* | 64.15 ± 0.81 bc* | 66.04 ± 1.28 c* | 68.79 ± 1.11 d* | Y = 26.00X2 + 6.82X + 61.81 | 0.996 |
| OCR (mg g−1 h−1) | 0.24 ± 0.01 | 0.16 ± 0.01 a* | 0.17 ± 0.01 ab* | 0.18 ± 0.01 b* | 0.20 ± 0.01 c* | 0.22 ± 0.01 d* | Y = 0.214X2 + 0.064X + 0.16 | 0.997 |
| AER (mg g−1 h−1) | 0.030 ± 0.002 | 0.017 ± 0.001 a* | 0.019 ± 0.002 ab* | 0.020 ± 0.001 ab* | 0.023 ± 0.002 b* | 0.027 ± 0.002 c* | Y = 0.035X2 + 0.009X + 0.017 | 0.997 |
| O:N ratio | 6.99 ± 0.37 | 8.06 ± 0.29 b* | 7.85 ± 0.45 ab* | 7.67 ± 0.58 ab* | 7.55 ± 0.68 ab* | 7.09 ± 0.51 a | Y = −3.142X2 − 0.982X + 8.029 | 0.969 |
| Item | 0.00 FA | 0.00 FA+Cu | 0.10 FA+Cu | 0.20 FA+Cu | 0.30 FA+Cu | 0.40 FA+Cu | Regression Equation | R2 |
|---|---|---|---|---|---|---|---|---|
| Na+, K+-ATPase (U mg−1 protein) | 6.61 ± 0.45 | 4.09 ± 0.14 a* | 4.83 ± 0.25 b* | 5.52 ± 0.29 c* | 5.63 ± 0.31 c* | 6.07 ± 0.38 d* | Y = −8.43X2 + 8.13X + 4.11 | 0.981 |
| GOT (U g−1 protein) | 440.10 ± 26.01 | 266.94 ± 13.46 a* | 320.54 ± 15.12 b* | 320.74 ± 24.03 b* | 354.36 ± 18.91 c* | 376.31 ± 13.12 c* | Y = −221.40X2 + 339.50X + 273.10 | 0.936 |
| GPT (U g−1 protein) | 146.52 ± 8.72 | 79.24 ± 3.37 a* | 89.70 ± 5.10 b* | 104.07 ± 3.71 c* | 104.18 ± 5.27 c* | 113.08 ± 4.38 d* | Y = −124.10X2 + 131.80X + 79.13 | 0.963 |
| O2·− (U g−1 protein) | 23.43 ± 1.63 | 32.92 ± 1.99 c* | 29.72 ± 1.41 b* | 27.58 ± 1.63 ab* | 28.11 ± 1.61 ab* | 26.38 ± 1.66 a* | Y = 40.07X2 − 30.71X + 32.68 | 0.932 |
| ·OH (U mg−1 protein) | 17.78 ± 0.68 | 22.47 ± 1.13 c* | 20.28 ± 1.26 b* | 18.69 ± 0.83 ab | 18.98 ± 1.00 ab | 17.51 ± 1.08 a | Y = 23.71X2 − 20.70X + 22.30 | 0.937 |
| MDA (nmol mg−1 protein) | 4.87 ± 0.25 | 6.81 ± 0.24 c* | 6.03 ± 0.30 b* | 5.99 ± 0.38 b* | 5.47 ± 0.34 a | 5.28 ± 0.31 a | Y = 5.00X2 − 5.62X + 6.74 | 0.946 |
| GSH (mg g−1 protein) | 10.47 ± 0.50 | 8.18 ± 0.27 a* | 8.32 ± 0.37 a* | 9.12 ± 0.53 b* | 9.61 ± 0.50 b | 10.59 ± 0.74 c | Y = 9.79X2 + 2.20X + 8.14 | 0.987 |
| SOD (U mg−1 protein) | 12.07 ± 0.75 | 9.87 ± 0.45 a* | 10.69 ± 0.52 ab | 10.62 ± 0.58 ab | 11.72 ± 0.87 bc | 12.87 ± 0.95 c | Y = 13.07X2 + 1.80X + 10.00 | 0.957 |
| CAT (U mg−1 protein) | 4.44 ± 0.30 | 2.79 ± 0.20 a* | 3.49 ± 0.18 b* | 4.11 ± 0.23 c | 4.82 ± 0.26 c | 5.53 ± 0.31 c* | Y = 6.81X + 2.79 | 0.999 |
| GPx (U mg−1 protein) | 100.73 ± 7.26 | 119.20 ± 5.85 a* | 126.90 ± 7.05 ab* | 126.28 ± 7.20 ab* | 134.12 ± 8.48 b* | 138.98 ± 6.87 b* | Y = 47.00X + 119.60 | 0.928 |
| GST (U mg−1 protein) | 48.05 ± 2.37 | 33.14 ± 2.36 a* | 42.63 ± 1.39 b | 47.45 ± 2.85 bc | 46.62 ± 3.55 bc | 51.71 ± 3.44 c* | Y = −103.20X2 + 82.41X + 34.02 | 0.931 |
| Item | 0.00 FA | 0.00 FA+Cu | 0.10 FA+Cu | 0.20 FA+Cu | 0.30 FA+Cu | 0.40 FA+Cu | Regression Equation | R2 |
|---|---|---|---|---|---|---|---|---|
| Hct (%) | 0.48 ± 0.02 | 0.42 ± 0.02 a* | 0.43 ± 0.02 a* | 0.45 ± 0.02 ab | 0.46 ± 0.02 ab | 0.49 ± 0.03 b | Y = 0.214X2 + 0.084X + 0.420 | 0.984 |
| RBC (1012 L−1) | 2.44 ± 0.11 | 2.24 ± 0.13 a | 2.25 ± 0.12 a | 2.32 ± 0.14 ab | 2.38 ± 0.15 ab | 2.49 ± 0.13 b | Y = 1.36X2 + 0.087X + 2.24 | 0.994 |
| HbC (g L−1) | 100.58 ± 2.40 | 82.66 ± 4.54 a* | 85.95 ± 3.46 a* | 89.40 ± 3.85 ab* | 94.37 ± 4.43 bc | 99.22 ± 5.04 c | Y = 32.28X2 + 27.76X + 82.72 | 0.999 |
| MCV (fL cell−1) | 197.31 ± 8.35 | 186.50 ± 10.59 a | 190.76 ± 11.60 a | 193.75 ± 11.64 a | 194.96 ± 11.90 a | 196.50 ± 10.54 a | Y = −64.28X2 + 50.71X + 186.20 | 0.989 |
| MCH (pg cell−1) | 41.23 ± 2.41 | 36.93 ± 1.50 a* | 38.21 ± 1.36 ab | 38.57 ± 1.50 ab | 39.76 ± 2.66 b | 39.91 ± 1.27 b | Y = −10.21X2 + 11.59X + 36.97 | 0.968 |
| MCHC (g L−1) | 208.85 ± 4.98 | 198.37 ± 10.90 a | 200.58 ± 8.07 a | 199.39 ± 8.58 a | 204.07 ± 9.58 a | 203.43 ± 10.33 a | Y = −14.28X2 + 19.71X + 197.90 | 0.828 |
| Item | 0.00 FA | 0.00 FA+Cu | 0.10 FA+Cu | 0.20 FA+Cu | 0.30 FA+Cu | 0.40 FA+Cu | Regression Equation | R2 |
|---|---|---|---|---|---|---|---|---|
| LDH (U g−1 hemoglobin) | 118.20 ± 8.12 | 95.95 ± 6.14 a* | 109.78 ± 6.16 b | 108.52 ± 7.32 b | 111.50 ± 8.60 b | 127.19 ± 8.87 c | Y = 64.20X + 97.74 | 0.831 |
| GOT (U g−1 hemoglobin) | 21.13 ± 1.32 | 16.45 ± 1.07 a* | 19.27 ± 1.10 b | 21.32 ± 1.60 bc | 22.87 ± 1.48 c | 22.12 ± 1.74 c | Y = −54.57X2 + 36.76X + 16.32 | 0.989 |
| GPT (U g−1 hemoglobin) | 15.59 ± 1.04 | 12.57 ± 0.78 a* | 15.03 ± 0.90 b | 15.60 ± 1.11 b | 16.42 ± 1.02 b | 15.91 ± 1.00 b | Y = −40.64X2 + 24.32X + 12.67 | 0.974 |
| Na+, K+-ATPase (U mg−1 hemoglobin) | 1.52 ± 0.05 | 1.14 ± 0.07 a* | 1.36 ± 0.06 b | 1.39 ± 0.07 b | 1.51 ± 0.10 b | 1.67 ± 0.11 b | Y = 1.21X + 1.17 | 0.954 |
| Item | 0.00 FA | 0.00 FA+Cu | 0.10 FA+Cu | 0.20 FA+Cu | 0.30 FA+Cu | 0.40 FA+Cu | Regression Equation | R2 |
|---|---|---|---|---|---|---|---|---|
| O2·− (U g−1 protein) | 26.18 ± 1.87 | 35.61 ± 2.24 c* | 34.58 ± 1.82 bc* | 32.56 ± 2.22 b* | 31.62 ± 1.20 ab* | 29.48 ± 2.16 a* | Y = −15.22X + 35.81 | 0.984 |
| H2O2 (μmol g−1 protein) | 40.43 ± 1.91 | 57.24 ± 3.82 c* | 56.36 ± 3.74 c* | 53.53 ± 2.64 bc* | 50.11 ± 2.56 ab* | 47.88 ± 3.03 a* | Y = −24.97X + 58.01 | 0.972 |
| Met-Hb (g L−1) | 1.74 ± 0.10 | 2.02 ± 0.14 b* | 1.89 ± 0.09 b | 1.88 ± 0.07 b | 1.69 ± 0.08 a | 1.61 ± 0.10 a | Y = −1.02X + 2.02 | 0.950 |
| MDA (nmol mg−1 protein) | 1.64 ± 0.09 | 2.22 ± 0.10 c* | 2.10 ± 0.15 bc* | 2.04 ± 0.14 bc* | 2.00 ± 0.12 ab* | 1.86 ± 0.11 a* | Y = −0.82X + 2.21 | 0.961 |
| GSH (μmol g−1 protein) | 5.07 ± 0.31 | 3.63 ± 0.27 a* | 4.03 ± 0.16 b* | 4.32 ± 0.16 b* | 5.00 ± 0.19 c | 5.53 ± 0.31 d | Y = 4.77X + 3.55 | 0.980 |
| SOD (U mg−1 protein) | 68.95 ± 5.46 | 81.91 ± 6.29 b* | 79.07 ± 4.99 ab | 77.05 ± 3.51 ab | 73.24 ± 5.01 a | 72.20 ± 4.86 a | Y = −25.25X + 81.74 | 0.979 |
| CAT (U mg−1 protein) | 6.03 ± 0.33 | 4.10 ± 0.34 a* | 4.33 ± 0.26 a* | 4.89 ± 0.34 b* | 5.58 ± 0.32 c | 5.87 ± 0.44 c | Y = 4.79X + 4.00 | 0.974 |
| GPx (U mg−1 protein) | 66.85 ± 5.14 | 45.38 ± 2.27 a* | 48.49 ± 3.71 ab* | 52.64 ± 3.50 b* | 58.56 ± 3.53 c* | 62.90 ± 4.01 c | Y = 30.21X2 + 33.02X + 45.17 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, H.; Wu, S.; Ai, C.; Yang, Q.; Jia, J.; Xu, X.; Wu, M.; Jiang, J. Effects of Ferulic Acid on Respiratory Metabolism, Oxidative Lesions, and Apoptotic Parameters in Gills and Red Blood Cells of Carp (Cyprinus carpio Var. Jian) Response to Copper. Antioxidants 2024, 13, 314. https://doi.org/10.3390/antiox13030314
Li H, Liu H, Wu S, Ai C, Yang Q, Jia J, Xu X, Wu M, Jiang J. Effects of Ferulic Acid on Respiratory Metabolism, Oxidative Lesions, and Apoptotic Parameters in Gills and Red Blood Cells of Carp (Cyprinus carpio Var. Jian) Response to Copper. Antioxidants. 2024; 13(3):314. https://doi.org/10.3390/antiox13030314
Chicago/Turabian StyleLi, Huatao, Haijing Liu, Siyue Wu, Chengyan Ai, Qi Yang, Jingting Jia, Xiao Xu, Min Wu, and Jun Jiang. 2024. "Effects of Ferulic Acid on Respiratory Metabolism, Oxidative Lesions, and Apoptotic Parameters in Gills and Red Blood Cells of Carp (Cyprinus carpio Var. Jian) Response to Copper" Antioxidants 13, no. 3: 314. https://doi.org/10.3390/antiox13030314
APA StyleLi, H., Liu, H., Wu, S., Ai, C., Yang, Q., Jia, J., Xu, X., Wu, M., & Jiang, J. (2024). Effects of Ferulic Acid on Respiratory Metabolism, Oxidative Lesions, and Apoptotic Parameters in Gills and Red Blood Cells of Carp (Cyprinus carpio Var. Jian) Response to Copper. Antioxidants, 13(3), 314. https://doi.org/10.3390/antiox13030314







