A Comparison of the Antioxidant Potential and Metabolite Analysis of Marine Fungi Associated with the Red Algae Pterocladiella capillacea from Northern Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Marine Fungi
2.2. Preparation of Fungal Crude Extracts
2.3. Determination of Antioxidant Activity
2.3.1. Free Radical 1,1-Diphenyl-2-picryl-hydrazyl (DPPH)-Scavenging Test
2.3.2. 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radical-Scavenging Test
2.4. Determination of Total Phenolic Content
2.5. Determination of Total Flavonoid Content
2.6. Determination of Total Tannin Content
2.7. Antibacterial Activity
2.8. Brine Shrimp Assay
2.9. LC-HDMSE Analysis of Fungal Extracts
Metabolites Analysis
2.10. Statistical Analysis
3. Results
3.1. Antioxidant and Phytochemical Analysis
3.2. Brine Shrimp Lethal Assay
3.3. Antibacterial Activity
3.4. LC-HDMSE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakai, M. Current Research Status of Fish Immunostimulants. Aquaculture 1999, 172, 63–92. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Flegel, T.W. Historic Emergence, Impact, and Current Status of Shrimp Pathogens in Asia. J. Invertebr. Pathol. 2012, 110, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M. Plant Bioactive Molecules; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019; ISBN 1527526372. [Google Scholar]
- Jadhav, H.B.; Sablani, S.; Gogate, P.; Annapure, U.; Casanova, F.; Nayik, G.A.; Alaskar, K.; Sarwar, N.; Raina, I.A.; Ramniwas, S. Factors Governing Consumers’ Buying Behavior Concerning Nutraceutical Product. Food Sci. Nutr. 2023, 11, 4988–5003. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The Antiviral, Anti-inflammatory Effects of Natural Medicinal Herbs and Mushrooms and SARS-CoV-2 Infection. Nutrients 2020, 25, 2573. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, A.; Jaszek, M.; Stefaniuk, D.; Ciszewski, T.; Matuszewski, Ł. Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE 2018, 13, e0197044. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Harikrishnan, R.; Mukhopadhyay, A.; Ringø, E. Fungi and Actinobacteria: Alternative Probiotics for Sustainable Aquaculture. Fishes 2023, 8, 575. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs Over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wang, J.; Zhang, X.Y.; Nong, X.H.; Qi, S.H. New Furanone Derivatives and Alkaloids from the Co-Culture of Marine-Derived Fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2017, 14, e1600327. [Google Scholar] [CrossRef]
- Sugiharto, S.; Yudiarti, T.; Isroli, I. Functional Properties of Filamentous Fungi Isolated from the Indonesian Fermented Dried Cassava, with Particular Application on Poultry. Mycobiology 2015, 43, 415–422. [Google Scholar] [CrossRef]
- Alanzi, A.; Elhawary, E.A.; Ashour, M.L.; Moussa, A.Y. Aspergillus co-cultures: A Recent Insight into Their Secondary Metabolites and Microbial Interactions. Arch. Pharm. Res. 2023, 46, 273–298. [Google Scholar] [CrossRef]
- Sung, A.A.; Gromek, S.M.; Balunas, M.J. Upregulation and Identification of Antibiotic Activity of a Marine-Derived Streptomyces sp. via Co-Cultures with Human Pathogens. Mar. Drugs 2017, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Fischer, T.; Klassen, S.; Hamacher, A.; Roth, Y.O.; Kassack, M.U.; Roth, E.H. A New Cytotoxic Steroid from Co-Fermentation of Two Marine Alga-Derived Micro-organisms. Nat. Prod. Res. 2014, 28, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wang, R.; Xia, T.; Zhang, S.; Ma, S.; Guo, Z. Natural Products and Biological Activity from Actinomycetes Associated with Marine Algae. Molecules 2023, 28, 5138. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.W.; Hoffmeister, D.; Maggio-Hall, L.A.; Murillo, R.; Glasner, J.D.; Keller, N.P. Genomic Mining for Aspergillus Natural Products. Chem. Biol. 2006, 13, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine Natural Products of Fungal Origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Bellik, Y.; Boukraâ, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: An update. Molecules 2013, 18, 322–353. [Google Scholar] [CrossRef] [PubMed]
- Dutt, R.; Garg, V.; Khatri, N.; Madan, A.K. Phytochemicals in anticancer drug development. Anti-Cancer Agents Med. Chem. 2019, 19, 172–183. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Wang, J.; Ding, W.; Li, C.; Huang, S.; She, Z.; Lin, Y. A new polysubstituted benzaldehyde from the co-culture broth of two marine fungi (strains nos. E33 and K38). Chem. Nat. Compd. 2013, 49, 799–802. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef]
- Diender, M.; Parera Olm, I.; Sousa, D.Z. Synthetic co-cultures: Novel avenues for bio-based processes. Curr. Opin. Biotechnol. 2021, 67, 72–79. [Google Scholar] [CrossRef]
- Li, S.; Xiao, J.; Sun, T.; Yu, F.; Zhang, K.; Feng, Y.; Xu, C.; Wang, B.; Cheng, L. Synthetic microbial consortia with programmable ecological interactions. Methods Ecol. Evol. 2022, 13, 1608–1621. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Proksch, P. Endophytes and associated marine derived fungi—Ecological and chemical perspectives. Fungal Divers. 2012, 57, 45–83. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Deutsch, Y.; Gur, L.; Berman Frank, I.; Ezra, D. Endophytes From Algae, a Potential Source for New Biologically Active Metabolites for Disease Management in Aquaculture. Front. Mar. Sci. 2021, 8, 636636. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Neil, D.M.; Peeler, E.J.; Shields, J.D.; Small, H.J.; Flegel, T.W.; Vlak, J.M.; Jones, B.; Morado, F.; Moss, S.; et al. Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J. Invertebr. Pathol. 2012, 110, 141–157. [Google Scholar] [CrossRef] [PubMed]
- De Felício, R.; Pavão, G.B.; Oliveira, A.L.L.; Erbert, C.; Conti, R.; Pupo, M.T.; Furtado, N.A.J.C.; Ferreira, E.G.; Costa-Lotufo, L.V.; Young, M.C.M.; et al. Antibacterial, antifungal and cytotoxic activities exhibited by endophytic fungi from the Brazilian marine red alga Bostrychia tenella (Ceramiales). Rev. Bras. Farmacogn. 2015, 25, 641–650. [Google Scholar] [CrossRef]
- Wiegand, S.; Jogler, M.; Boedeker, C.; Pinto, D.; Vollmers, J.; Rivas-Marin, E.; Kohn, T.; Peeters, S.H.; Heuer, A.; Rast, P.; et al. Cultivation and functional characterization of 79 planctomycetes uncovers their unique biology. Nat. Microbiol. 2020, 5, 126–140. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Martínez Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of Probiotics in Aquaculture. Int. Sch. Res. Not. 2012, 2012, 916845. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Seedevi, P.; Rajan, D.K. Potential uses of fungal polysaccharides as immunostimulants in fish and shrimp aquaculture: A review. Aquaculture 2019, 500, 250–263. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, S.; Meena, D.K.; Sarkar, U.K. Application of Probiotics in Shrimp Aquaculture: Importance, Mechanisms of Action, and Methods of Administration. Rev. Fish. Sci. Aquacult. 2016, 24, 342–368. [Google Scholar] [CrossRef]
- Cha, H.-J.; Chiang, M.W.L.; Guo, S.-Y.; Lin, S.-M.; Pang, K.-L. Culturable Fungal Community of Pterocladiella capillacea in Keelung, Taiwan: Effects of Surface Sterilization Method and Isolation Medium. J. Fungi 2021, 7, 651. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Woźniak, A. The Effect of Lamium album Extract on Cultivated Human Corneal Epithelial Cells (10.014 pRSV-T). J. Ophthalmic Vis. Res. 2015, 10, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hayakawa, S.; Chuamanochan, M.; Fujimoto, M.; Innun, A.; Izumori, K. Antioxidant effects of Maillard reaction products obtained from ovalbumin and different D-aldohexoses. Biosci. Biotechnol. Biochem. 2006, 70, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Orak, H.H. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape cultivars and their correlations. Sci. Hortic. 2017, 111, 235–241. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Lieberman, M. A Brine Shrimp Bioassay for Measuring Toxicity and Remediation of Chemicals. J. Chem. Educ. 1999, 76, 1689. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Nariya, P.B.; Bhalodia, N.R.; Shukla, V.J.; Acharya, R.; Nariya, M.B. In vitro evaluation of antioxidant activity of Cordia dichotoma (Forst f.) bark. Ayu 2013, 34, 124–128. [Google Scholar] [CrossRef]
- Ancerewicz, J.; Miglavaca, E.; Carrupt, P.A.; Testa, B.; Bree, F.; Zinin, R.; Tillement, J.-P.; Tillement, S.; Guyot, D.; Chauvet-Monges, A.-M.; et al. Structure property relationship of trimetadizine derivatives and model compounds as potential antioxidants. Free. Radic. Biol. Med. 1998, 25, 113–120. [Google Scholar] [CrossRef]
- Min, D.B. Lipid oxidation of edible oil. In Food Lipids: Chemistry, Nutrition and Biotechnology; Akoh, K., Min, D.B., Eds.; Marcel Dekkar: New York, NY, USA, 1998. [Google Scholar]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J. Automatic method for determination of total antioxidant capacity using 2,2-diphenyl-1-picrylhydrazyl assay. Anal. Chim. Acta 2006, 558, 310–318. [Google Scholar] [CrossRef]
- Polášek, M.; Skala, P.; Opletal, L.; Jahodář, L. Rapid automated assay of anti-oxidation/radical-scavenging activity of natural substances by sequential injection technique (SIA) using spectrophotometric detection. Anal. Bioanal. Chem. 2004, 379, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.P.; Gillespie, T.J.; Porreca, F. Peptide fragments derived from the β-chain of hemoglobin (hemorphins) are centrally active in vivo. Peptides 1989, 10, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.; Mallouchos, A.; Komaitis, M. Investigation of the antioxidant behavior of air- and freeze-dried aromatic plant materials in relation to their phenolic content and vegetative cycle. J. Agric. Food Chem. 2008, 56, 5743–5752. [Google Scholar] [CrossRef] [PubMed]
- Soquetta, M.B.; Terra, L.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Kešelj, K.; Pavkov, I.; Radojcin, M.; Stamenković, Z. Comparison of energy consumption in the convective and freeze drying of raspberries. J. Process Energy Agric. 2017, 21, 192–196. [Google Scholar] [CrossRef]
- Weiguang, Y.; Hazel, Y.W. Effects of drying and extraction conditions on the biochemical activity of selected herbs. HortScience 2011, 46, 70–73. [Google Scholar]
- Stępień, A.E.; Gorzelany, J.; Matłok, N.; Lech, K.; Figiel, A. The effect of drying methods on the energy consumption, bioactive potential and color of dried leaves of Pink Rock Rose (Cistus creticus). J. Food Sci. Technol. 2019, 56, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.G.; Gonçalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014, 146, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Gramza, A.; Pawlak-Lemańska, K.; Korczak, J.; Wasowicz, E.; Rudzińska, M. Tea extracts as free radical scavengers. Pol. J. Environ. Stud. 2004, 14, 861–867. [Google Scholar]
- Srivastava, M.P.; Tiwari, R.; Sharma, N. Assessment of phenol and flavonoid content in the plant materials. J. New Biol. Rep. 2013, 2, 163–166. [Google Scholar]
- Tshivhandekano, I.; Ntushelo, K.; Ngezimana, W.; Tshikalange, T.E.; Mudau, F.N. Chemical compositions and antimicrobial activities of Athrixia phylicoides DC. (bush tea), Monsonia burkeana (special tea) and synergistic effects of both combined herbal teas. Asian Pac. J. Trop. Med. 2014, 7, S448–S453. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.R.; David, J.M.; Borges, R.D.S.; Ferreira, S.L.C.; David, J.P.; Reis, P.S.D.; Bruns, R.E. Determination of flavanones in orange juices obtained from different sources by HPLC/DAD. J. Anal. Methods Chem. 2014, 2014, 296838. [Google Scholar] [CrossRef]
- Bakhari, N.A.; Abdullah, A.R.; Hasnah, O.; Nordin, N.H. The relationship between phenolic, tannin and flavonoid content with the antioxidant activity of Pereskia bleo (Kunth). In Proceedings of the 2010 International Conference on Science and Social Research (CSSR), Kuala Lumpur, Malaysia, 5–7 December 2010; pp. 494–498. [Google Scholar]
- Stévant, P.; Indergård, E.; Ólafsdóttir, A.; Marfaing, H.; Larssen, W.E.; Fleurence, J.; Roleda, M.Y.; Rustad, T.; Slizyte, R.; Nordtvedt, T.S. Effects of drying on the nutrient content and physico-chemical and sensory characteristics of the edible kelp Saccharina latissima. J. Appl. Phycol. 2018, 30, 2587–2599. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 143. [Google Scholar] [CrossRef]
- Chao, J.; Dai, Y.; Cheng, H.Y.; Lam, W.; Cheng, Y.C.; Li, K.; Peng, W.H.; Pao, L.H.; Hsieh, M.T.; Qin, X.M.; et al. Improving the concentrations of the active components in the herbal tea ingredient, Uraria crinita: The effect of post-harvest oven-drying processing. Sci. Rep. 2017, 7, 38763. [Google Scholar] [CrossRef]
- Ghelichkhani, G.; Modaresi, M.H.; Rashidi, L.; Shariatifar, N.; Homapour, M.; Arabameri, M. Effect of the spray and freeze dryers on the bioactive compounds of olive leaf aqueous extract by chemometrics of HCA and PCA. J. Food Meas. Charact. 2019, 13, 2751–2763. [Google Scholar] [CrossRef]
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of solvent evaporation method on phenolic compounds and the antioxidant activity of Moringa oleifera cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Becker, J.V.; Armstrong, G.O.; van der Merwe, M.J.; Lambrechts, M.G.; Vivier, M.A.; Pretorius, I.S. Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res. 2003, 4, 79–85. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.U.H. Novel Hypotensive Agents, Niazimin A, Niazimin B, Niazicin A and Niazicin B from Moringa oleifera: Isolation of First Naturally Occurring Carbamates. J. Chem. Soc. Perkin Trans. 1994, 3035–3040. [Google Scholar] [CrossRef]
- Anjum, F.; Bukhari, S.A.; Shahid, M.; Anwar, S.; Afzal, M.; Afzal, N. Comparative evaluation of antioxidant potential of parasitic plant collected from different hosts. J. Food Process. Technol. 2013, 4, 228. [Google Scholar] [CrossRef]
- Monge, A.N.; Sigelman, D.W.; Temple, R.J.; Chahal, H.S. Use of US Food and Drug Administration Expedited Drug Development and Review Programs by Orphan and Nonorphan Novel Drugs Approved from 2008 to 2021. JAMA Netw. Open 2022, 5, e2239336. [Google Scholar] [CrossRef]
- Long, Z.; Xuli, L.; Shengtao, Y.; Ying, Z.; Fanke, Z.; Shaohao, X.; Yupo, C.; Wei, Z. The anti-inflammatory activity of GABA-enriched Moringa oleifera leaves produced by fermentation with Lactobacillus plantarum LK-1. Front. Nutr. 2023, 10, 346. [Google Scholar] [CrossRef]
- Samidurai, A.; Xi, L.; Das, A.; Iness, A.N.; Vigneshwar, N.G.; Li, P.L.; Singla, D.K.; Muniyan, S.; Batra, S.K.; Kukreja, R.C. Role of phosphodiesterase 1 in the pathophysiology of diseases and potential therapeutic opportunities. Pharmacol. Ther. 2021, 226, 107858. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. Salt stimulation and tolerance in an intertidal stem-succulent halophyte. J. Plant Nutr. 2005, 28, 1365–1374. [Google Scholar] [CrossRef]
- Yun, T.; Jing, T.; Zang, X.; Zhou, D.; Li, K.; Zhao, Y.; Wang, W.; Xie, J. Antimicrobial mechanisms and secondary metabolite profiles of Streptomyces hygroscopicus subsp. hygroscopicus 5–4 against banana fusarium wilt disease using metabolomics. Front. Microbiol. 2023, 14, 1159534. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Ayatollahi, S.A.; Kobarfard, F.; Staniak, M.; Stępień-Warda, A.; Czopek, K.; Sen, S.; Acharya, K.; Matthews, K.; et al. Chemical Composition, Biological Activity, and Health-Promoting Effects of Withania somnifera for Pharma-Food Industry Applications. J. Food Qual. 2021, 2021, 8985179. [Google Scholar] [CrossRef]
- Amiri, F.; Moghadam, A.; Tahmasebi, A.; Niazi, A. Identification of key genes involved in secondary metabolite biosynthesis in Digitalis purpurea. PLoS ONE 2023, 18, e0277293. [Google Scholar] [CrossRef]
- Fidrianny, I.; Rahmiyani, I.; Wirasutisna, K.R. Antioxidant capacities from various leaves extracts of four varieties mangoes using DPPH, ABTS assays and correlation with total phenolic, flavonoid, carotenoid. Int. J. Pharm. Pharm. Sci. 2013, 5, 189–194. [Google Scholar]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Figueroa, M.E.; Davy, A.J. Salt stimulation of growth and photosynthesis in an extreme halophyte, Arthrocnemum macrostachyum. Plant Biol. 2010, 12, 79–87. [Google Scholar] [CrossRef]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Phenolic acids from plants: Extraction and application to human health. Nat. Prod. Commun. 2018, 58, 389–417. [Google Scholar]
- Jitan, S.A.; AlKhoori, S.; Ochsenkühn, M.; Amin, S.A.; Yousef, L.F. Ethanol/water extracts from halophyte species Arthrocnemum macrostachyum and Tetraena qatarensis. Cogent Chem. 2018, 4, 1536311. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from Southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef]
- Ali, A.; Alzeyoudi, S.A.R.; Almutawa, S.A.; Alnajjar, A.N.; Vijayan, R. Molecular basis of the therapeutic properties of hemorphins. Pharmacol. Res. 2020, 158, 104855. [Google Scholar] [CrossRef] [PubMed]
- Panella, N.A.; Dolan, M.C.; Karchesy, J.J.; Xiong, Y.; Peralta-Cruz, J.; Khasawneh, M.; Montenieri, J.A.; Maupin, G.O. Use of novel compounds for pest control: Insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar. J. Med. Entomol. 2005, 42, 352–358. [Google Scholar] [CrossRef]
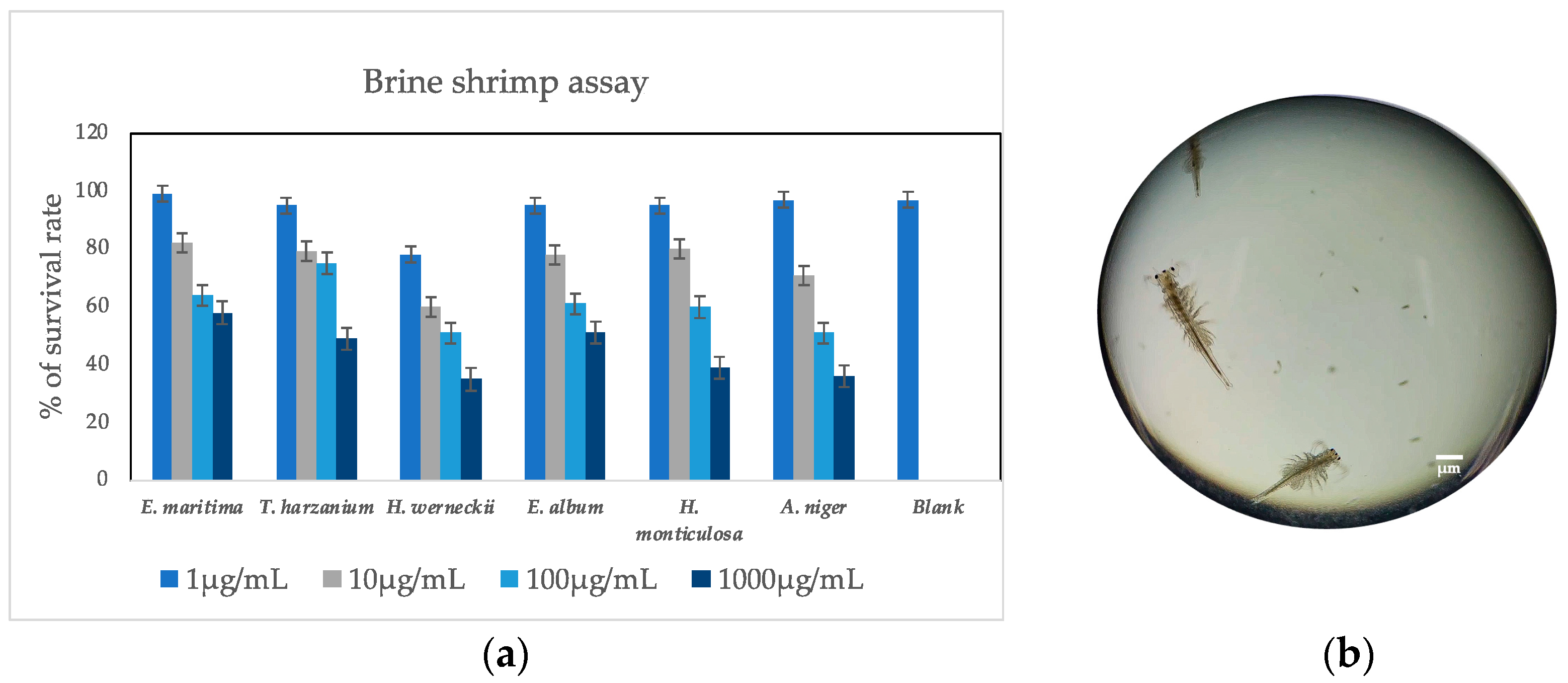
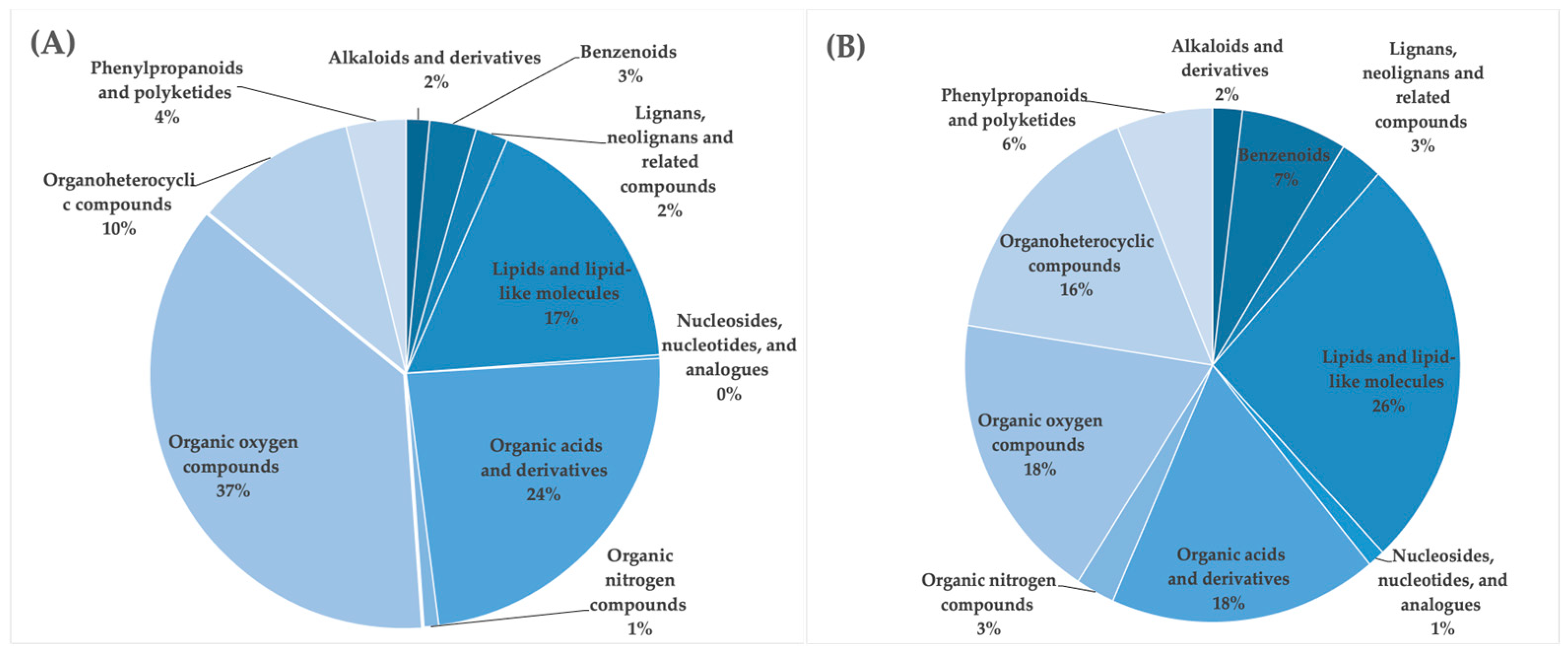

| Samples | ABTS+ Scavenging Activity (IC50 µg/mL) | DPPH Scavenging Activity (IC50 µg/mL) | Total Phenolic Content (mg/100 g) | Total Flavonoid Content (mg/100 g) | Total Tannin Content (mg/100 g) |
|---|---|---|---|---|---|
| Emericellopsis maritima | 38.01 ± 0.2 | 16.5 ± 1.2 | 85.007 ± 2.6 | 26. ± 04 | 8.03 ± 1.4 |
| Engyodontium album | 52.00 ± 1.4 | 26.8 ± 0.24 | 64.02 ± 1.4 | 32 ± 001 | 4.002 ± 2.01 |
| Hypomontagnella monticulosa | 78.64 ± 2.6 | 34.7 ± 2.1 | 23.004 ± 2.8 | 18.24 ± 02 | 16.03 ± 4.1 |
| Hortaea werneckii | 81.02 ± 1.12 | 35.6 ± 4.8 | 48.03 ± 0.2 | 34.01 ± 01 | 7.031 ± 0.026 |
| Trichoderma harzianum | 25.01 ± 3.18 | 12.2 ± 2.6 | 93.01 ± 1.8 | 31.32 ± 02 | 9.002 ± 2.5 |
| Aspergillus sp.7 | 76.24 ± 4.6 | 41.01 ± 1.4 | 65.02 ± 2.6 | 43.06 ± 002 | 24.1 ± 4.2 |
| Ascorbic acid | 7.03 ± 2.8 |
| Species | Bacillus subtilis | Vibrio parahaemolyticus | Escherichia coli | Staphylococcus aureus | Enterobacter aeruginosa |
|---|---|---|---|---|---|
| Emericellopsis maritima | 0.01 | 1.4 | 0.2 | 3.1 | 0.2 |
| Engyodontium album | 1.2 | 0.6 | 0.8 | 1.0 | 0 |
| Hypomontagnella monticulosa | 0 | 0.2 | 0 | 1.2 | 0 |
| Hortaea werneckii | 1.4 | 1.2 | 0.1 | 0 | 0 |
| Trichoderma harzianum | 1.2 | 2.4 | 1.2 | 0.4 | 0.01 |
| Aspergillus sp.7 | 0.2 | 0 | 0 | 0 | 0 |
| Compounds | E. maritima | E. album | H. monticulosa | H. werneckii | T. harzianum | A. niger | Compound ID |
|---|---|---|---|---|---|---|---|
| Niazicin A | 155,202.77 | 673.01 | 7161.57 | 2464.28 | 124,721 | 69.81158 | HMDB0303690 |
| Chembl4211493 | 97,804.71 | 173.16 | 120.93 | 46.04 | 119.58 | 52.16 | HMDB0257612 |
| Sucrose octaacetate | 73,594.50 | 27,778.63 | 38,632.07 | 109,316.67 | 71,510.63 | 30,874.62 | HMDB0029893 |
| Hemorphin-4 | 62,948.70 | 233.88 | 205.95 | 68.27 | 201.67 | 144.13 | HMDB0059788 |
| Phenyl-agarose | 16,089.59 | 2968.72 | 3263.43 | 7227.09 | 8201.72 | 3438.86 | HMDB0256427 |
| (3b,20R,22R)-3,20,27-Trihydroxy-1-oxowitha-5,24-dienolide 3-glucoside | 12,936.76 | 496.91 | 241.33 | 601.10 | 85.78 | 310.62 | HMDB0033573 |
| Digitoxigenin bisdigitoxide | 12,787.91 | 426.99 | 638.27 | 1280.91 | 3525.74 | 845.21 | HMDB0251275 |
| LysoPS (16:0/0:0) | 11,497.57 | 219.47 | 3363.01 | 221.33 | 474.59 | 890.39 | HMDB0240605 |
| Sinalbine | 11,455.53 | 247.90 | 1259.21 | 20,336.59 | 781.93 | 155.90 | HMDB0303664 |
| Olodaterol | 7307.81 | 3584.23 | 2275.44 | 14,503.53 | 4670.76 | 4774.74 | HMDB0255957 |
| Motolimod | 6348.01 | 423.97 | 1483.55 | 2991.55 | 1955.24 | 1470.58 | HMDB0254904 |
| Docosanamide | 5797.60 | 576.58 | 661.097 | 1660.71 | 637.03 | 551.65 | HMDB0000583 |
| N-Nitrosofenfluramine | 5669.05 | 3123.30 | 6295.30 | 28,770.25 | 5804.03 | 7980.27 | HMDB0255206 |
| Peonidin acetyl 3,5-diglucoside | 5362.16 | 2356.22 | 2802.88 | 8467.53 | 4177.09 | 2270.36 | HMDB0301895 |
| Ophiopogonin C’ | 5341.08 | 429.59 | 191.01 | 518.264 | 2366.44 | 309.52 | HMDB0029312 |
| Aminopentol | 5167.53 | 4490.50 | 14,032.33 | 17,448.69 | 2373.39 | 10,994.10 | HMDB0248328 |
| Ribociclib | 4802.41 | 3.59 | 6.48 | 4.73 | 11.04 | 21.46 | HMDB0257211 |
| Suvorexant | 4624.75 | 1166.79 | 236.07 | 4488.59 | 811.25 | 364.64 | HMDB0258640 |
| Janthitrem G | 4280.60 | 5900.46 | 1006.90 | 11,179.17 | 1748.32 | 1830.12 | HMDB0030531 |
| Labadoside | 3695.32 | 37.06 | 126.02 | 65.39318 | 4414.33 | 331.43 | HMDB0036397 |
| 1-Sinapoyl-2,2′-diferuloylgentiobiose | 3672.46 | 7.76 | 30.44 | 43.49 | 1617.97 | 1.75 | HMDB0301721 |
| Digitoxigenin 3-[glucosyl-(1->6)-glucosyl-(1->4)-2,6-dideoxyribohexoside] | 3437.48 | 2702.59 | 2050.30 | 11,713.11 | 4860.62 | 1283.45 | HMDB0034321 |
| Boviquinone 4 | 3387.657 | 57.35 | 65.96 | 153.14 | 74.39 | 89.42 | HMDB0030057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannan, J.; Pang, K.-L.; Ho, Y.-N.; Hsu, P.-H.; Chen, L.-L. A Comparison of the Antioxidant Potential and Metabolite Analysis of Marine Fungi Associated with the Red Algae Pterocladiella capillacea from Northern Taiwan. Antioxidants 2024, 13, 336. https://doi.org/10.3390/antiox13030336
Kannan J, Pang K-L, Ho Y-N, Hsu P-H, Chen L-L. A Comparison of the Antioxidant Potential and Metabolite Analysis of Marine Fungi Associated with the Red Algae Pterocladiella capillacea from Northern Taiwan. Antioxidants. 2024; 13(3):336. https://doi.org/10.3390/antiox13030336
Chicago/Turabian StyleKannan, Jiji, Ka-Lai Pang, Ying-Ning Ho, Pang-Hung Hsu, and Li-Li Chen. 2024. "A Comparison of the Antioxidant Potential and Metabolite Analysis of Marine Fungi Associated with the Red Algae Pterocladiella capillacea from Northern Taiwan" Antioxidants 13, no. 3: 336. https://doi.org/10.3390/antiox13030336
APA StyleKannan, J., Pang, K.-L., Ho, Y.-N., Hsu, P.-H., & Chen, L.-L. (2024). A Comparison of the Antioxidant Potential and Metabolite Analysis of Marine Fungi Associated with the Red Algae Pterocladiella capillacea from Northern Taiwan. Antioxidants, 13(3), 336. https://doi.org/10.3390/antiox13030336








