Oral Curcumin–Thioketal–Inulin Conjugate Micelles against Radiation–Induced Enteritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of CTI Conjugate
2.3. Physicochemical Characterizations of CTI Micelles
2.4. In Vitro Antioxidant Properties
2.5. Cytotoxicity Assay
2.6. In Vitro Cellular Uptake
2.7. In Vitro γ-ray Damage Evaluation
2.8. In Vitro Radioprotection Assay
2.9. Apoptosis Assay
2.10. Intracellular ROS-Scavenging Assay
2.11. Detection of γH2AX
2.12. Colony Formation Assay
2.13. Radiation Enteritis Model
2.14. Evaluation of WAI-Induced Intestinal Injury
2.15. Evaluation of Intestinal Permeability
2.16. Determination of Inflammatory Cytokines and Redox-Related Indicators
2.17. Behavioral Evaluation
2.18. Microbiome Analysis
2.19. Statistical Analysis
3. Results and Discussion
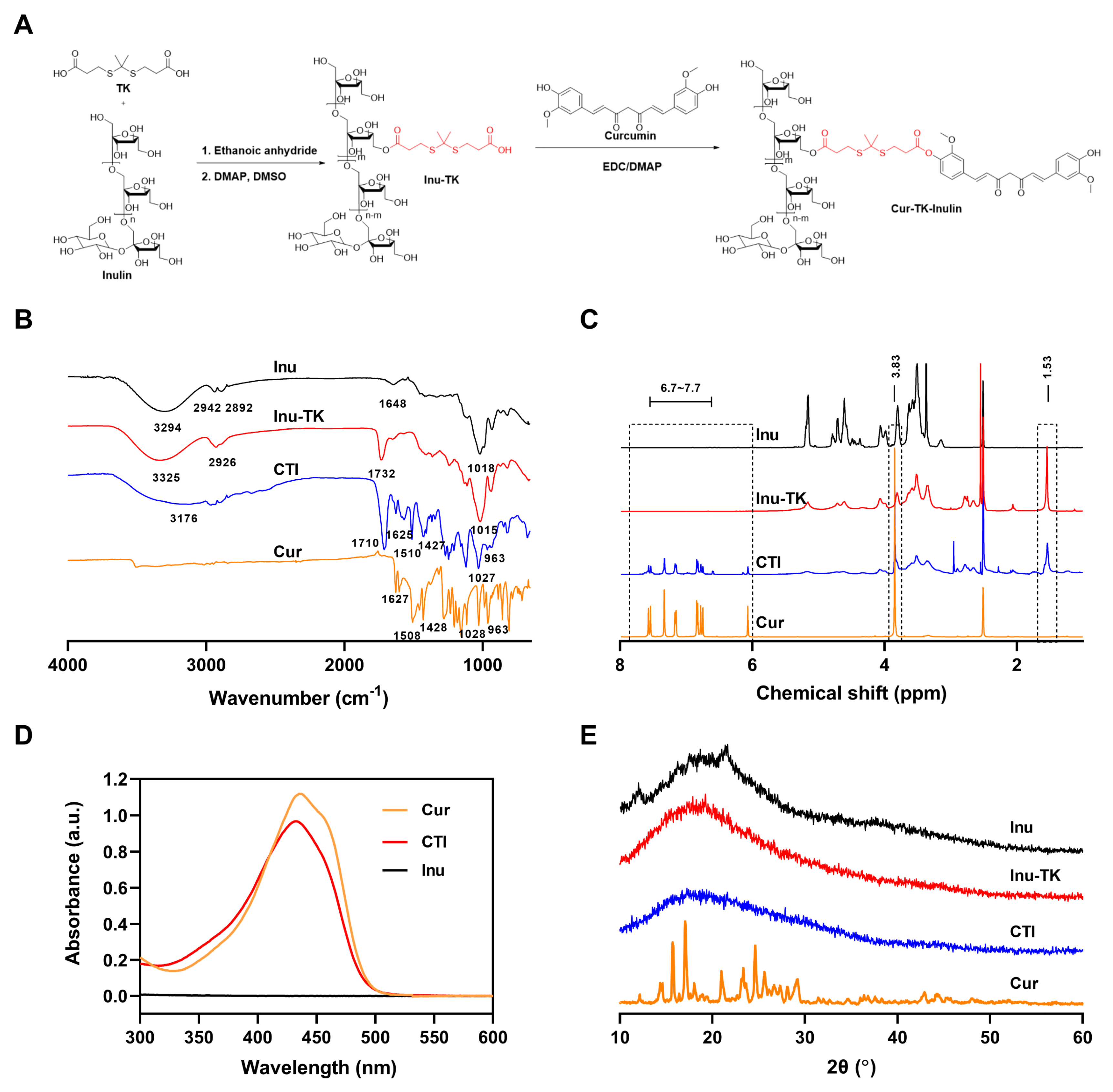
3.1. Characteristics of Cur–TK–Inu Conjugate
3.2. Characteristics of CTI Micelles
3.3. Stability of CTI Micelles
3.4. ROS-Sensitive Property of CTI Micelles
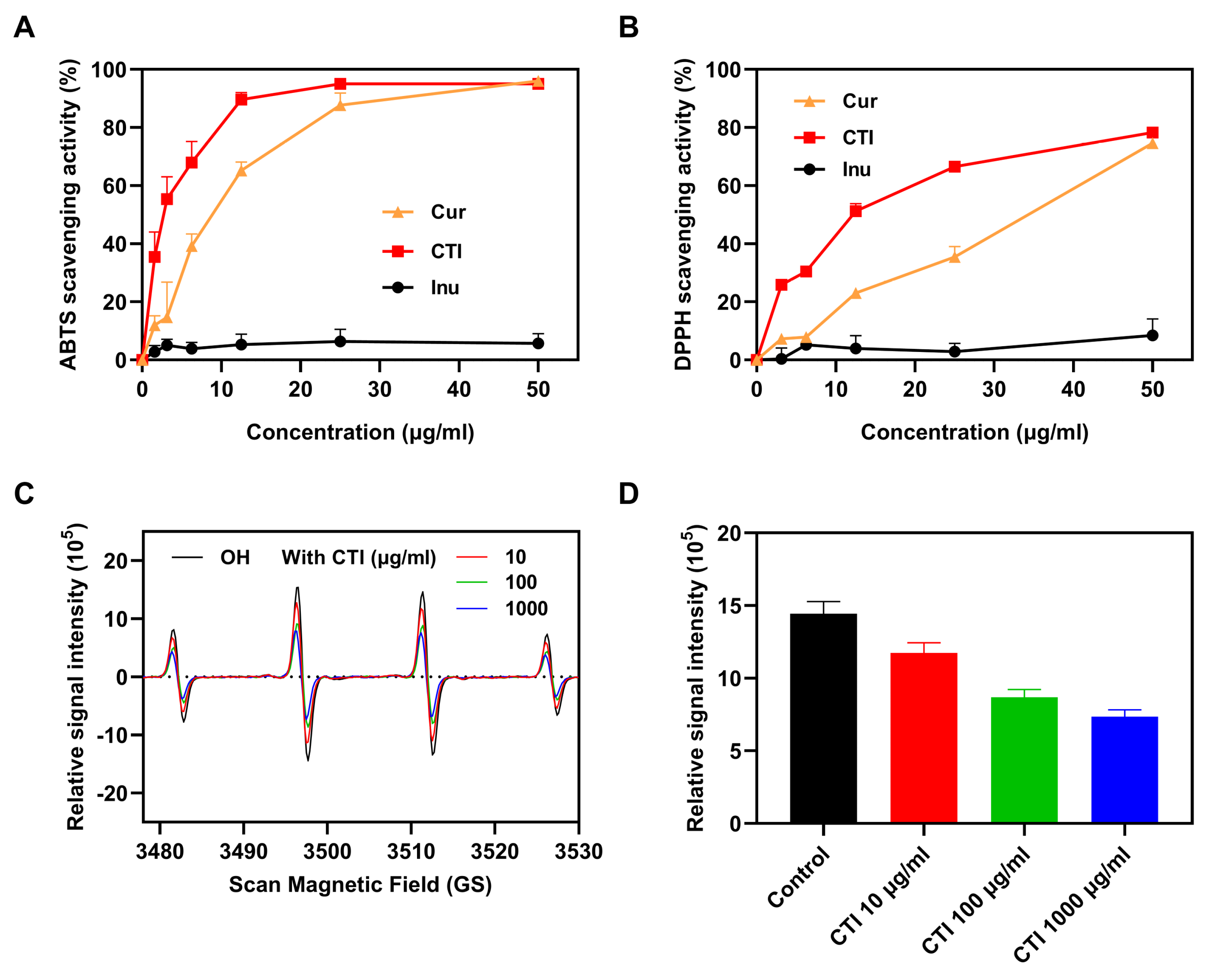
3.5. In Vitro Antioxidant Ability of CTI
3.6. CTI Mitigates Cytotoxicity and Enhances Cell Uptake
3.7. CTI Protects Intestinal Cells from Radiation-Induced Damage
3.8. CTI Ameliorates Severe Radiation-Induced Intestinal Injury
3.9. CTI Reduces the Expression of Inflammatory Cytokines and Improves Redox-Related Indicators
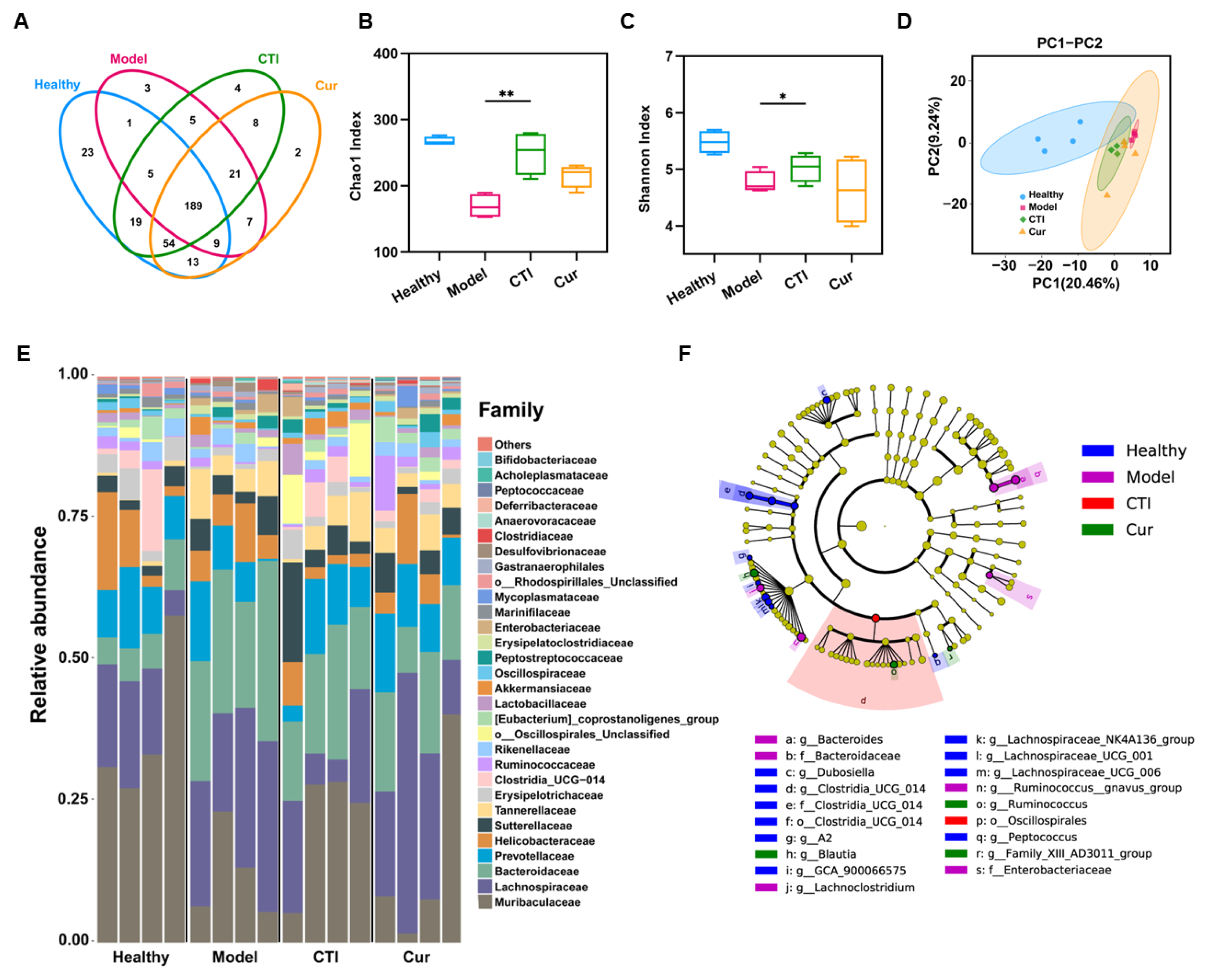
3.10. CTI Maintains the Diversity of Gut Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, W.; Zhang, X.; Mei, L.; Zhou, R.; Yin, W.; Wang, Q.; Gu, Z.; Zhao, Y. Stimuli-Responsive Small-on-Large Nanoradiosensitizer for Enhanced Tumor Penetration and Radiotherapy Sensitization. ACS Nano 2020, 14, 10001–10017. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Her, S.; Jaffray, D.A. Radiotherapy for Cancer: Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, M.; Tang, X.; Li, S.; Zhang, J.; Lu, L.; Wang, Q.; Yang, B. Intestinal delivery of ROS-scavenging carbonized polymer dots for full-course treatment of acute and chronic radiation enteritis. Appl. Mater. Today 2022, 28, 101544. [Google Scholar] [CrossRef]
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J. Radiation enteropathy—Pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, C.; Wang, N.; Zhu, S.; Mei, L.; Zhang, X.; Yong, Y.; Li, L.; Chen, C.; Huang, C.; et al. Graphdiyne nanoradioprotector with efficient free radical scavenging ability for mitigating radiation-induced gastrointestinal tract damage. Biomaterials 2020, 244, 119940. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, M.; Wang, C.; Zhu, S.; Niu, W.; Yong, Y.; Zhao, L.; Gu, Z. External use of Nano-graphdiyne hydrogel for skin radioprotection via both physically shielding of Low-energy X-ray and chemically scavenging of Broad-spectrum free radicals. Chem. Eng. J. 2022, 430, 132866. [Google Scholar] [CrossRef]
- Pamujula, S.; Kishore, V.; Rider, B.; Fermin, C.; Graves, R.; Agrawal, K.; Mandal, T. Radioprotection in mice following oral delivery of amifostine nanoparticles. Int. J. Biol. Macromol. 2005, 81, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhong, D.; Ouyang, J.; He, J.; Qi, Y.; Chen, W.; Zhang, X.; Tao, W.; Zhou, M. Microalgae-based oral microcarriers for gut microbiota homeostasis and intestinal protection in cancer radiotherapy. Nat. Commun. 2022, 13, 1413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; He, J.; Cui, J.; Wang, R.; Tang, Z.; Yu, H.; Zhou, M. Oral Microalgae-Nano Integrated System against Radiation-Induced Injury. ACS Nano 2023, 17, 10560–10576. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, L.; He, L.; Xu, Q.; Zhang, Z.; Zhang, Z.; Zhang, X.; Fan, R.; Ma, W.; Sun, Y.; et al. A strategy for attenuation of acute radiation-induced lung injury using crocetin from gardenia fruit. Biomed. Pharmacother. 2022, 149, 112899. [Google Scholar] [CrossRef]
- Tiwari, M.; Dixit, B.; Parvez, S.; Agrawala, P.K. EGCG, a tea polyphenol, as a potential mitigator of hematopoietic radiation injury in mice. Biomed. Pharmacother. 2017, 88, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pang, X.; Zhu, X.; Meng, Z.; Chen, X.; Zhang, J.; Ding, Q.; Li, Q.; Dou, G.; Ma, B. Lycium barbarum mitigates radiation injury via regulation of the immune function, gut microbiota, and related metabolites. Biomed. Pharmacother. 2021, 139, 111654. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, C.; Lu, W.; Xu, L.; Kuang, L.; Hua, D. ROS-sensitive Crocin-loaded chitosan microspheres for lung targeting and attenuation of radiation-induced lung injury. Carbohydr. Polym. 2023, 307, 120628. [Google Scholar] [CrossRef] [PubMed]
- Khayatan, D.; Razavi, S.M.; Arab, Z.N.; Niknejad, A.H.; Nouri, K.; Momtaz, S.; Gumpricht, E.; Jamialahmadi, T.; Abdolghaffari, A.H.; Barreto, G.E.; et al. Protective effects of curcumin against traumatic brain injury. Biomed. Pharmacother. 2022, 154, 113621. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ding, X.; Khan, I.M.; Yue, L.; Zhang, Y.; Wang, Z. Preparation and characterization of curcumin/chitosan conjugate as an efficient photodynamic antibacterial agent. Carbohydr. Polym. 2023, 313, 120852. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Galani, V.; Tsekeris, P.; Kyritsis, A.P.; Alexiou, G.A. Radiosensitization and Radioprotection by Curcumin in Glioblastoma and Other Cancers. Biomedicines 2022, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Miao, L.; Guo, Y.; Yuan, R.; Li, X.; Wang, X.; Lin, X.; Tian, H. Oral Codelivery of WR-1065 Using Curcumin-Linked ROS-Sensitive Nanoparticles for Synergistic Radioprotection. ACS Biomater. Sci. Eng. 2021, 7, 2496–2507. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhuang, B.; Huang, Y.; Liu, Y.; Yuan, B.; Wang, W.; Yuan, T.; Du, L.; Jin, Y. Inhaled curcumin mesoporous polydopamine nanoparticles against radiation pneumonitis. Acta Pharm. Sin. B 2022, 12, 2522–2532. [Google Scholar] [CrossRef]
- Lei, L.; Tu, Q.; Zhang, X.; Xiang, S.; Xiao, B.; Zhai, S.; Yu, H.; Tang, L.; Guo, B.; Chen, X.; et al. Stimulus-responsive curcumin-based polydopamine nanoparticles for targeting Parkinson’s disease by modulating α-synuclein aggregation and reactive oxygen species. Chem. Eng. J. 2023, 461, 141606. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, K.; Zhang, Y.; Sun, Z.; Song, B.; Wang, Y.; Zhang, B.; Chen, X.; Hu, D.; Wen, J.; et al. Hyaluronic acid-modified curcumin-copper complex nano delivery system for rapid healing of bacterial prostatitis. Carbohydr. Polym. 2023, 310, 120668. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.R.; James, N.R.; Kumar, P.R.A.; Raj, D.K. Galactosylated alginate-curcumin micelles for enhanced delivery of curcumin to hepatocytes. Int. J. Biol. Macromol. 2016, 86, 1–9. [Google Scholar] [PubMed]
- Luo, W.; Bai, L.; Zhang, J.; Li, Z.; Liu, Y.; Tang, X.; Xia, P.; Xu, M.; Shi, A.; Liu, X.; et al. Polysaccharides-based nanocarriers enhance the anti-inflammatory effect of curcumin. Carbohydr. Polym. 2023, 311, 120718. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Yang, Z.; Zeng, K.; Huang, Z.; Hou, X.; Zhao, H. Preparation and evaluation of water-soluble chondroitin sulfate-curcumin conjugate. Mater. Lett. 2023, 351, 135035. [Google Scholar] [CrossRef]
- Gruskiene, R.; Lavelli, V.; Sereikaite, J. Application of inulin for the formulation and delivery of bioactive molecules and live cells. Carbohydr. Polym. 2024, 327, 121670. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Zhang, C.; Patil, P.J.; Mehmood, A.; Li, X.; Bilal, M.; Haider, J.; Ahmad, S. Potential applications of hydrophobically modified inulin as an active ingredient in functional foods and drugs—A review. Carbohydr. Polym. 2021, 252, 117176. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, B.; Muellner, V.; Hlatky, K.; Tallian, C.; Vielnascher, R.; Guebitz, G.M.; Wirth, M.; Gabor, F. Chemically modified inulin for intestinal drug delivery—A new dual bioactivity concept for inflammatory bowel disease treatment. Carbohydr. Polym. 2021, 252, 117091. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sun, J.; Williams, P.A.; Yang, J.; Zhang, S. Octenyl-succinylated inulins for the delivery of hydrophobic drug. Int. J. Biol. Macromol. 2022, 221, 1112–1120. [Google Scholar] [CrossRef]
- Licciardi, M.; Scialabba, C.; Sardo, C.; Cavallaro, G.; Giammona, G. Amphiphilic inulin graft co-polymers as self-assembling micelles for doxorubicin delivery. J. Mater. Chem. B. 2014, 2, 4262–4271. [Google Scholar] [CrossRef]
- Shen, J.; Jiao, W.; Chen, Z.; Wang, C.; Song, X.; Ma, L.; Tang, Z.; Yan, W.; Xie, H.; Yuan, B.; et al. Injectable multifunctional chitosan/dextran-based hydrogel accelerates wound healing in combined radiation and burn injury. Carbohydr. Polym. 2023, 316, 121024. [Google Scholar] [CrossRef] [PubMed]
- Shan, P.; Liao, J.; Li, J.; Wang, C.; Zhou, J.; Mei, L.; Dai, Y.; Wang, Q.; Yin, W. H2S-releasing adhesive hydrogel as oral radioprotectant for gastrointestinal tract radioprotection. Chin. Chem. Lett. 2023, 35, 108545. [Google Scholar] [CrossRef]
- Liu, D.; Zhuang, B.; Wei, M.; Yuan, T.; Li, J.; Deng, P.; Du, L.; Yuan, B.; Jin, Y. Oral konjac glucomannan for prevention of ionizing radiation-induced injury by regulating gut microbiota and increasing short chain fatty acids. Int. J. Biol. Macromol. 2023, 240, 124402. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jiao, W.; Tang, Z.; Wang, C.; Li, Q.; Wei, M.; Song, S.; Du, L.; Jin, Y. Quercetin inclusion complex gels ameliorate radiation-induced brain injury by regulating gut microbiota. Biomed. Pharmacother. 2023, 158, 114142. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Yang, H.; Gao, S.; Li, L.; Fu, X.; Wei, Q. Research progress on self-assembled nanodrug delivery systems. J. Mater. Chem. B 2022, 10, 1908–1922. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, Y.; Zhao, M.; Gao, W.; Zhang, H.; Li, S.; He, B.; Pu, Y. A reactive oxygen species-responsive prodrug micelle with efficient cellular uptake and excellent bioavailability. J. Mater. Chem. B 2018, 6, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Vakilzadeh, H.; Varshosaz, J.; Dinari, M.; Mirian, M.; Hajhashemi, V.; Shamaeizadeh, N.; Sadeghi, H.M.-M. Smart redox-sensitive micelles based on chitosan for dasatinib delivery in suppressing inflammatory diseases. Int. J. Biol. Macromol. 2023, 229, 696–712. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, L.; Qin, W.; Loy, D.A.; Wu, Z.; Chen, H.; Zhang, Q. Preparation, characterization and antioxidant properties of curcumin encapsulated chitosan/lignosulfonate micelles. Carbohydr. Polym. 2022, 281, 119080. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, Y.; Yang, C.; Liu, X.; Huangfu, Y.; Zhang, C.; Huang, P.; Dong, A.; Liu, J.; Liu, J.; et al. Bioinspired and Inflammation-Modulatory Glycopeptide Hydrogels for Radiation-Induced Chronic Skin Injury Repair. Adv. Healthc. Mater. 2023, 12, e2201671. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Tang, H.; Zhang, Z.; Zhou, X.; Xu, W. ROS-Responsive and pH-Sensitive Aminothiols Dual-Prodrug for Radiation Enteritis. Antioxidants 2022, 11, 2145. [Google Scholar] [CrossRef]
- Zhong, D.; Zhang, D.; Chen, W.; He, J.; Ren, C.; Zhang, X.; Kong, N.; Tao, W.; Zhou, M. Orally deliverable strategy based on microalgal biomass for intestinal disease treatment. Sci. Adv. 2021, 7, eabi9265. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.; Cao, Y.; Lu, W.; Kuang, L.; Hua, D. Strategy for Highly Efficient Radioprotection by a Selenium-Containing Polymeric Drug with Low Toxicity and Long Circulation. ACS Appl. Mater. Inter. 2020, 12, 44534–44540. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Mu, X.; Sun, S.; Yang, H.; Ouyang, L.; Zhang, S.; Guo, J.; Meng, J.; Liu, Y.; Ma, H.; et al. Establishing bilateral modulation of radiation induced redox damage via biocatalytic single atom engineering at Au clusters. Chem. Eng. J. 2022, 445, 136793. [Google Scholar] [CrossRef]
- Lu, L.; Jiang, M.; Zhu, C.; He, J.; Fan, S. Amelioration of whole abdominal irradiation-induced intestinal injury in mice with 3,3′-Diindolylmethane (DIM). Free Radic. Biol. Med. 2019, 130, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lu, H.; Li, M.; Tian, Y. Probiotic Consortia and their Metabolites Protect Intestine Against Radiation Injury by Improving Intestinal Epithelial Homeostasis. Int. J. Biol. Macromol. 2023, 117 (Suppl. S2), e269. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Jang, W.I.; Park, S.; Cho, S.S.; Lee, S.B.; Kim, M.-J.; Park, S.; Shim, S.; Jang, H. Metallothionein 2 activation by pravastatin reinforces epithelial integrity and ameliorates radiation-induced enteropathy. EBioMedicine 2021, 73, 103641. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, J.; Yang, Z.L.; You, H. Extracellular superoxide dismutase increased the therapeutic potential of human mesenchymal stromal cells in radiation pulmonary fibrosis. Cytotherapy 2017, 19, 586–602. [Google Scholar] [CrossRef]
- Cao, K.; Lei, X.; Liu, H.; Zhao, H.; Guo, J.; Chen, Y.; Xu, Y.; Cheng, Y.; Liu, C.; Cui, J.; et al. Polydatin alleviated radiation-induced lung injury through activation of Sirt3 and inhibition of epithelial-mesenchymal transition. J. Cell. Mol. Med. 2017, 21, 3264–3276. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Xue, F.; Liu, W.; Kuang, Y.; Gu, B.; Song, S.; Chen, H. In situ forming oxygen/ROS-responsive niche-like hydrogel enabling gelation-triggered chemotherapy and inhibition of metastasis. Bioact. Mater. 2023, 21, 86–96. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Jiao, W.; Yuan, B.; Xie, H.; Chen, Z.; Wei, M.; Sun, Y.; Wu, Y.; Zhang, F.; Li, Z.; et al. Oral Curcumin–Thioketal–Inulin Conjugate Micelles against Radiation–Induced Enteritis. Antioxidants 2024, 13, 417. https://doi.org/10.3390/antiox13040417
Shen J, Jiao W, Yuan B, Xie H, Chen Z, Wei M, Sun Y, Wu Y, Zhang F, Li Z, et al. Oral Curcumin–Thioketal–Inulin Conjugate Micelles against Radiation–Induced Enteritis. Antioxidants. 2024; 13(4):417. https://doi.org/10.3390/antiox13040417
Chicago/Turabian StyleShen, Jintao, Wencheng Jiao, Bochuan Yuan, Hua Xie, Ziyuan Chen, Meng Wei, Yingbao Sun, Yanping Wu, Feng Zhang, Zhangyu Li, and et al. 2024. "Oral Curcumin–Thioketal–Inulin Conjugate Micelles against Radiation–Induced Enteritis" Antioxidants 13, no. 4: 417. https://doi.org/10.3390/antiox13040417
APA StyleShen, J., Jiao, W., Yuan, B., Xie, H., Chen, Z., Wei, M., Sun, Y., Wu, Y., Zhang, F., Li, Z., Jin, X., Du, L., & Jin, Y. (2024). Oral Curcumin–Thioketal–Inulin Conjugate Micelles against Radiation–Induced Enteritis. Antioxidants, 13(4), 417. https://doi.org/10.3390/antiox13040417






