Harmony in Motion: Unraveling the Nexus of Sports, Plant-Based Nutrition, and Antioxidants for Peak Performance
Abstract
:1. Introduction
1.1. Background
1.2. Objectives and Scope of the Study
2. Antioxidants: An Overview
2.1. Definition and Types of Antioxidants
2.1.1. Vitamin C (Ascorbic Acid)
2.1.2. Vitamin E (Tocopherols and Tocotrienols)
2.1.3. Beta-Carotene
2.1.4. Polyphenols
2.1.5. Selenium
2.2. Importance of Antioxidants in Human Health
2.3. Dietary Sources of Antioxidants
3. Sports and Oxidative Stress
3.1. Introduction to Oxidative Stress in Sports
3.2. Mechanisms of Oxidative Stress during Physical Activity
3.3. Impact of Oxidative Stress on Athletic Performance
4. The Role of Plants in Sports Nutrition
4.1. Plant-Based Diets for Athletes
4.2. Nutrient-Rich Plants for Sports Nutrition
| Nutrient | Plant-Based Sports Diet | Non-Plant-Based Sports Diet | References |
|---|---|---|---|
| Protein | Legumes, Tofu, Tempeh, Seitan, Quinoa | Chicken, Fish, Lean Meat, Eggs | [206,207] |
| Omega-3 Fatty Acids | Flaxseeds, Chia Seeds, Walnuts, Algal Oil | Fatty Fish (Salmon, Mackerel), Fish Oil Supplements | [208,209] |
| Iron | Lentils, Chickpeas, Spinach, Pumpkin Seeds | Red Meat, Poultry, Fish, Fortified Cereals | [210,211] |
| Calcium | Kale, Bok Choy, Tofu (Calcium-set), Fortified Plant Milk | Dairy Products, Fortified Dairy Alternatives | [212,213] |
| Vitamin B12 | Fortified Foods (Plant Milk, Breakfast Cereals), B12 Supplements | Animal Products (Meat, Dairy, Eggs) | [214,215] |
| Zinc | Lentils, Chickpeas, Pumpkin Seeds, Cashews | Meat, Shellfish, Dairy Products | [216,217] |
| Vitamin D | Fortified Plant Milk, Fortified Orange Juice, Sun Exposure | Fatty Fish (Salmon, Tuna), Fortified Dairy Products | [218,219] |
| Fiber | Whole Grains, Legumes, Nuts, Seeds, Fruits, Vegetables | Limited in Animal Products | [220,221] |
| Antioxidants | Berries, Dark Leafy Greens, Nuts, Seeds | Not as Prominent in Traditional Sports Diets | [222,223] |
| Carbohydrates | Whole Grains (Brown Rice, Quinoa), Sweet Potatoes, Fruits | Pasta, Bread, Rice (White) | [224,225] |
4.3. Plant Compounds and Their Potential Benefits in Sports
5. Antioxidants in Plant-Based Foods
5.1. Overview of Antioxidants in Plants
| Study Title | Methodology | Sample Size | Main Findings | Reference |
|---|---|---|---|---|
| The Effect of Pomegranate Juice Supplementation on Strength and Soreness after Eccentric Exercise | Randomized, double-blind, placebo-controlled crossover trial | 17 healthy, physically active, resistance-trained men | In resistance-trained people, twice-daily pomegranate juice supplementation lowers muscular soreness in the elbow flexor but not in the knee extensor muscles. | [253] |
| Effects of a single dose of beetroot juice on cycling time trial performance at ventilatory thresholds intensity in male triathletes | Randomized, double-blind, placebo-controlled crossover trial | 12 well-trained, male triathletes (aged 21-47 yr) | Acute BJ supplementation does not support an improvement in the variables examined. Higher doses are needed for improving time trial performance in male triathletes during a cycle ergometer test. | [254] |
| Influence of tart cherry juice on indices of recovery following marathon running | Randomized, double-blind, placebo-controlled trial | 20 volunteers, male (n = 13) and female (n = 7). | Demonstrated that the cherry juice reduced oxidative stress and inflammation and hence increases the rate of recovery. | [255] |
| Dietary antioxidant restriction affects the inflammatory response in athletes | Observational study | 17 healthy endurance-trained male adults aged 18–35 years | A diet rich in carotenoids may be beneficial to combat exercise-induced oxidative stress in athletes performing exercise. | [256] |
| Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running | Randomized, controlled trial | 25 healthy adults | Daily blueberry consumption for 6 weeks increases NK cell counts, and acute ingestion reduces oxidative stress and increases anti-inflammatory cytokines. | [257] |
| Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage | Randomized, triple-blind, placebo-controlled trial | 20 healthy, untrained men | The green tea extract supplementation has positive effects on muscle recovery after strenuous exercise. | [258] |
| Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS) | Randomized, double-blind, controlled trial | 17 healthy adults | Oral curcumin likely reduces pain associated with DOMS with some evidence for enhanced recovery of muscle performance. | [259] |
| A 12-Week Randomized Double-Blind Placebo-Controlled Clinical Trial, Evaluating the Effect of Supplementation with a Spinach Extract on Skeletal Muscle Fitness in Adults Older Than 50 Years of Age | Double-blind, placebo-controlled randomized trial | 51 participants | In subjects, moderate-intensity strength training combined with daily supplementation for 12 weeks with a natural extract of Spinacia oleracea L. improved muscle-related variables and muscle quality | [260] |
| Effect of Exercise on Oxidative Stress: A 12-Month Randomized, Controlled Trial | 12-months randomized control trial (RCT) | 173 overweight women | Aerobic exercise, when accompanied by relatively marked gains in aerobic fitness, decreases oxidative stress among previously sedentary older women. | [261] |
| Antioxidant and anti-nociceptive effects of Phyllanthus amarus on improving exercise recovery in sedentary men: a randomized crossover (double-blind) design. | Randomized, double-blind, controlled trial | 12 participants | Acute Phyllanthus amarus supplementation reduced oxidative stress and muscle soreness induced by high-intensity exercise | [262] |
| Consumption of an Anthocyanin-Rich Extract Made from New Zealand Blackcurrants Prior to Exercise May Assist Recovery from Oxidative Stress and Maintains Circulating Neutrophil Function: A Pilot Study | Experimental design | 12 participants | Consumption of blackcurrant anthocyanin-rich extract (BAE) 1 h prior to exercise facilitated recovery from exercise-induced oxidative stress and preserved circulating neutrophil function. | [263] |
| A double-blind, randomized, placebo-controlled trial on the effect of Ashwagandha (Withania somnifera dunal.) root extract in improving cardiorespiratory endurance and recovery in healthy athletic adults | Double-blind, randomized, placebo-controlled trial | 50 endurance athletes | Ashwagandha root extract can successfully enhance cardiorespiratory endurance and improve the quality of life in healthy athletic adults | [264] |
| Effects of Six-Week Ginkgo biloba Supplementation on Aerobic Performance, Blood Pro/Antioxidant Balance, and Serum Brain-Derived Neurotrophic Factor in Physically Active Men | Double-blind, placebo-controlled Trial | 18 active young men | Ginkgo biloba extract provide improvements in endurance performance and blood antioxidant capacity, and elicit somewhat better neuroprotection through increased exercise-induced production of BDNF. | [265] |
| Evaluation of the Efficacy of Supplementation with Planox® Lemon verbena Extract in Improving Oxidative Stress and Muscle Damage: A Randomized Double-Blind Controlled Trial | Randomized double-blind controlled trial | 30 males and 30 females | Lemon verbena extract is a safe and edible natural plant extract that can reduce muscle damage and soreness after exercise. | [266] |
5.2. Specific Antioxidants Found in Common Plant Foods
5.3. Synergistic Effects of Antioxidants in Plants
6. Practical Applications for Athletes
6.1. Incorporating Plant-Based Foods into Athletes’ Diets
6.2. Developing Antioxidant-Rich Meal Plans for Training and Recovery
| Food Group | Food Options | Key Antioxidants | Benefits for Athletes | Additional Nutrients | Reference |
|---|---|---|---|---|---|
| Protein Powerhouses | Lentils | Phenolic acids, Flavonoids | Muscle repair, satiety, sustained energy | Fiber, Iron, Folate | [194,318] |
| Tempeh | Isoflavones, Lunasin | Muscle building, immune function | Iron, Calcium, Prebiotics | [319,320] | |
| Quinoa | Quercetin, Kaempferol | Reduced inflammation, improved recovery | Fiber, Magnesium, Iron | [224,321] | |
| Tofu | Genistein, Daidzein | Bone health, muscle preservation | Calcium, Iron, Manganese | [322,323] | |
| Nuts and Seeds (almonds, chia, hemp) | Vitamin E, Selenium | Reduced oxidative stress, cell protection | Healthy fats, Fiber, Minerals | [324,325] | |
| Fuel for Performance (high in complex carbs) | Brown rice | Anthocyanins, Phenolic acids | Sustained energy release, blood sugar control | Fiber, Manganese, B vitamins | [326,327] |
| Sweet potatoes | Beta-carotene, Chlorogenic acid | Improved blood flow, muscle endurance | Vitamin A, Potassium, Fiber | [328,329] | |
| Oats | Avenanthramides, Ferulic acid | Reduced inflammation, improved recovery | Fiber, beta-glucan, Magnesium | [330,331] | |
| Vitamin & Mineral Champions | Berries (blueberries, strawberries) | Anthocyanins, Ellagic acid | Improved cognitive function, reduced muscle soreness | Vitamin C, Fiber, Potassium | [332,333,334] |
| Leafy greens (kale, spinach) | Vitamin C, Lutein | Bone health, immune function, eye health | Vitamin K, Folate, Calcium | [335,336] | |
| Cruciferous vegetables (broccoli, cauliflower) | Glucosinolates, Sulforaphane | Detoxification, cancer prevention | Fiber, Vitamin C, Potassium | [337,338] | |
| Fortified plant milks (calcium, vitamin D) | Calcium, Vitamin D | Bone health, immune function | Vitamin B12, Riboflavin | [339,340] | |
| Omega-3 Superstars | Chia seeds | Alpha-linolenic acid (ALA) | Brain health, reduced inflammation | Fiber, Protein, Calcium | [341,342] |
| Algae oil (DHA/EPA supplements) | Docosahexaenoic acid (DHA), Eicosapentaenoic acid (EPA) | Cognitive function, muscle recovery, anti-inflammatory properties | Vitamin E | [343,344] | |
| Natural healer | Turmeric | Curcumin | Anti-inflammatory properties | Flavonoids | [345,346] |
6.3. Considerations for Different Types of Sports
7. Challenges and Limitations
8. Future Directions and Research Opportunities
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastro Hepat. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Vandoni, M.; Calcaterra, V.; Carnevale Pellino, V.; De Silvestri, A.; Marin, L.; Zuccotti, G.V.; Tranfaglia, V.; Giuriato, M.; Codella, R.; Lovecchio, N. “Fitness and Fatness” in children and adolescents: An Italian cross-sectional study. Children 2021, 8, 762. [Google Scholar] [CrossRef] [PubMed]
- Janeckova, K.; Hamrik, Z.; Matusova, M.; Badura, P. “I am going out!”–lifestyle sports and physical activity in adolescents. BMC Public Health 2021, 21, 1079. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.S.; Butler, N. Sports participation and emotional wellbeing in adolescents. Lancet 1996, 347, 1789–1792. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Angeles-Valencia, M.; Morales-Gonzalez, A.; Madrigal-Santillan, E.O.; Morales-Martinez, M.; Madrigal-Bujaidar, E.; Alvarez-Gonzalez, I.; Gutierrez-Salinas, J.; Esquivel-Chirino, C.; Chamorro-Cevallos, G.; et al. Oxidative Stress, Mitochondrial Function and Adaptation to Exercise: New Perspectives in Nutrition. Life 2021, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Suzuki, K.; Castell, L. A short overview of changes in inflammatory cytokines and oxidative stress in response to physical activity and antioxidant supplementation. Antioxidants 2020, 9, 886. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, J.C. The role of reactive oxygen species and antioxidants in oxidative stress. Int. J. Res. 2016, 3, 1–8. [Google Scholar]
- Keshari, A.K.; Verma, A.K.; Kumar, T.; Srivastava, R. Oxidative stress: A review. Int. J. Sci. Technoledge 2015, 3, 155. [Google Scholar]
- Slattery, K.; Bentley, D.; Coutts, A.J. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: Implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. 2015, 45, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Antioxidants in exercise nutrition. Sports Med. 2001, 31, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, I. Training, changes in nutritional requirements and dietary support of physical exercise. Nutr. Skelet. Muscle 2019, 151–182. [Google Scholar] [CrossRef]
- Lynch, H.; Johnston, C.; Wharton, C. Plant-Based Diets: Considerations for Environmental Impact, Protein Quality, and Exercise Performance. Nutrients 2018, 10, 1841. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.; Nguyen, C.; Shetty, M.; Oppezzo, M.; Barrack, M.; Fredericson, M. Popular Dietary Trends’ Impact on Athletic Performance: A Critical Analysis Review. Nutrients 2023, 15, 3511. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: A randomized trial using a metabolomics approach. PLoS ONE 2013, 8, e72215. [Google Scholar] [CrossRef] [PubMed]
- Kostrakiewicz-Gieralt, K. Plants, algae, cyanobacteria and fungi in Diet of vegan and vegetarian sportsmen-a systematic review. Cent. Eur. J. Sport. Sci. Med. 2022, 37, 23–43. [Google Scholar] [CrossRef]
- Shashirekha, M.; Mallikarjuna, S.; Rajarathnam, S. Status of bioactive compounds in foods, with focus on fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- FG Cicero, A.; Colletti, A. Effects of carotenoids on health: Are all the same? Results from clinical trials. Curr. Pharm. Des. 2017, 23, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous vegetables, isothiocyanates, and bladder cancer prevention. Mol. Nutr. Food Res. 2018, 62, 1800079. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.; Klintman, M. The practice approach in practice: Lessons for civil society organizations (CSOs) that work towards sustainable food consumption in Sweden. Sustain. Prod. Consum. 2021, 26, 480–492. [Google Scholar] [CrossRef]
- Thurecht, R. Factors Influencing the Food Choices of high-Performance Athletes. Bachelor’s Thesis, University of the Sunshine Coast, Queensland, Australia, 2022. [Google Scholar]
- López-Martínez, M.I.; Miguel, M.; Garcés-Rimón, M. Protein and sport: Alternative sources and strategies for bioactive and sustainable sports nutrition. Front. Nutr. 2022, 9, 926043. [Google Scholar] [CrossRef] [PubMed]
- Khonina, M. Beyond Food as Fuel: Women Athletes’ Relationship to Food, Sporting Cultures, and Body Ideals. Master’s Thesis, Simon Fraser University, Burnaby, BC, Canada, 2021. [Google Scholar]
- Jowett, S.; Lavallee, D. Social Psychology in Sport; Human Kinetics: Champaign, IL, USA, 2007; Volume 10. [Google Scholar]
- Tanner, R.; Gore, C. Physiological Tests for Elite Athletes; Human Kinetics: Champaign, IL, USA, 2013. [Google Scholar]
- Ackland, T.R.; Elliott, B.; Bloomfield, J. Applied Anatomy and Biomechanics in Sport; Human Kinetics: Champaign IL USA, 2009. [Google Scholar]
- Ober, C.; Sinatra, S.T.; Zucker, M. Earthing: The Most Important Health Discovery Ever? Basic Health Publications, Inc.: Laguna Beach, CA, USA, 2010.
- Schiattarella, G.G.; Hill, J.A. Therapeutic targeting of autophagy in cardiovascular disease. J. Mol. Cell. Cardiol. 2016, 95, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P. Nutrition and Athlete Immune Health: New Perspectives on an Old Paradigm. Sports Med. 2019, 49, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Sands, D.C.; Morris, C.E.; Dratz, E.A.; Pilgeram, A.L. Elevating optimal human nutrition to a central goal of plant breeding and production of plant-based foods. Plant Sci. 2009, 177, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Kandylis, P.; Dimitrellou, D.; Salamoura, C.; Zakynthinos, G.; Proestos, C. Innovative and fortified food: Probiotics, prebiotics, GMOs, and superfood. In Preparation and Processing of Religious and Cultural Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–129. [Google Scholar]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Choudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative stress and antioxidants in disease and cancer: A review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Coutinho, O.P. Free radicals in the regulation of damage and cell death--basic mechanisms and prevention. Drug Discov. Ther. 2010, 4, 144–167. [Google Scholar] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H. IOC consensus statement: Dietary supplements and the high-performance athlete. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A. The Role of Dietary Antioxidants in Exercise-Induced Oxidative Stress and Athletic Performance. Doctoral Dissertation, Auckland University of Technology, Auckland, New Zealand, 2011. [Google Scholar]
- Patani, A.; Balram, D.; Yadav, V.K.; Lian, K.-Y.; Patel, A.; Sahoo, D.K. Harnessing the power of nutritional antioxidants against adrenal hormone imbalance-associated oxidative stress. Front. Endocrinol. 2023, 14, 1271521. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.R.; Biklé, A. What Your Food Ate: How to Restore Our Land and Reclaim Our Health; WW Norton & Company: New York, NY, USA, 2022. [Google Scholar]
- Recknagel, R.O.; Glende, E.A.; Britton, R.S. Free radical damage and lipid peroxidation. In Hepatotoxicology; CRC Press: Boca Raton, FL, USA, 2020; pp. 401–436. [Google Scholar]
- Khemka, S.; Reddy, A.; Garcia, R.I.; Jacobs, M.; Reddy, R.P.; Roghani, A.K.; Pattoor, V.; Basu, T.; Sehar, U.; Reddy, P.H. Role of diet and exercise in aging, Alzheimer’s disease, and other chronic diseases. Ageing Res. Rev. 2023, 91, 102091. [Google Scholar] [CrossRef] [PubMed]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Buren, L.V.; Wagner, E.; Wiseman, S.; Put, F.V.D.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.J.F.c. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Sathe, S.K.; Monaghan, E.K.; Kshirsagar, H.H.; Venkatachalam, M. Chemical composition of edible nut seeds and its implications in human health. In Tree Nuts: Composition, Phytochemicals, and Health Effects; Routledge: London, UK, 2009; pp. 11–35. [Google Scholar]
- Dixit, V.; Kamal, S.W.J.; Chole, P.B.; Dayal, D.; Chaubey, K.K.; Pal, A.K.; Xavier, J.; Manjunath, B.T.; Bachheti, R.K. Functional Foods: Exploring the Health Benefits of Bioactive Compounds from Plant and Animal Sources. J. Food Qual. 2023, 2023, 5546753. [Google Scholar] [CrossRef]
- Heiras-Palazuelos, M.J.; Ochoa-Lugo, M.I.; Gutiérrez-Dorado, R.; López-Valenzuela, J.A.; Mora-Rochín, S.; Milán-Carrillo, J.; Garzón-Tiznado, J.A.; Reyes-Moreno, C. Technological properties, antioxidant activity and total phenolic and flavonoid content of pigmented chickpea (Cicer arietinum L.) cultivars. Int. J. Food Sci. Nutr. 2013, 64, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic profiles and antioxidant activity of germinated legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lietz, G.; Seal, C.J. Phenolic, apparent antioxidant and nutritional composition of quinoa (Chenopodium quinoa Willd.) seeds. Int. J. Food Sci. Technol. 2021, 56, 3245–3254. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Rahman, H.; Thushar, S.; Singh, R.K. Healthy and resilient cereals and pseudo-cereals for marginal agriculture: Molecular advances for improving nutrient bioavailability. Front. Genet. 2020, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kim, D.O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Giada, M. Food phenolic compounds: Main classes, sources and their antioxidant power. Oxidative Stress. Chronic Degener. Dis. A Role Antioxid. 2013, 2013, 87–112. [Google Scholar]
- Celebioglu, A.; Uyar, T. Antioxidant vitamin E/cyclodextrin inclusion complex electrospun nanofibers: Enhanced water solubility, prolonged shelf life, and photostability of vitamin E. J. Agric. Food Chem. 2017, 65, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Vitamin C in stem cell biology: Impact on extracellular matrix homeostasis and epigenetics. Stem Cells Int. 2017, 2017, 8936156. [Google Scholar] [CrossRef] [PubMed]
- Moores, J. Vitamin C: A wound healing perspective. Br. J. Community Nurs. 2013, 18, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M. Nutritional support to maintain proper immune status during intense training. Nutr. Coach. Strategy Modul. Train. Effic. 2013, 75, 85–97. [Google Scholar]
- Ashour, T.J.; Ali, C.; Youssef, H.M. The effects of strenuous exercise and nutrition on the immune functions of elite athletes. Eur. J. Phys. Educ. Sport. Sci. 2017, 3, 126–141. [Google Scholar]
- Zeb, A. A comprehensive review on different classes of polyphenolic compounds present in edible oils. Food Res. Int. 2021, 143, 110312. [Google Scholar] [CrossRef] [PubMed]
- Przykaza, K.; Nikolaichuk, H.; Kozub, A.; Tomaszewska-Gras, J.; Peršurić, Ž.; Pavelić, S.K.; Fornal, E. Newly marketed seed oils. What we can learn from the current status of authentication of edible oils. Food Control 2021, 130, 108349. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and exercise performance: With a focus on vitamin E and C supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Devi, M.B. Vegetables as a potential source of nutraceuticals and phytochemicals: A review. Int. J. Med. Pharm. Sci. 2015, 5, 1–14. [Google Scholar]
- Garden-Robinson, J. Carotenoids in green vegetables and health aspects. In Pigments in Fruits and Vegetables; Springer: New York, NY, USA, 2015; pp. 229–246. [Google Scholar]
- Mushtaq, M.; Anwar, F. A Centum of Valuable Plant Bioactives; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Ebadi, M.; Mohammadi, M.; Pezeshki, A.; Jafari, S.M. Health Benefits of Beta-Carotene. In Handbook of Food Bioactive Ingredients: Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–26. [Google Scholar]
- Bloomer, R.J. Effect of exercise on oxidative stress biomarkers. Adv. Clin. Chem. 2008, 46, 1–50. [Google Scholar] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Angeles-Valencia, M.; Anguiano-Robledo, L.; González-López, L.L.; Sosa-Gómez, A.; Fregoso-Aguilar, T.; Esquivel-Chirino, C.; Ruiz-Velazco-Benítez, Y.A. Phytochemicals and modulation of exercise-induced oxidative stress: A novel overview of antioxidants. Am. J. Transl. Res. 2022, 14, 8292. [Google Scholar] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Lattanzio, V. Bioactive polyphenols: Their role in quality and storability of fruit and vegetables. J. Appl. Bot. 2003, 77, 128–146. [Google Scholar]
- Sarker, U.; Oba, S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Sci. Rep. 2019, 9, 18233. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Crozier, A. Dietary flavonoids and phenolic compounds. Plant Phenolics Hum. Health Biochem. Nutr. Pharmacol. 2010, 1, 1–50. [Google Scholar]
- Maoto, M.M.; Beswa, D.; Jideani, A.I. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef]
- dos Santos, M.; da Silva Júnior, F.M.R.; Muccillo-Baisch, A.L. Selenium content of Brazilian foods: A review of the literature values. J. Food Compos. Anal. 2017, 58, 10–15. [Google Scholar] [CrossRef]
- Moskwa, J.; Naliwajko, S.K.; Puścion-Jakubik, A.; Soroczyńska, J.; Socha, K.; Koch, W.; Markiewicz-Żukowska, R. In Vitro Assessment of the Bioaccessibility of Zn, Ca, Mg, and Se from Various Types of Nuts. Foods 2023, 12, 4453. [Google Scholar] [CrossRef]
- Spallholz, J.E. Selenium and glutathione peroxidase: Essential nutrient and antioxidant component of the immune system. In Antioxidant Nutrients and Immune Functions; Springer: Berlin/Heidelberg, Germany, 1990; pp. 145–158. [Google Scholar]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-dependent antioxidant enzymes: Actions and properties of selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Córdova Martínez, A.; Seco-Calvo, J. The role of selenium mineral trace element in exercise: Antioxidant defense system, muscle performance, hormone response, and athletic performance. A systematic review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef] [PubMed]
- Nocella, C.; Cammisotto, V.; Pigozzi, F.; Borrione, P.; Fossati, C.; D’Amico, A.; Cangemi, R.; Peruzzi, M.; Gobbi, G.; Ettorre, E. Impairment between oxidant and antioxidant systems: Short-and long-term implications for athletes’ health. Nutrients 2019, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Bast, A.; Hanekamp, J.C. Toxicology: What Everyone should Know; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Asejeje, F.O.; Ogunro, O.B. Deciphering the mechanisms, biochemistry, physiology, and social habits in the process of aging Aging process: Deciphering the mechanisms, biochemistry, physiology, and social habits. Arch. Gerontol. Geriatr. Plus 2023, 1, 100003. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sierra, T.; Eugenio-Pérez, D.; Sánchez-Chinchillas, A.; Pedraza-Chaverri, J. Role of food-derived antioxidants against cisplatin induced-nephrotoxicity. Food Chem. Toxicol. 2018, 120, 230–242. [Google Scholar] [CrossRef]
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional antioxidants and adaptive cell responses: An update. Curr. Mol. Med. 2011, 11, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Cram, D.L. Causes and Consequences of Oxidative Stress in a Cooperatively Breeding Bird; University of Exeter (United Kingdom): Exeter, UK, 2013. [Google Scholar]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.S.; Azam, M.; Basra, M.A.R. Impact of natural antioxidants on biological systems. Life Sci. 2020, 4, 139–162. [Google Scholar]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Sadiq, I.Z. Free radicals and oxidative stress: Signaling mechanisms, redox basis for human diseases, and cell cycle regulation. Curr. Mol. Med. 2023, 23, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial oxidative stress—A causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K. Oxidative shielding or oxidative stress? J. Pharmacol. Exp. Ther. 2012, 342, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Maccarrone, M. Chronic inflammatory disorders and their redox control: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Sign 2011, 15, 2605–2641. [Google Scholar] [CrossRef] [PubMed]
- Boehm, J.K.; Kubzansky, L.D. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychol. Bull. 2012, 138, 655. [Google Scholar] [CrossRef]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.-C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Bio Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.; Ou, N.; Lu, Q.-B. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci. Rep. 2013, 3, 3169. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Ryan, L. Improving public health?: The role of antioxidant-rich fruit and vegetable beverages. Food Res. Int. 2011, 44, 3135–3148. [Google Scholar] [CrossRef]
- Fuertes, P.O. Recent Developments in Antioxidants from Natural Sources; IntechOpen: London, UK, 2023. [Google Scholar]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D. Superfoods: The Food and Medicine of the Future; North Atlantic Books: Berkeley, CA, USA, 2009. [Google Scholar]
- Agrawal, S. Investigating chemical attributes, nutritional significance, and bioactive elements in horticultural wonders. Pharma Innov. J. 2023, 12, 228–233. [Google Scholar]
- Gündeşli, M.A.; Korkmaz, N.; Okatan, V. Polyphenol content and antioxidant capacity of berries: A review. Int. J. Agric. For. Life Sci. 2019, 3, 350–361. [Google Scholar]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and human health—A focus on oxidative stress, inflammation and disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.P.; Priti; Dikshit, H.; Aski, M.; Sangwan, S.; Stobdan, T.; Singh, A.; Kumar, R.R.; Praveen, S. Microgreens: A Novel Food for Nutritional Security. In Conceptualizing Plant-Based Nutrition: Bioresources, Nutrients Repertoire and Bioavailability; Springer: Berlin/Heidelberg, Germany, 2022; pp. 123–156. [Google Scholar]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables–the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar]
- Owis, A. Broccoli; the green beauty: A review. J. Pharm. Sci. Res. 2015, 7, 696. [Google Scholar]
- Ahamad, S.; Vinod, B.; Menaka, M.; Kumar, A.; Kumar, M. Nutraceutical Insights: Broccoli’s Bioactive Bounty in Health Promotion and Disease Prevention. Biot. Res. Today 2023, 5, 808–810. [Google Scholar]
- Ros, E.; Singh, A.; O’Keefe, J.H. Nuts: Natural pleiotropic nutraceuticals. Nutrients 2021, 13, 3269. [Google Scholar] [CrossRef] [PubMed]
- Rosengarten, F., Jr. The Book of Edible Nuts; Courier Corporation: North Chelmsford, MA, USA, 2004. [Google Scholar]
- Cardoso, B.R.; Duarte, G.B.S.; Reis, B.Z.; Cozzolino, S.M. Brazil nuts: Nutritional composition, health benefits and safety aspects. Food Res. Int. 2017, 100, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Cicia, G.; Del Giudice, T.; Sacchi, R.; Vecchio, R. Consumers’ perceptions and preferences for bitterness in vegetable foods: The case of extra-virgin olive oil and brassicaceae—A narrative review. Nutrients 2019, 11, 1164. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R.; Appel, C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Etkin, N.L. Edible Medicines: An Ethnopharmacology of Food; University of Arizona Press: Tucson, AZ, USA, 2008. [Google Scholar]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in sports and exercise: Tracking health, performance, and recovery in athletes. J. Strength. Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.N.d.; Lima, L.C.F. The association between physical exercise and Reactive Oxygen Species (ROS) production. J. Sports Med. Doping Stud. 2015, 5, 1–7. [Google Scholar]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Sign 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.-M.; Bătrînu, M.-G.; Vari, C.E. Positive aspects of oxidative stress at different levels of the human body: A review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Reactive oxygen species and cellular defense system. In Free Radicals in Human Health and Disease; Springer: Berlin/Heidelberg, Germany, 2015; pp. 17–29. [Google Scholar]
- Jordan, A.C.; Perry, C.G.; Cheng, A.J. Promoting a pro-oxidant state in skeletal muscle: Potential dietary, environmental, and exercise interventions for enhancing endurance-training adaptations. Free Radic. Bio Med. 2021, 176, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical activity in the prevention and treatment of coronary artery disease. J. Am. Heart Assoc. 2018, 7, e007725. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.M.; Adhihetty, P.J.; Leeuwenburgh, C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J. Physiol. 2016, 594, 5105–5123. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Tripaldi, R.; Davì, G.; Santilli, F. Oxidative stress modulation through habitual physical activity. Curr. Pharm. Des. 2016, 22, 3648–3680. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.R. Exercise-Induced Oxidative Stress, Muscle Damage and Inflammation in Prolonged High-Intensity Intermittent Exercise: Effect of Quercetin. Ph.D. Thesis, University of Hertfordshire, Hatfield, UK, 2020. [Google Scholar]
- Di Meo, S.; Napolitano, G.; Venditti, P. Mediators of physical activity protection against ROS-linked skeletal muscle damage. Int. J. Mol. Sci. 2019, 20, 3024. [Google Scholar] [CrossRef] [PubMed]
- Feflea, I.; Gherdan, J.; Stupariu, M. Theoretical aspects of the impact of geographical factors on the basketball game. Geosport Soc. 2023, 19, 132–144. [Google Scholar] [CrossRef]
- Egan, B.; Sharples, A.P. Molecular responses to acute exercise and their relevance for adaptations in skeletal muscle to exercise training. Physiol. Rev. 2023, 103, 2057–2170. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. Anaerobic metabolism during exercise. In Exercise Metabolism; Springer: Berlin/Heidelberg, Germany, 2022; pp. 51–70. [Google Scholar]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-l.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Farkhad, N.K.; Mahmoudian-Sani, M.R.; Shirzad, H. Antioxidants as a double-edged sword in the treatment of cancer. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Bhattacharjee, S.; Bhattacharjee, S. ROS and oxidative stress: Origin and implication. In Reactive Oxygen Species in Plant Biology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–31. [Google Scholar]
- Gomez-Cabrera, M.C.; Carretero, A.; Millan-Domingo, F.; Garcia-Dominguez, E.; Correas, A.G.; Olaso-Gonzalez, G.; Viña, J. Redox-related biomarkers in physical exercise. Redox Biol. 2021, 42, 101956. [Google Scholar] [CrossRef] [PubMed]
- Dekerle, J.; de Souza, K.M.; de Lucas, R.D.; Guglielmo, L.G.A.; Greco, C.C.; Denadai, B.S. Exercise tolerance can be enhanced through a change in work rate within the severe intensity domain: Work above critical power is not constant. PLoS ONE 2015, 10, e0138428. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.E.; Hodges, N.J.; Bosch, J.A.; Aldred, S. Prolonged depletion of antioxidant capacity after ultraendurance exercise. Med. Sci. Sports Exerc. 2011, 43, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport. Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Hadžović-Džuvo, A.; Valjevac, A.; Lepara, O.; Pjanić, S.; Hadžimuratović, A.; Mekić, A. Oxidative stress status in elite athletes engaged in different sport disciplines. Bosn. J. Basic. Med. Sci. 2014, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Pervaiz, S. Mitochondria: Redox metabolism and dysfunction. Biochem. Res. Int. 2012, 2012, 896751. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, S.; Al Azzawi, T.N.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The Key Roles of ROS and RNS as a Signaling Molecule in Plant–Microbe Interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Jaimes, E.A. Mitochondria and reactive oxygen species: Physiology and pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306–6344. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.; Perrotta, R.; Graziano, A.; Calabrese, E.J.; Calabrese, V. Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: Mitochondria as a “chi”. Immun. Ageing 2013, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R.; Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R843–R863. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Alvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodriguez-Manas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef] [PubMed]
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.A.; Modesti, A. Oxidative stress in exercise training: The involvement of inflammation and peripheral signals. Free Radic. Res. 2019, 53, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport. Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodriguez-Manas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Jamurtas, A.Z.; Paschalis, V.; Fatouros, I.G.; Koutedakis, Y.; Kouretas, D. The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: Magnitude and time-course considerations. Sports Med. 2008, 38, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. The Physiology of Training for High Performance. Appl. Physiol. Nutr. Metab. 2015, 40, 753–754. [Google Scholar]
- Jackson, M.J. Free radicals generated by contracting muscle: By-products of metabolism or key regulators of muscle function? Free Radic. Biol. Med. 2008, 44, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P.; Klotz, L.O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, J.A. Invited review: Physiological and pathophysiological responses to intermittent hypoxia. J. Appl. Physiol. (1985) 2001, 90, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M. Nutritional needs for exercise in the heat. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I.; Halson, S.; Burke, L.M.; Balague, G.; Farrow, D. An Integrated, Multifactorial Approach to Periodization for Optimal Performance in Individual and Team Sports. Int. J. Sports Physiol. Perform. 2018, 13, 538–561. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Ni, S.; Li, D.; Liu, J.; Chu, H.Y.; Zhang, N.; Sun, M.; Li, N.; Ren, Q. Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation. Nat. Commun. 2022, 13, 4241. [Google Scholar] [CrossRef]
- Meyer, N.L.; Reguant-Closa, A.; Nemecek, T. Sustainable diets for athletes. Curr. Nutr. Rep. 2020, 9, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Ruscigno, M. Plant-Based Sports Nutrition: Expert Fueling Strategies for Training, Recovery, and Performance, 1st ed.; Human Kinetics Publishers: Champaign, IL, USA, 2020; p. 344. [Google Scholar]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Kaparapu, J.; Pragada, P.M.; Geddada, M.N.R. Fruits and Vegetables and its Nutritional Benefits. In Functional Foods and Nutraceuticals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 241–260. [Google Scholar] [CrossRef]
- Mishra, S.K.; Ishfaq, P.M.; Tripathi, S.; Gupta, N. Fruits as Boosters of the Immune System. In Plants and Phytomolecules for Immunomodulation: Recent Trends and Advances; Springer: Berlin/Heidelberg, Germany, 2022; pp. 391–411. [Google Scholar]
- Wang, S.; Melnyk, J.P.; Tsao, R.; Marcone, M.F. How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Res. Int. 2011, 44, 14–22. [Google Scholar] [CrossRef]
- Orlich, M.J.; Jaceldo-Siegl, K.; Sabaté, J.; Fan, J.; Singh, P.N.; Fraser, G.E. Patterns of food consumption among vegetarians and non-vegetarians. Brit, J. Nutr. 2014, 112, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Sabate, J.; Soret, S. Sustainability of plant-based diets: Back to the future. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 476S–482S. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A. Biofortification of Plant-Based Food Products and Applications to the Athlete. Doctoral Dissertation, University of Saskatchewan Saskatoon, Saskatoon, SK, Canada, 2023. [Google Scholar]
- West, S.; Monteyne, A.J.; van der Heijden, I.; Stephens, F.B.; Wall, B.T. Nutritional Considerations for the Vegan Athlete. Adv. Nutr. 2023, 14, 774–795. [Google Scholar] [CrossRef] [PubMed]
- Hooley, D.; Nobis, N. A moral argument for veganism. In Philosophy Comes to Dinner; Routledge: London, UK, 2015; pp. 92–108. [Google Scholar]
- Rogerson, D. Vegan diets: Practical advice for athletes and exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Adegbola, P.; Aderibigbe, I.; Hammed, W.; Omotayo, T. Antioxidant and anti-inflammatory medicinal plants have potential role in the treatment of cardiovascular disease: A review. Am. J. Cardiovasc. Dis. 2017, 7, 19. [Google Scholar] [PubMed]
- Young-Dorn, M. Perceived Challenges and Strategies Associated with Sustaining a Plant-Based Diet: A Qualitative Study with Successful and Unsuccessful Plant-Based Diet Converters with a History of CVD. Doctoral Dissertation, Northcentral University, Scottsdale, Arizona, 2020. [Google Scholar]
- Xia, Z.; Cholewa, J.M.; Dardevet, D.; Huang, T.; Zhao, Y.; Shang, H.; Yang, Y.; Ding, X.; Zhang, C.; Wang, H.; et al. Effects of oat protein supplementation on skeletal muscle damage, inflammation and performance recovery following downhill running in untrained collegiate men. Food Funct. 2018, 9, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Ciona, D. The Efficacy of Lentils as a Pre-Exercise Meal for Athletes of High Intensity Soccer-Specific Intermittent Exercise. Doctoral Dissertation, University of Saskatchewan, Saskatoon, SK, Canada, 2013. [Google Scholar]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ. Almonds (Prunus dulcis Mill. DA webb): A source of nutrients and health-promoting compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Alcorta, A.; Porta, A.; Tarrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 2012, 1820, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R.; Fresan, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef] [PubMed]
- Falchetti, A.; Cavati, G.; Valenti, R.; Mingiano, C.; Cosso, R.; Gennari, L.; Chiodini, I.; Merlotti, D. The effects of vegetarian diets on bone health: A literature review. Front. Endocrinol. (Lausanne) 2022, 13, 899375. [Google Scholar] [CrossRef] [PubMed]
- Theobald, H.E. Dietary calcium and health. Nutr. Bull. 2005, 30, 237–277. [Google Scholar] [CrossRef]
- Lorente-Cebrian, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Martinez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in wild plants, nuts and seeds. Asia Pac. J. Clin. Nutr. 2002, 11, S163–S173. [Google Scholar] [CrossRef]
- Martínez-Medina, G.A.; Carranza-Méndez, R.; Amaya-Chantaca, D.P.; Ilyna, A.; Gaviria-Acosta, E.; Hoyos-Concha, J.L.; Chávez-González, M.L.; Govea-Salas, M.; Prado-Barragán, L.A.; Aguilar-González, C.N. Bioactive Peptides from Food Industrial Wastes. In Bioactive Peptides; CRC Press: Boca Raton, FL, USA, 2021; pp. 169–203. [Google Scholar] [CrossRef]
- Hernandez, E.M.; de Jong, L. Applications of omega-3 fats in foods. In Omega-3 Oils; Elsevier: Amsterdam, The Netherlands, 2011; pp. 151–176. [Google Scholar]
- Saunders, A.V.; Davis, B.C.; Garg, M.L. Omega-3 polyunsaturated fatty acids and vegetarian diets. Med. J. Aust. 2013, 199, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ismail, T.; Layla, A.; Hussain, M.; Qamar, M. An Overview of Plant-Based Protein Rich Products. In Plant Protein Foods; Springer: Berlin/Heidelberg, Germany, 2022; pp. 27–60. [Google Scholar]
- Delgado, A.M.; Vaz Almeida, M.D.; Parisi, S.; Delgado, A.M.; Parisi, S.; Vaz Almeida, M.D. Fish, meat and other animal protein sources. In Chemistry of the Mediterranean Diet; Springer: Berlin/Heidelberg, Germany, 2017; pp. 177–207. [Google Scholar]
- He, K. Fish, long-chain omega-3 polyunsaturated fatty acids and prevention of cardiovascular disease—Eat fish or take fish oil supplement? Prog. Cardiovasc. Dis. 2009, 52, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Platel, K.; Srinivasan, K. Bioavailability of micronutrients from plant foods: An update. Crit. Rev. Food Sci. Nutr. 2016, 56, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Taneja, D.; Rai, S.; Yadav, K. Evaluation of promotion of iron-rich foods for the prevention of nutritional anemia in India. Indian J. Public Health 2020, 64, 236. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L.J. Multiple sources of dietary calcium—Some aspects of its essentiality. Regul. Toxicol. Pharmacol. 2004, 39, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Proulx, W.R.; Heaney, R.J.T.A.j.o.c.n. Choices for achieving adequate dietary calcium with a vegetarian diet. Am. J. Clin. Nutr. 1999, 70, 543s–548s. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Geisel, J. Vegetarian lifestyle and monitoring of vitamin B-12 status. Clin. Chim. Acta 2002, 326, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.-K.; Hannibal, L.; Hettich, M.; Behringer, S.; Spiekerkoetter, U.; Steinborn, C.; Gründemann, C.; Zimmermann-Klemd, A.M.; Müller, A.; Simmet, T.J.N. Vitamin B12 status upon short-term intervention with a vegan diet—A randomized controlled trial in healthy participants. Nutrients 2019, 11, 2815. [Google Scholar] [CrossRef] [PubMed]
- Fedha, M.S. Physicochemical characterization and food application potential of pumpkin (Cucurbita sp.) fruit and seed kernel flours. Doctoral Dissertation, Omo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2014. [Google Scholar]
- Bilandžić, N.; Sedak, M.; Đokić, M.; Varenina, I.; Kolanović, B.S.; Božić, Đ.; Brstilo, M.; Šimić, B.J. Determination of zinc concentrations in foods of animal origin, fish and shellfish from Croatia and assessment of their contribution to dietary intake. J. Food Compos. Anal. 2014, 35, 61–66. [Google Scholar] [CrossRef]
- Moulas, A.N.; Vaiou, M.J.J.o.b. Vitamin D fortification of foods and prospective health outcomes. J. Biotechnol. 2018, 285, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2021, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.M.; Mollá, E.; Benítez, V. Sources of fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–146. [Google Scholar]
- Pastell, H.; Putkonen, T.; Rita, H. Dietary fibre in legumes, seeds, vegetables, fruits and mushrooms: Comparing traditional and semi-automated filtration techniques. J. Food Compos. Anal. 2019, 75, 1–7. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Natural antioxidants of plant origin. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 90, pp. 1–81. [Google Scholar]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.J.F.S.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Jancurová, M.; Minarovicová, L.; Dandar, A. Quinoa–A review. Czech J. Food Sci. 2009, 27, 71–79. [Google Scholar] [CrossRef]
- Shintani, T. The Good Carbohydrate Revolution: A Proven Program for Low-Maintenance Weight Loss and Optimum Health; Simon and Schuster: New York, NY, USA, 2003. [Google Scholar]
- Kussmann, M.; Abe Cunha, D.H.; Berciano, S. Bioactive compounds for human and planetary health. Front. Nutr. 2023, 10, 1193848. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.H.; Mithen, R.F. Plant science and human nutrition: Challenges in assessing health-promoting properties of phytochemicals. Plant Cell 2011, 23, 2483–2497. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; Hayes, L.D.; Milic, M.; Padulo, J. Herbal medicine for sports: A review. J. Int. Soc. Sports Nutr. 2018, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An overview of bioactive flavonoids from citrus fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Hever, J. Plant-Based Diets: A Physician’s Guide. Perm. J. 2016, 20, 15–082. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.K.P.; Massey, A.R.; Pinnamaneni, S.R.; Reddivari, L.; Reardon, K.F. Grain and sweet sorghum (Sorghum bicolor L. Moench) serves as a novel source of bioactive compounds for human health. Crit. Rev. Food Sci. Nutr. 2018, 58, 2867–2881. [Google Scholar] [CrossRef] [PubMed]
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent advances in biologically active compounds in herbs and spices: A review of the most effective antioxidant and anti-inflammatory active principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fan, D.; Huang, J.-l.; Zuo, T. The gut microbiome: Linking dietary fiber to inflammatory diseases. Med. Microecol. 2022, 14, 100070. [Google Scholar] [CrossRef]
- Scheer, V.; Tiller, N.B.; Doutreleau, S.; Khodaee, M.; Knechtle, B.; Pasternak, A.; Rojas-Valverde, D. Potential long-term health problems associated with ultra-endurance running: A narrative review. Sports Med. 2022, 52, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mandal, S.K. Current developments on anti-inflammatory natural medicines. Asian J. Pharm. Clin. Res. 2018, 11, 61. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef] [PubMed]
- Hausswirth, C.; Le Meur, Y. Physiological and nutritional aspects of post-exercise recovery: Specific recommendations for female athletes. Sports Med. 2011, 41, 861–882. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, H.N.; Sağır, S.G.; Hataş, Ö.; Smolarczyk, M.; Akalan, C. Physical, physiological and psychological profiles of elite Turkish taekwondo athletes. Biomed. Hum. Kinet. 2020, 12, 187–196. [Google Scholar] [CrossRef]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Resveratrol and exercise. Biomed. Rep. 2016, 5, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Tech. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Kuti, J.O. 244 Antioxidant Activity of Opuntia Cactus Pears. HortScience 2000, 35, 433B. [Google Scholar] [CrossRef]
- Sharma, S.; Shree, B.; Sharma, D.; Kumar, S.; Kumar, V.; Sharma, R.; Saini, R. Vegetable microgreens: The gleam of next generation super foods, their genetic enhancement, health benefits and processing approaches. Food Res. Int. 2022, 155, 111038. [Google Scholar] [CrossRef] [PubMed]
- Maury, G.L.; Rodríguez, D.M.; Hendrix, S.; Arranz, J.C.E.; Boix, Y.F.; Pacheco, A.O.; Díaz, J.G.; Morris-Quevedo, H.J.; Dubois, A.F.; Aleman, E.I.; et al. Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Sachithanandam, V.; Lalitha, P.; Parthiban, A.; Mageswaran, T.; Manmadhan, K.; Sridhar, R. A Review on Antidiabetic Properties of Indian Mangrove Plants with Reference to Island Ecosystem. Evid. Based Complement. Altern. Med. 2019, 2019, 4305148. [Google Scholar] [CrossRef] [PubMed]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens-A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Trombold, J.R.; Reinfeld, A.S.; Casler, J.R.; Coyle, E.F. The effect of pomegranate juice supplementation on strength and soreness after eccentric exercise. J. Strength Cond. Res. 2011, 25, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Castaño, M.V.; Palau-Salvà, G.; Cuenca, E.; Muñoz-González, A.; García-Fernández, P.; del Carmen Lozano-Estevan, M.; Veiga-Herreros, P.; Maté-Muñoz, J.L.; Domínguez, R. Effects of a single dose of beetroot juice on cycling time trial performance at ventilatory thresholds intensity in male triathletes. J. Int. Soc. Sports Nutr. 2018, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.; Brouner, J.; Jewell, A.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, B.A.; Callister, R.; Watson, T.A.; Garg, M.L. Dietary antioxidant restriction affects the inflammatory response in athletes. Br. J. Nutr. 2010, 103, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, L.S.; Nieman, D.C.; Dumke, C.L.; Shooter, L.A.; Henson, D.A.; Utter, A.C.; Milne, G.; McAnulty, S.R. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl. Physiol. Nutr. Metab. 2011, 36, 976–984. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.; Machado, Á.S.; Souza, M.A.; Mello-Carpes, P.B.; Carpes, F.P. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol. Behav. 2018, 194, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Nicol, L.M.; Rowlands, D.S.; Fazakerly, R.; Kellett, J. Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS). Eur. J. Appl. Physiol. 2015, 115, 1769–1777. [Google Scholar] [CrossRef]
- Pérez-Piñero, S.; Ávila-Gandía, V.; Rubio Arias, J.A.; Muñoz-Carrillo, J.C.; Losada-Zafrilla, P.; López-Román, F.J. A 12-Week Randomized Double-Blind Placebo-Controlled Clinical Trial, Evaluating the Effect of Supplementation with a Spinach Extract on Skeletal Muscle Fitness in Adults Older Than 50 Years of Age. Nutrients 2021, 13, 4373. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Gross, M.D.; Potter, J.D.; Schmitz, K.H.; Duggan, C.; McTiernan, A.; Ulrich, C.M. Effect of exercise on oxidative stress: A 12-month randomized, controlled trial. Med. Sci. Sports Exerc. 2010, 42, 1448. [Google Scholar] [CrossRef] [PubMed]
- Roengrit, T.; Wannanon, P.; Prasertsri, P.; Kanpetta, Y.; Sripanidkulchai, B.-o.; Leelayuwat, N. Antioxidant and anti-nociceptive effects of Phyllanthus amarus on improving exercise recovery in sedentary men: A randomized crossover (double-blind) design. J. Int. Soc. Sports Nutr. 2014, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.D.; Lyall, K.A.; Roberts, J.M.; Perthaner, A.; Wells, R.W.; Cooney, J.M.; Jensen, D.J.; Burr, N.S.; Hurst, S.M. Consumption of an anthocyanin-rich extract made from New Zealand blackcurrants prior to exercise may assist recovery from oxidative stress and maintains circulating neutrophil function: A pilot study. Front. Nutr. 2019, 6, 73. [Google Scholar] [CrossRef]
- Tiwari, S.; Gupta, S.K.; Pathak, A.K. A double-blind, randomized, placebo-controlled trial on the effect of Ashwagandha (Withania somnifera dunal.) root extract in improving cardiorespiratory endurance and recovery in healthy athletic adults. J. Ethnopharmacol. 2021, 272, 113929. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Krępa, E.; Kłapcińska, B.; Pokora, I.; Domaszewski, P.; Kempa, K.; Podgórski, T. Effects of six-week Ginkgo biloba supplementation on aerobic performance, blood pro/antioxidant balance, and serum brain-derived neurotrophic factor in physically active men. Nutrients 2017, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Hsu, Y.-J.; Ho, C.-S.; Chang, C.-H.; Liu, C.-W.; Huang, C.-C.; Chiang, W.-D. Evaluation of the efficacy of supplementation with Planox® lemon verbena extract in improving oxidative stress and muscle damage: A randomized double-blind controlled trial. Int. J. Med. 2021, 18, 2641. [Google Scholar] [CrossRef] [PubMed]
- Dini, C.; Zaro, M.J.; Viña, S.Z. Bioactivity and Functionality of Anthocyanins: A Review. Curr. Bioact. Compd. 2019, 15, 507–523. [Google Scholar] [CrossRef]
- Goncalves, A.C.; Nunes, A.R.; Falcao, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Faoro, F. Health-promoting effects of grape bioactive phytochemicals. In Complementary and Alternative Therapies and the Aging Population; Elsevier: Amsterdam, The Netherlands, 2009; pp. 445–474. [Google Scholar]
- Jaglan, P.; Buttar, H.S.; Al-bawareed, O.; Chibisov, S. Potential health benefits of selected fruits: Apples, blueberries, grapes, guavas, mangos, pomegranates, and tomatoes. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 359–370. [Google Scholar]
- Butnariu, M.; Butu, A. Chemical composition of vegetables and their products. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 627–692. [Google Scholar]
- Crozier, A.; Yokota, T.; Jaganath, I.B.; Marks, S.; Saltmarsh, M.; Clifford, M.N. Secondary Metabolites in Fruits, Vegetables, Beverages and Other Plant-based Dietary Components. In Plant Secondary Metabolites; Blackwell Pub Oxford: Ames, IA, USA, 2006; pp. 208–302. [Google Scholar] [CrossRef]
- Olaniran, A.F.; Folorunsho, J.O.; Akinsanola, B.A.; Taiwo, A.E.; Iranloye, Y.M.; Okonkwo, C.E.; Osemwegie, O.O. Application of Astaxanthin and Carotenoids Derived from Algae for the Production of Nutraceuticals, Pharmaceuticals, Additives, Food Supplement and Feed. In Next-Generation Algae; Wiley: Hoboken, NJ, USA, 2023; pp. 95–124. [Google Scholar] [CrossRef]
- Sandei, L. Lycopene and Tomatoes. In Lycopene and Tomatoes in Human Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2018; pp. 149–178. [Google Scholar] [CrossRef]
- Sharma, S.; Katoch, V.; Kumar, S.; Chatterjee, S. Functional relationship of vegetable colors and bioactive compounds: Implications in human health. J. Nutr. Biochem. 2021, 92, 108615. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Mühling, K.H. Plant-derived sulfur containing natural products produced as a response to biotic and abiotic stresses: A review of their structural diversity and medicinal importance. J. Appl. Bot. Food Qual. 2019, 92, 204–215. [Google Scholar] [CrossRef]
- Herr, I.; Buchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. Whole grains and pulses: A comparison of the nutritional and health benefits. J. Agric. Food Chem. 2014, 62, 7029–7049. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Mahanty, J.; Kumar, S.; Singh, H.; Sharma, A. An Insight into the Functional Benefit of Phenolic Acids from Whole Grains: An Update. Curr. Nutr. Food Sci. 2023, 19, 906–921. [Google Scholar] [CrossRef]
- Sofi, S.A.; Ahmed, N.; Farooq, A.; Rafiq, S.; Zargar, S.M.; Kamran, F.; Dar, T.A.; Mir, S.A.; Dar, B.N.; Mousavi Khaneghah, A. Nutritional and bioactive characteristics of buckwheat, and its potential for developing gluten-free products: An updated overview. Food Sci. Nutr. 2023, 11, 2256–2276. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional constituents of pseudo cereals and their potential use in food systems: A review. Trends Food Sci. Tech. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Szabo, Z.; Koczka, V.; Marosvolgyi, T.; Szabo, E.; Frank, E.; Polyak, E.; Fekete, K.; Erdelyi, A.; Verzar, Z.; Figler, M. Possible Biochemical Processes Underlying the Positive Health Effects of Plant-Based Diets-A Narrative Review. Nutrients 2021, 13, 2593. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S. The Biochemistry, Chemistry and Physiology of the Isoflavones in Soybeans and their Food Products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elsoud, M.A.; Hashem, N.M.; Nour El-Din, A.N.M.; Kamel, K.I.; Hassan, G.A. Soybean isoflavone affects in rabbits: Effects on metabolism, antioxidant capacity, hormonal balance and reproductive performance. Anim. Reprod. Sci. 2019, 203, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deng, Z.; Liu, R.; Zhang, H.; Zhu, H.; Jiang, L.; Tsao, R. A comprehensive profiling of free, conjugated and bound phenolics and lipophilic antioxidants in red and green lentil processing by-products. Food Chem. 2020, 325, 126925. [Google Scholar] [CrossRef] [PubMed]
- El Haddad, N.; En-Nahli, Y.; Choukri, H.; Aloui, K.; Mentag, R.; El-Baouchi, A.; Hejjaoui, K.; Rajendran, K.; Smouni, A.; Maalouf, F.; et al. Metabolic Mechanisms Underlying Heat and Drought Tolerance in Lentil Accessions: Implications for Stress Tolerance Breeding. Plants 2023, 12, 3962. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free Radic. Bio Med. 2021, 176, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J.; Jackson, K.G.; Minihane, A.M. Green tea (Camellia sinensis) catechins and vascular function. Br. J. Nutr. 2009, 102, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- Rubab, S.; Rizwani, G.H.; Bahadur, S.; Shah, M.; Alsamadany, H.; Alzahrani, Y.; Alghamdi, S.A.; Anwar, Y.; Shuaib, M.; Shah, A.A.; et al. Neuropharmacological potential of various morphological parts of Camellia sinensis L. Saudi J. Biol. Sci. 2020, 27, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary Flavonoids for Immunoregulation and Cancer: Food Design for Targeting Disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci. Tech. 2021, 117, 15–33. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Carotenes and xanthophylls as antioxidants. In Handbook of Antioxidants for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 17–50. [Google Scholar]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New concepts and paradigms for the protective effects of plant-based food components in relation to food complexity. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 293–312. [Google Scholar]
- Plamada, D.; Teleky, B.-E.; Nemes, S.A.; Mitrea, L.; Szabo, K.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Ciont, C.; Martău, G.A. Plant-based dairy alternatives—A future direction to the milky way. Foods 2023, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Castell, L.M.; Casa, D.J.; Close, G.L.; Costa, R.J.S.; Desbrow, B.; Halson, S.L.; Lis, D.M.; Melin, A.K.; Peeling, P.; et al. International Association of Athletics Federations Consensus Statement 2019: Nutrition for Athletics. Int. J. Sport. Nutr. Exerc. Metab. 2019, 29, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.; Hingst, J. The Athlete’S Guide to Sports Supplements; Human Kinetics: Champaign, IL, USA, 2013; p. 304. [Google Scholar]
- Burke, L.M.; Loucks, A.B.; Broad, N. Energy and carbohydrate for training and recovery. J. Sports Sci. 2006, 24, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Pelosi, E.; Giampieri, F.; Battino, M. The VegPlate for Sports: A Plant-Based Food Guide for Athletes. Nutrients 2023, 15, 1746. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M. Practical nutritional recommendations for the athlete. Nestle Nutr. Inst. Workshop Ser. 2011, 69, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Hannon, M.P.; Unnithan, V.; Morton, J.P.; Close, G.L. Nutritional strategies to support young athletes. In Strength and Conditioning for Young Athletes; Routledge: London, UK, 2019; pp. 300–335. [Google Scholar] [CrossRef]
- Meccariello, R.; D’Angelo, S. Impact of Polyphenolic-Food on Longevity: An Elixir of Life. An Overview. Antioxidants 2021, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Ranchordas, M.K.; Dawson, J.T.; Russell, M. Practical nutritional recovery strategies for elite soccer players when limited time separates repeated matches. J. Int. Soc. Sports Nutr. 2017, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Heaton, L.E.; Davis, J.K.; Rawson, E.S.; Nuccio, R.P.; Witard, O.C.; Stein, K.W.; Baar, K.; Carter, J.M.; Baker, L.B. Selected In-Season Nutritional Strategies to Enhance Recovery for Team Sport Athletes: A Practical Overview. Sports Med. 2017, 47, 2201–2218. [Google Scholar] [CrossRef] [PubMed]
- Bozonet, S.M.; Carr, A.C.; Pullar, J.M.; Vissers, M.C. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 2015, 7, 2574–2588. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C Content in Fruits: Biosynthesis and Regulation. Front. Plant Sci. 2018, 9, 2006. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Jagim, A.; Hagele, A.; Jäger, R. Plant Proteins and Exercise: What Role Can Plant Proteins Have in Promoting Adaptations to Exercise? Nutrients 2021, 13, 1962. [Google Scholar] [CrossRef] [PubMed]
- Décombaz, J. Nutrition and recovery of muscle energy stores after exercise. Schweiz. Z. Fur Sportmed. Und Sport. 2003, 51, 31–38. [Google Scholar]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Deis, L.; Quiroga, A.M.; De Rosas, M.I. Coloured Compounds in Fruits and Vegetables and Health. In Psychiatry and Neuroscience Update; Springer: Berlin/Heidelberg, Germany, 2021; pp. 343–358. [Google Scholar] [CrossRef]
- Gush, L.; Shah, S.; Gilani, F. Macronutrients and micronutrients. In A Prescription for Healthy Living; Elsevier: Amsterdam, The Netherlands, 2021; pp. 255–273. [Google Scholar]
- Kaale, L.D.; Siddiq, M.; Hooper, S.J.L.S. Lentil (Lens culinaris Medik) as nutrient-rich and versatile food legume: A review. Open Access 2023, 5, e169. [Google Scholar]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R.J.M. Fermented soy products and their potential health benefits: A review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Ahnan-Winarno, A.D.; Cordeiro, L.; Winarno, F.G.; Gibbons, J.; Xiao, H. Tempeh: A semicentennial review on its health benefits, fermentation, safety, processing, sustainability, and affordability. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1717–1767. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Fujita, T.; Ishii, T.; Ueno, N.J. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010, 119, 1300–1306. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Goldin, B.R.; Saul, N.; Gualtieri, L.; Barakat, S.; Adlercreutz, H.J. Tofu and soy drinks contain phytoestrogens. J. Am. Diet. Assoc. 1994, 94, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Paz, S.; Rubio, C.; Gutiérrez, Á.J.; González-Weller, D.; Hardisson, A. Dietary Intake of Essential Elements (Na, K, Mg, Ca, Mn, Zn, Fe, Cu, Mo, Co) from Tofu Consumption. Biol. Trace Element Res. 2021, 199, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Mishra, S. Nutritional Value of Product and Its Enrichment Using Super Seeds (Pumpkin Seeds, Chia Seeds, Sunflower Seeds and Almond) in Application of Ready to Eat Upma. Food Sci. Technol. 2020, 21, 48–54. [Google Scholar]
- Dodevska, M.; Kukic Markovic, J.; Sofrenic, I.; Tesevic, V.; Jankovic, M.; Djordjevic, B.; Ivanovic, N. Similarities and differences in the nutritional composition of nuts and seeds in Serbia. Front. Nutr. 2022, 9, 1003125. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Karbalaii, M.T.; Jaafar, H.Z.; Rahmat, A.J. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 2018, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.D.; Subhasree, R.; Bhakyaraj, R.; Vidhyalakshmi, R.J.M. Brown rice-beyond the color reviving a lost health food—A review. Magnesium 2009, 187, 67–72. [Google Scholar]
- Grace, M.H.; Yousef, G.G.; Gustafson, S.J.; Truong, V.-D.; Yencho, G.C.; Lila, M. Phytochemical changes in phenolics, anthocyanins, ascorbic acid, and carotenoids associated with sweetpotato storage and impacts on bioactive properties. Food Chem. 2014, 145, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Suzuno, H.; Sugiyama, N.; Innami, S.; Tadokoro, T.; Maekawa, A. Nutritive evaluation on chemical components of leaves, stalks and stems of sweet potatoes (Ipomoea batatas poir). Food Chem. 2000, 68, 359–367. [Google Scholar] [CrossRef]
- Bratt, K.; Sunnerheim, K.; Bryngelsson, S.; Fagerlund, A.; Engman, L.; Andersson, R.E.; Dimberg, L.H. Avenanthramides in oats (Avena sativa L.) and structure− antioxidant activity relationships. J. Agric. Food Chem. 2003, 51, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Maria, J.M. Nutrient profile of fermented oats. Int. J. Food Sci. Nutr. 2017, 2, 69–71. [Google Scholar]
- Cervantes, L.; Martinez-Ferri, E.; Soria, C.; Ariza, M.T. Bioavailability of phenolic compounds in strawberry, raspberry and blueberry: Insights for breeding programs. Food Biosci. 2020, 37, 100680. [Google Scholar] [CrossRef]
- Pereira, C.C.; da Silva, E.d.N.; de Souza, A.O.; Vieira, M.A.; Ribeiro, A.S.; Cadore, S.J. Evaluation of the bioaccessibility of minerals from blackberries, raspberries, blueberries and strawberries. J. Food Compos. Anal. 2018, 68, 73–78. [Google Scholar] [CrossRef]
- Keservani, R.K.; Sharma, A.K.; Kesharwani, R.K. Medicinal effect of nutraceutical fruits for the cognition and brain health. Scientifica 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, S.; Shekhar, C. Nutritional components in green leafy vegetables: A review. J. Pharmacogn. Phytochem. 2020, 9, 2498–2502. [Google Scholar]
- Randhawa, M.A.; Khan, A.A.; Javed, M.S.; Sajid, M.W. Green leafy vegetables: A health promoting source. In Handbook of Fertility; Elsevier: Amsterdam, The Netherlands, 2015; pp. 205–220. [Google Scholar]
- Manchali, S.; Murthy, K.N.C.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Murillo, G.; Mehta, R.G. Cruciferous vegetables and cancer prevention. Nutr. Cancer 2001, 41, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Henry, C.J.; Dwyer, J.T. Proposed nutrient standards for plant-based beverages intended as milk alternatives. Front. Nutr. 2021, 8, 796. [Google Scholar] [CrossRef] [PubMed]
- Dukariya, G.; Shah, S.; Singh, G.; Kumar, A.J. Soybean and its products: Nutritional and health benefits. J. Nut. Sci Health Diet. 2020, 1, 22–29. [Google Scholar]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, S.; Klein, C.; Pfeffer, A.; Rasińska, J.; Stahn, L.; Knuth, K.; Abuelnor, B.; Panzel, A.E.C.; Rex, A.; Koch, S.J. Chia seeds as a potential cognitive booster in the APP23 Alzheimer’s disease model. Sci. Rep. 2020, 10, 18215. [Google Scholar] [CrossRef] [PubMed]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. Omega-3 fatty acids for nutrition and medicine: Considering microalgae oil as a vegetarian source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Corder, K.E.; Newsham, K.R.; McDaniel, J.L.; Ezekiel, U.R.; Weiss, E.P. Effects of short-term docosahexaenoic acid supplementation on markers of inflammation after eccentric strength exercise in women. J. Sports Sci. Med. 2016, 15, 176. [Google Scholar] [PubMed]
- Gupta, S.C.; Kismali, G.; Aggarwal, B.B. Curcumin, a component of turmeric: From farm to pharmacy. Biofactors 2013, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Rolls, B.J.; Drewnowski, A.; Ledikwe, J.H. Changing the energy density of the diet as a strategy for weight management. J. Am. Diet. Assoc. 2005, 105, S98–S103. [Google Scholar] [CrossRef] [PubMed]
- Clifford, J.; Maloney, K. Nutrition for Athletes; Colorado State University: Fort Collins, CO, USA, 2015. [Google Scholar]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Farajian, P.; Kavouras, S.; Yannakoulia, M.; Sidossis, L.S. Dietary intake and nutritional practices of elite Greek aquatic athletes. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Venderley, A.M.; Campbell, W.W. Vegetarian diets: Nutritional considerations for athletes. Sports Med. 2006, 36, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Ahnen, R.T.; Jonnalagadda, S.S.; Slavin, J.L. Role of plant protein in nutrition, wellness, and health. Nutr. Rev. 2019, 77, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.J. Nuts and novel biomarkers of cardiovascular disease. Am. J. Clin. Nutr. 2009, 89, 1649S–1656S. [Google Scholar] [CrossRef] [PubMed]
- Bingham, M.E.; Borkan, M.E.; Quatromoni, P.A. Sports nutrition advice for adolescent athletes: A time to focus on food. Am. J. Lifestyle Med. 2015, 9, 398–402. [Google Scholar] [CrossRef]
- Lis, D.M.; Kings, D.; Larson-Meyer, D.E. Dietary practices adopted by track-and-field athletes: Gluten-free, low FODMAP, vegetarian, and fasting. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Petersen, K.S.; Kris-Etherton, P.M.; Rogers, C.J. Role of dietary spices in modulating inflammation and oxidative stress. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Academic Press: Cambridge, MA, USA, 2022; pp. 545–580. [Google Scholar]
- Karakol, P.; Kapi, E. Use of selected antioxidant-rich spices and herbs in foods. In Antioxidants-Benefits, Sources, Mechanisms of Action; IntechOpen: London, UK, 2021. [Google Scholar]
- Lima, M.; Costa, R.; Rodrigues, I.; Lameiras, J.; Botelho, G. A Narrative Review of Alternative Protein Sources: Highlights on Meat, Fish, Egg and Dairy Analogues. Foods 2022, 11, 2053. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.K.; Kumar, S. Meat Analogues: Plant based alternatives to meat products- A review. Int. J. Food Ferment. Technol. 2015, 5, 107. [Google Scholar] [CrossRef]
- Santos, H.O.; Price, J.C.; Bueno, A.A. Beyond Fish Oil Supplementation: The Effects of Alternative Plant Sources of Omega-3 Polyunsaturated Fatty Acids upon Lipid Indexes and Cardiometabolic Biomarkers-An Overview. Nutrients 2020, 12, 3159. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.R.; Severino, P.; Ferreira, C.S.; Zielinska, A.; Santini, A.; Souto, S.B.; Souto, E.B. Linseed Essential Oil—Source of Lipids as Active Ingredients for Pharmaceuticals and Nutraceuticals. Curr. Med. Chem. 2019, 26, 4537–4558. [Google Scholar] [CrossRef] [PubMed]
- Holway, F.E.; Spriet, L.L. Sport-specific nutrition: Practical strategies for team sports. In Food, Nutrition and Sports Performance III; Routledge: London, UK, 2013; pp. 115–125. [Google Scholar]
- Heaney, R.P. Effect of calcium on skeletal development, bone loss, and risk of fractures. Am. J. Med. 1991, 91, 23S–28S. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K. Eating patterns, diet quality and energy balance A perspective about applications and future directions for the food industry. Physiol. Behav. 2014, 134, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B. Strategies for reducing or preventing the generation of oxidative stress. Oxid. Med. Cell Longev. 2011, 2011, 194586. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]



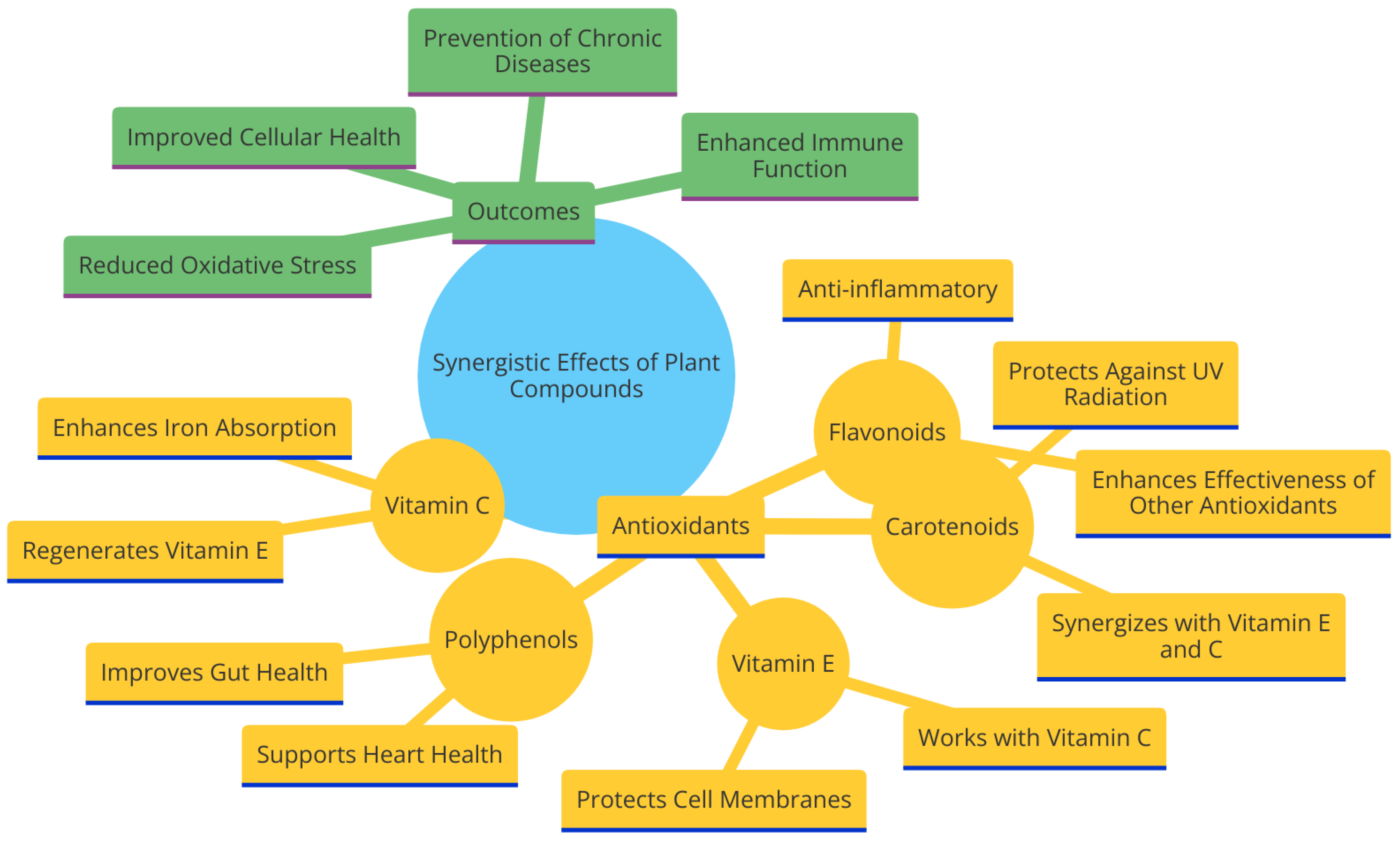
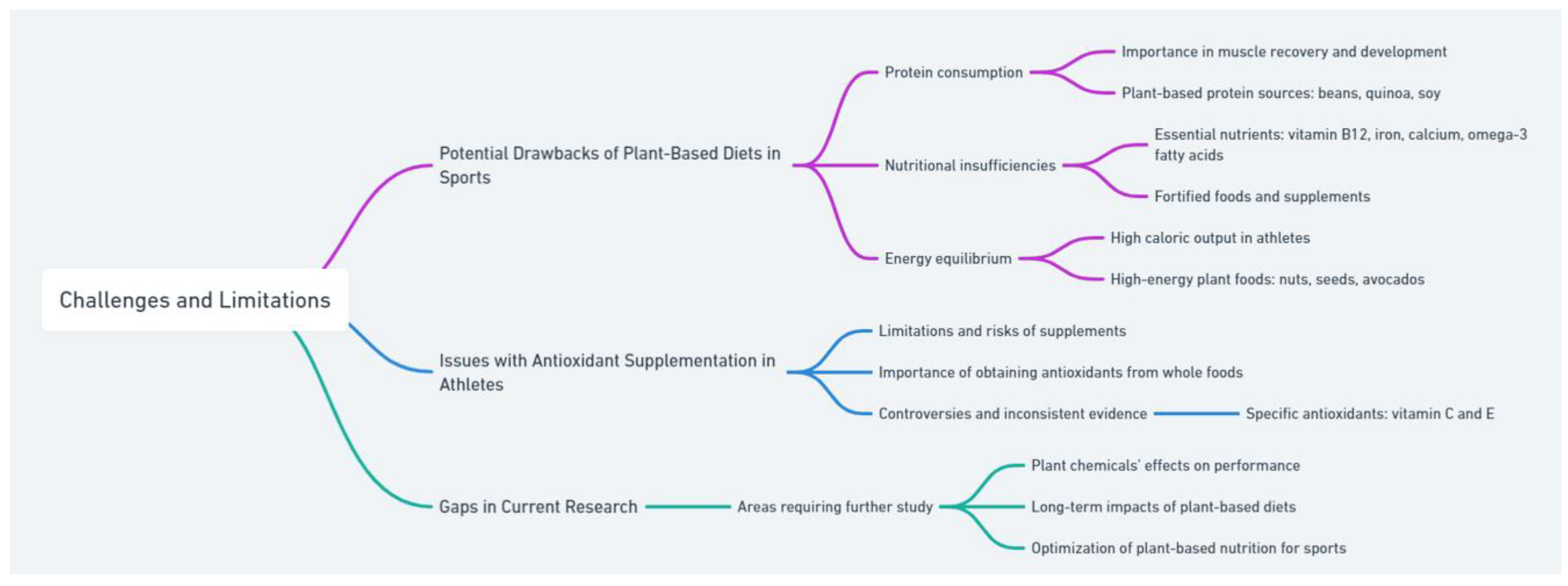
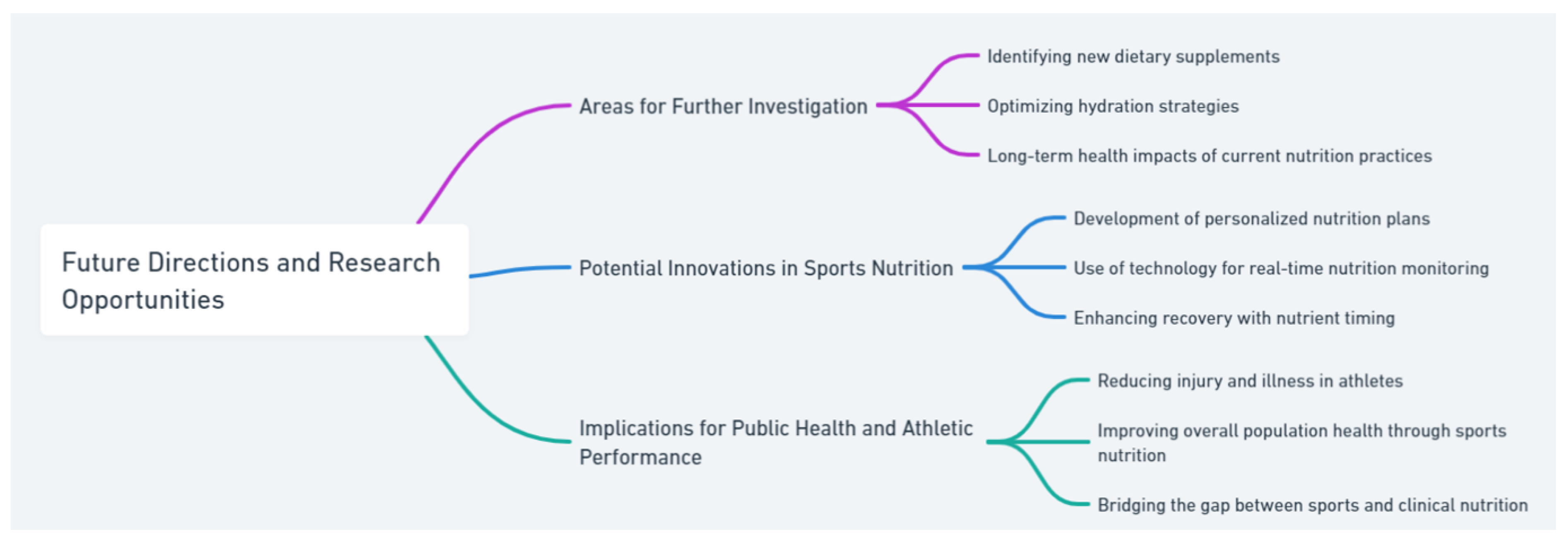
| Plant Food Category | Specific Plant Foods | Antioxidants Present | References |
|---|---|---|---|
| Fruits | Berries (Blueberries, Strawberries, Raspberries), Citrus fruits (Oranges, Grapefruits, Lemons), Apples, Pears, Cherries | Anthocyanins, Vitamin C, Quercetin, Flavonoids | [45,46,47] |
| Vegetables | Leafy greens (Spinach, Kale, Swiss Chard), Cruciferous vegetables (Broccoli, Cauliflower), Bell peppers, Tomatoes, Carrots | Quercetin, Polyphenols, Lutein, Zeaxanthin, Vitamin E, Glucosinolates, Sulforaphane, Indoles, Vitamin C, Vitamin A, Lycopene, Beta-carotene, | [48,49] |
| Nuts and Seeds | Almonds, Walnuts, Chia Seeds, Flaxseeds, Sunflower seeds, Pumpkin seeds | Vitamin E, Omega-3 fatty acids, Polyphenols, Selenium, Vitamin E | [50,51] |
| Legumes | Chickpeas, Lentils, Black beans, Peanuts, Pinto beans, Kidney beans | Flavonoids, Resveratrol, Coenzyme Q10, Isoflavones | [52,53] |
| Whole Grains | Quinoa, Brown rice, Oats, Barley, Whole wheat | Vitamin E, Selenium, Polyphenols, Ferulic Acid, Beta-glucans | [54,55] |
| Food Group | Antioxidant-Rich Examples | Serving Recommendations | Benefits | References |
|---|---|---|---|---|
| Fruits (5 servings per day) | Berries (blueberries, strawberries, raspberries), citrus fruits (oranges, grapefruits, kiwis), pomegranates, pineapple, mangoes, apples, pears | 1–2 servings per meal, snack on fruits in between | Rich in vitamin C, flavonoids, anthocyanins; boost immunity, reduce inflammation, protect against muscle damage | [348,349] |
| Vegetables (5 servings per day) | Cruciferous vegetables (broccoli, kale, Brussels sprouts), leafy greens (spinach, swiss chard, collard greens), bell peppers, sweet potatoes, carrots, onions, tomatoes | 1–2 servings per meal, incorporate vegetables into snacks | Abundant in carotenoids, vitamin C, and other phytonutrients; enhance cell health, improve antioxidant defenses, support recovery | [348,350] |
| Legumes (2–3 servings per week) | Lentils, black beans, chickpeas, kidney beans, soybeans | 1/2 cup cooked legumes per meal, incorporate into salads, soups, and dips | Excellent source of plant-based protein, fiber, and various antioxidants; contribute to muscle building, satiety, and overall wellbeing | [351,352] |
| Nuts and seeds (1–2 servings per day) | Walnuts, almonds, chia seeds, flaxseeds, hemp seeds | 1/4 cup nuts or 2 tablespoons seeds per day, sprinkle on yogurt, salads, or add to smoothies | High in polyunsaturated fats, vitamin E, and minerals; provide sustained energy, promote cell health, aid in recovery | [351,353] |
| Whole grains (3–5 servings per day) | Quinoa, brown rice, whole-wheat bread, oats, barley | 1/2 cup cooked grains per meal, choose whole-grain bread and cereals | Rich in fiber, vitamin E, and B vitamins; support gut health, regulate blood sugar, improve energy levels | [354,355] |
| Spices and herbs (daily) | Turmeric, ginger, garlic, cinnamon, parsley, rosemary, oregano | Add to cooking, sprinkle on meals, use in teas and infusions | Contain powerful antioxidants and anti-inflammatory compounds; enhance flavor, boost digestion, and provide additional health benefits | [356,357] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayaz, A.; Zaman, W.; Radák, Z.; Gu, Y. Harmony in Motion: Unraveling the Nexus of Sports, Plant-Based Nutrition, and Antioxidants for Peak Performance. Antioxidants 2024, 13, 437. https://doi.org/10.3390/antiox13040437
Ayaz A, Zaman W, Radák Z, Gu Y. Harmony in Motion: Unraveling the Nexus of Sports, Plant-Based Nutrition, and Antioxidants for Peak Performance. Antioxidants. 2024; 13(4):437. https://doi.org/10.3390/antiox13040437
Chicago/Turabian StyleAyaz, Asma, Wajid Zaman, Zsolt Radák, and Yaodong Gu. 2024. "Harmony in Motion: Unraveling the Nexus of Sports, Plant-Based Nutrition, and Antioxidants for Peak Performance" Antioxidants 13, no. 4: 437. https://doi.org/10.3390/antiox13040437
APA StyleAyaz, A., Zaman, W., Radák, Z., & Gu, Y. (2024). Harmony in Motion: Unraveling the Nexus of Sports, Plant-Based Nutrition, and Antioxidants for Peak Performance. Antioxidants, 13(4), 437. https://doi.org/10.3390/antiox13040437