Effects of Regular Exercise on the Biochemical, Oxidative, and Inflammatory Profiles and Quality of Life in Older Spaniards with Metabolic Syndrome
Abstract
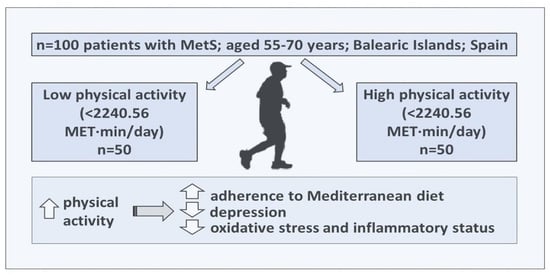
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric Parameters
2.3. Plasma and Urine Isolation, and Biochemical and Hematological Parameters
2.4. Quality of Life Parameters
2.5. Inflammatory and Oxidative Stress Biomarkers
2.6. Statistics
3. Results
3.1. Anthropometric and Hematological Parameters
3.2. Quality of Life Parameters
3.3. Inflammatory Biomarkers
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nolan, P.B.; Carrick-Ranson, G.; Stinear, J.W.; Reading, S.A.; Dalleck, L.C. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Prev. Med. Rep. 2017, 7, 211–215. [Google Scholar] [CrossRef]
- Cano-Ibáñez, N.; Gea, A.; Martínez-González, M.A.; Salas-Salvadó, J.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Aros, F.; Wärnberg, J.; et al. Dietary Diversity and Nutritional Adequacy among an Older Spanish Population with Metabolic Syndrome in the PREDIMED-Plus Study: A Cross-Sectional Analysis. Nutrients 2019, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- de Loos, A.D.; Jiskoot, G.; Beerthuizen, A.; Busschbach, J.; Laven, J. Metabolic health during a randomized controlled lifestyle intervention in women with PCOS. Eur. J. Endocrinol. 2021, 186, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Marchiani, S.; Corona, G.; Maggi, M. Metabolic Syndrome and Reproduction. Int. J. Mol. Sci. 2021, 22, 1988. [Google Scholar] [CrossRef]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.H. Novel Insights into the Pathogenesis and Management of the Metabolic Syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Gómez-Sánchez, M.; Gómez-Sánchez, L.; Patino-Alonso, M.C.; Alonso-Domínguez, R.; Sánchez-Aguadero, N.; Recio-Rodríguez, J.I.; González-Sánchez, J.; García-Ortiz, L.; Gómez-Marcos, M.A. Relationship of healthy vascular aging with lifestyle and metabolic syndrome in the general Spanish population. The EVA study. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 854–861. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet. Child Adolesc. Health 2022, 6, 158–170. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 2 April 2024).
- Chen, M.Z.; Wong, M.W.K.; Lim, J.Y.; Merchant, R.A. Frailty and Quality of Life in Older Adults with Metabolic Syndrome—Findings from the Healthy Older People Everyday (HOPE) Study. J. Nutr. Health Aging 2021, 25, 637–644. [Google Scholar] [CrossRef]
- Subías-Perié, J.; Navarrete-Villanueva, D.; Fernández-García, Á.I.; Moradell, A.; Gesteiro, E.; Pérez-Gómez, J.; Ara, I.; Vicente-Rodríguez, G.; Casajús, J.A.; Gómez-Cabello, A. Prevalence of Metabolic Syndrome and Association with Physical Activity and Frailty Status in Spanish Older Adults with Decreased Functional Capacity: A Cross-Sectional Study. Nutrients 2022, 14, 2302. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.M.; Hasler, B.P.; Kamarck, T.W.; Muldoon, M.F.; Manuck, S.B. Social Jetlag, Chronotype, and Cardiometabolic Risk. J. Clin. Endocrinol. Metab. 2015, 100, 4612–4620. [Google Scholar] [CrossRef] [PubMed]
- Same, R.V.; Feldman, D.I.; Shah, N.; Martin, S.S.; Al Rifai, M.; Blaha, M.J.; Graham, G.; Ahmed, H.M. Relationship Between Sedentary Behavior and Cardiovascular Risk. Curr. Cardiol. Rep. 2016, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, J.; Yin, Z.; Wang, L.; Peng, L. The association between depression and metabolic syndrome and its components: A bidirectional two-sample Mendelian randomization study. Transl. Psychiatry 2021, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Carretero Gómez, J.; Arévalo Lorido, J.C.; Gómez Huelgas, R.; Sánchez Vidal, M.T.; Suárez Tembra, M.; Varela Aguilar, J.M.; Munielo Voces, I.; Fernández Pérez, E.; Fernández Rodríguez, J.M.; Ena Muñoz, J. Prevalence of obesity according to Edmonton staging in the Internal Medicine consultations. Results of the OBEMI study. Rev. Clin. Esp. 2017, 217, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Barcones-Molero, M.F.; Sánchez-Villegas, A.; Martínez-González, M.A.; Bes-Rastrollo, M.; Martínez-Urbistondo, M.; Santabárbara, J.; Martínez, J.A. The influence of obesity and weight gain on quality of life according to the SF-36 for individuals of the dynamic follow-up cohort of the University of Navarra. Rev. Clin. Esp. 2018, 218, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Delgado, A.; López-García, E.; Martínez-González, M.A.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gómez, A.M.; Wärnberg, J.; et al. Health-related quality of life in individuals with metabolic syndrome: A cross-sectional study. Med. Fam. Semer. 2020, 46, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Fuentes, F.; Vilahur, G.; Badimon, L.; Palomo, I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediat. Inflamm. 2013, 2013, 136584. [Google Scholar] [CrossRef]
- Boccardi, V.; Mancinetti, F.; Baroni, M.; Cecchetti, R.; Bastiani, P.; Ruggiero, C.; Mecocci, P. Metabolic Score for Insulin Resistance (METS-IR) and Circulating Cytokines in Older Persons: The Role of Gender and Body Mass Index. Nutrients 2022, 14, 3228. [Google Scholar] [CrossRef]
- O’Hagan, R.; Berg, A.R.; Hong, C.G.; Parel, P.M.; Mehta, N.N.; Teague, H.L. Systemic consequences of abnormal cholesterol handling: Interdependent pathways of inflammation and dyslipidemia. Front. Immunol. 2022, 13, 972140. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Savaş, E.M.; Oğuz, S.H.; Samadi, A.; Yılmaz Işıkhan, S.; Ünlütürk, U.; Lay, İ.; Gürlek, A. Apoptosis Inhibitor of Macrophage, Monocyte Chemotactic Protein-1, and C-Reactive Protein Levels Are Increased in Patients with Metabolic Syndrome: A Pilot Study. Metab. Syndr. Relat. Disord. 2020, 18, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S. Compendium of Physical Activities: Classification of energy costs of human physical activities. Med. Sci. Sport. Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Sanz Fernández, J.; Navarro, M.E.; Vázquez Valverde, C. Adaptación Española del Inventario Para la Depresión de Beck-II (BDI-II): Propiedades Psicométricas en Estudiantes Universitarios; Análisis y Modification de Conducta; Universidad Complutense de Madrid: Madrid, Spain, 2003; Volume 29, pp. 239–288. ISSN 0211-7339. ISSN-e 2173-6855. [Google Scholar]
- Alonso, J.; Regidor, E.; Barrio, G.; Prieto, L.; Rodriguez, C.; de la Fuente, L. Population reference values of the Spanish version of the Health Questionnaire SF-36. Med. Clin. 1998, 111, 410–416. [Google Scholar]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arellano, A.; Martínez-González, M.A.; Ramallal, R.; Salas-Salvadó, J.; Hébert, J.R.; Corella, D.; Shivappa, N.; Forga, L.; Schröder, H.; Muñoz-Bravo, C.; et al. Dietary inflammatory index and all-cause mortality in large cohorts: The SUN and PREDIMED studies. Clin. Nutr. 2019, 38, 1221–1231. [Google Scholar] [CrossRef]
- Álvarez-Álvarez, I.; Martínez-González, M.Á.; Sánchez-Tainta, A.; Corella, D.; Díaz-López, A.; Fitó, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; et al. Adherence to an Energy-restricted Mediterranean Diet Score and Prevalence of Cardiovascular Risk Factors in the PREDIMED-Plus: A Cross-sectional Study. Rev. Esp. Cardiol. (Engl. Ed). 2019, 72, 925–934. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Topolski, T.D.; LoGerfo, J.; Patrick, D.L.; Williams, B.; Walwick, J.; Patrick, M.B. The Rapid Assessment of Physical Activity (RAPA) Among Older Adults. Prev. Chronic Dis. 2006, 3, A118. [Google Scholar]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Poulsen, H.E.; Weimann, A.; Henriksen, T.; Kjær, L.K.; Larsen, E.L.; Carlsson, E.R.; Christensen, C.K.; Brandslund, I.; Fenger, M. Oxidatively generated modifications to nucleic acids in vivo: Measurement in urine and plasma. Free Radic. Biol. Med. 2019, 145, 336–341. [Google Scholar] [CrossRef]
- Liang, M.; Pan, Y.; Zhong, T.; Zeng, Y.; Cheng, A.S.K. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: A systematic review and network meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Sequi-Dominguez, I.; Alvarez-Bueno, C.; Martinez-Vizcaino, V.; Fernandez-Rodriguez, R.; del Saz Lara, A.; Cavero-Redondo, I. Effectiveness of Mobile Health Interventions Promoting Physical Activity and Lifestyle Interventions to Reduce Cardiovascular Risk Among Individuals With Metabolic Syndrome: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e17790. [Google Scholar] [CrossRef]
- Qian, K.; Dong, H.; Qian, J.; Gong, J. Effect of glycosylated hemoglobin protein molecule in treating diabetes. Cell. Mol. Biol. 2020, 66, 45–48. [Google Scholar] [CrossRef]
- de Almeida Mendes, M.; da Silva, I.; Ramires, V.; Reichert, F.; Martins, R.; Ferreira, R.; Tomasi, E. Metabolic equivalent of task (METs) thresholds as an indicator of physical activity intensity. PLoS ONE 2018, 13, e0200701. [Google Scholar]
- Zhang, D.; Liu, X.; Liu, Y.; Sun, X.; Wang, B.; Ren, Y.; Zhao, Y.; Zhou, J.; Han, C.; Yin, L.; et al. Leisure-time physical activity and incident metabolic syndrome: A systematic review and dose-response meta-analysis of cohort studies. Metabolism 2017, 75, 36–44. [Google Scholar] [CrossRef]
- Serrano-Sánchez, J.A.; Fernández-Rodríguez, M.J.; Sanchis-Moysi, J.; del Cristo Rodríguez-Pérez, M.; Marcelino-Rodríguez, I.; de León, A.C. Domain and intensity of physical activity are associated with metabolic syndrome: A population-based study. PLoS ONE 2019, 14, e0219798. [Google Scholar] [CrossRef]
- Gallardo-Alfaro, L.; Del Mar Bibiloni, M.; Mascaró, C.M.; Montemayor, S.; Ruiz-Canela, M.; Salas-Salvad, J.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients 2020, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Alfaro, L.; del Mar Bibiloni, M.; Bouzas, C.; Mascaró, C.M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Schröder, H.; Martínez, J.A.; Alonso-Gómez, Á.M.; et al. Physical activity and metabolic syndrome severity among older adults at cardiovascular risk: 1-Year trends. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2870–2886. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Kerling, A.; Protte, G.; Bayerle, P.; Stenner, H.T.; Rolff, S.; Sundermeier, T.; Kück, M.; Ensslen, R.; Nachbar, L.; et al. Telemonitoring-supported exercise training, metabolic syndrome severity, and work ability in company employees: A randomised controlled trial. Lancet. Public Health 2019, 4, e343–e352. [Google Scholar] [CrossRef]
- Cerletti, P.; Keidel, D.; Imboden, M.; Schindler, C.; Probst-Hensch, N. The modifying role of physical activity in the cross-sectional and longitudinal association of health-related quality of life with physiological functioning-based latent classes and metabolic syndrome. Health Qual. Life Outcomes 2020, 18, 345. [Google Scholar] [CrossRef] [PubMed]
- Zupkauskiene, J.; Lauceviciene, I.; Navickas, P.; Ryliskyte, L.; Puronaite, R.; Badariene, J.; Laucevicius, A. Changes in health-related quality of life, motivation for physical activity, and the levels of anxiety and depression after individualized aerobic training in subjects with metabolic syndrome. Hell. J. Cardiol. 2022, 66, 41–51. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.S.; Krogh-Madsen, R.; Petersen, G.L.; Hansen, Å.M.; Pedersen, B.K.; Lund, R.; Bruunsgaard, H. Cardiorespiratory fitness and the metabolic syndrome: Roles of inflammation and abdominal obesity. PLoS ONE 2018, 13, e0194991. [Google Scholar] [CrossRef] [PubMed]
- Alizaei Yousefabadi, H.; Niyazi, A.; Alaee, S.; Fathi, M.; Mohammad Rahimi, G.R. Anti-Inflammatory Effects of Exercise on Metabolic Syndrome Patients: A Systematic Review and Meta-Analysis. Biol. Res. Nurs. 2021, 23, 280–292. [Google Scholar] [CrossRef]
- Galozzi, P.; Bindoli, S.; Doria, A.; Sfriso, P. The revisited role of interleukin-1 alpha and beta in autoimmune and inflammatory disorders and in comorbidities. Autoimmun. Rev. 2021, 20, 102785. [Google Scholar] [CrossRef]
- Bjerre, M. Osteoprotegerin (OPG) as a biomarker for diabetic cardiovascular complications. Springerplus 2013, 2, 658. [Google Scholar] [CrossRef]
- Bøgh, H.L.; Stanislaus, S.; Kjærstad, H.L.; Sletved, K.S.O.; Forman, J.L.; Poulsen, H.E.; Vinberg, M.; Kessing, L.V.; Coello, K. Associations between levels of oxidative nucleoside damage and cardiovascular risk in patients newly diagnosed with bipolar disorder and their unaffected relatives. Transl. Psychiatry 2022, 12, 327. [Google Scholar] [CrossRef]
| Low Physical Activity n = 50 | High Physical Activity n = 50 | p-Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Weight (kg) | 88.0 (14.5) | 87.3 (12.8) | 0.661 |
| Waist (cm) | 111.2 (10.2) | 111.2 (10.0) | 0.984 |
| Hip (cm) | 113.7 (10.1) | 111.9 (8.6) | 0.116 |
| Height (cm) | 162.8 (9.3) | 162.9 (9.3) | 0.945 |
| BMI (kg/m2) | 33.1 (3.7) | 32.8 (3.5) | 0.574 |
| Systolic blood pressure (mmHg) | 140.6 (19.4) | 140.2 (16.3) | 0.834 |
| Diastolic blood pressure (mmHg) | 82.1 (11.0) | 80.9 (10.4) | 0.358 |
| Glycemia (mg/dL) | 124.4 (44.8) | 111.7 (22.4) | 0.003 |
| HbA1c (%) | 6.45 (1.4) | 5.99 (0.75) | 0.001 |
| Triglycerides (mg/dL) | 155.2 (79.0) | 147.4 (64.3) | 0.377 |
| HDL-cholesterol (mg/dL) | 43.7 (10.2) | 44.7 (10.6) | 0.447 |
| Total cholesterol (mg/dL) | 185.3 (38.9) | 184.4 (34.1) | 0.842 |
| AST (U/L) | 20.5 (6.9) | 20.7 (5.4) | 0.783 |
| ALT (U/L) | 22.1 (9.3) | 21.9 (8.4) | 0.878 |
| GGT (U/L) | 34.2 (27.8) | 30.4 (16.4) | 0.191 |
| Physical activity (MET·min/day) | 1023.3 (708.8) | 4743.1 (2870.1) | <0.001 |
| Low Physical Activity n = 50 | High physical Activity n = 50 | p-Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Hematocrit (%) | 43.0 (4.0) | 43.0 (4.1) | 0.952 |
| Erythrocytes (106/mm3) | 4.80 (0.5) | 4.78 (0.5) | 0.683 |
| Leukocytes (103/mm3) | 7.59 (1.9) | 7.12 (1.6) | 0.030 |
| Neutrophils (103/mm3) | 4.75 (4.8) | 4.00 (1.6) | 0.083 |
| Lymphocytes (103/mm3) | 2.46 (0.8) | 2.54 (2.3) | 0.710 |
| Monocytes (103/mm3) | 0.64 (0.2) | 0.62 (0.2) | 0.389 |
| Eosinophils (103/mm3) | 0.30 (0.6) | 0.23 (0.2) | 0.174 |
| Basophils (103/mm3) | 0.095 (0.4) | 0.046 (0.03) | 0.131 |
| Low Physical Activity n = 50 | High Physical Activity n = 50 | p-Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Hours of sleep | 7.22 (1.3) | 7.31 (1.3) | 0.568 |
| Chair test | 11.3 (4.6) | 12.6 (4.4) | 0.027 |
| BDI-II | 7.81 (6.7) | 5.27 (5.1) | 0.001 |
| AMD | 6.92 (2.2) | 8.07 (2.6) | <0.001 |
| DII | 0.681 (2.1) | −0.148 (2.0) | 0.003 |
| HRQoL physical dimensions | 45.5 (8.3) | 48.0 (8.0) | 0.039 |
| HRQoL mental dimensions | 48.8 (10.8) | 50.1 (10.6) | 0.437 |
| n (%) | n (%) | ||
| RAPA | <0.001 | ||
| Sedentary or inactive | 40 (79.9) | 23 (45.9) | |
| Moderately active | 7 (14.9) | 14 (28.1) | |
| Active | 3 (5.2) | 13 (25.9) |
| Low Physical Activity n = 50 | High Physical Activity n = 50 | p-Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| MCP1 (pg/mL) | 236.3 (87.5) | 231.3 (83.9) | 0.790 |
| TNF-α (pg/mL) | 4.42 (1.9) | 2.99 (1.6) | 0.003 |
| OPG (pg/mL) | 34.3 (15.3) | 19.4 (11.9) | <0.001 |
| IL-1β (pg/mL) | 12.6 (7.2) | 8.96 (4.0) | 0.003 |
| IL-6 (pg/mL) | 7.49 (5.1) | 3.22 (2.3) | 0.002 |
| IL-10 (pg/mL) | 1.33 (0.7) | 1.08 (0.2) | 0.310 |
| IL-15 (pg/mL) | 6.86 (3.8) | 8.06 (4.8) | 0.327 |
| Resistin (ng/mL) | 7.17 (8.9) | 3.35 (1.8) | 0.025 |
| Leptin (ng/mL) | 11.03 (8.6) | 11.9 (14.7) | 0.815 |
| Ghrelin (pg/mL) | 307.7 (57.4) | 289.6 (51.2) | 0.212 |
| 8-OxoGuo/Creatinine (nM/mM) | 2.15 (0.6) | 1.86 (0.4) | 0.006 |
| 8-oxodG/Creatinine (nM/mM) | 1.51 (0.6) | 1.39 (0.7) | 0.322 |
| Low Physical Activity n = 50 | High Physical Activity n = 50 | p-Value | ||
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Cholesterol | Crude OR | 1.00 (ref.) | 1.219 (0.751–1.978) | 0.423 |
| Adjusted OR | 1.00 (ref.) | 1.366 (0.828–2.252) | 0.222 | |
| Triglycerides | Crude OR | 1.00 (ref.) | 0.986 (0.610–1.593) | 0.954 |
| Adjusted OR | 1.00 (ref.) | 0.964 (0.591–1.573) | 0.883 | |
| Glycemia | Crude OR | 1.00 (ref.) | 0.640 (0.396–1.034) | 0.068 |
| Adjusted OR | 1.00 (ref.) | 0.579 (0.352–0.951) | 0.031 | |
| Systolic blood pressure | Crude OR | 1.00 (ref.) | 1.045 (0.648–1.686) | 0.856 |
| Adjusted OR | 1.00 (ref.) | 1.045 (0.644–1.697) | 0.857 | |
| Diastolic blood pressure | Crude OR | 1.00 (ref.) | 0.648 (0.401–1.048) | 0.077 |
| Adjusted OR | 1.00 (ref.) | 0.612 (0.371–1.007) | 0.053 | |
| MCP1 | Crude OR | 1.00 (ref.) | 0.874 (0.367–2.079) | 0.761 |
| Adjusted OR | 1.00 (ref.) | 0.757 (0.298–1.922) | 0.558 | |
| TNF-α | Crude OR | 1.00 (ref.) | 0.295 (0.99–0.881) | 0.029 |
| Adjusted OR | 1.00 (ref.) | 0.258 (0.076–0.878) | 0.030 | |
| OPG | Crude OR | 1.00 (ref.) | 0.160 (0.050–0.512) | 0.002 |
| Adjusted OR | 1.00 (ref.) | 0.164 (0.049–0.552) | 0.004 | |
| IL-1β | Crude OR | 1.00 (ref.) | 0.338 (0.140–0.814) | 0.016 |
| Adjusted OR | 1.00 (ref.) | 0.266 (0.102–0.691) | 0.007 | |
| IL-6 | Crude OR | 1.00 (ref.) | 0.208 (0.052–0.830) | 0.026 |
| Adjusted OR | 1.00 (ref.) | 0.172 (0.034–0.877) | 0.034 | |
| IL-10 | Crude OR | 1.00 (ref.) | 1.000 (0.167–5.985) | 1.000 |
| Adjusted OR | 1.00 (ref.) | 0.408 (0.028–6.013) | 0.514 | |
| IL-15 | Crude OR | 1.00 (ref.) | 1.058 (0.350–3.193) | 0.921 |
| Adjusted OR | 1.00 (ref.) | 1.021 (0.280–3.719) | 0.975 | |
| Resistin | Crude OR | 1.00 (ref.) | 0.601 (0.206–1.752) | 0.351 |
| Adjusted OR | 1.00 (ref.) | 0.611 (0.197–1.896) | 0.394 | |
| Leptin | Crude OR | 1.00 (ref.) | 0.701 (0.218–2.261) | 0.552 |
| Adjusted OR | 1.00 (ref.) | 0.763 (0.211–2.759) | 0.680 | |
| Ghrelin | Crude OR | 1.00 (ref.) | 0.505 (0.177–1.441) | 0.202 |
| Adjusted OR | 1.00 (ref.) | 0.557 (0.175–1.777) | 0.323 | |
| 8-OxoGuo/Creatinine | Crude OR | 1.00 (ref.) | 0.585 (0.277–1.236) | 0.160 |
| Adjusted OR | 1.00 (ref.) | 0.601 (0.277–1.303) | 0.197 | |
| 8-oxodG/Creatinine | Crude OR | 1.00 (ref.) | 0.586 (0.284–1.209) | 0.148 |
| Adjusted OR | 1.00 (ref.) | 0.577 (0.272–1.223) | 0.152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monserrat-Mesquida, M.; Quetglas-Llabrés, M.M.; Bouzas, C.; García, S.; Mateos, D.; Ugarriza, L.; Gómez, C.; Tur, J.A.; Sureda, A. Effects of Regular Exercise on the Biochemical, Oxidative, and Inflammatory Profiles and Quality of Life in Older Spaniards with Metabolic Syndrome. Antioxidants 2024, 13, 450. https://doi.org/10.3390/antiox13040450
Monserrat-Mesquida M, Quetglas-Llabrés MM, Bouzas C, García S, Mateos D, Ugarriza L, Gómez C, Tur JA, Sureda A. Effects of Regular Exercise on the Biochemical, Oxidative, and Inflammatory Profiles and Quality of Life in Older Spaniards with Metabolic Syndrome. Antioxidants. 2024; 13(4):450. https://doi.org/10.3390/antiox13040450
Chicago/Turabian StyleMonserrat-Mesquida, Margalida, Maria Magdalena Quetglas-Llabrés, Cristina Bouzas, Silvia García, David Mateos, Lucía Ugarriza, Cristina Gómez, Josep A. Tur, and Antoni Sureda. 2024. "Effects of Regular Exercise on the Biochemical, Oxidative, and Inflammatory Profiles and Quality of Life in Older Spaniards with Metabolic Syndrome" Antioxidants 13, no. 4: 450. https://doi.org/10.3390/antiox13040450