Effects of Phytosterol Ester Supplementation on Egg Characteristics, Eggshell Ultrastructure, Antioxidant Capacity, Liver Function and Hepatic Metabolites of Laying Hens during Peak Laying Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Diets
2.2. Management
2.3. Determination of Performance Parameters
2.4. Determination of Egg Quality
2.5. Sample Collection
2.6. Determination of Amino Acid Analysis in Egg Yolk
2.7. Observation and Determination of Eggshell Ultrastructure
2.8. Determination of Lipid Metabolites and Antioxidant Capacity
2.9. Histology
2.10. Non-Targeted Profiling of Metabolites in Liver
2.11. Statistical Analysis
3. Results
3.1. Performance Parameters
3.2. Egg Quality
3.3. Ultrastructure of Eggshell
3.4. Amino Acid Profile of Egg Yolk
3.5. Phytosterol Esters Improved Fatty Liver Hemorrhagic Syndrome in Laying Hens
3.6. Antioxidant Capacity
3.7. Phytosterol Esters Alter Liver Metabolites in Laying Hens
3.7.1. Comparative Analysis of Samples
3.7.2. Analysis of Differential Metabolites
3.7.3. Metabolic Set Analysis
3.7.4. Metabolic Pathway Analysis
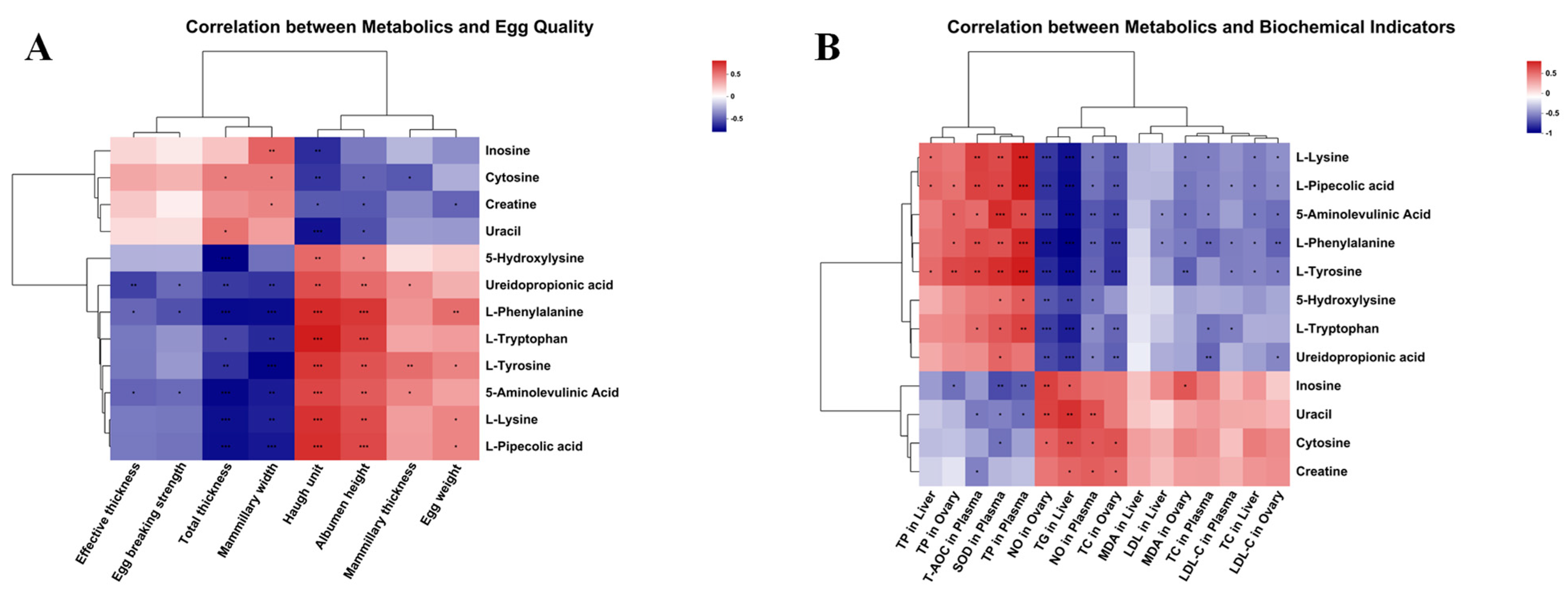
3.8. Correlation Analysis
4. Discussion
4.1. Phytosterol Esters Improve Egg Characteristics in Laying Hens by Promoting Amino Acid Anabolism
4.2. Phytosterol Esters Effectively Reduced Fatty Liver in Laying Hens
4.3. Phytosterol Esters Improve Antioxidant Capacity in Laying Hens
4.4. Analysis of Changes in Metabolic Pathway by Liver Untargeted Metabolomics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, Y.F.; Gao, X.N.; Xu, D.N.; Li, M.C.; Gao, Z.S.; Tang, Z.H.; Mhlambi, N.H.; Wang, W.J.; Fan, W.T.; Shi, X.Z. Protective Effect of the New Prepared Atractylodes Macrocephala Koidz Polysaccharide on Fatty Liver Hemorrhagic Syndrome in Laying Hens. Poult. Sci. 2021, 100, 938–948. [Google Scholar] [CrossRef]
- Shini, A.; Shini, S.; Bryden, W.L. Fatty Liver Haemorrhagic Syndrome Occurrence in Laying Hens: Impact of Production System. Avian Pathol. 2019, 48, 25–34. [Google Scholar] [CrossRef]
- Choi, Y.I.; Ahn, H.J.; Lee, B.K.; Oh, S.T.; An, B.K.; Kang, C.W. Nutritional and Hormonal Induction of Fatty Liver Syndrome and Effects of Dietary Lipotropic Factors in Egg-Type Male Chicks. Asian-Australas. J. Anim. Sci. 2012, 25, 1145–1152. [Google Scholar] [CrossRef]
- Yang, F.; Ruan, J.M.; Wang, T.C.; Luo, J.R.; Cao, H.B.; Song, Y.L.; Huang, J.Z.; Hu, G.L. Improving Effect of Dietary Soybean Phospholipids Supplement on Hepatic and Serum Indexes Relevant to Fatty Liver Hemorrhagic Syndrome in Laying Hens. Anim. Sci. J. 2017, 88, 1860–1869. [Google Scholar] [CrossRef]
- Duan, Q.B.; Yu, P.T. Causes and control measures of sudden death syndrome in broilers. Modern Rural Sci. Technol. 2019, 6, 41. [Google Scholar]
- Ding, J.X.; He, S.J.; Liu, D.Y.; Li, J. The effect of diet supplemented with selenium-enriched yeast on production performance of Hy-Line Brown layers in summer season. Heilongjiang J. Anim. Sci. Vet. Med. 2018, 14, 148–151. [Google Scholar]
- Wang, J.; Qian, X.; Gao, Q.; Lv, C.M.; Xu, J.; Jin, H.B.; Zhu, H. Quercetin Increases the Antioxidant Capacity of the Ovary in Menopausal Rats and in Ovarian Granulosa Cell Culture In Vitro. J. Ovarian Res. 2018, 11, 51. [Google Scholar] [CrossRef]
- Peters, A.E.; Mihalas, B.P.; Bromfield, E.G.; Roman, S.D.; Nixon, B.; Sutherland, J.M. Autophagy in Female Fertility: A Role in Oxidative Stress and Aging. Antioxid. Redox Sign. 2020, 32, 550–568. [Google Scholar] [CrossRef]
- Deng, T.; Huang, Y.; He, B.Q.; Li, J.X.; Cao, Y.P. Investigation progress of preparation methods of phytosterol esters. Cereals Oils 2014, 27, 13–17. [Google Scholar]
- Cheng, Q.W.; Meng, L.L.; Feng, L.; Xu, J.R.; Teng, Z.M. Study on the Preparation of Phytosterols Stearic. Food Res. Dev. 2016, 37, 128–131. [Google Scholar]
- Xu, Q.Q.; Jin, W.B.; Su, B.G.; Yang, Y.W.; Ren, Q.L. Progress in the Chemical Synthesis, Separation and Purification of Phytosterol Esters. J. Chin. Cereals Oils Assoc. 2014, 29, 120–128. [Google Scholar]
- Zhao, Y.; Tang, G.S.; Hou, Y.Y.; Niu, S.J.; Gao, Y.G.; Han, X.; Zhang, Y.Y.; Shen, Y.L.; Zhang, L.X. Research on synthesis technology of phytosterol esters. J. Food Saf. Qual. 2015, 6, 585–590. [Google Scholar]
- Qing, Y.Q. Effects of Dietary Supplement with Phy-Sterol Esters on Production Performance, Egg Quality and Serum Biochemical Indexes of Quail. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2023. [Google Scholar]
- Peng, J.; Chen, L.P.; Bei, Y.J.; Ding, X.Y.; Zhou, F. Physiological functions and application in animal production of phytosterols. Feed Res. 2021, 44, 152–154. [Google Scholar]
- Pollak, O.J. Reduction of Blood Cholesterol in Man. Circulation 1953, 7, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J. Effects of Maternal Dietary Supplementation of Phytosterol Esters in Mammalian during Gestation on Muscle Development of Its Offspring. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar]
- Fernandes, P.; Cabral, J.M.S. Phytosterols: Applications and Recovery Methods. Bioresour. Technol. 2007, 98, 2335–2350. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ferdosh, S.; Akanda, M.J.H.; Ghafoor, K.; Rukshana, A.H.; Ali, M.E.; Yunus, K.; Fauzi, M.B.; Hadijah, S.; Shaarani, S.M.; et al. Techniques for the Extraction of Phytosterols and Their Benefits in Human Health: A Review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar]
- Zhang, X.; Chen, S.M.; Wu, N.; Wang, T.; Pei, X.W.; Jiang, L.Z.; Han, C.P.; Yu, D.Y. Selective Modification of MCM-41 Immobilized Lipase and Its Application in Sterol Ester Synthesis. Food Sci. 2019, 40, 192. [Google Scholar]
- Guo, Y. Plant Sterol Ester of Alpha-Linolenic Improved Nonalcoholic Fatty Liver Disease by Inhibiting Endoplasmic Reticulum Stress. Master’s Thesis, Shanxi Medical University, Shanxi, China, 2021. [Google Scholar]
- Li, X.Y.; Zheng, M.M.; Guo, Y.; Wang, L.Q.; Xue, T.L.; Han, H. Protective of Plant Sterol Ester of α-Linolenic Acid from Non-Alcojolice Fatty Liver DiseaseI by Inhibiting Oxidative Stress. Acta Nutr. Sin. 2020, 42, 575–580. [Google Scholar]
- Wang, L.Q.; Zheng, M.M.; Xue, T.L.; Li, J.; Pei, L.Y.; Han, H. Plant Sterol Ester of α-Linolenic Acid Improves Liver Fibrosis by Regulating TGF-β1/Smad Signaling Psthway in Mice. Acta Nutr. Sin. 2021, 43, 236–241. [Google Scholar]
- Zhang, X.F.; Xue, Y.T.; Zhang, D.J. Advances in Research on Phytosterols Protecting Gastric Mucosa and Anti-Gastrointestinal Tumors. Genom. Appl. Biol. 2020, 39, 2444–2450. [Google Scholar]
- Xue, Y.T.; Zhang, X.F.; Zhang, D.J. Research progress of the blood lipid lowering effect of phytosterol. West China J. Pharm. Sci. 2019, 34, 92–97. [Google Scholar]
- Wang, X.K.; Chen, W.; Zhang, T.J.; Yi, Z.G.; Gu, Y.L.; Li, T. Research and application prospect of phytosterols(esters). China Surfactant Deterg. Cosmet. 2023, 53, 445–452. [Google Scholar]
- Zhang, N.; Yang, K.L.; Zhang, S.; Hao, J.Y.; Deng, S.T.; Yang, W.G.; Liu, S.P.; Sun, J.L.; Fang, R.J. Effects of Phytosterol Ester on Laying Performance, Egg Quality, Liver Antioxidant Capacity and Yolk Precursor Synthesis of Laying Hens during Late Laying Period. Chin. J. Anim. Nutr. 2023, 35, 7123–7137. [Google Scholar]
- Ding, X.Q.; Yuan, C.C.; Huang, Y.B.; Jiang, L.; Qian, L.C. Effects of Phytosterol Supplementation on Growth Performance, Serum Lipid, Proinflammatory Cytokines, Intestinal Morphology, and Meat Quality of White Feather Broilers. Poult. Sci. 2021, 100, 101096. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.Y.; Sun, X.J.; Hu, Z.Y.; Xiao, R. Comparison of the anaesthetic effects of three anaesthetic methods on Leghorn chickens. Lab. Anim. Comp. Med. 2016, 36, 137–140. [Google Scholar]
- Fathi, M.M.; El-Dlebshany, A.E.; El-Deen, M.B.; Radwan, L.M.; Rayan, G.N. Effect of Long-Term Selection for Egg Production on Eggshell Quality of Japanese Quail (Coturnix japonica). Poult. Sci. 2016, 95, 2570–2575. [Google Scholar] [CrossRef] [PubMed]
- Bain, M.M. Eggshell Strength: A Relationship between the Mechanism of Failure and the Ultrastructural Organization of the Mammillary Layer. Br. Poult. Sci. 1992, 33, 303–319. [Google Scholar] [CrossRef]
- Solomon, S.E. Structural Variations in the Mammillary Layer. In Egg & Eggshell Quality; Iowa State University Press: Ames, IO, USA, 1997; pp. 50–66. [Google Scholar]
- Li, C.; Al-Dalali, S.; Zhou, H.; Xu, B. Influence of Curing on the Metabolite Profile of Water-Boiled Salted Duck. Food Chem. 2022, 397, 133752. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Hu, J.; Yang, G.; Wang, Z.; Sun, J. Dissection of the Response Mechanism of Alfalfa under Phosphite Stress Based on Metabolomic and Transcriptomic Data. Plant Physiol. Biochem. 2022, 192, 35–49. [Google Scholar] [CrossRef]
- Kong, X.; Guo, Z.; Yao, Y.; Xia, L.; Liu, R.; Song, H.; Zhang, S. Acetic Acid Alters Rhizosphere Microbes and Metabolic Composition to Improve Willows Drought Resistance. Sci. Total Environ. 2022, 844, 157132. [Google Scholar] [CrossRef]
- Yuan, C.C.; Fan, J.H.; Jiang, L.; Ye, W.X.; Chen, Z.; Wu, W.Z.; Huang, Q.X.; Qian, L.C. Integrated Analysis of Gut Microbiome and Liver Metabolome to Evaluate the Effects of Fecal Microbiota Transplantation on Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Injury in Mice. Nutrients 2023, 15, 1149. [Google Scholar] [CrossRef]
- Hu, Q.L.; Huang, D.; Ping, F.J. Physiological functions of phytosterols and their research and application in animal production. Feed Res. 2014, 21, 42–45. [Google Scholar]
- Zhou, B.L. Applications of phytosterols. China Oils Fats 1992, 4, 33–38. [Google Scholar]
- Qian, L.C.; Jiang, L.; Ye, W.X.; Yu, D.Y.; Wang, Z.G.; Han, X.Y.; Zhao, P.J. A Method of Preparing Phytosterol Esters for Feeding, Phytosterol Esters and Their Applications. CN202211230864.3, 10 October 2022. [Google Scholar]
- Yuan, C. Research on the Mechanism of L-Arginine on the Regulation Offeed Intake and Tissue Protein Metabolism in Laying Hens. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2016. [Google Scholar]
- Zhu, Y.X. Key technology of forest ecological farming for Hyland Brown laying hens. Livest. Vet. Sci. Technol. Inform. 2020, 10, 188. [Google Scholar]
- Zhou, C.J. Biosafety Evaluation of Phytosterol as Feed Additivein Laying Hens. Master’s Thesis, Yangzhou University, Yangzhou, China, 2014. [Google Scholar]
- Zhou, C.J.; Shi, S.R.; Tong, H.B.; Zou, J.M.; Wang, Z.Y. Phytosterol: Effects on Production Performance, Blood Routine Parameters and Serum Biochemical Parameters of Laying Hens. Chin. J. Anim. Nutr. 2013, 25, 1099–1104. [Google Scholar]
- Wang, L.C.; Gu, W.T.; Zhou, Y.M.; Wan, T. Effects of Phytosterols on Performance, Cholesterol Content in Egg Yolk and Reproductive Hormones in Serum of Laying Hens in Late Period of Laying. J. Chin. Cereals Oils Assoc. 2008, 23, 166–171. [Google Scholar]
- Gu, W.T. Application of Phytosterols and Its Mechanisms in Regulation of Cholesterol Metabolism in Meat-Strain Ducks. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2007. [Google Scholar]
- Chang, L.L.; Shen, Y.R.; Zhou, C.J.; Wang, Z.Y.; Tong, H.B.; Zou, J.M.; Shi, S.R. Effects of High-Dose Phytosterol on Performance, Egg Quality and Lipid Metabolism of Laying Hens. China J. Anim. Nutr. 2014, 26, 1652–1659. [Google Scholar]
- Yuan, C.; Song, H.H.; Zhang, X.Y.; Jiang, Y.J.; Zhang, A.T.; Azzam, M.M.; Zou, X.T. Effect of Expanded Cottonseed Meal on Laying Performance, Egg Quality, Concentrations of Free Gossypol in Tissue, Serum and Egg of Laying Hens. Anim. Sci. J. 2014, 85, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N. Dietary Manganese Supplementation Modulated Eggshell Quality in Laying Hens. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2018. [Google Scholar]
- Zhang, Y.N.; Zhang, H.J.; Wu, S.G.; Wang, J.; Qi, G.H. Dietary Manganese Supplementation Affects Mammillary Knobs of Eggshell Ultrastructure in Laying Hens. Poult. Sci. 2018, 97, 1253–1262. [Google Scholar] [CrossRef]
- Ketta, M.; Tůmová, E. Eggshell Structure, Measurements, and Quality-Affecting Factors in Laying Hens: A Review. Czech J. Anim. Sci. 2016, 61, 299–309. [Google Scholar] [CrossRef]
- Athanasiadou, D.; Jiang, W.; Goldbaum, D.; Saleem, A.; Basu, K.; Pacella, M.S.; Böhm, C.F.; Chromik, R.R.; Hincke, M.T.; Rodríguez-Navarro, A.B. Nanostructure, Osteopontin, and Mechanical Properties of Calcitic Avian Eggshell. Sci. Adv. 2018, 4, eaar3219. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.L. Regulation Mechanism of Glucosamine on Eggshell Quality in Laying Hens. Master’s Thesis, Guizhou University, Guiyang, China, 2019. [Google Scholar]
- Xiao, J.F. Effect of Dietary Manganese Sources and Supplemental Levels on Eggshell Quality of Laying Hens. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2014. [Google Scholar]
- Ma, Y.X.; Wu, Y.X.; Shao, R.; Miao, L. Stigmasterol inhibits the contraction of rat prostatic stromal cells by inhibiting calcium influx. Tianjin J. Tradit. Chin. Med. 2022, 39, 1445–1451. [Google Scholar]
- Zhao, Z.B. Cholesterol Attenuated HCC Progression through Modulating IP3Rs Mediated Calcium Release. Master’s Thesis, Chongqing Medical University, Chongqing, China, 2019. [Google Scholar]
- Wang, G. Cholesterol Mediates by the Inhibitory Effect of Shear Force on Calcium-Activated Chloride Channel TMEM16A Current. Master’s Thesis, China Medical University, Shenyang, China, 2022. [Google Scholar]
- Zhou, H.Y. The Mechanisms of Phytosterol Ester on Non-Alcoholic Fatty Liver Disease Metabolism. Master’s Thesis, Shanghai Jiao Tong University, Shanghai, China, 2017. [Google Scholar]
- Chen, Z.F.; Gao, F.; Xu, H.B. Advances in the mechanism of phytosterols affecting cholesterol metabolism. J. Environ. Hyg. 2008, 35, 360–363. [Google Scholar]
- Liu, X.; Pan, Y.; Shen, Y.; Liu, H.; Zhao, X.; Li, J.; Ma, N. Protective Effects of Abrus Cantoniensis Hance on the Fatty Liver Hemorrhagic Syndrome in Laying Hens Based on Liver Metabolomics and Gut Microbiota. Front. Vet. Sci. 2022, 9, 862006. [Google Scholar] [CrossRef] [PubMed]
- De Smet, E.; Mensink, R.P.; Plat, J. Effects of Plant Sterols and Stanols on Intestinal Cholesterol Metabolism: Suggested Mechanisms from Past to Present: Molecular Nutrition & Food Research. Mol. Nutr. Food Res. 2012, 56, 1058–1072. [Google Scholar] [PubMed]
- Plat, J.; Baumgartner, S.; Mensink, R.P. Mechanisms Underlying the Health Benefits of Plant Sterol and Stanol Ester Consumption. J. AOAC Int. 2015, 98, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Wu, P.; Yang, W.; Zhou, Y. Effect of Different Phytosterols on Lipid Metabolism of Laying Hens. J. Chin. Cereals Oils Assoc. 2012, 27, 85–89. [Google Scholar]
- Jia, D.H.; Zhou, Y.M.; Wang, T. Effects of Phytosterol on Cholesterol and Protein Level and Antioxidation Enzyme Activity in Serum of Broilers. J. Chin. Cereals Oils Assoc. 2007, 22, 88–93. [Google Scholar]
- Sun, L. A Primary Research of The Application of Phytostreols in Livestock Production. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2014. [Google Scholar]
- Liu, W.W. Study on Blood-Fat-Lowering Effects of Phytosterol Esters in Hyperlipidemia Rats. Master’s Thesis, Zhejiang University, Hangzhou, China, 2007. [Google Scholar]
- Wang, Z.Y. Effects of 25-(OH)D3, Vitamin C, 4,7-dihydroxyisoflavoneon Chicken eggshell Quality and Calcium Metabolism. Master’s Thesis, Huzhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Newmeyer, D.D.; Ferguson-Miller, S. Mitochondria: Releasing Power for Life and Unleashing the Machineries of Death. Cell 2003, 112, 481–490. [Google Scholar] [CrossRef]
- Panda, A.K.; Rao, S.S.R.; Raju, M.V.; Sharma, S.S. Effect of Probiotic (Lactobacillus sporogenes) Feeding on Egg Productionand Quality, Yolk Cholesterol and Humoralimmune Response of White Leghorn Layerbreeders. J. Sci. Food Agric. 2008, 88, 43–47. [Google Scholar] [CrossRef]
- Surai, P.F.; Sparks, N.H.C. Tissue-Specific Fatty Acid and α-Tocopherol Profiles in Male Chickens Depending on Dietary Tuna Oil and Vitamin E Provision. Poult. Sci. 2000, 79, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Reynard, M.; Savory, C.J. Stress-Induced Oviposition Delays in Laying Hens: Duration and Consequences for Eggshell Quality. Brit. Poult. Sci. 1999, 40, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Sun, F.G.; Huang, Z.Y.; Pan, Z.C.; Li, Z.H.; Zou, S.L. Effects of phytosterol on growth performance, physical indicators, muscle composition and hepatic biochemical index of Micropterus salmoides. J. Aquacult. 2020, 41, 26–30. [Google Scholar]
- Yuan, C.; Ding, X.; Jiang, L.; Ye, W.; Xu, J.; Qian, L. Effects of Dietary Phytosterols Supplementation on Serum Parameters, Nutrient Digestibility and Digestive Enzyme of White Feather Broilers. Ital. J. Anim. Sci. 2021, 20, 2102–2109. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Li, Z.H.; Gong, H.; Sun, F.G.; Zou, S.L. Effects of Phytosterol on Growth Performance and Serum Biochemical indexes of Qingyuan Partridge Chicken. Guangdong Feed 2019, 28, 26–29. [Google Scholar]
- Li, Z.H.; Huang, Z.Y.; Pan, Z.C.; Sun, F.G.; Zhao, H.H. Effects of Phytosterol on Growth Performance, Serum Lipid Metabolism Indicators and Hepatopancreas Antioxidant Indicators of Tilapia (Oreochromis niloticus). Chin. J. Anim. Nutr. 2019, 31, 5866–5872. [Google Scholar]
- Luceri, C.; Bigagli, E.; Femia, A.P.; Caderni, G.; Giovannelli, L.; Lodovici, M. Aging Related Changes in Circulating Reactive Oxygen Species (ROS) and Protein Carbonyls Are Indicative of Liver Oxidative Injury. Toxicol. Rep. 2018, 5, 141–145. [Google Scholar] [CrossRef]
- Bullwinkle, T.J.; Ibba, M. Emergence and Evolution. In Aminoacyl-tRNA Synthetases in Biology and Medicine; Kim, S., Ed.; Topics in Current Chemistry; Springer: Dordrecht, The Netherlands, 2014; pp. 43–87. [Google Scholar]
- Gomez, M.A.R.; Ibba, M. Aminoacyl-tRNA Synthetases. RNA 2020, 26, 910–936. [Google Scholar] [CrossRef]
- Ibba, M.; Söll, D. Aminoacyl-tRNAs: Setting the Limits of the Genetic Code. Genes Dev. 2004, 18, 731–738. [Google Scholar] [CrossRef]
- Takénaka, A.; Moras, D. Correlation between Equi-Partition of Aminoacyl-tRNA Synthetases and Amino-Acid Biosynthesis Pathways. Nucleic Acids Res. 2020, 48, 3277–3285. [Google Scholar] [CrossRef]



| Ingredients (%) | Content | Nutrient Composition | Content |
|---|---|---|---|
| Corn | 61 | ME 2 (kcal·kg−1) | 2629.9 |
| Soybean meal | 25.5 | Crude protein (%) | 16.38 |
| Soybean oil | 1.5 | Calcium (%) | 3.14 |
| Limestone | 8 | Available phosphorus (%) | 0.33 |
| Premix 1 | 4 | Lysine (%) | 1.18 |
| Total | 100 | Methionine (%) | 0.45 |
| Threonine (%) | 0.63 | ||
| Cystine (%) | 0.27 |
| Items | Phytosterol Esters/(mg/kg) | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | |||
| Feed intake (g/d/hen) | 119.37 | 115.39 | 119.39 | 121.20 | 0.79 | 0.052 |
| Laying rate (%) | 88.47 | 89.44 | 89.25 | 90.51 | 0.01 | 0.6 |
| Feed-to-egg ratio (feed/kg egg) | 2.16 | 2.17 | 2.19 | 2.16 | 0.01 | 0.915 |
| Average egg weight (g) | 60.49 b | 60.26 b | 62.80 a | 62.98 a | 0.34 | <0.001 |
| Items | Phytosterol Esters/(mg/kg) | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | |||
| Egg breaking strength (kgf) | 5.33 a | 5.06 ab | 4.61 c | 4.78 bc | 0.09 | 0.011 |
| Albumen height (mm) | 6.32 c | 8.65 b | 9.59 a | 8.51 b | 0.29 | <0.001 |
| Haugh unit (B) | 76.86 b | 93.36 a | 94.79 a | 89.47 a | 1.78 | <0.001 |
| Yolk color depth | 6.87 | 6.93 | 7.03 | 6.97 | 0.05 | 0.74 |
| Eggshell thickness (mm) | 0.42 | 0.42 | 0.43 | 0.43 | 0.00 | 0.169 |
| Items (μm) | Phytosterol Esters/(mg/kg) | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | |||
| Total thickness | 3723.24 a | 3661.89 ab | 3631.99 b | 3638.07 b | 12.28 | 0.026 |
| Effective thickness | 2801.20 a | 2784.18 ab | 2684.57 bc | 2616.92 c | 21.74 | 0.004 |
| Mammillary thickness | 742.62 b | 822.04 b | 929.54 a | 931.85 a | 20.68 | 0.001 |
| Mammillary width | 1056.03 a | 804.65 b | 606.11 c | 653.75 c | 41.42 | <0.001 |
| Items (μg/g) | Phytosterol Esters/(mg/kg) | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | |||
| Valine | 20.84 | 22.43 | 22.02 | 20.72 | 0.40 | 0.382 |
| Glycine | 5.54 | 4.79 | 4.80 | 5.49 | 0.19 | 0.336 |
| Alanine | 22.89 | 22.99 | 23.05 | 24.23 | 0.42 | 0.695 |
| Serine | 28.64 | 26.37 | 28.84 | 29.47 | 0.84 | 0.652 |
| Proline | 22.79 | 21.64 | 21.25 | 23.50 | 0.58 | 0.551 |
| Threonine | 12.44 | 12.33 | 12.70 | 12.80 | 0.09 | 0.236 |
| Isoleucine | 19.69 b | 20.47 ab | 21.15 ab | 22.33 a | 0.38 | 0.01 |
| Leucine | 25.65 b | 25.85 ab | 26.80 ab | 26.93 a | 0.22 | 0.029 |
| Asparagine | 12.30 | 12.29 | 12.37 | 12.56 | 0.06 | 0.336 |
| Aspartic | 23.43 | 23.59 | 23.87 | 24.25 | 0.19 | 0.472 |
| Homocysteine | 11.47 | 11.43 | 12.98 | 12.91 | 0.42 | 0.412 |
| Glutamine | 24.59 | 23.01 | 25.51 | 25.19 | 0.51 | 0.337 |
| Lysine | 1.25 | 1.44 | 0.61 | 0.95 | 0.17 | 0.370 |
| Glutamic | 53.95 | 53.79 | 54.69 | 55.92 | 0.49 | 0.455 |
| Methionine | 12.79 | 13.32 | 14.60 | 14.39 | 0.34 | 0.186 |
| Histidine | 5.37 | 5.81 | 6.2 | 4.28 | 0.44 | 0.466 |
| Phenylalanine | 12.31 b | 13.41 ab | 13.70 ab | 14.90 a | 0.43 | 0.047 |
| Arginine | 18.95 | 19.17 | 17.75 | 16.95 | 0.42 | 0.214 |
| Tyrosine | 7.62 | 8.97 | 10.18 | 8.67 | 0.74 | 0.738 |
| Tryptophan | 22.66 | 22.39 | 23.36 | 23.61 | 0.24 | 0.233 |
| Items (ng/g) | Phytosterol Esters/(mg/kg) | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | |||
| L-Phenylalanine | 852.00 b | 1236.67 a | 1208.57 a | 1193.57 a | 32.07 | <0.001 |
| Leucyl-Lysine | 77.48 b | 196.00 a | 168.27 a | 152.86 ab | 11.06 | 0.008 |
| Glutamyl-Leucyl-Arginine | 19.59 b | 48.33 a | 47.83 a | 41.29 ab | 3.48 | 0.046 |
| Aspartyl-Isoleucine | 63.12 b | 112.82 a | 102.66 ab | 101.90 ab | 5.62 | 0.044 |
| L-Tryptophan | 548.20 b | 781.33 a | 726.57 a | 687.93 ab | 22.75 | 0.014 |
| L-Tyrosine | 599.40 b | 736.00 a | 706.86 ab | 741.36 a | 17.38 | 0.033 |
| Items 1 | Phytosterol Esters/(mg/kg) | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | |||
| TP (g/gprot) | 13.84 c | 15.55 bc | 18.89 a | 16.86 ab | 0.54 | 0.002 |
| ALB (g/gprot) | 4.46 | 3.21 | 2.95 | 2.54 | 0.30 | 0.116 |
| AKP (IU/gprot) | 206.65 a | 190.63 b | 180.95 c | 175.05 c | 2.83 | <0.001 |
| LDL-C (mmol/gprot) | 0.08 a | 0.07 a | 0.04 b | 0.03 c | 0.00 | <0.001 |
| HDL-C (mmol/gprot) | 3.26 | 4.02 | 4.26 | 6.25 | 0.41 | 0.052 |
| TC (mmol/gprot) | 17.80 a | 14.26 a | 6.28 b | 3.73 b | 1.43 | <0.001 |
| TG (mmol/gprot) | 11.45 a | 3.33 b | 2.38 b | 2.89 b | 0.81 | <0.001 |
| Items 1 | Phytosterol Esters/(mg/kg) | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | |||
| Liver | ||||||
| CAT (U/mgprot) | 67.46 | 70.30 | 71.06 | 69.61 | 0.69 | 0.303 |
| SOD (U/mgprot) | 153.20 | 154.54 | 146.54 | 151.98 | 2.65 | 0.752 |
| T-AOC (U/mgprot) | 4.35 | 4.80 | 4.99 | 5.13 | 0.12 | 0.081 |
| MDA (nmol/mgprot) | 0.53 b | 0.47 b | 0.34 ab | 0.37 a | 0.03 | 0.044 |
| NO (umol/gprot) | 1.58 | 1.05 | 0.87 | 0.82 | 0.13 | 0.141 |
| Ovary | ||||||
| T-AOC (U/mgprot) | 3.73 | 4.04 | 2.94 | 2.94 | 0.25 | 0.300 |
| MDA (nmol/mgprot) | 2.61 d | 2.16 c | 1.33 b | 0.94 a | 0.15 | <0.001 |
| NO (umol/gprot) | 8.35 a | 4.54 bc | 5.07 b | 3.95 c | 0.39 | <0.001 |
| Plasma | ||||||
| SOD (U/mL) | 22.95 b | 23.17 a | 23.20 a | 23.32 a | 0.04 | 0.009 |
| T-AOC (U/mL) | 3.67 b | 4.50 a | 4.08 ab | 4.48 a | 0.10 | 0.001 |
| MDA (nmol/mL) | 1.28 | 1.12 | 1.17 | 0.82 | 0.07 | 0.090 |
| NO (umol/L) | 9.53 a | 7.49 b | 7.38 b | 7.15 b | 0.29 | 0.004 |
| Num | First Category | Second Category | Pathway Description | Ratio 1 | p-Value |
|---|---|---|---|---|---|
| 1 | Genetic Information Processing | Translation | Aminoacyl-tRNA biosynthesis | 4/23 | <0.001 |
| 2 | Metabolism | Amino acid metabolism | Phenylalanine, tyrosine, and tryptophan biosynthesis | 3/23 | <0.001 |
| 3 | Metabolism | Amino acid metabolism | Glycine, serine, and threonine metabolism | 3/23 | 0.002 |
| 4 | Metabolism | Amino acid metabolism | Lysine degradation | 3/23 | 0.002 |
| 5 | Metabolism | Global and overview maps | Nucleotide metabolism | 3/23 | 0.004 |
| 6 | Metabolism | Nucleotide metabolism | Pyrimidine metabolism | 3/23 | 0.005 |
| 7 | Environmental Information Processing | Membrane transport | ABC transporters | 4/23 | 0.006 |
| 8 | Metabolism | Metabolism of cofactors and vitamins | Pantothenate and CoA biosynthesis | 2/23 | 0.011 |
| 9 | Metabolism | Metabolism of other amino acids | beta-Alanine metabolism | 2/23 | 0.012 |
| 10 | Metabolism | Amino acid metabolism | Phenylalanine metabolism | 2/23 | 0.029 |
| 11 | Organismal Systems | Endocrine system | Melanogenesis | 1/23 | 0.032 |
| 12 | Metabolism | Metabolism of other amino acids | D-Amino acid metabolism | 2/23 | 0.049 |
| Items | Liver | Plasma | Ovary | |||
|---|---|---|---|---|---|---|
| MDA | SOD | T-AOC | NO | MDA | NO | |
| Egg weight | −0.302 | 0.368 | 0.296 | −0.465 * | −0.525 ** | −0.560 ** |
| Egg breaking strength | 0.381 | −0.188 | −0.403 | 0.459 * | 0.546 ** | 0.444 * |
| Albumen height | −0.423 * | 0.388 | 0.297 | −0.632 ** | −0.584 ** | −0.648 ** |
| Haugh unit | −0.265 | 0.347 | 0.404 | −0.634 ** | −0.529 ** | −0.677 ** |
| Items | Liver | |||
|---|---|---|---|---|
| TP | TC | TG | LDL | |
| Egg weight | 0.468 * | −0.380 | −0.708 ** | −0.525 ** |
| Egg breaking strength | −0.475 * | 0.525 ** | 0.542 ** | 0.585 ** |
| Albumen height | 0.641 ** | −0.478 * | −0.784 ** | −0.538 ** |
| Haugh unit | 0.619 ** | −0.331 | −0.793 ** | −0.362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Ma, X.; Chen, R.; Fan, J.; Ye, W.; Chen, Z.; Huang, Q.; Qian, L. Effects of Phytosterol Ester Supplementation on Egg Characteristics, Eggshell Ultrastructure, Antioxidant Capacity, Liver Function and Hepatic Metabolites of Laying Hens during Peak Laying Period. Antioxidants 2024, 13, 458. https://doi.org/10.3390/antiox13040458
Wu W, Ma X, Chen R, Fan J, Ye W, Chen Z, Huang Q, Qian L. Effects of Phytosterol Ester Supplementation on Egg Characteristics, Eggshell Ultrastructure, Antioxidant Capacity, Liver Function and Hepatic Metabolites of Laying Hens during Peak Laying Period. Antioxidants. 2024; 13(4):458. https://doi.org/10.3390/antiox13040458
Chicago/Turabian StyleWu, Wenzi, Xin Ma, Rui Chen, Jinghui Fan, Wenxin Ye, Zhuo Chen, Qixin Huang, and Lichun Qian. 2024. "Effects of Phytosterol Ester Supplementation on Egg Characteristics, Eggshell Ultrastructure, Antioxidant Capacity, Liver Function and Hepatic Metabolites of Laying Hens during Peak Laying Period" Antioxidants 13, no. 4: 458. https://doi.org/10.3390/antiox13040458





