Porcine Brain Enzyme Hydrolysate Enhances Immune Function and Antioxidant Defense via Modulation of Gut Microbiota in a Cyclophosphamide-Induced Immunodeficiency Model
Abstract
:1. Introduction
2. Methods
2.1. Production of PBEH
2.2. Animal Care and Immunosuppressive Animal Model Induced using CTX
2.3. Isolation of Splenocytes and Their Proliferation Induced using Lipopolysaccharides (LPS)
2.4. Natural Killer Cell Activity
2.5. Histology of the Spleen and Large Intestines
2.6. Serum Short-Chain Fatty Acid (SCFA) Concentrations and Gut Microbiome
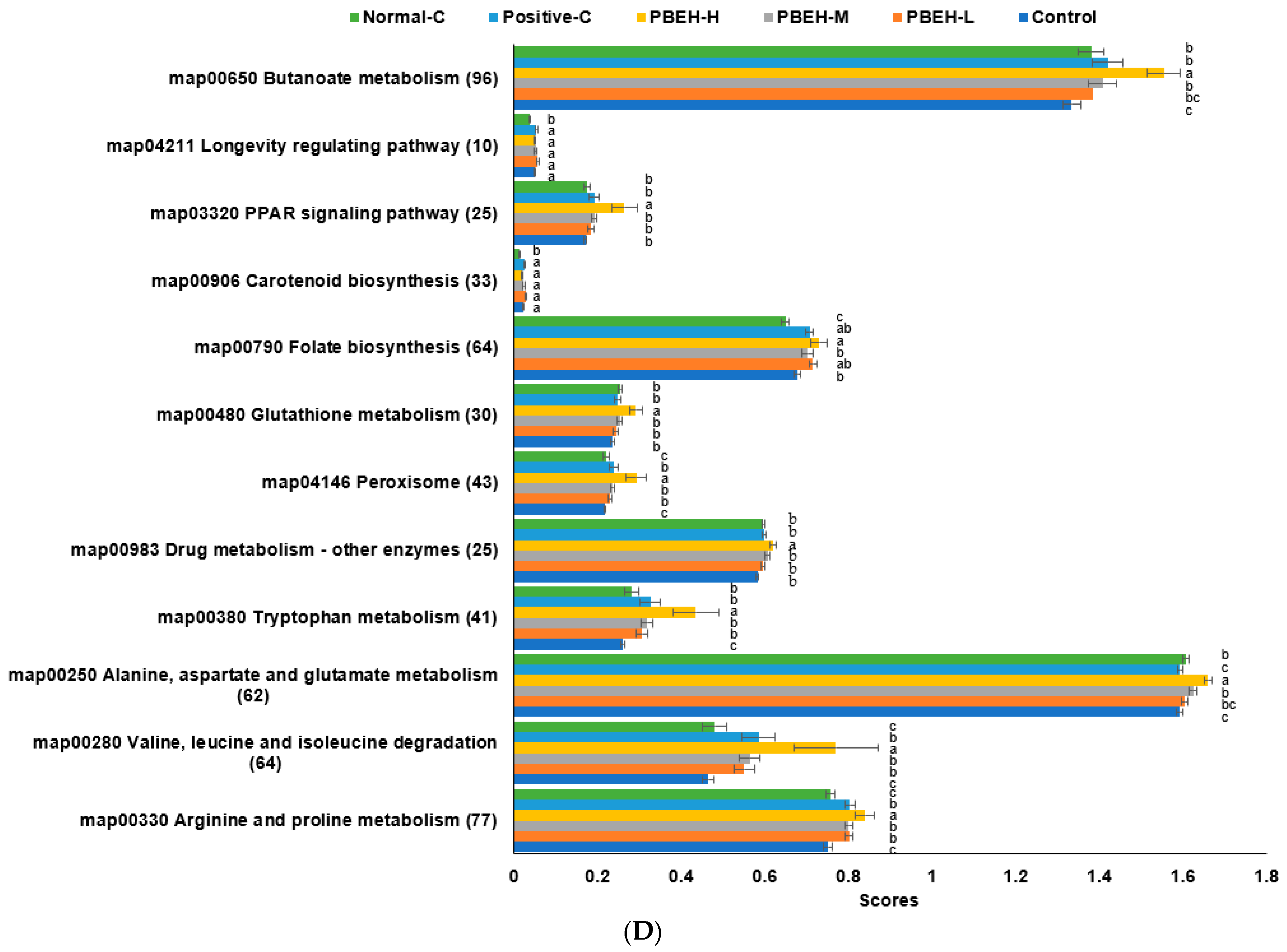
2.7. Metagenome Functions Related to Metabolism using PICRUSt2 Pipeline Analysis
2.8. Statistical Analysis
3. Results
3.1. Body Weight and Immunity-Related Organ Weight Changes
3.2. Liver Damage Index
3.3. WBC Count and Its Components
3.4. Splenocyte Proliferation and NK Cell Activity
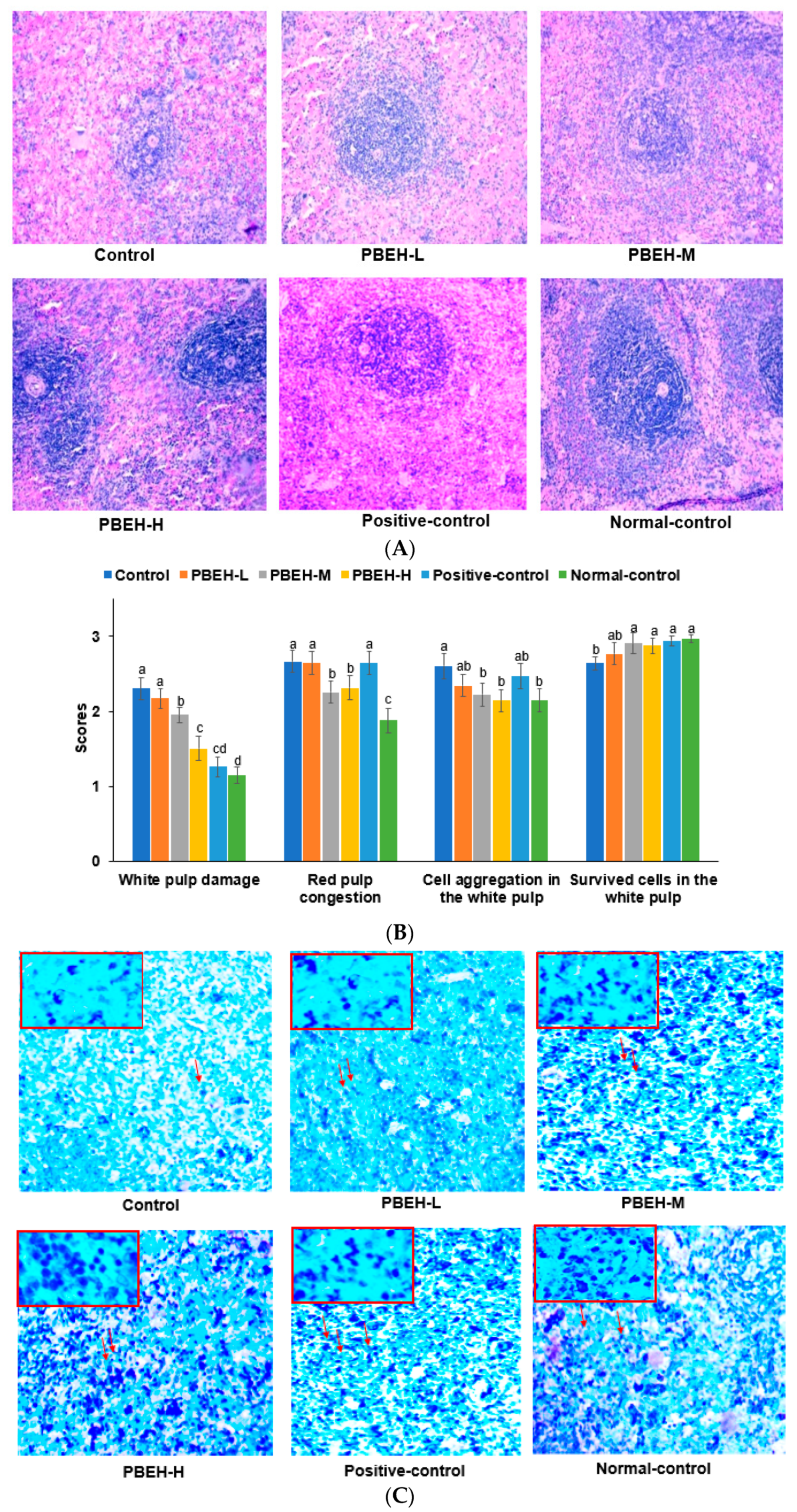
3.5. Spleen Morphology
3.6. Serum immunoglobulin and Cytokine Cincentrationss
3.7. Cytokine Contents in the Splenocytes
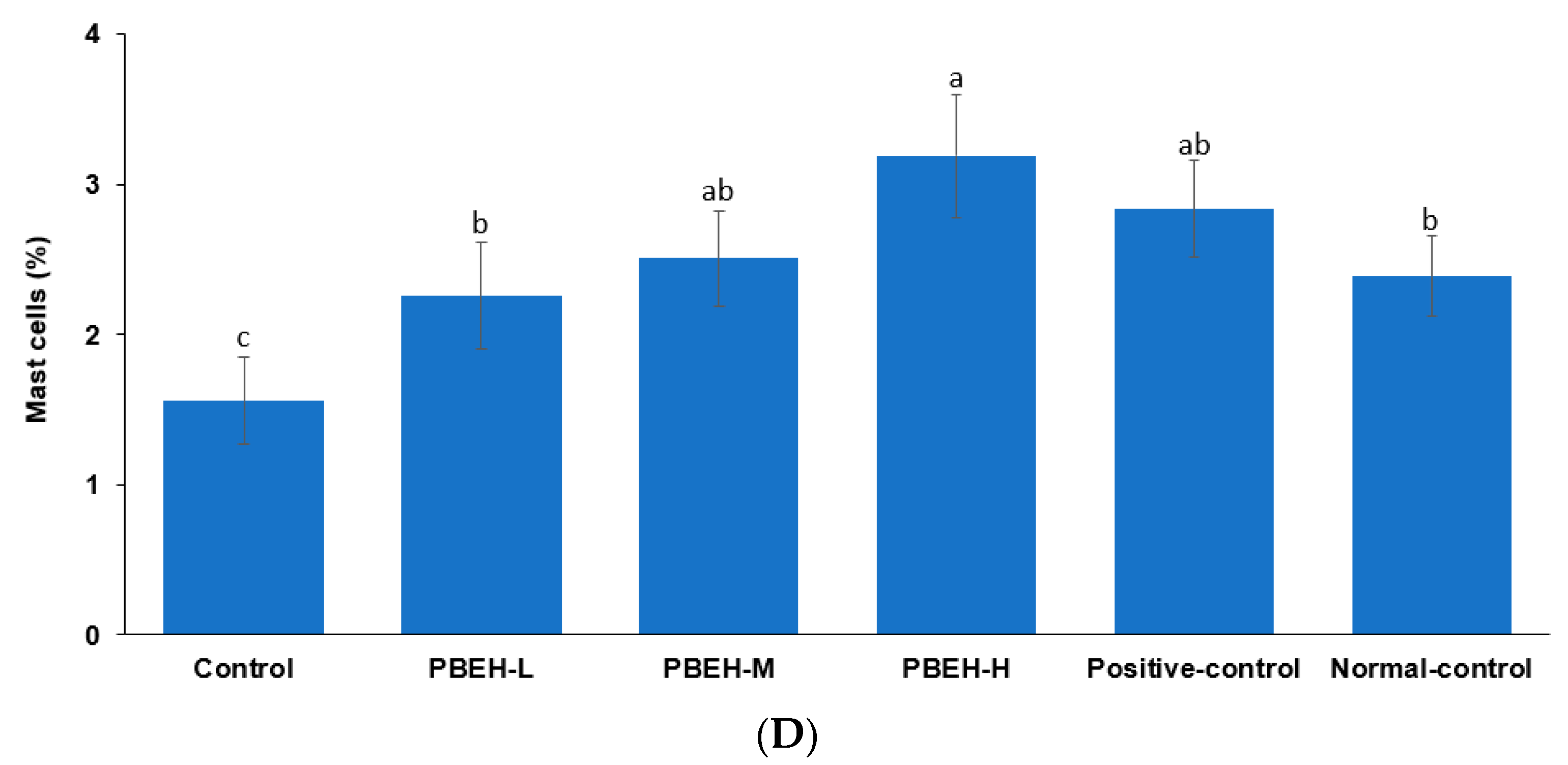
3.8. Large Intestine Morphology
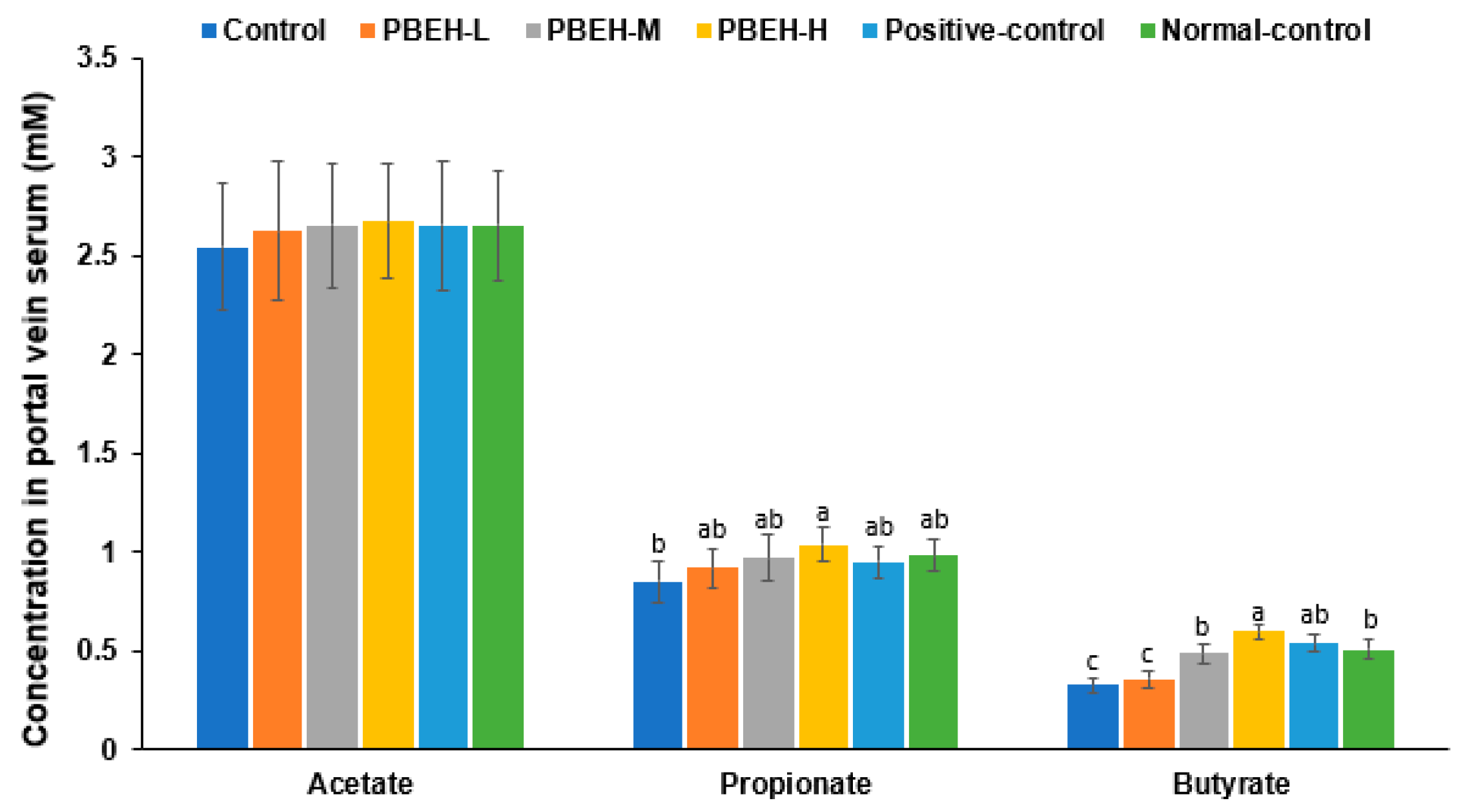
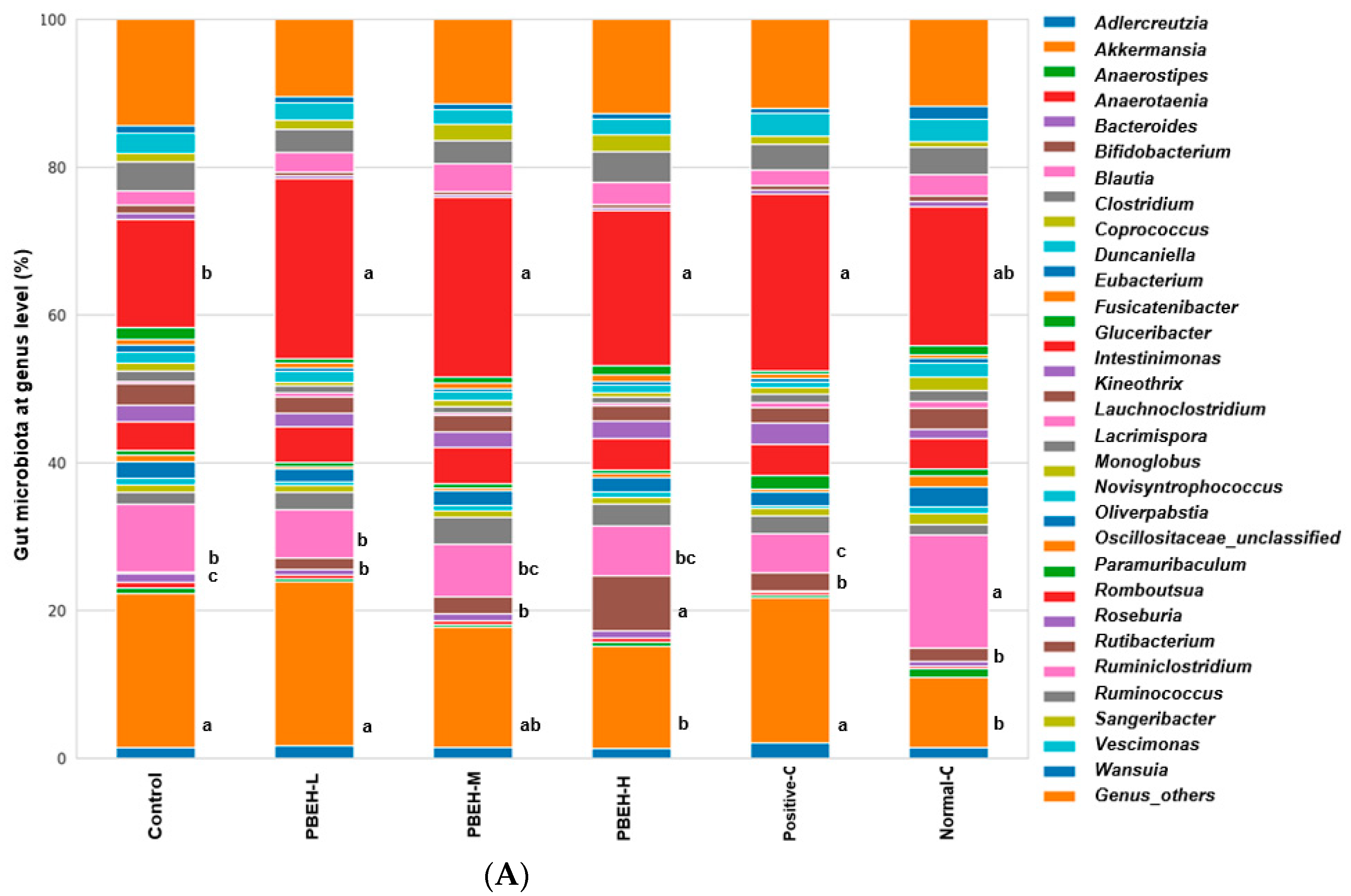
3.9. SCFAs in the Portal Vein and Gut Microbiota in the cecum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ryan, T.; Coakley, J.D.; Martin-Loeches, I. Defects in innate and adaptive immunity in patients with sepsis and health care associated infection. Ann. Transl. Med. 2017, 5, 447. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Schwartz, B. The relationship between nutrition and the immune system. Front. Nutr. 2022, 9, 1082500. [Google Scholar] [CrossRef] [PubMed]
- Bellocchi, C.; Carandina, A.; Montinaro, B.; Targetti, E.; Furlan, L.; Rodrigues, G.D.; Tobaldini, E.; Montano, N. The Interplay between Autonomic Nervous System and Inflammation across Systemic Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 2449. [Google Scholar] [CrossRef]
- Meisel, C.; Schwab, J.M.; Prass, K.; Meisel, A.; Dirnagl, U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 2005, 6, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kim, M.J.; Kim, M.J.; Wu, X.; Yang, H.J.; Yuan, H.; Huang, S.; Yoon, S.M.; Kim, K.N.; Park, S. Long-Term Effect of Porcine Brain Enzyme Hydrolysate Intake on Scopolamine-Induced Memory Impairment in Rats. Int. J. Mol. Sci. 2022, 23, 3361. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Liu, J.; Wang, Y.; Zhou, Q.; Wang, S.; Wang, X. Ginsenoside Rg3 improves cyclophosphamide-induced immunocompetence in Balb/c mice. Int. Immunopharmacol. 2019, 72, 98–111. [Google Scholar] [CrossRef]
- Riaz, M.; Rahman, N.U.; Zia-Ul-Haq, M.; Jaffar, H.Z.E.; Manea, R. Ginseng: A dietary supplement as immune-modulator in various diseases. Trends Food Sci. Technol. 2019, 83, 12–30. [Google Scholar] [CrossRef]
- Yang, H.L.; Yang, T.Y.; Gowrisankar, Y.V.; Liao, C.H.; Liao, J.W.; Huang, P.J.; Hseu, Y.C. Suppression of LPS-Induced Inflammation by Chalcone Flavokawain A through Activation of Nrf2/ARE-Mediated Antioxidant Genes and Inhibition of ROS/NFκB Signaling Pathways in Primary Splenocytes. Oxid. Med. Cell Longev. 2020, 2020, 3476212. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.K.S.; Lee, J.H.; Lee, M.J.; Park, J.Y.; Kang, K.S. Multitargeted Herbal Prescription So Shiho Tang: A Scoping Review on Biomarkers for the Evaluation of Therapeutic Effects. Pharmaceuticals 2023, 16, 1371. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, J.; Bu, W.; Zhang, W.; Duan, B.; Wang, X.; Yao, L.; Li, Z.; Li, J. A New Role for the Spleen: Aggravation of the Systemic Inflammatory Response in Rats with Severe Acute Pancreatitis. Am. J. Pathol. 2019, 189, 2233–2245. [Google Scholar] [CrossRef] [PubMed]
- Bagalagel, A.; Diri, R.; Noor, A.; Almasri, D.; Bakhsh, H.; Kutbi, H.; Al-Gayyar, M. Curative effects of fucoidan on acetic acid induced ulcerative colitis in rats via modulating aryl hydrocarbon receptor and phosphodiesterase-4. BMC Complement. Med. Ther. 2022, 22, 196. [Google Scholar] [CrossRef]
- Berlin, P.; Reiner, J.; Wobar, J.; Bannert, K.; Glass, Ä.; Walter, M.; Bastian, M.; Willenberg, H.S.; Vollmar, B.; Klar, E.; et al. Villus Growth, Increased Intestinal Epithelial Sodium Selectivity, and Hyperaldosteronism Are Mechanisms of Adaptation in a Murine Model of Short Bowel Syndrome. Dig. Dis. Sci. 2019, 64, 1158–1170. [Google Scholar] [CrossRef]
- Popeijus, H.E.; Zwaan, W.; Tayyeb, J.Z.; Plat, J. Potential Contribution of Short Chain Fatty Acids to Hepatic Apolipoprotein A-I Production. Int. J. Mol. Sci. 2021, 22, 5986. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yuan, H.; Zhang, T.; Wu, X.; Huang, S.K.; Cho, S.M. Long-term silk peptide intake promotes skeletal muscle mass, reduces inflammation, and modulates gut microbiota in middle-aged female rats. Biomed. Pharmacother. 2021, 137, 111415. [Google Scholar] [CrossRef]
- Yang, H.J.; Zhang, T.; Wu, X.G.; Kim, M.J.; Kim, Y.H.; Yang, E.S.; Yoon, Y.S.; Park, S. Aqueous Blackcurrant Extract Improves Insulin Sensitivity and Secretion and Modulates the Gut Microbiome in Non-Obese Type 2 Diabetic Rats. Antioxidants 2021, 10, 756. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Park, S.; Kang, S. Association between Polygenetic Risk Scores of Low Immunity and Interactions between These Scores and Moderate Fat Intake in a Large Cohort. Nutrients 2021, 13, 2849. [Google Scholar] [CrossRef]
- Björkström, N.K.; Strunz, B.; Ljunggren, H.-G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2022, 22, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, D.-S.; Jung, Y.; Sung, N.-Y.; Kim, M.; Han, I.-J.; Nho, E.Y.; Hong, J.H.; Lee, J.-K.; Boo, M.; et al. Immune-Enhancing Effect of Sargassum horneri on Cyclophosphamide-Induced Immunosuppression in BALB/c Mice and Primary Cultured Splenocytes. Molecules 2022, 27, 8253. [Google Scholar] [CrossRef] [PubMed]
- Meyts, I.; Bousfiha, A.; Duff, C.; Singh, S.; Lau, Y.L.; Condino-Neto, A.; Bezrodnik, L.; Ali, A.; Adeli, M.; Drabwell, J. Primary Immunodeficiencies: A Decade of Progress and a Promising Future. Front. Immunol. 2020, 11, 625753. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, Z.; Wu, Q.; Chen, J.; Liu, X.; Zhang, J.; Luo, Y.; Sun, W.; Huang, Z.; Zhang, J.; et al. Immunological modulation of the Th1/Th2 shift by ionizing radiation in tumors (Review). Int. J. Oncol. 2021, 59, 50. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K. Curcumin and its analogues: A potential natural compound against HIV infection and AIDS. Food Funct. 2015, 6, 3412–3419. [Google Scholar] [CrossRef]
- Khazeei Tabari, M.A.; Iranpanah, A.; Bahramsoltani, R.; Rahimi, R. Flavonoids as Promising Antiviral Agents against SARS-CoV-2 Infection: A Mechanistic Review. Molecules 2021, 26, 3900. [Google Scholar] [CrossRef]
- Im, K.; Kim, J.; Min, H. Ginseng, the natural effectual antiviral: Protective effects of Korean Red Ginseng against viral infection. J. Ginseng Res. 2016, 40, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Kyung, J.-S.; Kim, S.W.; In, G.; Park, C.-K.; Hyun, S.H. Immunoenhancing Effects of Korean Red Ginseng Powder in BALB/c Mice Treated with Cyclophosphamide. Food Suppl. Biomater. Health 2021, 1, e46. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Fan, M.; Zhang, Y.; Liang, Z.; Zhang, L.; Gao, X.; He, X.; Li, X.; Zhao, D.; et al. Prevention effect of total ginsenosides and ginseng extract from Panax ginseng on cyclophosphamide-induced immunosuppression in mice. Phytother. Res. 2023, 37, 3583–3601. [Google Scholar] [CrossRef]
- Wang, S.; Huang, S.; Ye, Q.; Zeng, X.; Yu, H.; Qi, D.; Qiao, S. Prevention of Cyclophosphamide-Induced Immunosuppression in Mice with the Antimicrobial Peptide Sublancin. J. Immunol. Res. 2018, 2018, 4353580. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.L.; Fan, W.; Chao, J.; Hou, Y.Q.; Yin, Y.L.; Zhu, H.L.; Meng, G.Q.; Che, Z.Q. Dietary L-arginine supplementation alleviates immunosuppression induced by cyclophosphamide in weaned pigs. Amino Acids 2009, 37, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Ebokaiwe, A.P.; Obasi, D.O.; Njoku, R.C.C.; Osawe, S.; Olusanya, O.; Kalu, W.O. Cyclophosphamide instigated hepatic-renal oxidative/inflammatory stress aggravates immunosuppressive indoleamine 2,3-dioxygenase in male rats: Abatement by quercetin. Toxicology 2021, 464, 153027. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, T.; Liu, C.; Shan, X.; Xu, Z.; Hong, H.; Lin, H.; Chen, J.; Sun, H. Cyclophosphamide induced physiological and biochemical changes in mice with an emphasis on sensitivity analysis. Ecotoxicol. Environ. Saf. 2021, 211, 111889. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, J.; Schubert, R.; Schwan, C.; Müller, K.; Böhles, H.J.; Zielen, S. Antioxidative Capacity in Patients with CommonVariable Immunodeficiency. J. Clin. Immunol. 2000, 20, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Tanir Basaranoglu, S.; Cekic, S.; Kirhan, E.; Dirican, M.; Kilic, S.S. Oxidative stress in common variable immunodeficiency. Eur. J. Inflamm. 2021, 19, 20587392211002411. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Zhang, T.; Park, S. The impact of gut microbiome enterotypes on ulcerative colitis: Identifying key bacterial species and revealing species co-occurrence networks using machine learning. Gut Microbes 2024, 16, 2292254. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2023, 19, 275–293. [Google Scholar] [CrossRef]
- Romano, M.; Fanelli, G.; Albany, C.J.; Giganti, G.; Lombardi, G. Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity. Front. Immunol. 2019, 10, 433537. [Google Scholar] [CrossRef]

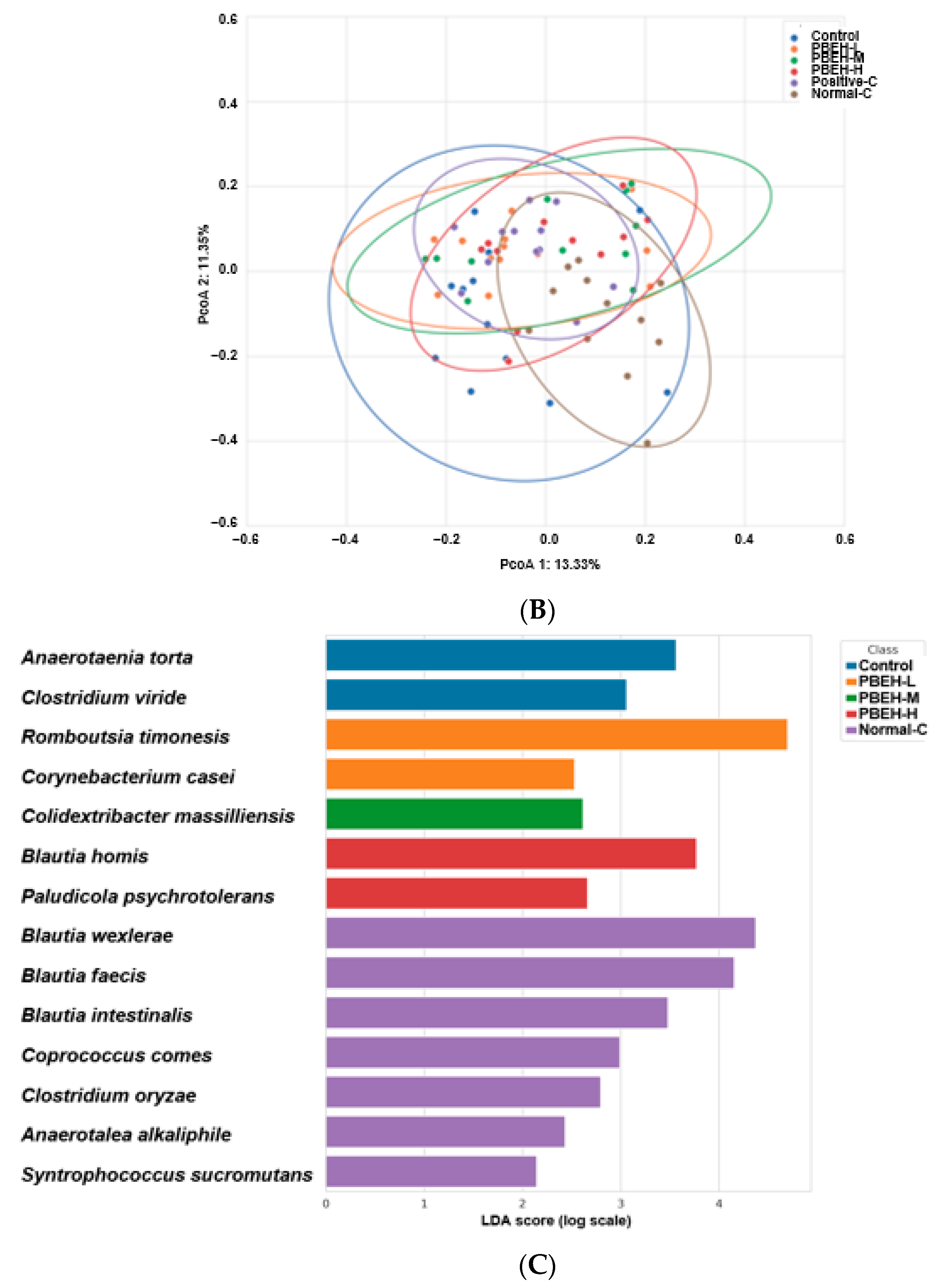
| Control | PBEH-L | PBEH-M | PBEH-H | Positive-Control | Normal-Control | |
|---|---|---|---|---|---|---|
| Body weight gain (g) | 175 ± 20.3 c | 203 ± 19.5 b | 185 ± 20.4 bc | 194 ± 21.4 b | 179 ± 24 c | 222 ± 19.7 a |
| Food intake (g/day) | 24 ± 1.7 b | 26 ± 0.9 a | 25.1 ± 0.6 ab | 25 ± 0.8 ab | 24.1 ± 1.2 b | 26.5 ± 1.1 a |
| Spleen index | 1.89 ± 0.44 c | 2.07 ± 0.3 bc | 2.02 ± 0.28 bc | 2.16 ± 0.36 b | 2.1 ± 0.42 bc | 2.75 ± 0.49 a |
| Thymus index | 1.52 ± 0.48 b | 1.5 ± 0.49 b | 1.38 ± 0.17 b | 1.4 ± 0.25 b | 1.41 ± 0.33 b | 1.84 ± 0.29 a |
| Hepatic lipid peroxide contents (nmol/mg of tissue) | 0.63 ± 0.05 a | 0.58 ± 0.06 ab | 0.54 ± 0.06 b | 0.47 ± 0.05 c | 0.54 ± 0.05 b | 0.48 ± 0.05 c |
| Serum ALT (IU/L) | 18.8 ± 0.71 a | 16.1 ± 1.48 b | 12.4 ± 0.57 c | 16 ± 0.74 b | 13 ± 0.93 c | 12 ± 1.52 c |
| Serum AST (IU/L) | 28 ± 0.52 a | 27.7 ± 0.7 a | 26.1 ± 0.54 b | 27.1 ± 1.38 ab | 28.4 ± 0.88 a | 22.6 ± 0.79 c |
| Control | PBEH-L | PBEH-M | PBEH-H | Positive-Control | Normal-Control | |
|---|---|---|---|---|---|---|
| WBC (109/L) | 2.82 ± 0.55 b | 3.11 ± 1.01 b | 3 ± 0.51 b | 3.13 ± 0.73 b | 3.18 ± 0.95 b | 7.05 ± 1.86 a |
| Lymphocyte (109/L) | 1.51 ± 0.54 c | 1.88 ± 0.61 bc | 1.78 ± 0.45 bc | 1.91 ± 0.5 b | 1.93 ± 0.73 b | 5.18 ± 1.55 a |
| Granulocytes (109/L) | 1.17 ± 0.22 b | 1.1 ± 0.46 b | 1.09 ± 0.25 b | 1.08 ± 0.25 b | 1.12 ± 0.31 b | 1.65 ± 0.53 a |
| Mid white blood cell (109/L) | 0.13 ± 0.04 b | 0.13 ± 0.03 b | 0.13 ± 0.03 b | 0.13 ± 0.04 b | 0.12 ± 0.03 b | 0.22 ± 0.08 a |
| Control | PBEH-L | PBEH-M | PBEH-H | Positive-Control | Normal-Control | |
|---|---|---|---|---|---|---|
| Splenocyte proliferation without LPS induction (%) | 80.6 ± 19.3 | 84.2 ± 17.4 | 91.7 ± 34.7 | 95.9 ± 24.6 | 98.2 ± 11 | 100 ± 27.1 |
| Splenocyte proliferation with LPS induction (10 ng/mL) | 72.4 ± 11.5 b | 79.1 ± 7.5 b | 75.2 ± 11.5 b | 89.6 ± 7.6 a | 87.4 ± 10 a | 100 ± 16.7 a |
| Lipid peroxide contents in splenocytes | 0.93 ± 0.08 a | 0.81 ± 0.08 b | 0.76 ± 0.07 b | 0.64 ± 0.06 c | 0.82 ± 0.08 b | 0.76 ± 0.08 b |
| Natural killer cell activity(%) | 51.4 ± 9.1 c | 81.7 ± 11.5 b | 73 ± 15.5 b | 93.6 ± 15 a | 76.6 ± 7.6 b | 100 ± 14.4 a |
| Control | PBEH-L | PBEH-M | PBEH-H | Positive-Control | Normal-Control | |
|---|---|---|---|---|---|---|
| IgA (μg/mL) | 91.7 ± 5.64 c | 95.7 ± 5.14 c | 106.4 ± 6.32 ab | 103.7 ± 6.14 b | 102.1 ± 3.95 b | 114.6 ± 5.47 a |
| IgG (mg/mL) | 4.85 ± 0.34 c | 4.97 ± 0.41 c | 5.61 ± 0.38 b | 6.03 ± 0.38 ab | 5.78 ± 0.43 ab | 6.38 ± 0.39 a |
| IgM (mg/mL) | 0.72 ± 0.04 b | 0.78 ± 0.05 b | 0.87 ± 0.04 a | 0.89 ± 0.06 a | 0.84 ± 0.08 a | 0.91 ± 0.07 a |
| IL-1β (pg/mL) | 17.6 ± 0.92 a | 17.6 ± 1.13 a | 15.7 ± 0.68 b | 15.7 ± 0.97 b | 15 ± 0.84 b | 18.5 ± 1.15 a |
| IL-4 (pg/mL) | 29 ± 1.55 a | 27.5 ± 1.69 ab | 29.8 ± 1.67 a | 29.3 ± 1.43 ab | 31.3 ± 2 a | 26.6 ± 1.38 b |
| IL-6 (pg/mL) | 11.4 ± 0.9 b | 12.7 ± 1.01 ab | 13.4 ± 1.1 a | 12.6 ± 1.3 ab | 11.6 ± 0.8 b | 9.4 ± 0.99 c |
| IFN-γ (pg/mL) | 13.5 ± 0.48 b | 14.5 ± 0.39 ab | 14.2 ± 0.54 ab | 15.1 ± 0.73 a | 14.8 ± 0.72 a | 14.8 ± 0.42 a |
| Th1/Th2 ratio | 0.46 ± 0.02 c | 0.53 ± 0.02 b | 0.48 ± 0.02 c | 0.52 ± 0.03 b | 0.47 ± 0.03 c | 0.56 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Yang, H.J.; Zhang, T.; Li, C.; Kim, M.J.; Kim, K.-N.; Park, S. Porcine Brain Enzyme Hydrolysate Enhances Immune Function and Antioxidant Defense via Modulation of Gut Microbiota in a Cyclophosphamide-Induced Immunodeficiency Model. Antioxidants 2024, 13, 476. https://doi.org/10.3390/antiox13040476
Yue Y, Yang HJ, Zhang T, Li C, Kim MJ, Kim K-N, Park S. Porcine Brain Enzyme Hydrolysate Enhances Immune Function and Antioxidant Defense via Modulation of Gut Microbiota in a Cyclophosphamide-Induced Immunodeficiency Model. Antioxidants. 2024; 13(4):476. https://doi.org/10.3390/antiox13040476
Chicago/Turabian StyleYue, Yu, Hye Jeong Yang, Ting Zhang, Chen Li, Min Jung Kim, Keun-Nam Kim, and Sunmin Park. 2024. "Porcine Brain Enzyme Hydrolysate Enhances Immune Function and Antioxidant Defense via Modulation of Gut Microbiota in a Cyclophosphamide-Induced Immunodeficiency Model" Antioxidants 13, no. 4: 476. https://doi.org/10.3390/antiox13040476
APA StyleYue, Y., Yang, H. J., Zhang, T., Li, C., Kim, M. J., Kim, K.-N., & Park, S. (2024). Porcine Brain Enzyme Hydrolysate Enhances Immune Function and Antioxidant Defense via Modulation of Gut Microbiota in a Cyclophosphamide-Induced Immunodeficiency Model. Antioxidants, 13(4), 476. https://doi.org/10.3390/antiox13040476








