Nanoemulsions Based on Soluble Chenopodin/Alginate Complex for Colonic Delivery of Quercetin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Chenopodin
2.3. Preparation of Soluble Chenopodin/Alginate Complex (QPA)
2.4. Nanoemulsion Preparation
2.5. Characterization of NEs
2.5.1. Size, Polydispersity Index, and Zeta Potential
2.5.2. Encapsulation Efficiency (EE)
2.6. Stability Tests
2.7. Differential Scanning Calorimetry (DSC)
2.8. Fourier Transform Infrared (ATR–FTIR) Spectroscopy
2.9. Scanning Transmission Electron Microscopy (STEM)
2.10. Release Profile in PBS 1X and Krebs Buffer
2.11. Release in the Presence of Colonic Bacteria
2.12. Evaluation of Cytotoxicity
2.13. Evaluation of Antioxidant Capacity
2.13.1. ABTS and ORAC Assays
2.13.2. Antioxidant Capacity in the HT-29 Cell Line
2.14. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Chenopodin
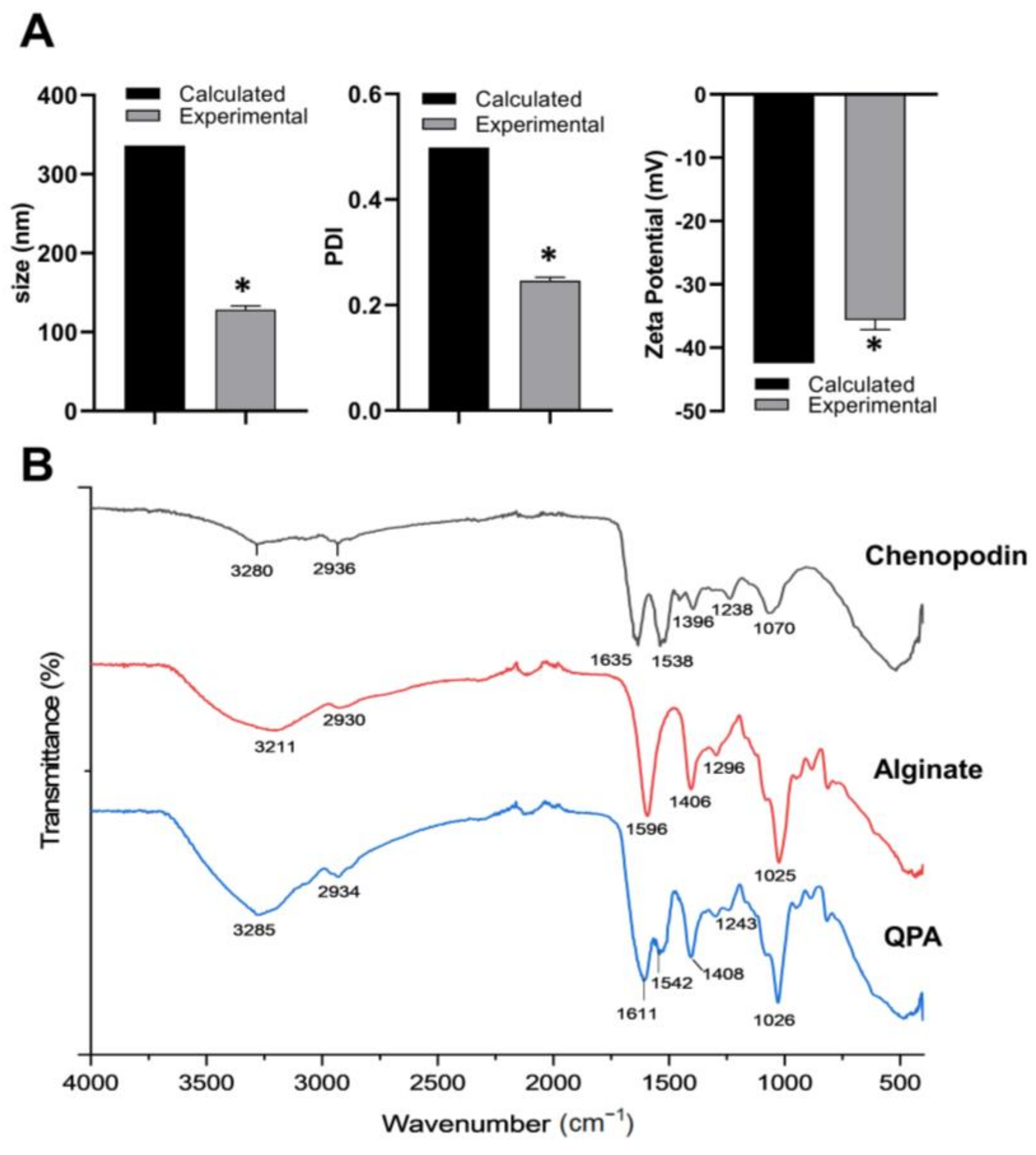
3.2. Preparation of Chenopodin/Alginate Soluble Complex (QPA)
3.3. Preparation and Characterization of NEs
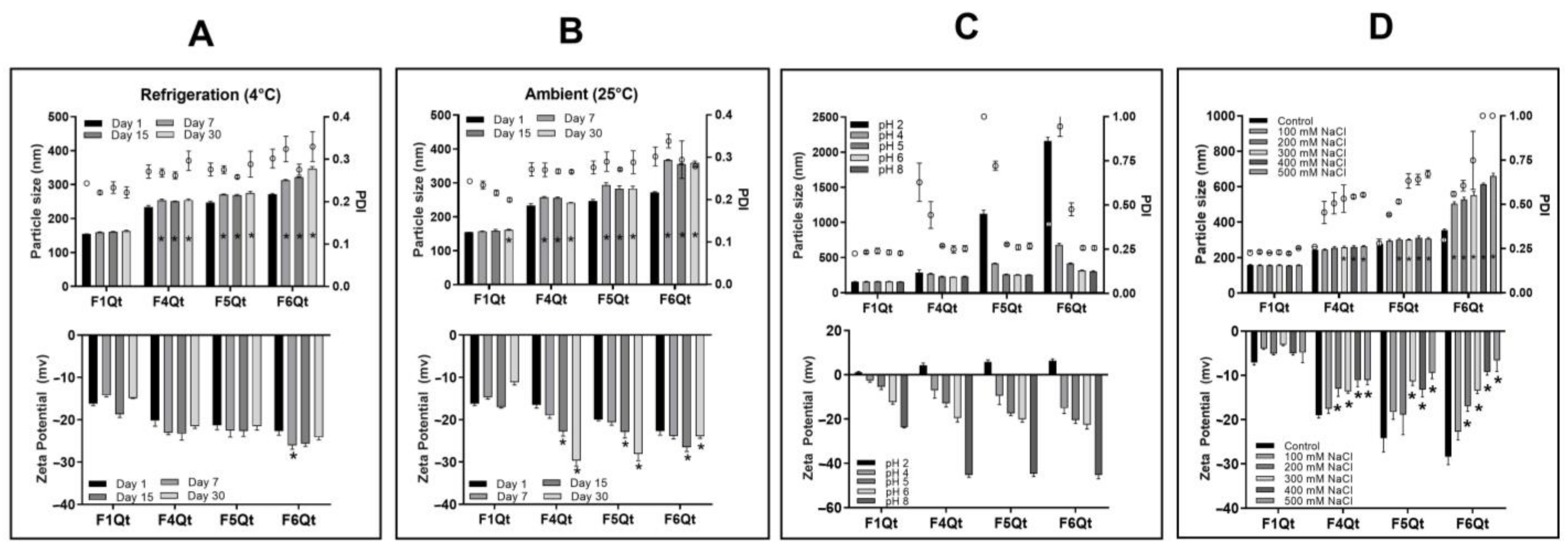
3.4. Stability of NEs at Different Times, Temperatures, pH Levels, and NaCl Concentrations
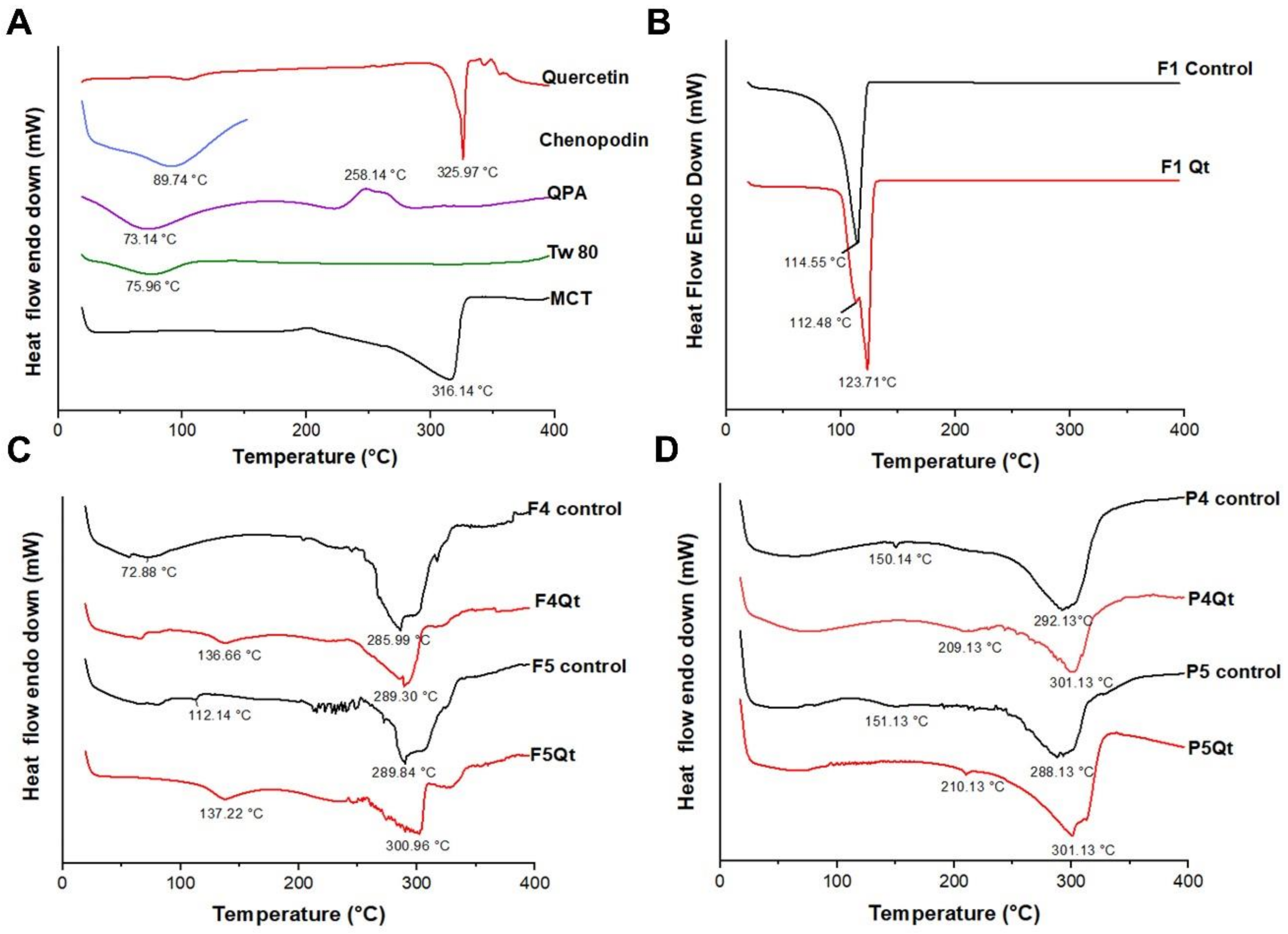
3.5. DSC Analysis
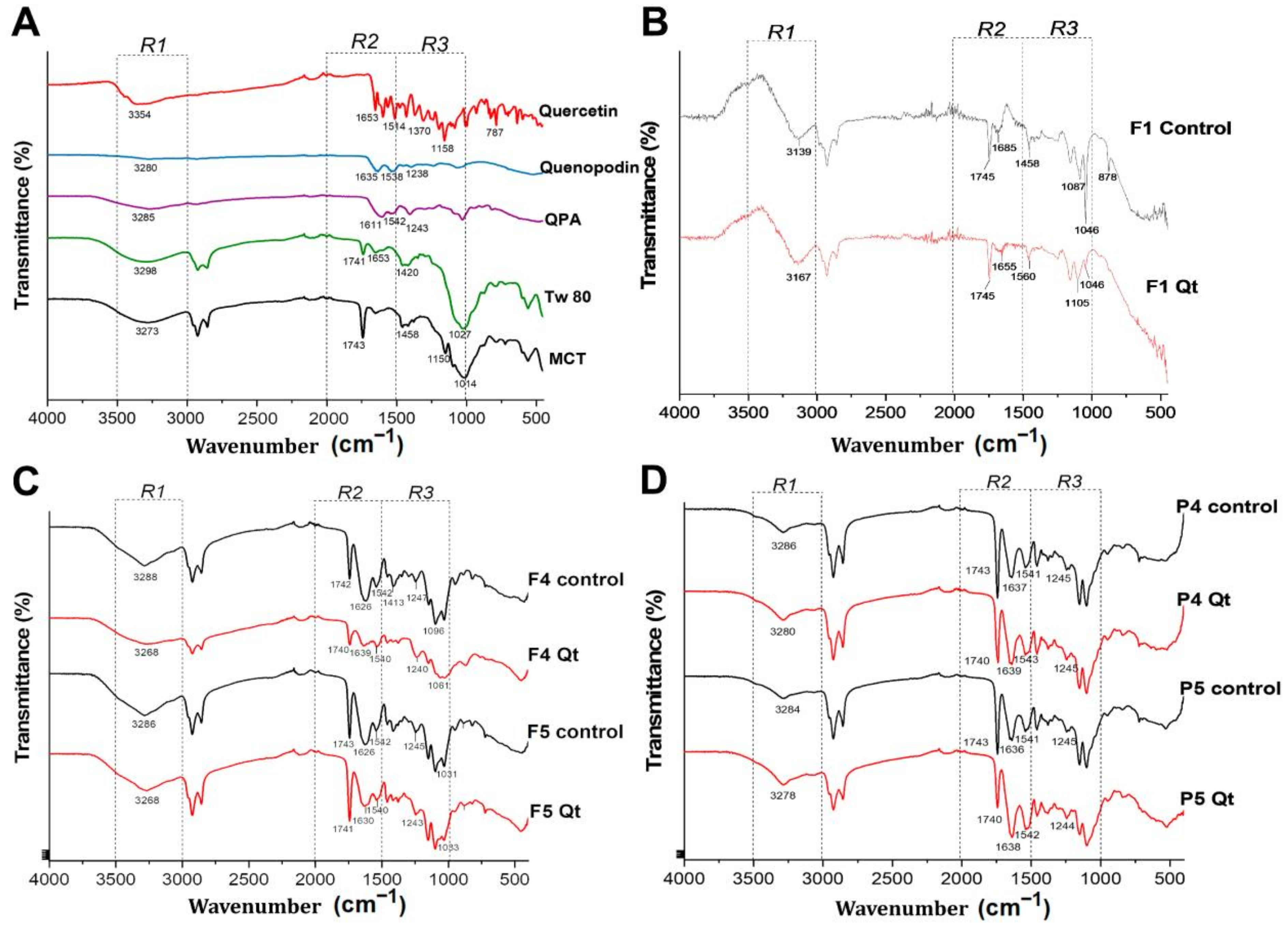
3.6. ATR–FTIR Analysis
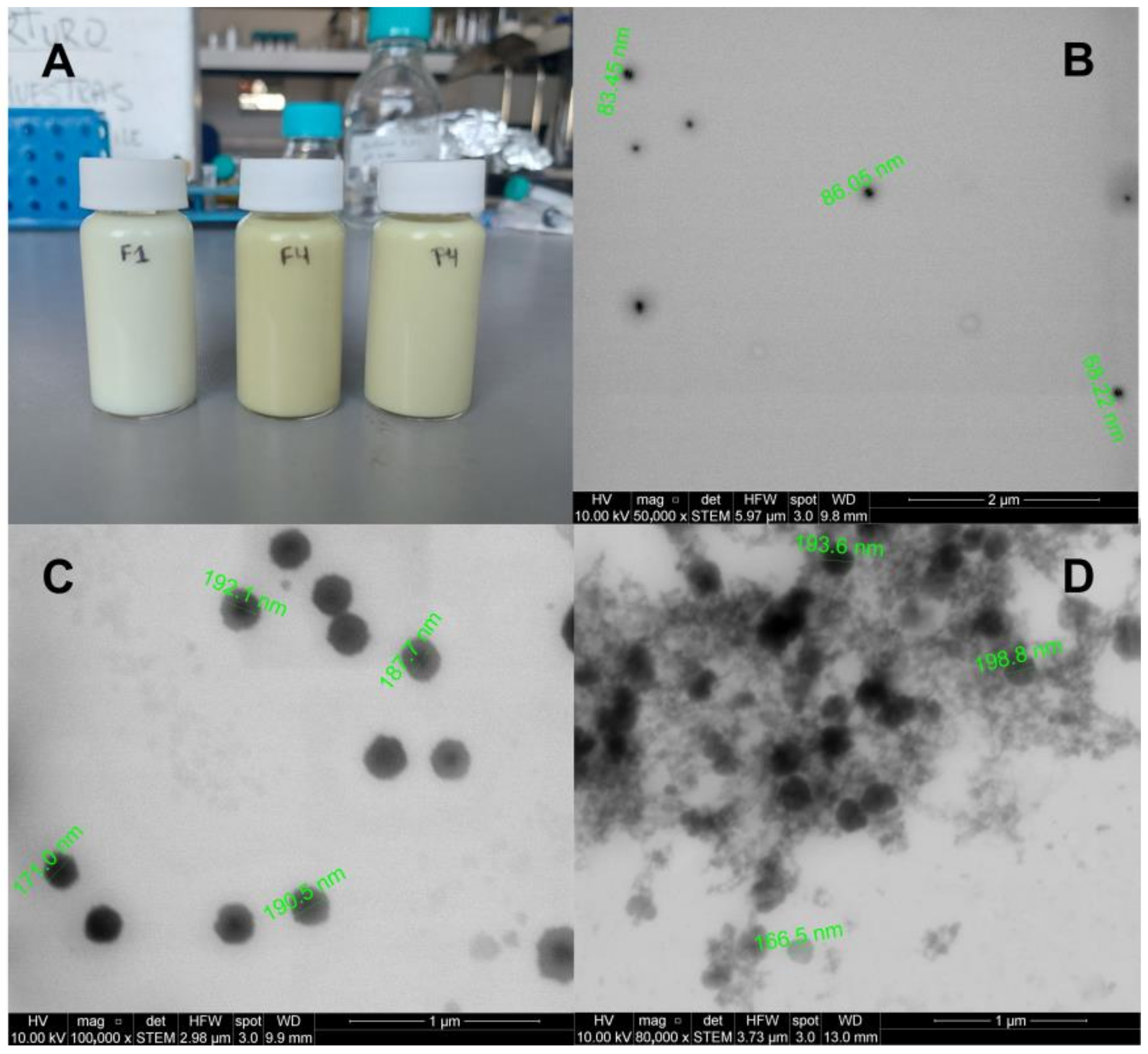
3.7. STEM Images of NEs
3.8. Dissolution Studies in Simulated Colonic Media
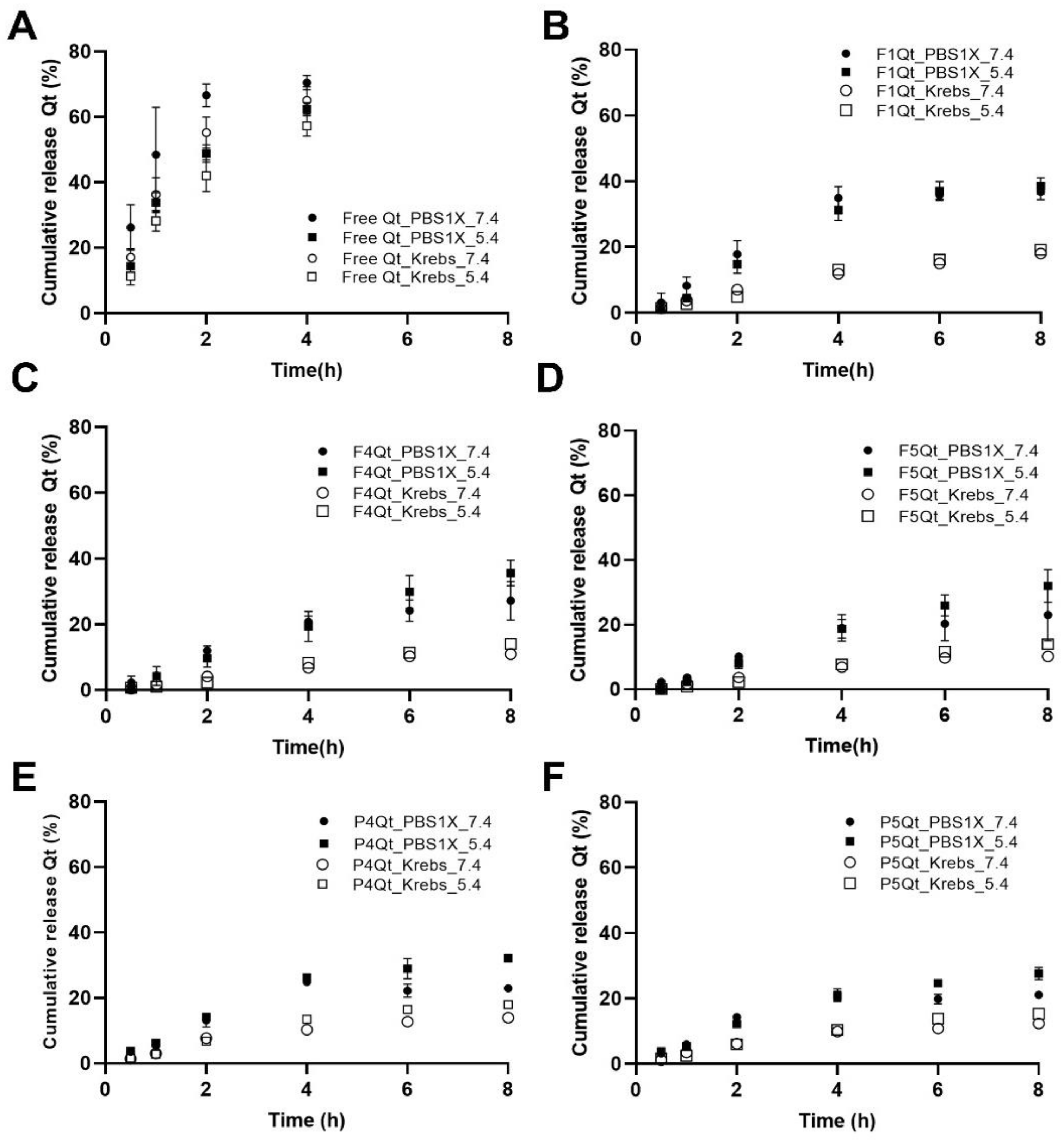
3.8.1. Comparison between Compendial (PBS1X) and Biorelevant (Krebs) Buffers
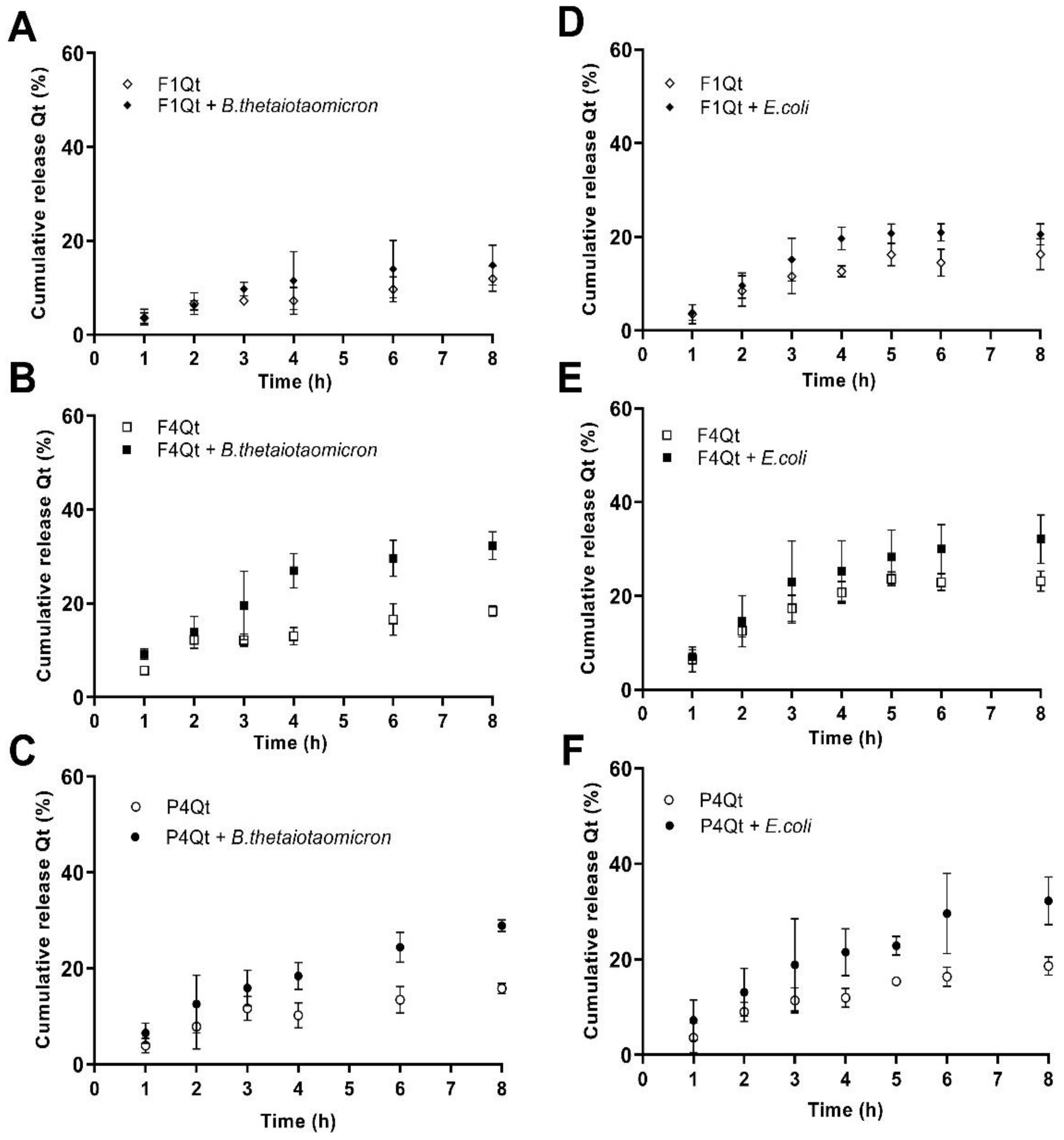
3.8.2. Release in Presence of B. thetaiotaomicron and E. coli
3.9. Cytotoxicity of NEs in HT-29 Cell Line
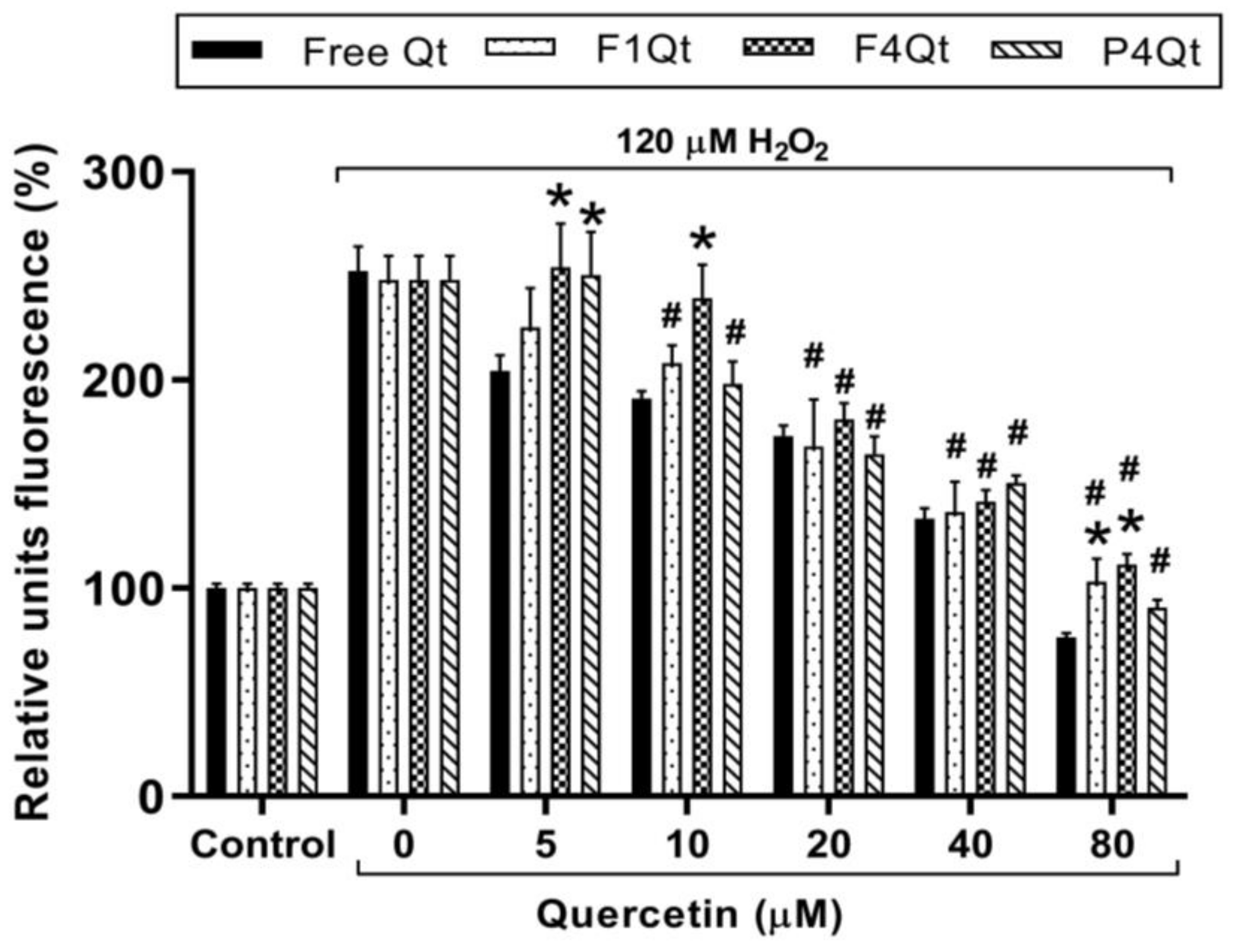
3.10. Antioxidant Capacity of Qt and NEs Measured via ABTS and ORAC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Dong, Z.; Liu, K.; Lu, Y.; Wu, W.; Qi, J.; Chen, Z. Targeting Strategies of Oral Nano-Delivery Systems for Treating Inflammatory Bowel Disease. Int. J. Pharm. 2021, 600, 120461. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhang, M.; Liu, K. Colon-Targeted Drug Delivery of Polysaccharide-Based Nanocarriers for Synergistic Treatment of Inflammatory Bowel Disease: A Review. Carbohydr. Polym. 2021, 272, 118530. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, S.; Chen, C.; Ming, Y.; Du, J.; Mu, J.; Luo, F.; Huang, D.; Wang, N.; Lin, Z.; et al. Colon-Targeted Oral Nanoparticles Based on ROS-Scavenging Hydroxyethyl Starch-Curcumin Conjugates for Efficient Inflammatory Bowel Disease Therapy. Int. J. Pharm. 2022, 623, 121884. [Google Scholar] [CrossRef] [PubMed]
- Yener, S.; Akbulut, K.G.; Karakuş, R.; Erdoğan, D.; Acartürk, F. Development of Melatonin Loaded Pectin Nanoparticles for the Treatment of Inflammatory Bowel Disease: In Vitro and in Vivo Studies. J. Drug Deliv. Sci. Technol. 2022, 67, 102861. [Google Scholar] [CrossRef]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef] [PubMed]
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Yanping, C.; Hongqing, W.; Zhipeng, G.; Kaiqi, Z.; et al. Phytochemicals and Inflammatory Bowel Disease: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1321–1345. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.; Ma, Y.; Cannup, B.; Xie, D.; Jung, Y.; Zhang, J.; Yang, C.; Gao, F.; Merlin, D.; Xiao, B. Oral Delivery of Natural Active Small Molecules by Polymeric Nanoparticles for the Treatment of Inflammatory Bowel Diseases. Adv. Drug Deliv. Rev. 2021, 176, 113887. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ali, H.; Khan, S.; Khan, S.A.; Weigmann, B. Advances in Orally-Delivered pH-Sensitive Nanocarrier Systems; an Optimistic Approach for the Treatment of Inflammatory Bowel Disease. Int. J. Pharm. 2019, 558, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yue, W.; Luo, Y.; Luo, Q.; Liu, S.; Chen, H.; Qin, W.; Zhang, Q. Preparation and Stability Characterization of Soybean Protein Isolate/Sodium Alginate Complexes-Based Nanoemulsions Using High-Pressure Homogenization. LWT 2022, 154, 112607. [Google Scholar] [CrossRef]
- Lim, S.B.; Banerjee, A.; Önyüksel, H. Improvement of Drug Safety by the Use of Lipid-Based Nanocarriers. J. Control. Release 2012, 163, 34–45. [Google Scholar] [CrossRef]
- Beloqui, A.; Memvanga, P.B.; Coco, R.; Reimondez-Troitiño, S.; Alhouayek, M.; Muccioli, G.G.; Alonso, M.J.; Csaba, N.; De La Fuente, M.; Préat, V. A Comparative Study of Curcumin-Loaded Lipid-Based Nanocarriers in the Treatment of Inflammatory Bowel Disease. Colloids Surf. B Biointerfaces 2016, 143, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol Nanoemulsions Incorporated in Quinoa Protein/Chitosan Edible Films; Antifungal Effect in Cherry Tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Karthik, P.; Saravana Raj, A.; Pavithra, J. Natural Compound-Based Interfacial Stabilization of Nanoemulsions. In Bio-Based Nanoemulsions for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 71–89. ISBN 978-0-323-89846-1. [Google Scholar]
- López-Monterrubio, D.I.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Influence of β-Carotene Concentration on the Physicochemical Properties, Degradation and Antioxidant Activity of Nanoemulsions Stabilized by Whey Protein Hydrolyzate-Pectin Soluble Complexes. LWT 2021, 143, 111148. [Google Scholar] [CrossRef]
- Romo, I.; Abugoch, L.; Tapia, C. Soluble Complexes between Chenopodins and Alginate/Chitosan: Intermolecular Interactions and Structural-Physicochemical Properties. Carbohydr. Polym. 2020, 227, 115334. [Google Scholar] [CrossRef] [PubMed]
- Arazo, M.; Jaque, N.; Caro, N.; Abugoch, L.; Tapia, C. Development of a Scalable Procedure by a Discontinuous Crossflow DF/UF to Obtain a Concentrate of Chenopodin from a Dead-End Centrifugal UF at Bench Scale. Food Chem. 2020, 313, 126154. [Google Scholar] [CrossRef] [PubMed]
- Arazo Rusindo, M.C. Nuevas Nanoemulsiones Basadas en Complejos Solubles de Quenopodina-Quitosano y Quenopodina-Alginato Como Matrices de Liberación Controlada de Quercetina a Nivel Colónico. Ph.D. Thesis, Universidad de Chile, Santiago, Chile, 2021. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hermia, J. Constant Pressure Blocking Filtration Laws—Application to Power-Law Non-Newtonian Fluids. Eng. Phys. 1982, 60, 183–187. [Google Scholar]
- Samadi, A.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Eufrasio-da-silva, T. Ameliorating Quercetin Constraints in Cancer Therapy with pH-Responsive Agarose-Polyvinylpyrrolidone-Hydroxyapatite Nanocomposite Encapsulated in Double Nanoemulsion. Int. J. Biol. Macromol. 2021, 182, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.; Araujo, V.; Caro, N.; Gotteland, M.; Abugoch, L.; Tapia, C. Spray Freeze-Drying as an Alternative to the Ionic Gelation Method to Produce Chitosan and Alginate Nano-Particles Targeted to the Colon. J. Pharm. Sci. 2015, 104, 4373–4385. [Google Scholar] [CrossRef]
- Tapia, C.; Escobar, Z.; Costa, E.; Sapag-Hagar, J.; Valenzuela, F.; Basualto, C.; Nella Gai, M.; Yazdani-Pedram, M. Comparative Studies on Polyelectrolyte Complexes and Mixtures of Chitosan–Alginate and Chitosan–Carrageenan as Prolonged Diltiazem Clorhydrate Release Systems. Eur. J. Pharm. Biopharm. 2004, 57, 65–75. [Google Scholar] [CrossRef]
- Zhang, L.; Alfano, J.; Race, D.; Davé, R.N. Zero-Order Release of Poorly Water-Soluble Drug from Polymeric Films Made via Aqueous Slurry Casting. Eur. J. Pharm. Sci. 2018, 117, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Intiquilla, A.; Jiménez-Aliaga, K.; Iris Zavaleta, A.; Gamboa, A.; Caro, N.; Diaz, M.; Gotteland, M.; Abugoch, L.; Tapia, C. Nanoencapsulation of Antioxidant Peptides from Lupinus Mutabilis in Chitosan Nanoparticles Obtained by Ionic Gelling and Spray Freeze Drying Intended for Colonic Delivery. Food Biosci. 2022, 50, 102055. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the Probe 2‘,7‘-Dichiorofluorescin as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Ng, K.S.Y.; Dunstan, D.E.; Martin, G.J.O. Influence of Diafiltration on Flux Decline during Skim Milk Ultrafiltration. Int. Dairy J. 2018, 87, 67–74. [Google Scholar] [CrossRef]
- Abugoch, L.; Castro, E.; Tapia, C.; Añón, M.C.; Gajardo, P.; Villarroel, A. Stability of Quinoa Flour Proteins (Chenopodium Quinoa Willd.) during Storage. Int. J. Food Sci. Technol. 2009, 44, 2013–2020. [Google Scholar] [CrossRef]
- Brinegar, C.; Goundan, S. Isolation and Characterization of Chenopodin, the 11S Seed Storage Protein of Quinoa (Chenopodium Quinoa). J. Agric. Food Chem. 1993, 41, 182–185. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, R.; Yuan, W. Composition and Secondary Structure of Proteins Isolated from Six Different Quinoa Varieties from China. J. Cereal Sci. 2020, 95, 103036. [Google Scholar] [CrossRef]
- Shi, L.S.; Yang, X.Y.; Gong, T.; Hu, C.Y.; Shen, Y.H.; Meng, Y.H. Ultrasonic Treatment Improves Physical and Oxidative Stabilities of Walnut Protein Isolate-Based Emulsion by Changing Protein Structure. LWT 2023, 173, 114269. [Google Scholar] [CrossRef]
- Duhoranimana, E.; Karangwa, E.; Lai, L.; Xu, X.; Yu, J.; Xia, S.; Zhang, X.; Muhoza, B.; Habinshuti, I. Effect of Sodium Carboxymethyl Cellulose on Complex Coacervates Formation with Gelatin: Coacervates Characterization, Stabilization and Formation Mechanism. Food Hydrocoll. 2017, 69, 111–120. [Google Scholar] [CrossRef]
- Xiao, C.; Liu, H.; Lu, Y.; Zhang, L. Blend Films from Sodium Alginate and Gelatin Solutions. J. Macromol. Sci. Part A 2001, 38, 317–328. [Google Scholar] [CrossRef]
- Ji, R.; Cui, H.; Duhoranimana, E.; Hayat, K.; Yu, J.; Hussain, S.; Usman Tahir, M.; Zhang, X.; Ho, C.-T. Co-Encapsulation of L-Ascorbic Acid and Quercetin by Gelatin/Sodium Carboxymethyl Cellulose Coacervates Using Different Interlayer Oils. Food Res. Int. 2021, 145, 110411. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.G.; Gupta, R.; Timilsena, Y.P.; Adhikari, B. Optimisation of the Complex Coacervation between Canola Protein Isolate and Chitosan. J. Food Eng. 2016, 191, 58–66. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) Based Microemulsions: Preclinical Drug Delivery, Toxicity and Antimicrobial Applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; González-Aguilar, G.A.; Martín-Belloso, O. Interfacial Activity of Phenolic-Rich Extracts from Avocado Fruit Waste: Influence on the Colloidal and Oxidative Stability of Emulsions and Nanoemulsions. Innov. Food Sci. Emerg. Technol. 2021, 69, 102665. [Google Scholar] [CrossRef]
- Roldan-Cruz, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Assessing the Stability of Tween 80-Based O/W Emulsions with Cyclic Voltammetry and Electrical Impedance Spectroscopy. Colloids Surf. Physicochem. Eng. Asp. 2016, 511, 145–152. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Balandrán-Quintana, R.R.; Valdés-Covarrubias, M.Á.; Cabellos, J.L. Potential of Quercetin in Combination with Antioxidants of Different Polarity Incorporated in Oil-in-Water Nanoemulsions to Control Enzymatic Browning of Apples. J. Mol. Struct. 2022, 1254, 132372. [Google Scholar] [CrossRef]
- Marhamati, M.; Ranjbar, G.; Rezaie, M. Effects of Emulsifiers on the Physicochemical Stability of Oil-in-Water Nanoemulsions: A Critical Review. J. Mol. Liq. 2021, 340, 117218. [Google Scholar] [CrossRef]
- Liao, L.; Julian McClements, D.; Chen, X.; Zhu, Y.; Liu, Y.; Liang, R.; Zou, L.; Liu, W. Dietary Proteins as Excipient Ingredients for Improving the Solubility, Stability, and Bioaccessibility of Quercetin: Role of Intermolecular Interactions. Food Res. Int. 2022, 161, 111806. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Raikos, V.; Duthie, G.; Ranawana, V. Comparing the Efficiency of Different Food-Grade Emulsifiers to Form and Stabilise Orange Oil-in-Water Beverage Emulsions: Influence of Emulsifier Concentration and Storage Time. Int. J. Food Sci. Technol. 2017, 52, 348–358. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.-H. Soy Glycinin as Food-Grade Pickering Stabilizers: Part. I. Structural Characteristics, Emulsifying Properties and Adsorption/Arrangement at Interface. Food Hydrocoll. 2016, 60, 606–619. [Google Scholar] [CrossRef]
- Basalious, E.B.; Shawky, N.; Badr-Eldin, S.M. SNEDDS Containing Bioenhancers for Improvement of Dissolution and Oral Absorption of Lacidipine. I: Development and Optimization. Int. J. Pharm. 2010, 391, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Abugoch, L.E.; Romero, N.; Tapia, C.A.; Silva, J.; Rivera, M. Study of Some Physicochemical and Functional Properties of Quinoa (Chenopodium Quinoa Willd) Protein Isolates. J. Agric. Food Chem. 2008, 56, 4745–4750. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving Emulsion Formation, Stability and Performance Using Mixed Emulsifiers: A Review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ju, X.; Aluko, R.E.; Zou, Y.; Wang, Z.; Liu, M.; He, R. Rice Bran Protein-Based Nanoemulsion Carrier for Improving Stability and Bioavailability of Quercetin. Food Hydrocoll. 2020, 108, 106042. [Google Scholar] [CrossRef]
- Iqbal, R.; Mehmood, Z.; Baig, A.; Khalid, N. Formulation and Characterization of Food Grade O/W Nanoemulsions Encapsulating Quercetin and Curcumin: Insights on Enhancing Solubility Characteristics. Food Bioprod. Process. 2020, 123, 304–311. [Google Scholar] [CrossRef]
- Bäther, S.; Hundschell, C.S.; Kieserling, H.; Wagemans, A.M. Impact of the Solvent Properties on Molecular Interactions and Phase Behaviour of Alginate-Gelatin Systems. Colloids Surf. Physicochem. Eng. Asp. 2023, 656, 130455. [Google Scholar] [CrossRef]
- Moon, H.; Lertpatipanpong, P.; Hong, Y.; Kim, C.-T.; Baek, S.J. Nano-Encapsulated Quercetin by Soluble Soybean Polysaccharide/Chitosan Enhances Anti-Cancer, Anti-Inflammation, and Anti-Oxidant Activities. J. Funct. Foods 2021, 87, 104756. [Google Scholar] [CrossRef]
- Kumar, A.; Kanwar, R.; Mehta, S.K. Development of Phosphatidylcholine/Tween 80 Based Biocompatible Clove Oil-in-Water Nanoemulsion as a Green Nanocarrier for Controlled Herbicide Delivery. Environ. Pollut. 2022, 293, 118558. [Google Scholar] [CrossRef]
- Zhang, X.; Song, R.; Liu, X.; Xu, Y.; Wei, R. Fabrication of Vitamin D3 Nanoemulsions Stabilized by Tween 80 and Span 80 as a Composite Surface-Active Surfactant: Characterization and Stability. Colloids Surf. Physicochem. Eng. Asp. 2022, 645, 128873. [Google Scholar] [CrossRef]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of Insulin-Loaded Alginate Nanoparticles Produced by Ionotropic Pre-Gelation through DSC and FTIR Studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef]
- Jia, Y.; Yan, X.; Huang, Y.; Zhu, H.; Qi, B.; Li, Y. Different Interactions Driving the Binding of Soy Proteins (7S/11S) and Flavonoids (Quercetin/Rutin): Alterations in the Conformational and Functional Properties of Soy Proteins. Food Chem. 2022, 396, 133685. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Shu, B.; Lou, X.; Wang, K.; Zhai, Y.; Qu, Y.; Song, R.; Liu, F.; Dong, X.; Xu, H. Improvement of Stability and in Vitro Bioaccessibility of Nervonic Acid by Nonionic Surfactant in Protein-Based Nanoemulsions. Food Biosci. 2023, 51, 102299. [Google Scholar] [CrossRef]
- Hazra, M.; Dasgupta Mandal, D.; Mandal, T.; Bhuniya, S.; Ghosh, M. Designing Polymeric Microparticulate Drug Delivery System for Hydrophobic Drug Quercetin. Saudi Pharm. J. 2015, 23, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-F.; Zheng, G.-D.; Wang, W.-J.; Yin, Z.-P.; Chen, J.-G.; Li, J.-E.; Zhang, Q.-F. Physicochemical Properties and Bioavailability Comparison of Two Quercetin Loading Zein Nanoparticles with Outer Shell of Caseinate and Chitosan. Food Hydrocoll. 2021, 120, 106959. [Google Scholar] [CrossRef]
- Javani, R.; Hashemi, F.S.; Ghanbarzadeh, B.; Hamishehkar, H. Quercetin-Loaded Niosomal Nanoparticles Prepared by the Thin-Layer Hydration Method: Formulation Development, Colloidal Stability, and Structural Properties. LWT 2021, 141, 110865. [Google Scholar] [CrossRef]
- Liang, Q.; Sun, X.; Raza, H.; Aslam Khan, M.; Ma, H.; Ren, X. Fabrication and Characterization of Quercetin Loaded Casein Phosphopeptides-Chitosan Composite Nanoparticles by Ultrasound Treatment: Factor Optimization, Formation Mechanism, Physicochemical Stability and Antioxidant Activity. Ultrason. Sonochem. 2021, 80, 105830. [Google Scholar] [CrossRef] [PubMed]
- Walia, N.; Zhang, S.; Wismer, W.; Chen, L. A Low Energy Approach to Develop Nanoemulsion by Combining Pea Protein and Tween 80 and Its Application for Vitamin D Delivery. Food Hydrocoll. Health 2022, 2, 100078. [Google Scholar] [CrossRef]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of Flavonoids in Liposomal Delivery Systems: The Case of Quercetin, Kaempferol and Luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef]
- Yang, X.; Wu, D.; Du, Z.; Li, R.; Chen, X.; Li, X. Spectroscopy Study on the Interaction of Quercetin with Collagen. J. Agric. Food Chem. 2009, 57, 3431–3435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Wen, X.; Tao, H.; Wang, K.; Fu, R.; Tao, H.; Wang, F.; Chen, N.; Ni, Y. Conformational Changes and the Formation of New Bonds Achieving Robust Nanoemulsions by Electrostatic Interactions between Whey Protein Isolate and Chondroitin Sulfate. Food Hydrocoll. 2023, 136, 108263. [Google Scholar] [CrossRef]
- Pool, H.; Mendoza, S.; Xiao, H.; McClements, D.J. Encapsulation and Release of Hydrophobic Bioactive Components in Nanoemulsion-Based Delivery Systems: Impact of Physical Form on Quercetin Bioaccessibility. Food Funct. 2013, 4, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Acree, W.E. On the Solubility of Quercetin. J. Mol. Liq. 2014, 197, 157–159. [Google Scholar] [CrossRef]
- Colino, C.I.; Velez Gomez, D.; Alonso Horcajo, E.; Gutierrez-Millan, C. A Comparative Study of Liposomes and Chitosomes for Topical Quercetin Antioxidant Therapy. J. Drug Deliv. Sci. Technol. 2022, 68, 103094. [Google Scholar] [CrossRef]
- Souza De Castilho, T.; Matias, T.B.; Nicolini, K.P.; Nicolini, J. Study of Interaction between Metal Ions and Quercetin. Food Sci. Hum. Wellness 2018, 7, 215–219. [Google Scholar] [CrossRef]
- Vieira, W.T.; Nicollini, M.V.S.; da Silva, M.G.C.; de Oliveira Nascimento, L.; Vieira, M.G.A. Natural Polysaccharides and Proteins Applied to the Development of Gastroresistant Multiparticulate Systems for Anti-Inflammatory Drug Delivery—A Systematic Review. Eur. Polym. J. 2022, 172, 111205. [Google Scholar] [CrossRef]
- Andrade, D.; Maldonado-Bravo, F.; Alburquerque, A.; Pérez, C.; Gamboa, A.; Caro, N.; Díaz-Dosque, M.; Gotelland, M.; Abugoch, L.; Tapia, C. Nanoencapsulation of Maqui (Aristotelia chilensis) Extract in Chitosan–Tripolyphosphate and Chenopodin-Based Systems. Antioxidants 2024, 13, 273. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Hochart-Behra, A.-C.; Drobecq, H.; Tourret, M.; Dubreuil, L.; Behra-Miellet, J. Anti-Stress Proteins Produced by Bacteroides Thetaiotaomicron after Nutrient Starvation. Anaerobe 2014, 28, 18–23. [Google Scholar] [CrossRef]
- Minogue, T.D.; Daligault, H.A.; Davenport, K.W.; Bishop-Lilly, K.A.; Broomall, S.M.; Bruce, D.C.; Chain, P.S.; Chertkov, O.; Coyne, S.R.; Freitas, T.; et al. Complete Genome Assembly of Escherichia Coli ATCC 25922, a Serotype O6 Reference Strain. Genome Announc. 2014, 2, e00969-14. [Google Scholar] [CrossRef]
- Zhang, H.; Mou, J.; Ding, J.; Qin, W. Magneto-Controlled Potentiometric Assay for E. Coli Based on Cleavage of Peptide by Outer-Membrane Protease T. Electrochim. Acta 2021, 384, 138408. [Google Scholar] [CrossRef]
- Li, M.; Li, G.; Shang, Q.; Chen, X.; Liu, W.; Pi, X.; Zhu, L.; Yin, Y.; Yu, G.; Wang, X. In Vitro Fermentation of Alginate and Its Derivatives by Human Gut Microbiota. Anaerobe 2016, 39, 19–25. [Google Scholar] [CrossRef]
- Araujo, V.; Gamboa, A.; Caro, N.; Abugoch, L.; Gotteland, M.; Valenzuela, F.; Merchant, H.A.; Basit, A.W.; Tapia, C. Release of Prednisolone and Inulin from a New Calcium-Alginate Chitosan-Coated Matrix System for Colonic Delivery. J. Pharm. Sci. 2013, 102, 2748–2759. [Google Scholar] [CrossRef]
- Kaindl, U.; Eyberg, I.; Rohr-Udilova, N.; Heinzle, C.; Marian, B. The Dietary Antioxidants Resveratrol and Quercetin Protect Cells from Exogenous Pro-Oxidative Damage. Food Chem. Toxicol. 2008, 46, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.B.; Rajendiran, V.; Kasinathan, N.K.; Amrithalakshmi, P.; Venkatabalasubramanian, S.; Murali, M.R.; Devaraj, H.; Devaraj, S.N. Differential Cytotoxic Activity of Quercetin on Colonic Cancer Cells Depends on ROS Generation through COX-2 Expression. Food Chem. Toxicol. 2017, 106, 92–106. [Google Scholar] [CrossRef]
- Kee, J.-Y.; Han, Y.-H.; Kim, D.-S.; Mun, J.-G.; Park, J.; Jeong, M.-Y.; Um, J.-Y.; Hong, S.-H. Inhibitory Effect of Quercetin on Colorectal Lung Metastasis through Inducing Apoptosis, and Suppression of Metastatic Ability. Phytomedicine 2016, 23, 1680–1690. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Kogiannou, D.A.A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M.G.; Kaliora, A.C. Herbal Infusions; Their Phenolic Profile, Antioxidant and Anti-Inflammatory Effects in HT29 and PC3 Cells. Food Chem. Toxicol. 2013, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Galante, M.; De Flaviis, R.; Boeris, V.; Spelzini, D. Effects of the Enzymatic Hydrolysis Treatment on Functional and Antioxidant Properties of Quinoa Protein Acid-Induced Gels. LWT 2020, 118, 108845. [Google Scholar] [CrossRef]
- Felix, M.; Cermeño, M.; Romero, A.; FitzGerald, R.J. Characterisation of the Bioactive Properties and Microstructure of Chickpea Protein-Based Oil in Water Emulsions. Food Res. Int. 2019, 121, 577–585. [Google Scholar] [CrossRef]
- Martínez, J.H.; Velázquez, F.; Burrieza, H.P.; Martínez, K.D.; Paula Domínguez Rubio, A.; dos Santos Ferreira, C.; del Pilar Buera, M.; Pérez, O.E. Betanin Loaded Nanocarriers Based on Quinoa Seed 11S Globulin. Impact on the Protein Structure and Antioxidant Activity. Food Hydrocoll. 2019, 87, 880–890. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
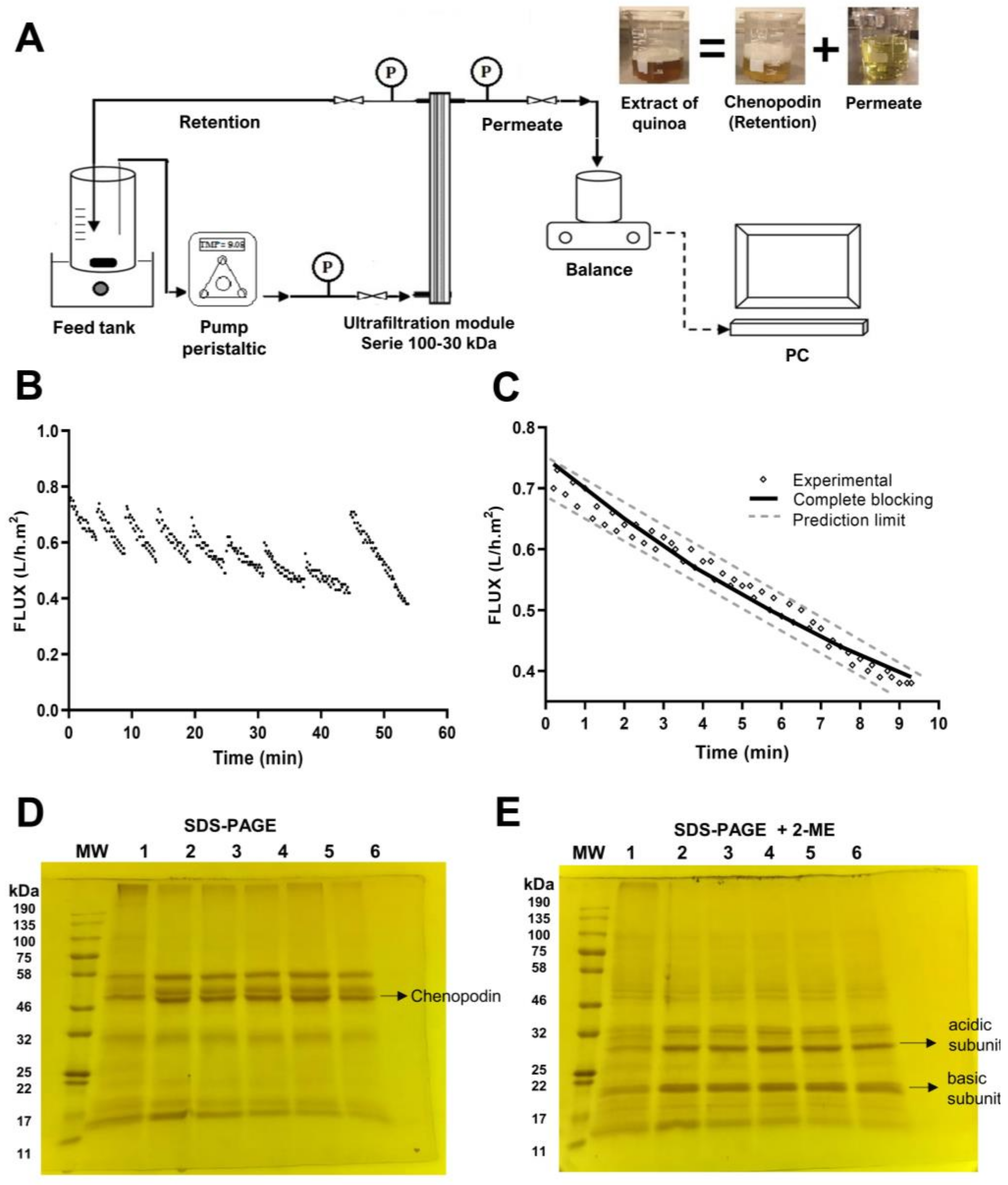

| Crossflow UF Method 1 * | Crossflow UF Method 2 this work | |||
|---|---|---|---|---|
| DF | Concentration | DF | Concentration | |
| 30 and 100 kDa | 100 kDa | 100–30 kDa | 100–30 kDa | |
| Feed volume (mL) | 200 | 200 | 200 | 400 |
| Operating time (h) | 1.32 | 0.06 | 0.76 | 0.16 |
| Final retained volume (mL) | 200 | 40 | 200 | 50 |
| Soluble proteins in final concentration stage (mg/mL) | 21.11 | 42.7 | ||
| Total protein in final concentrate (g) | 0.844 | 2.14 | ||
| Protein yield (% w/w) | 26.14 | 32.75 | ||
| NE Control | Ratio QPA/Tw80 | Size (nm) | PDI | Zeta Potential | EE (%) | LC (mg/mL) |
|---|---|---|---|---|---|---|
| F1 | 0/100 | 170.7 ± 1.5 a | 0.215 ± 0.006 a | −5.67 ± 0.58 a | N.D. | N.D. |
| F2 | 20/80 | 178.3 ± 2.5 b | 0.241 ± 0.013 b | −7.97 ± 0.26 b | N.D. | N.D. |
| F3 | 40/60 | 201.3 ± 3.5 c | 0.238 ± 0.010 b | −8.31 ± 0.55 b | N.D. | N.D. |
| F4 | 60/40 | 219.3 ± 1.5 d | 0.237 ± 0.009 b | −9.40 ± 1.58 b | N.D. | N.D. |
| F5 | 70/30 | 248.0 ± 10.8 e | 0.245 ± 0.022 b | −11.20 ± 1.00 bc | N.D. | N.D. |
| F6 | 80/20 | 263.0 ± 2.6 f | 0.252 ± 0.013 b | −14.50 ± 1.36 cd | N.D. | N.D. |
| NE-Qt | ||||||
| F1Qt | 0/100 | 160.1 ± 2.2 a | 0.232 ± 0.013 a | −13.03 ± 1.81 a | 90.78 ± 3.77 a | 0.91 ± 0.04 a |
| F2Qt | 20/80 | 175.3 ± 1.2 b | 0.282 ± 0.012 b | −18.33 ± 1.58 b | 89.83 ± 5.91 a | 0.90 ± 0.06 a |
| F3Qt | 40/60 | 202.3 ± 4.2 c | 0.270 ± 0.010 b | −19.00 ± 1.73 b | 88.90 ± 6.11 a | 0.89 ± 0.06 a |
| F4Qt | 60/40 | 226.8 ± 3.2 d | 0.249 ± 0.021 a | −20.57 ± 1.75 b | 87.66 ± 2.69 a | 0.88 ± 0.03 a |
| F5Qt | 70/30 | 257.8 ± 2.5 e | 0.261 ± 0.014 b | −22.13 ± 1.25 bc | 85.72 ± 3.82 a | 0.86 ± 0.04 a |
| F6Qt | 80/20 | 314.8 ± 7.8 f | 0.258 ± 0.012 b | −24.90 ± 1.65 cd | 86.00 ± 3.43 a | 0.86 ± 0.02 a |
| ABTS | ORAC | ||
|---|---|---|---|
| µmol TE/mg of Quercetin | IC50 (µg/mL) | µmol TE/mg of Quercetin | |
| Free Qt | 7.57 ± 0.19 a | 2.13 ± 0.01 a | 50.01 ± 2.23 a |
| F1Qt | 7.01 ± 0.09 b | 2.44 ± 0.05 b | 51.76 ± 1.25 a |
| F4Qt | 7.52 ± 0.10 a | 2.14 ± 0.08 a | 55.33 ± 2.70 b |
| P4Qt | 7.92 ± 0.40 ac | 2.11 ± 0.03 a | 56.85 ± 5.32 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intiquilla, A.; Arazo, M.; Gamboa, A.; Caro, N.; Gotteland, M.; Palomino-Calderón, A.; Abugoch, L.; Tapia, C. Nanoemulsions Based on Soluble Chenopodin/Alginate Complex for Colonic Delivery of Quercetin. Antioxidants 2024, 13, 658. https://doi.org/10.3390/antiox13060658
Intiquilla A, Arazo M, Gamboa A, Caro N, Gotteland M, Palomino-Calderón A, Abugoch L, Tapia C. Nanoemulsions Based on Soluble Chenopodin/Alginate Complex for Colonic Delivery of Quercetin. Antioxidants. 2024; 13(6):658. https://doi.org/10.3390/antiox13060658
Chicago/Turabian StyleIntiquilla, Arturo, Migdalia Arazo, Alexander Gamboa, Nelson Caro, Martin Gotteland, Alan Palomino-Calderón, Lilian Abugoch, and Cristian Tapia. 2024. "Nanoemulsions Based on Soluble Chenopodin/Alginate Complex for Colonic Delivery of Quercetin" Antioxidants 13, no. 6: 658. https://doi.org/10.3390/antiox13060658







