Antioxidant Activity and Oxidative Damage Associated with Seeding Surgery for Pearl Culture in the Winged Pearl Oyster Pteria sterna
Abstract
:1. Introduction
2. Materials and Methods
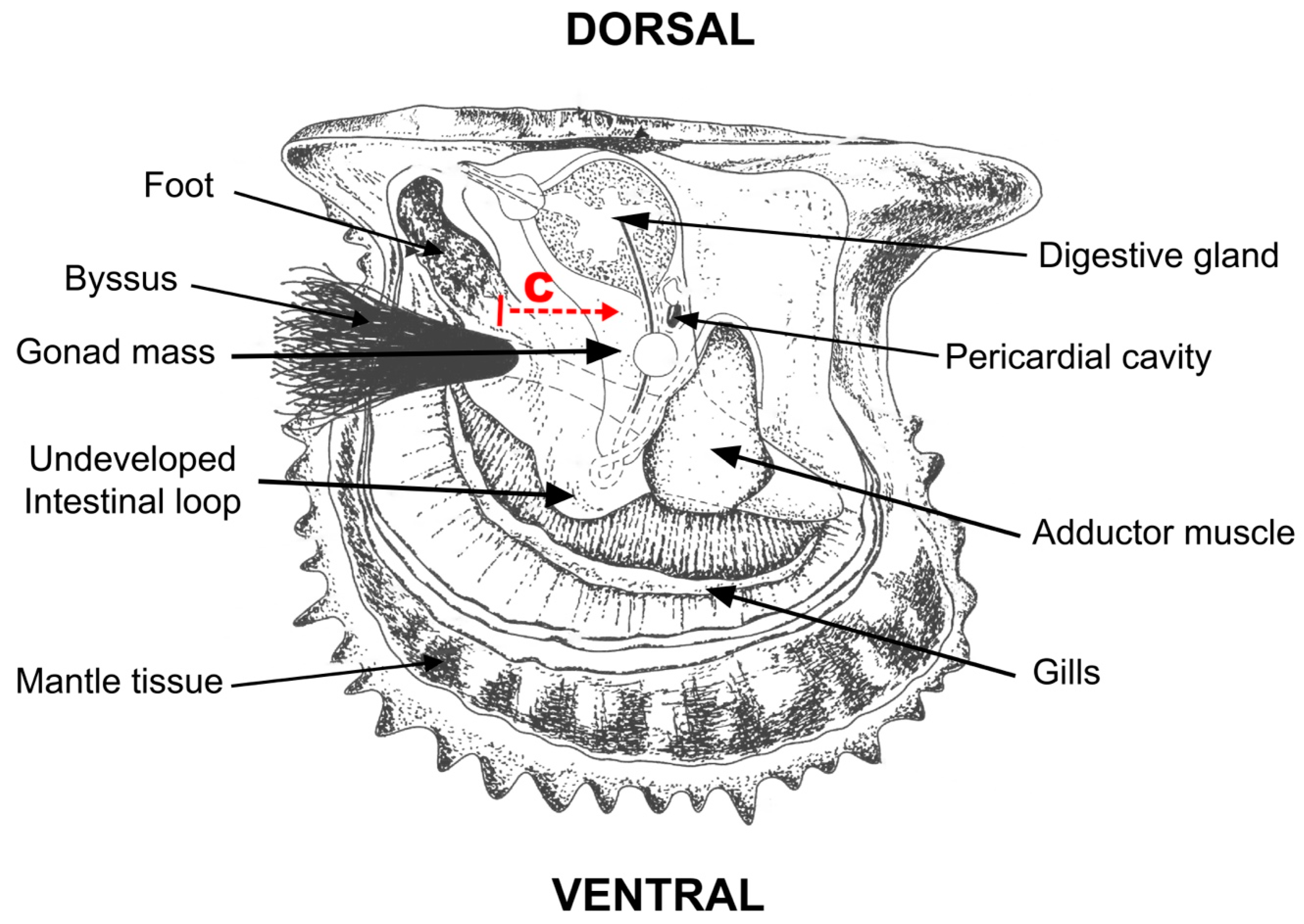
2.1. Experimental Design
2.2. Analysis of Antioxidant Activity
2.2.1. Superoxide Dismutase (SOD)
2.2.2. Catalase (CAT)
2.2.3. Glutathione Peroxidase (GPx)
2.3. Analysis of Oxidative Damage
2.4. Statistical Analysis
3. Results
3.1. Antioxidant Activity
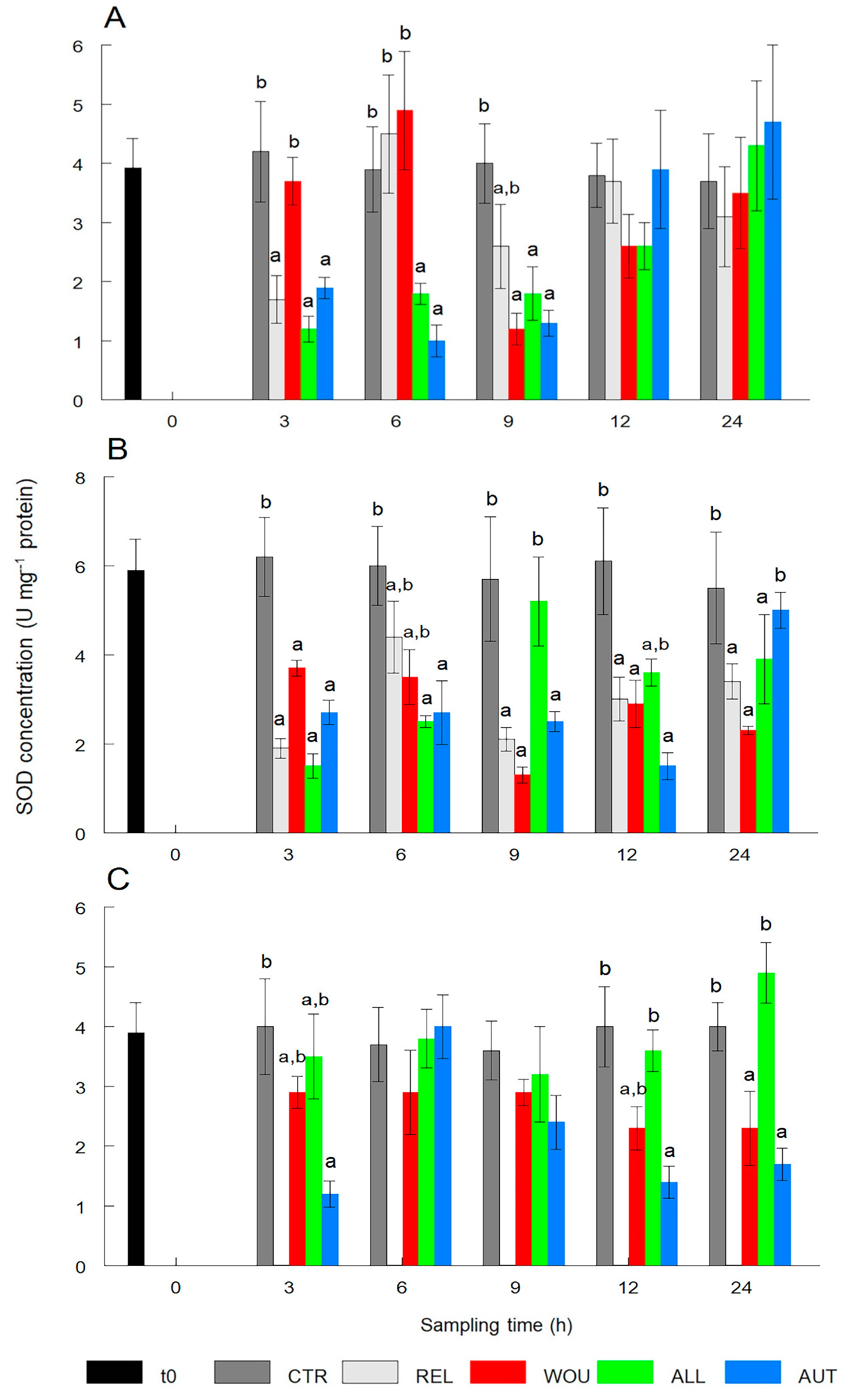
3.1.1. Superoxide Dismutase (SOD)
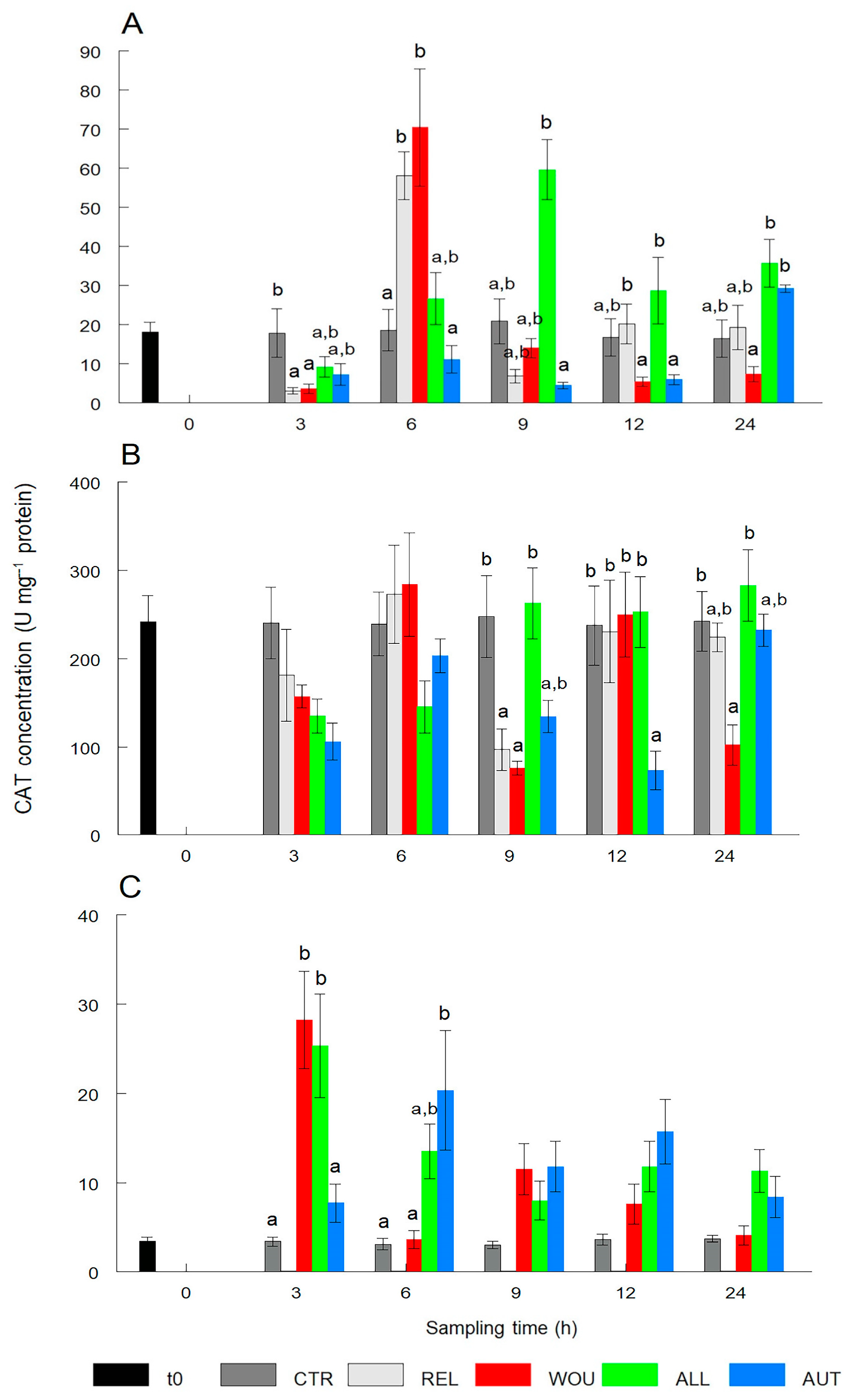
3.1.2. Catalase (CAT)
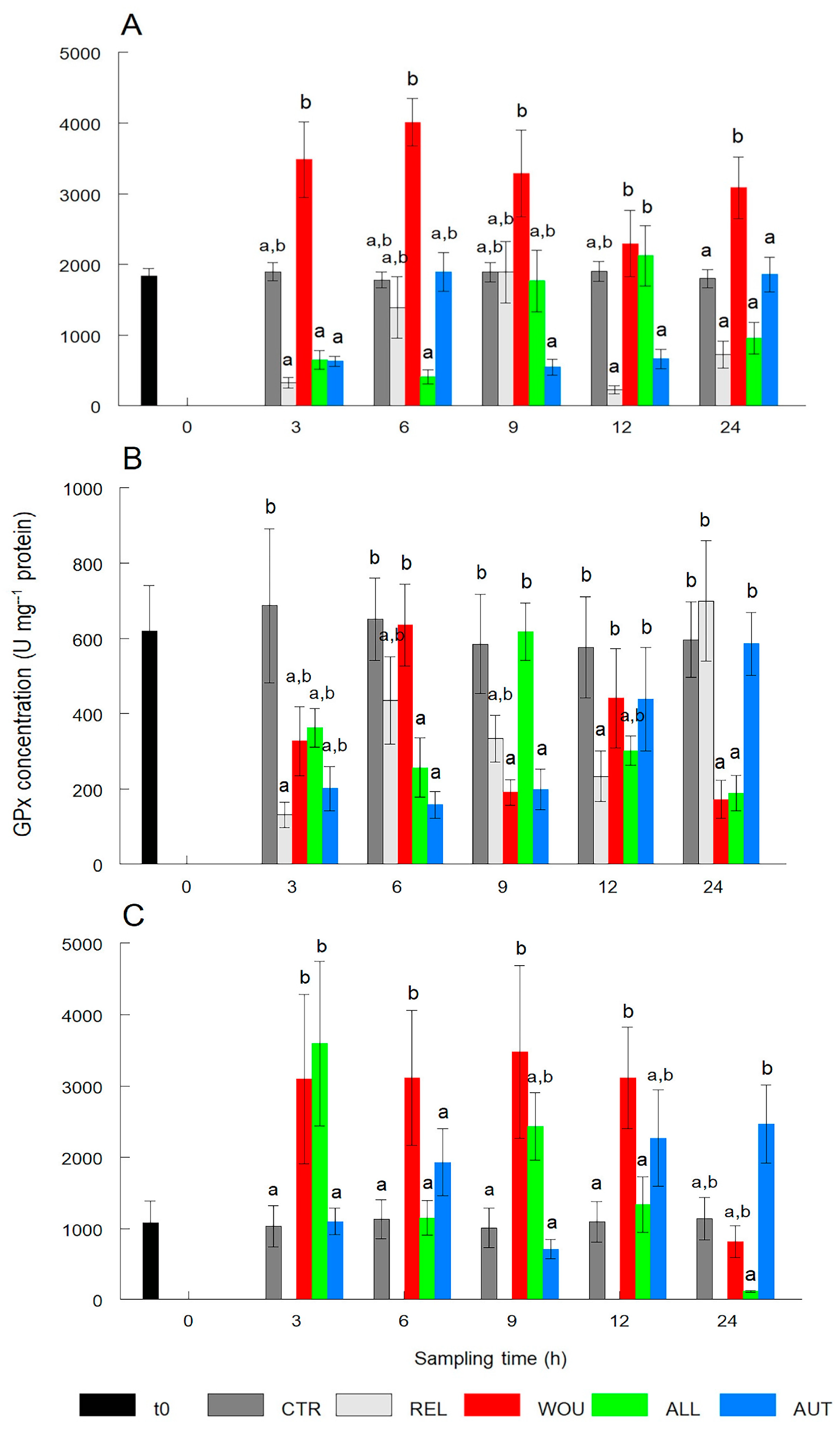
3.1.3. Glutathione Peroxidase (GPx)
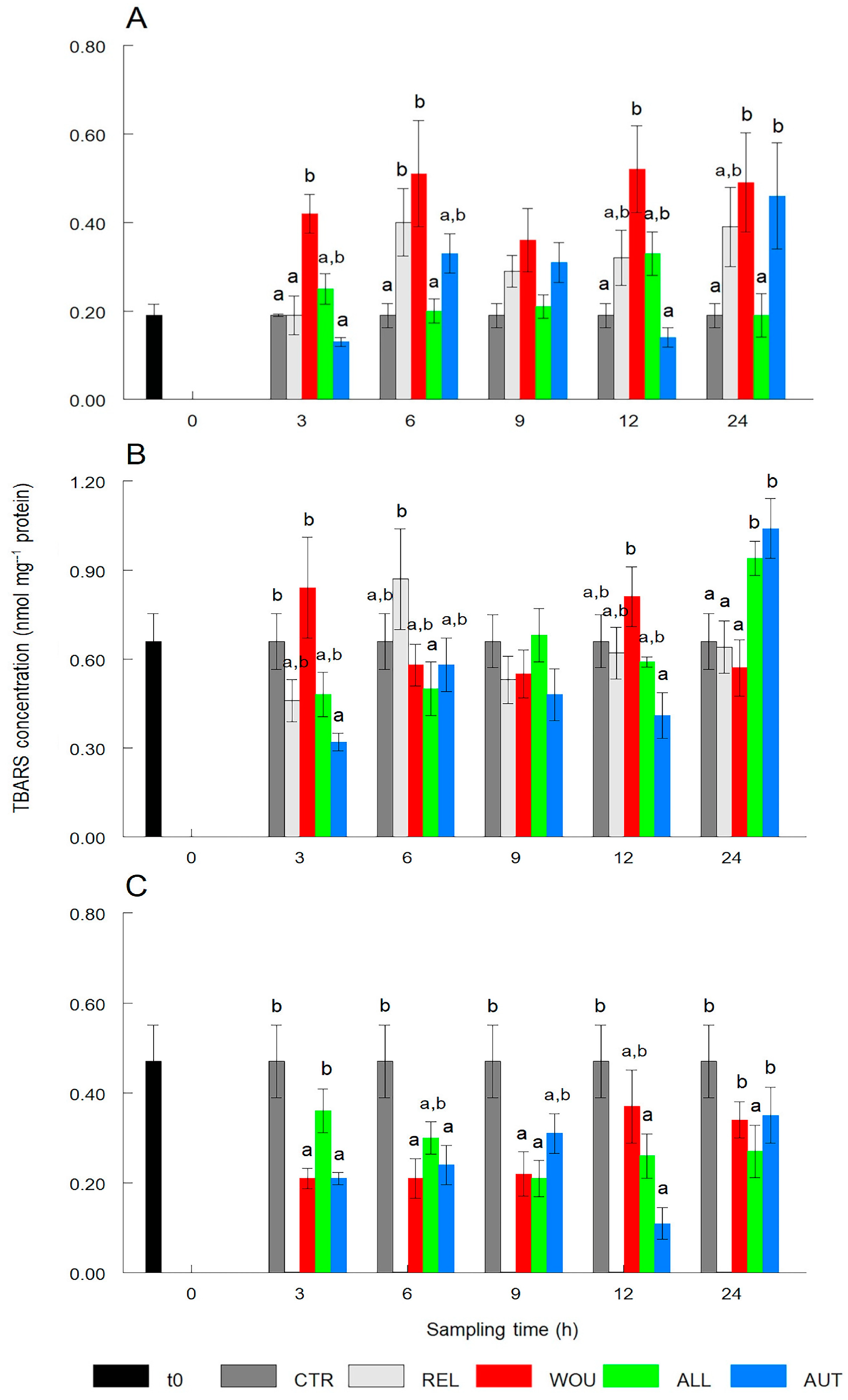
3.2. Oxidative Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDougall, C.; Moase, P.; Degnan, B.M. Host and Donor Influence on Pearls Produced by the Silver-lip Pearl Oyster, Pinctada maxima. Aquaculture 2016, 450, 313–320. [Google Scholar] [CrossRef]
- Saucedo, P.E.; McLaurin, D.; Lodeiros, C.; Freites, L.; León, L.; Cáceres-Puig, J.I.; Albuquerque, M.C.P.; Southgate, P.C.; Acosta-Salmon, H. Progress Towards Reestablishing Latin America as a Major Pearl Producing Region. A Review. Rev. Aquacult. 2023, 15, 242–260. [Google Scholar] [CrossRef]
- McGinty, E.L.; Evans, B.S.; Taylor, J.U.U.; Jerry, D.R. Xenografts and Pearl Production in Two Pearl Oyster Species, P. maxima and P. margaritifera: Effect on Pearl Quality and a Key to Understanding Genetic Contribution. Aquaculture 2010, 302, 175–181. [Google Scholar] [CrossRef]
- Fukushima, E.I.; Iwai, T.; Miura, C.; Celino, F.T.; Urasaki, S.; Miura, T. A Xenograft Mantle Transplantation Technique for Producing a Novel Pearl in an Akoya Oyster Host. Mar. Biotechnol. 2014, 16, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Salmon, H.; Southgate, P.C. Mantle Regeneration in the Pearl Oysters Pinctada fucata and Pinctada margaritifera. Aquaculture 2005, 246, 447–453. [Google Scholar] [CrossRef]
- Mamangkey, N.G.F.; Southgate, P.C. Regeneration of Excised Mantle Tissue by the Silver-lip Pearl Oyster, Pinctada maxima. Fish Shellfish Immunol. 2009, 27, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yang, S.; Cao, Y.; Zheng, Z.; Deng, Y.; Wang, Q.; Huang, R.; Du, X. Genome and Transcriptome Analyses Providing Insight Into the Immune Response of Pearl Oysters After Allograft and Xenograft Transplantations. Fish Shellfish Immunol. 2019, 90, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Adzigbli, L.; Wang, Z.; Li, J.; Deng, Y. Survival, Retention Rate and Immunity of the Black Shell-colored Stocks of Pearl Oyster Pinctada fucata martensii After Grafting Operation. Fish Shellfish Immunol. 2020, 98, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Fanb, S.; Liub, B.; Zhangb, B.; Sub, J.; Yu, D. Transcriptome Analysis of the Immune Reaction of the Pearl Oyster Pinctada fucata to Xenograft From Pinctada maxima. Fish Shellfish Immunol. 2017, 65, 331–345. [Google Scholar] [CrossRef]
- Wei, J.; Liu, B.; Fan, S.; Li, H.; Chen, M.; Zhang, B.; Su, J.; Meng, Z.; Yu, D. Differentially Expressed Immune-related Genes in Hemocytes of the Pearl Oyster Pinctada fucata against Allograft Identified by Transcriptome Analysis. Fish Shellfish Immunol. 2017, 62, 247–256. [Google Scholar] [CrossRef]
- Ma, X.; Deng, D.; Chen, W. Inhibitors and Activators of SOD, GSH-Px, and CAT. In Enzyme Inhibitors and Activators; IntechOpen: London, UK, 2017; Volume 29, pp. 207–224. [Google Scholar] [CrossRef]
- Canesi, L.; Auguste, M.; Balbi, T.; Prochazkova, P. Soluble Mediators of Innate Immunity in Annelids and Bivalve Mollusks: A Mini-review. Front. Immunol. 2022, 13, 1051155. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative Stress as a Mediator of Life History Trade-offs: Mechanisms, Measurements and Interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Istomina, A.; Yelovskaya, O.; Chelomin, V.; Karpenko, A.; Zvyagintsev, A. Antioxidant Activity of Far Eastern Bivalves in Their Natural Habitat. Mar. Environ. Res. 2021, 169, 105383. [Google Scholar] [CrossRef] [PubMed]
- Porte, C.; Sole, M.; Alborges, J.; Livingtone, D. Responses of Mixed-function Oxygenase and Antioxidant Enzyme System of Mytilus sp. to Organic Pollution. Comp. Biochem. Physiol. 2001, 100C, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Testa, C.P.; Gáspari, C.; Masutti, M.B.; Curi-Pedrosa, R.; de Almeida, E.A.; Filho, D.W. Oxidative Stress in the Mussel Mytella guyanensis From Polluted Mangroves on Santa Catarina Island, Brazil. Mar. Pollut. Bull. 2002, 44, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Geret, F.; Serafim, A.; Bebianno, M.J. Antioxidant Enzyme Activities, Metallothioneins and Lipid Peroxidation as Biomarkers in Ruditapes decussatus? Ecotoxicology 2003, 12, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Cantú-Medellín, N.; Olguín, N.O.; Méndez-Rodríguez, L.C.; Zenteno-Savín, T. Antioxidant Enzymes and Heavy Metal Levels in Tissues of the Black Chocolate Clam Megapitaria squalida. Arch. Environ. Contam. Toxicol. 2009, 56, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Nusetti, O.; Marcano, L.; Zapata, E.; Esclapés, M.; Nusetti, S.; Lodeiros, C. Respuestas Inmunológicas y de Enzimas Antioxidantes en la Ostra Perla Pinctada imbricata (Mollusca: Pteridae) Expuesta a Niveles Subletales de Fuel Oil n°6. Interciencia 2004, 29, 324–328, (In Spanish Abstract in English). [Google Scholar]
- Jing, G.; Li, Y.; Xie, L.; Zhang, R. Metal Accumulation and Enzyme Activities in Gills and Digestive Gland of Pearl Oyster (Pinctada fucata) Exposed to Copper. Comp. Biochem. Physiol. 2006, 144C, 184–190. [Google Scholar] [CrossRef]
- Anju, A.; Jeswin, J.; Thomas, P.C.; Paulton, M.P.; Vijayan, K.K. Molecular Cloning, Characterization and Expression Analysis of Cytoplasmic Cu/Zn-Superoxide Dismutase (SOD) From Pearl Oyster Pinctada fucata. Fish Shellfish Immunol. 2013, 34, 94650. [Google Scholar] [CrossRef]
- Kiefert, L.; McLaurin-Moreno, D.; Arizmendi-Castillo, E.; Hänni, H.A.; Elen, S. Cultured Pearls From the Gulf of California, Mexico. Gems Gemol. 2004, 40, 26–38. [Google Scholar] [CrossRef]
- Nava, M.; Arizmendi, E.; McLaurin, D. Evaluation of Success in the Seeding of Round Nuclei in Pteria sterna, A New Species in Pearl Culture. SPC Pearl Oyster Inf. Bull. 2000, 14, 12–16. [Google Scholar]
- Granados-Amores, A.; Saucedo, P.E.; Mazón-Suástegui, J.M.; Acosta-Salmón, H.; Zenteno-Savin, T.; Rodríguez-Jaramillo, C.; Campa-Córdova, A.I. Does Relaxation as a Sedative Therapy Previous to Pearl Production Affect Antioxidant response and Cause Oxidative Damage in the Winged Pearl Oyster Pteria sterna? Aquaculture 2018, 491, 295–301. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Measurement of Mn-SOD And Cu, Zn-SOD. In Experimental Protocols for Reactive Oxygen and Nitrogen Species; Taniguchi, N., Gutteridge, J., Eds.; Oxford University Press: London, UK, 2000; pp. 91–95. [Google Scholar]
- Aebi, H. Catalase in vivo. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Flohe, L.; Günzler, W.A. Assays of Glutathione Peroxidase. In Methods in Enzymology; Packer, L., Ed.; Oxygen Radicals in Biological Systems; Academic Press: New York, NY, USA, 1984; Volume 105, pp. 114–120. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yogi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 331–358. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.; Rolf, R. Introduction to Biostatistics, 2nd ed.; Dover Publications: Mineola, NY, USA, 2009. [Google Scholar]
- Regoli, F.; Nigro, M.; Orlando, E. Lysosomal and Antioxidant Responses to Metals in The Antarctic Scallop Adamussium colbecki. Aquat. Toxicol. 1998, 40, 375–392. [Google Scholar] [CrossRef]
- Campa-Córdova, A.I.; Nuñez, E.J.; Luna-González, A.; Romero-Geraldo, M.J.; Ascencio, F. Superoxide Dismutase Activity in Juvenile Litopenaeus vannamei and Nodipecten subnodosus Exposed to Toxic Dinoflagellate Prorocentrum lima. Comp. Biochem. Physiol. 2009, 149C, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Borković-Mitić, S.; Kovačević, T.B.; Perendija, B.R.; Despotović, S.; Gavrić, J.; Pavlović, S.; Saičić, Z. Superoxide Dismutase and Catalase Activities in the Digestive Gland and Gills of the Freshwater Bivalve Unio pictorum From the Sava River. Arch. Biol. Sci. 2011, 63, 185–192. [Google Scholar] [CrossRef]
- Soldatov, A.A.; Gostyukhina, O.L.; Borodina, A.V.; Golovina, I.V. Qualitative Composition of Carotenoids, Catalase and Superoxide Dismutase Activities in Tissues of the Bivalve Anadara inaequivalvis (Bruguiere, 1789). J. Evol. Biochem. Physiol. 2013, 49, 389–398. [Google Scholar] [CrossRef]
- Filho, W.D.; Tribess, T.; Gaspari, C.; Claudio, F.D.; Torres, M.A.; Magalhaes, A.R.M. Seasonal Changes in Antioxidant Defenses of the Digestive Gland of the Brown Mussel (Perna perna). Aquaculture 2001, 203, 149–158. [Google Scholar] [CrossRef]
- Cossu, C.; Doyotte, A.; Jacquin, M.C.; Babut, M.; Exinger, A.; Vasseur, P. Glutathione Reductase, Selenium-dependent Glutathione Peroxidase, Glutathione Levels, and Lipid Peroxidation in Freshwater Bivalve, Unio tumidus, As Biomarkers of Aquatic Contamination in Field Studies. Ecotox. Environ. Saf. 1997, 38, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Shi, S.; Cao, Y.; Yang, C.; Wang, Q.; Jiao, W.Y. Use of Mycophenolate Mofetil as Immunosuppressant During Pearl Production in Pearl Oyster Pinctada fucata martensii. Aquac. Res. 2021, 2, 4043–4049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados-Amores, A.; Campa-Córdova, Á.I.; Acosta-Salmón, H.; Angulo, C.; Zenteno-Savín, T.; Rodríguez-Jaramillo, C.; Saucedo, P.E. Antioxidant Activity and Oxidative Damage Associated with Seeding Surgery for Pearl Culture in the Winged Pearl Oyster Pteria sterna. Antioxidants 2024, 13, 723. https://doi.org/10.3390/antiox13060723
Granados-Amores A, Campa-Córdova ÁI, Acosta-Salmón H, Angulo C, Zenteno-Savín T, Rodríguez-Jaramillo C, Saucedo PE. Antioxidant Activity and Oxidative Damage Associated with Seeding Surgery for Pearl Culture in the Winged Pearl Oyster Pteria sterna. Antioxidants. 2024; 13(6):723. https://doi.org/10.3390/antiox13060723
Chicago/Turabian StyleGranados-Amores, Andrés, Ángel I. Campa-Córdova, Héctor Acosta-Salmón, Carlos Angulo, Tania Zenteno-Savín, Carmen Rodríguez-Jaramillo, and Pedro E. Saucedo. 2024. "Antioxidant Activity and Oxidative Damage Associated with Seeding Surgery for Pearl Culture in the Winged Pearl Oyster Pteria sterna" Antioxidants 13, no. 6: 723. https://doi.org/10.3390/antiox13060723




