Induction of Semaphorin 3A by Resveratrol and Pinostilbene via Activation of the AHR-NRF2 Axis in Human Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Cell Viability Test
2.4. Quantitative Real-Time PCR (qPCR) Analysis
2.5. Small Interfering RNA Transfection
2.6. Western Blot Analysis
2.7. Luciferase Assay on SEMA3A Promoter
2.8. ChIP-qPCR Assay
2.9. Statistical Analysis
3. Results
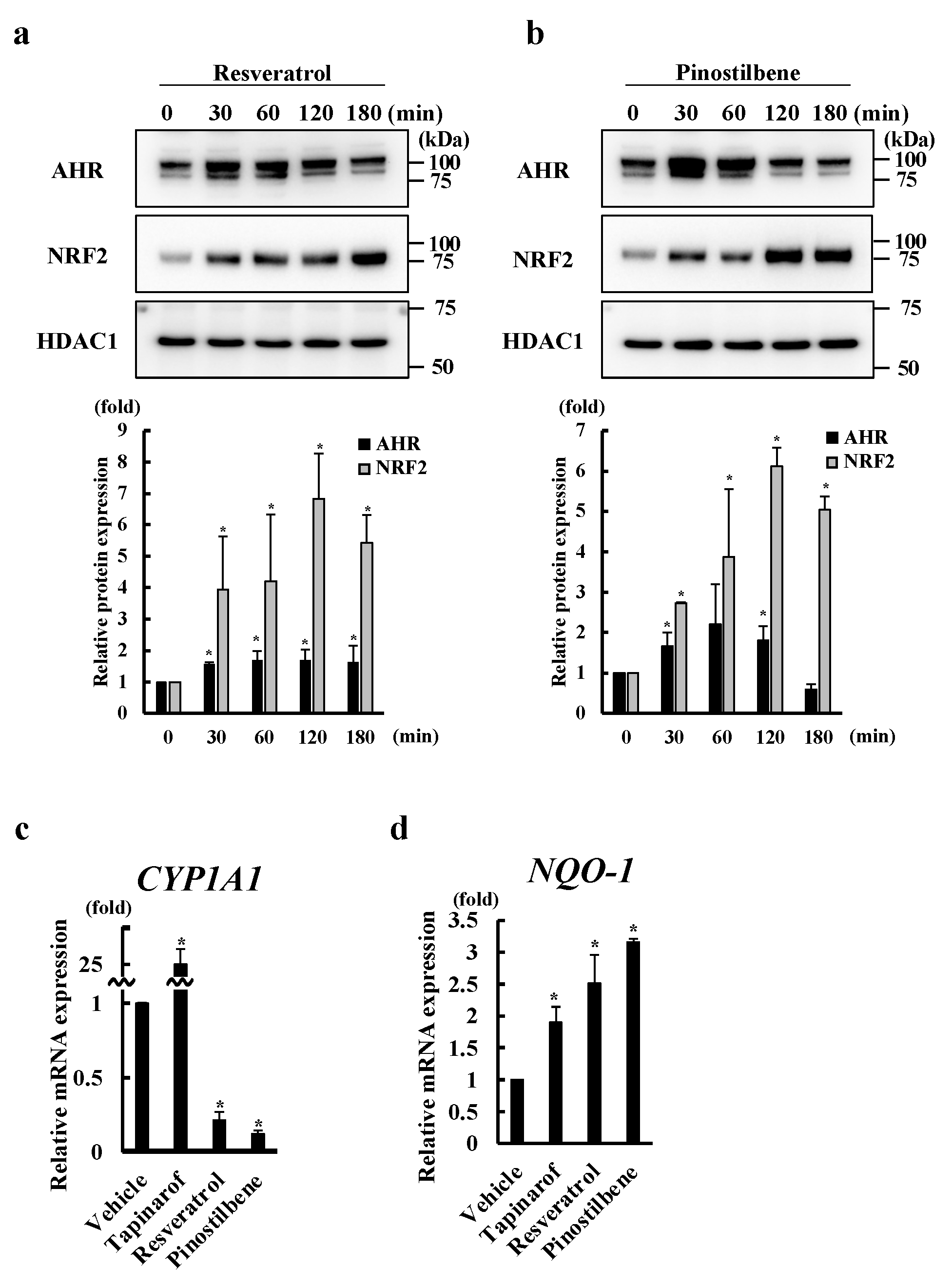
3.1. Resveratrol and Pinostilbene Upregulated SEMA3A in NHEKs
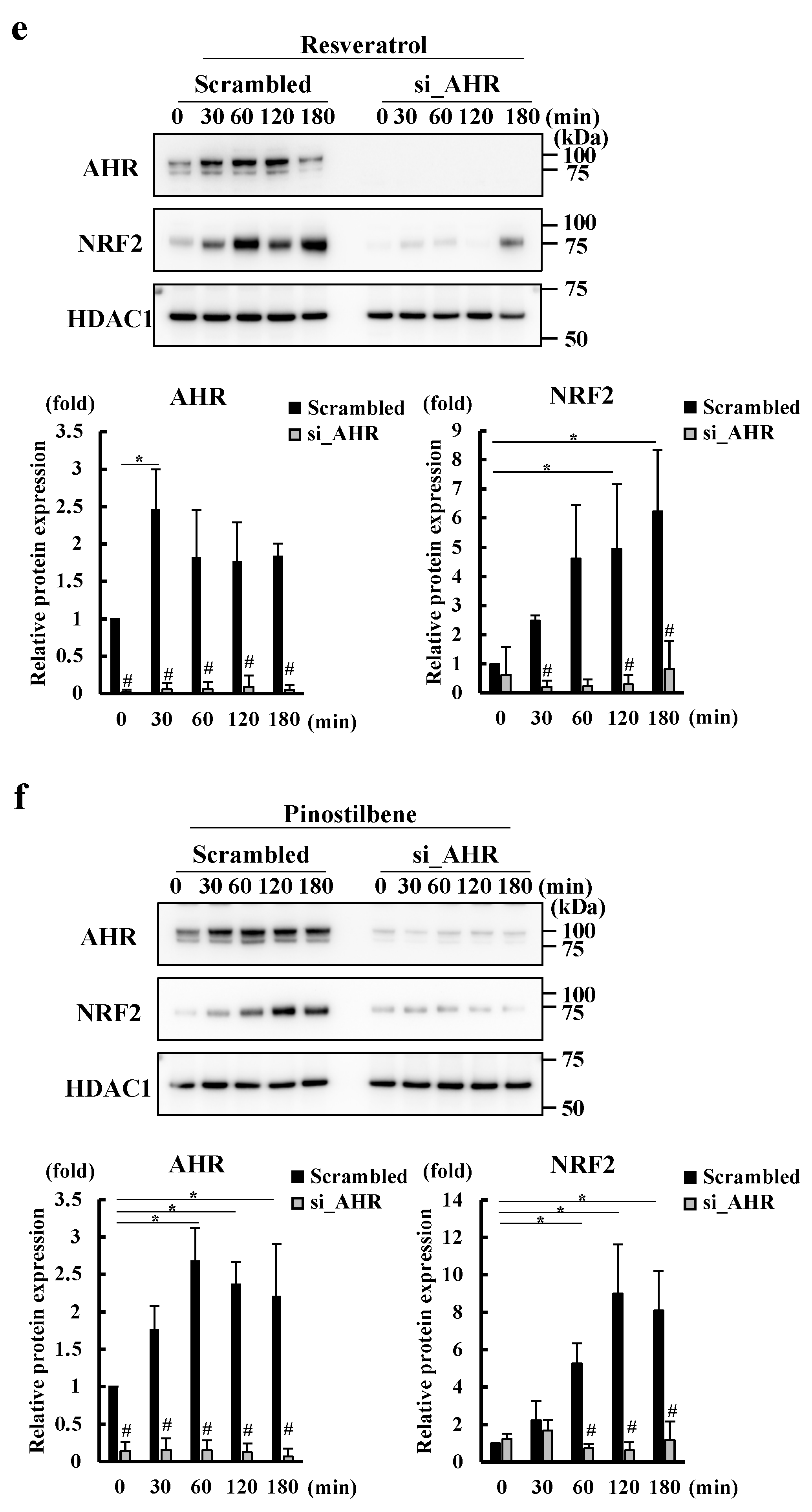
3.2. Resveratrol and Pinostilbene Activated the AHR-NRF2 Axis in NHEKs
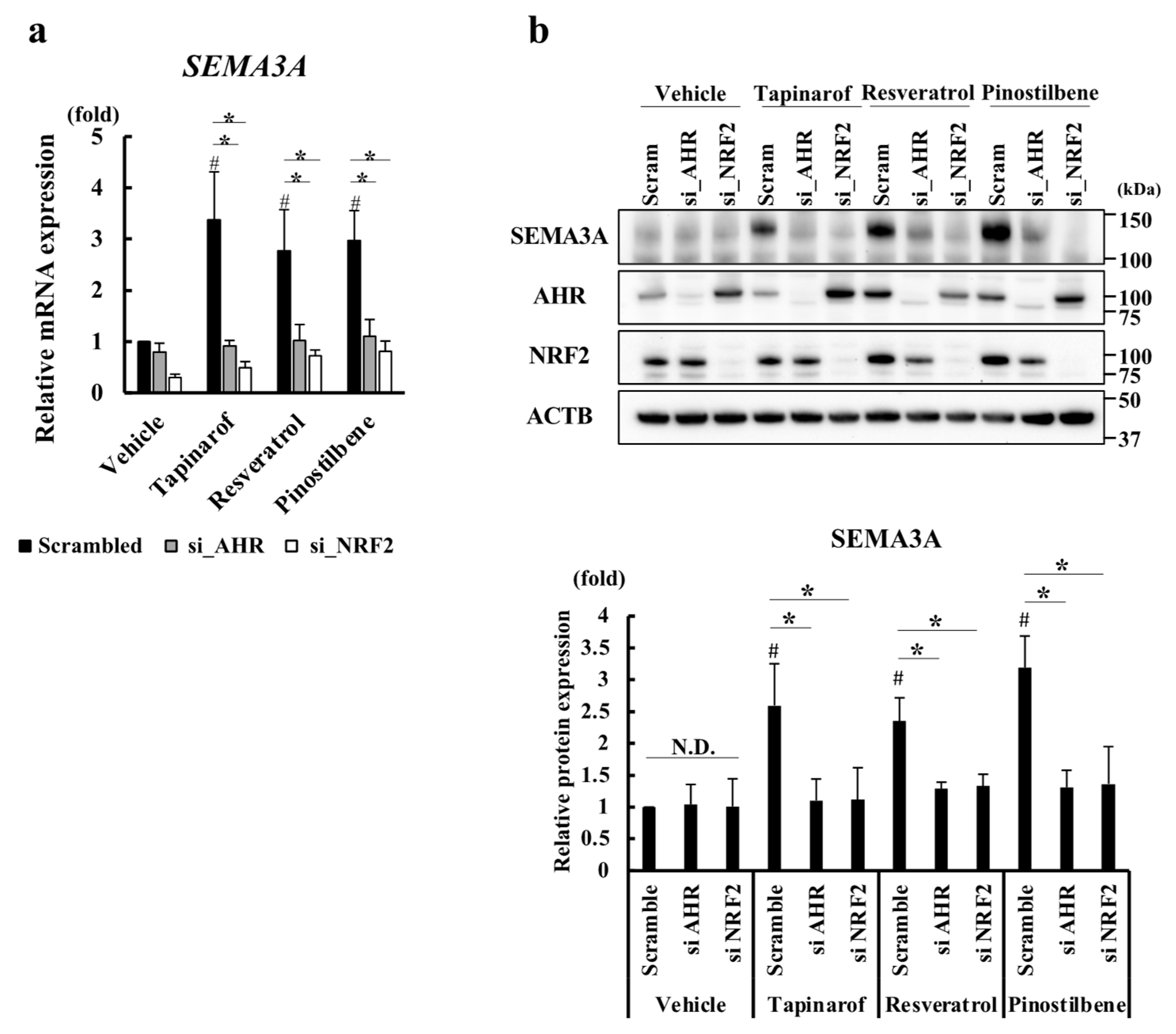
3.3. Resveratrol and Pinostilbene Upregulated SEMA3A via the AHR-NRF2 Axis in NHEKs
3.4. Resveratrol and Pinostilbene Increased the Promoter Activity of SEMA3A via NRF2 in NHEKs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zeidler, C.; Pereira, M.P.; Huet, F.; Misery, L.; Steinbrink, K.; Ständer, S. Pruritus in Autoimmune and Inflammatory Dermatoses. Front. Immunol. 2019, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Depner, M.; Karvonen, A.M.; Renz, H.; Braun-Fahrländer, C.; Schmausser-Hechfellner, E.; Pekkanen, J.; Riedler, J.; Dalphin, J.C.; et al. Phenotypes of Atopic Dermatitis Depending on the Timing of Onset and Progression in Childhood. JAMA Pediatr. 2017, 171, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Wang, A.R.; Li, W.Q.; Sevetson, E.; Block, J.K.; Qureshi, A.A. The Burden of Atopic Dermatitis: Summary of a Report for the National Eczema Association. J. Investig. Dermatol. 2017, 137, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Weisshaar, E.; Bentz, P.; Apfelbacher, C.; Haufe, E.; Heinrich, L.; Heratizadeh, A.; Abraham, S.; Harder, I.; Kleinheinz, A.; Wollenberg, A.; et al. TREATgermany study group. Itching in Atopic Dermatitis: Patient- and Physician-reported Outcomes in the German Atopic Dermatitis Registry TREATgermany. Acta Derm. Venereol. 2023, 103, adv00854. [Google Scholar] [CrossRef] [PubMed]
- Furue, K.; Ulzii, D.; Tanaka, Y.; Ito, T.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Pathogenic Implication of Epidermal Scratch Injury in Psoriasis and Atopic Dermatitis. J. Dermatol. 2020, 47, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic Dermatitis: Immune Deviation, Barrier Dysfunction, IgE Autoreactivity and New Therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and Recent Advances in the Pathophysiology of Atopic Dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune Communication Regulating Pruritus in Atopic Dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1875–1898. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.; Yosipovitch, G. A New Generation of Treatments for Itch. Acta Derm. Venereol. 2020, 100, adv00027. [Google Scholar] [CrossRef]
- Tominaga, M.; Takamori, K. Peripheral Itch Sensitization in Atopic Dermatitis. Allergol. Int. 2022, 71, 265–277. [Google Scholar] [CrossRef]
- Takahashi, S.; Ishida, A.; Kubo, A.; Kawasaki, H.; Ochiai, S.; Nakayama, M.; Koseki, H.; Amagai, M.; Okada, T. Homeostatic Pruning and Activity of Epidermal Nerves Are Dysregulated in Barrier-impaired Skin during Chronic Itch Development. Sci. Rep. 2019, 9, 8625. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Kim, B.; Luger, T.; Lerner, E.; Metz, M.; Adiri, R.; Canosa, J.M.; Cha, A.; Ständer, S. Similarities and Differences in Peripheral Itch and Pain Pathways in Atopic Dermatitis. J. Allergy Clin. Immunol. 2023, 153, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Tengara, S.; Kamo, A.; Ogawa, H.; Takamori, K. Psoralen-Ultraviolet A Therapy Alters Epidermal Sema3A and NGF Levels and Modulates Epidermal Innervation in Atopic Dermatitis. J. Dermatol. Sci. 2009, 55, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kamo, A.; Tominaga, M.; Tengara, S.; Ogawa, H.; Takamori, K. Inhibitory Effects of UV-based Therapy on Dry Skin-inducible Nerve Growth in Acetone-treated Mice. J. Dermatol. Sci. 2011, 62, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, E.P.; Rutto, K.V. Semaphorin 3A in the Immune System: Twenty Years of Study. Biochemistry 2022, 87, 640–657. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Rothenberg, M.E.; Sun, X.; Bachert, C.; Artis, D.; Zaheer, R.; Deniz, Y.; Rowe, P.; Cyr, S. Neuroimmune Interplay during Type 2 Inflammation: Symptoms, Mechanisms, and Therapeutic Targets in Atopic Diseases. J. Allergy Clin. Immunol. 2023, 153, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Kabata, H.; Artis, D. Neuro-immune Crosstalk and Allergic Inflammation. J. Clin. Investig. 2019, 129, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Negi, O.; Tominaga, M.; Tengara, S.; Kamo, A.; Taneda, K.; Suga, Y.; Ogawa, H.; Takamori, K. Topically Applied Semaphorin 3A Ointment Inhibits Scratching Behavior and Improves Skin Inflammation in NC/Nga Mice with Atopic Dermatitis. J. Dermatol. Sci. 2012, 66, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kamata, Y.; Tominaga, M.; Umehara, Y.; Yoshida, I.; Matsuoka, N.; Takamori, K. Extract of Scutellaria baicalensis Induces Semaphorin 3A Production in Human Epidermal Keratinocytes. PLoS ONE 2021, 16, e0250663. [Google Scholar] [CrossRef]
- Tsuji, G.; Yumine, A.; Yamamura, K.; Takemura, M.; Kido-Nakahara, M.; Ito, T.; Nakahara, T. The Therapeutic Aryl Hydrocarbon Receptor-Modulating Agent Tapinarof Regulates SEMA3A Expression in Human Keratinocytes through NRF2. J. Investig. Dermatol. 2024, 144, 710–713.e8. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An Environmental Contaminant, Benzo(a)pyrene, Induces Oxidative Stress-mediated Interleukin-8 Production in Human Keratinocytes via the Aryl Hydrocarbon Receptor Signaling Pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of Ketoconazole as an AhR-Nrf2 Activator in Cultured Human Keratinocytes: The Basis of its Anti-inflammatory Effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Newton, E.M.; Hsiao, J.; Shi, V.Y. Aryl Hydrocarbon Receptor/Nuclear Factor E2-related Factor 2 (AHR/NRF2) Signalling: A Novel Therapeutic Target for Atopic Dermatitis. Exp. Dermatol. 2022, 31, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Itoh, K.; Yamamoto, M.; Kensler, T.W. Enhanced Expression of the Transcription Factor Nrf2 by Cancer Chemopreventive Agents: Role of Antioxidant Response Element-like Sequences in the Nrf2 Promoter. Mol. Cell. Biol. 2002, 22, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Stein Gold, L.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the Treatment of Psoriasis: A Review of the Unique Mechanism of Action of a Novel Therapeutic Aryl Hydrocarbon Receptor-modulating Agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J. Investig. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Hashimoto-Hachiya, A.; Matsuda-Taniguchi, T.; Takai-Yumine, A.; Takemura, M.; Yan, X.; Furue, M.; Nakahara, T. Natural Compounds Tapinarof and Galactomyces Ferment Filtrate Downregulate IL-33 Expression via the AHR/IL-37 Axis in Human Keratinocytes. Front. Immunol. 2022, 13, 745997. [Google Scholar] [CrossRef] [PubMed]
- Pastorková, B.; Vrzalová, A.; Bachleda, P.; Dvořák, Z. Hydroxystilbenes and Methoxystilbenes Activate Human Aryl Hydrocarbon Receptor and Induce CYP1A Genes in Human Hepatoma Cells and Human Hepatocytes. Food Chem. Toxicol. 2017, 103, 122–132. [Google Scholar] [CrossRef]
- Mendonça, E.L.S.S.; Xavier, J.A.; Fragoso, M.B.T.; Silva, M.O.; Escodro, P.B.; Oliveira, A.C.M.; Tucci, P.; Saso, L.; Goulart, M.O.F. E-Stilbenes: General Chemical and Biological Aspects, Potential Pharmacological Activity Based on the Nrf2 Pathway. Pharmaceuticals 2024, 17, 232. [Google Scholar] [CrossRef]
- Casper, R.F.; Quesne, M.; Rogers, I.M.; Shirota, T.; Jolivet, A.; Milgrom, E.; Savouret, J.F. Resveratrol Has Antagonist Activity on the Aryl Hydrocarbon Receptor: Implications for Prevention of Dioxin Toxicity. Mol. Pharmacol. 1999, 56, 784–790. [Google Scholar] [PubMed]
- Krajka-Kuźniak, V.; Szaefer, H.; Stefański, T.; Sobiak, S.; Cichocki, M.; Baer-Dubowska, W. The Effect of Resveratrol and its Methylthio-derivatives on the Nrf2-ARE Pathway in Mouse Epidermis and HaCaT Keratinocytes. Cell. Mol. Biol. Lett. 2014, 19, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Vu, Y.H.; Hashimoto-Hachiya, A.; Takemura, M.; Yumine, A.; Mitamura, Y.; Nakahara, T.; Furue, M.; Tsuji, G. IL-24 Negatively Regulates Keratinocyte Differentiation Induced by Tapinarof, an Aryl Hydrocarbon Receptor Modulator: Implication in the Treatment of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 9412. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, H.P.; Daschner, P.J.; Yeh, G.C. Resveratrol Inhibits Transcription of CYP1A1 In Vitro by Preventing Activation of the Aryl Hydrocarbon Receptor. Cancer Res. 1998, 58, 5707–5712. [Google Scholar]
- Mikstacka, R.; Przybylska, D.; Rimando, A.M.; Baer-Dubowska, W. Inhibition of Human Recombinant Cytochromes P450 CYP1A1 and CYP1B1 by Trans-Resveratrol Methyl Ethers. Mol. Nutr. Food Res. 2007, 51, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Jones, C.; Perez, P.; Meunier, J.R. The Significance of Nrf2 Pathway in (Photo)-oxidative Stress Response in Melanocytes and Keratinocytes of the Human Epidermis. Pigment Cell Melanoma Res. 2008, 21, 79–88. [Google Scholar] [CrossRef]
- Bononi, I.; Tedeschi, P.; Mantovani, V.; Maietti, A.; Mazzoni, E.; Pancaldi, C.; Brandolini, V.; Tognon, M. Antioxidant Activity of Resveratrol Diastereomeric Forms Assayed in Fluorescent-Engineered Human Keratinocytes. Antioxidants 2022, 11, 196. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Kim, J.S. Carbon Monoxide Partially Mediates Protective Effect of Resveratrol Against UVB-Induced Oxidative Stress in Human Keratinocytes. Antioxidants 2019, 8, 432. [Google Scholar] [CrossRef]
- Shin, J.W.; Lee, H.S.; Na, J.I.; Huh, C.H.; Park, K.C.; Choi, H.R. Resveratrol Inhibits Particulate Matter-Induced Inflammatory Responses in Human Keratinocytes. Int. J. Mol. Sci. 2020, 21, 3446. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Resveratrol Attenuates HMGB1 Signaling and Inflammation in House Dust Mite-induced Atopic Dermatitis in Mice. Int. Immunopharmacol. 2014, 23, 617–623. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, J. Resveratrol Exerts Therapeutic Effects on Mice with Atopic Dermatitis. Wounds 2019, 31, 279–284. [Google Scholar] [PubMed]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef]
- Nene, S.; Devabattula, G.; Vambhurkar, G.; Tryphena, K.P.; Singh, P.K.; Khatri, D.K.; Godugu, C.; Srivastava, S. High mobility group box 1 cytokine targeted topical delivery of resveratrol embedded nanoemulgel for the management of atopic dermatitis. Drug Deliv Transl Res. 2024, 20, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Han, N.R.; Lee, J.S.; Jee, H.W.; Kim, J.H.; Kim, H.M.; Jeong, H.J. Effects of Resveratrol on Thymic Stromal Lymphopoietin Expression in Mast Cells. Medicina 2020, 57, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Yang, H.; Park, J.H.Y.; Kim, J.E.; Lee, K.W. Piceatannol, a metabolite of resveratrol, attenuates atopic dermatitis by targeting Janus kinase 1. Phytomedicine 2022, 99, 153981. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, C.D.; Hui, Y.; Chumanevich, A.P.; Robida, P.A.; Fuseler, J.W.; Sajish, M.; Nagarkatti, P.; Nagarkatti, M.; Oskeritzian, C.A. Resveratrol Protects against Skin Inflammation through Inhibition of Mast Cell, Sphingosine Kinase-1, Stat3 and NF-κB p65 Signaling Activation in Mice. Int. J. Mol. Sci. 2023, 24, 6707. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, X.; Cai, X.; Song, M.; Zheng, J.; Pan, C.; Qiu, P.; Zhang, L.; Zhou, S.; Tang, Z.; et al. Identification of Pinostilbene as a Major Colonic Metabolite of Pterostilbene and its Inhibitory Effects on Colon Cancer Cells. Mol. Nutr. Food Res. 2016, 60, 1924–1932. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Chin, M.C.; Lin, C.C.; His, Y.T.; Lo, Y.S.; Chuang, Y.C.; Chen, M.K. Pinostilbene Hydrate Suppresses Human Oral Cancer Cell Metastasis by Downregulation of Matrix Metalloproteinase-2 Through the Mitogen-Activated Protein Kinase Signaling Pathway. Cell. Physiol. Biochem. 2018, 50, 911–923. [Google Scholar] [CrossRef]
- Leláková, V.; Šmejkal, K.; Jakubczyk, K.; Veselý, O.; Landa, P.; Václavík, J.; Bobáľ, P.; Pížová, H.; Temml, V.; Steinacher, T.; et al. Parallel In Vitro and In Silico Investigations into Anti-inflammatory Effects of Non-prenylated Stilbenoids. Food Chem. 2019, 285, 431–440. [Google Scholar] [CrossRef]
- Chao, J.; Li, H.; Cheng, K.W.; Yu, M.S.; Chang, R.C.; Wang, M. Protective Effects of Pinostilbene, a Resveratrol Methylated Derivative, against 6-Hydroxydopamine-induced Neurotoxicity in SH-SY5Y Cells. J. Nutr. Biochem. 2010, 21, 482–489. [Google Scholar] [CrossRef]
- Chung, Y.C.; Hyun, C.G. Inhibitory Effects of Pinostilbene Hydrate on Melanogenesis in B16F10 Melanoma Cells via ERK and p38 Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4732. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Berger, A.; Böcker, A.; Busch, C.; Weiland, T.; Noor, S.; Leischner, C.; Schleicher, S.; Mayer, M.; Weiss, T.S.; et al. Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone proteins in human-derived hepatoblastoma cells. PLoS ONE 2013, 8, e73097. [Google Scholar] [CrossRef]
- Modoux, M.; Rolhion, N.; Lefevre, J.H.; Oeuvray, C.; Nádvorník, P.; Illes, P.; Emond, P.; Parc, Y.; Mani, S.; Dvorak, Z.; et al. Butyrate acts through HDAC inhibition to enhance aryl hydrocarbon receptor activation by gut microbiota-derived ligands. Gut Microbes 2022, 14, 2105637. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and Solubility of Trans-Resveratrol Are Strongly Influenced by pH and Temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yeo, S.C.; Chuang, X.F.; Lin, H.S. Determination of Pinostilbene in Rat Plasma by LC-MS/MS: Application to a Pharmacokinetic Study. J. Pharm. Biomed. Anal. 2016, 120, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Vaile, J.H.; Davis, P. Topical NSAIDs for Musculoskeletal Conditions. A Review of the Literature. Drugs 1998, 56, 783–799. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Martí, M.; Barba, C.; Carrer, V.; Rubio, L.; Coderch, L. Skin Permeation and Antioxidant Efficacy of Topically Applied Resveratrol. Arch. Dermatol. Res. 2017, 309, 423–431. [Google Scholar] [CrossRef]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Chiba, T.; Ito, T.; Nakahara, T.; Tsuji, G. Antioxidants for Healthy Skin: The Emerging Role of Aryl Hydrocarbon Receptors and Nuclear Factor-Erythroid 2-Related Factor-2. Nutrients 2017, 9, 223. [Google Scholar] [CrossRef]
- Mohammadi-Bardbori, A.; Bengtsson, J.; Rannug, U.; Rannug, A.; Wincent, E. Quercetin, Resveratrol, and Curcumin Are Indirect Activators of the Aryl Hydrocarbon Receptor (AHR). Chem. Res. Toxicol. 2012, 25, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Lulli, D.; Pascarella, A.; Maurelli, R.; Dellambra, E.; Potapovich, A.; Kostyuk, V.; De Luca, C.; Korkina, L. Resveratrol Enhances Solar UV-induced Responses in Normal Human Epidermal Keratinocytes. Photochem. Photobiol. 2012, 88, 1522–1530. [Google Scholar] [CrossRef]
- Park, S.L.; Justiniano, R.; Williams, J.D.; Cabello, C.M.; Qiao, S.; Wondrak, G.T. The Tryptophan-Derived Endogenous Aryl Hydrocarbon Receptor Ligand 6-Formylindolo[3,2-b]Carbazole Is a Nanomolar UVA Photosensitizer in Epidermal Keratinocytes. J. Investig. Dermatol. 2015, 135, 1649–1658. [Google Scholar] [CrossRef]
- Konstantinou, M.; Jendoubi, F.; Hegazy, S.; Bouznad, A.; Tauber, M.; Bulai-Livideanu, C.; Paul, C. Tapinarof-induced folliculitis: The paradigm of activation of the aryl hydrocarbon signaling pathway. J Am Acad Dermatol. 2021, 85, e37–e38. [Google Scholar] [CrossRef] [PubMed]
- Scheman, A.; King, E.; Kerchinsky, L.; Herbster, J. Case report: Contact allergy to tapinarof. Contact Dermatitis. 2024, 90, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Li, M.; Qu, M.L.; Li, X.; Pi, L.H.; Chen, Z.; Zhou, S.L.; Yi, X.Q.; Shi, X.J.; Wu, J.; et al. High Glucose Up-regulates Semaphorin 3A Expression via the mTOR Signaling Pathway in Keratinocytes: A Potential Mechanism and Therapeutic Target for Diabetic Small Fiber Neuropathy. Mol. Cell. Endocrinol. 2018, 472, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Watanabe, M.; Minami, N.; Yunizar, M.F.; Ichikawa, T. Semaphorin 3A: A Potential Target for Prevention and Treatment of Nickel Allergy. Commun. Biol. 2022, 5, 671. [Google Scholar] [CrossRef]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel Allergy and Allergic Contact Dermatitis: A Clinical Review of Immunology, Epidemiology, Exposure, and Treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, G.; Yumine, A.; Kawamura, K.; Takemura, M.; Nakahara, T. Induction of Semaphorin 3A by Resveratrol and Pinostilbene via Activation of the AHR-NRF2 Axis in Human Keratinocytes. Antioxidants 2024, 13, 732. https://doi.org/10.3390/antiox13060732
Tsuji G, Yumine A, Kawamura K, Takemura M, Nakahara T. Induction of Semaphorin 3A by Resveratrol and Pinostilbene via Activation of the AHR-NRF2 Axis in Human Keratinocytes. Antioxidants. 2024; 13(6):732. https://doi.org/10.3390/antiox13060732
Chicago/Turabian StyleTsuji, Gaku, Ayako Yumine, Koji Kawamura, Masaki Takemura, and Takeshi Nakahara. 2024. "Induction of Semaphorin 3A by Resveratrol and Pinostilbene via Activation of the AHR-NRF2 Axis in Human Keratinocytes" Antioxidants 13, no. 6: 732. https://doi.org/10.3390/antiox13060732
APA StyleTsuji, G., Yumine, A., Kawamura, K., Takemura, M., & Nakahara, T. (2024). Induction of Semaphorin 3A by Resveratrol and Pinostilbene via Activation of the AHR-NRF2 Axis in Human Keratinocytes. Antioxidants, 13(6), 732. https://doi.org/10.3390/antiox13060732






