Syringaresinol Attenuates α-Melanocyte-Stimulating Hormone-Induced Reactive Oxygen Species Generation and Melanogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Melanin Assay and Cell Viability Assay
2.4. Mushroom Tyrosinase Inhibition Assay
2.5. DPPH Assay and Intracellular ROS Level
2.6. Mitochondrial Superoxide Assay
2.7. RNA Sample Preparation and Real-Time PCR
| Forward β-actin | 5′-AGG GAA ATC GTG CGT GAC AT-3′ |
| Reverse β-actin | 5′-GGA AAA GAG CCT CAG GGC AT-3′ |
| Forward Tyrosinase | 5′-GGG CCC AAA TTG TAC AGA GA-3′ |
| Reverse Tyrosinase | 5′-ATG GGT GTT GAC CCA TTG TT-3′ |
| Forward TRP-1 | 5′-GTT CAA TGG CCA GGT CAG GA-3′ |
| Reverse TRP-1 | 5′-CAG ACA AGA AGC AAC CCC GA-3′ |
| Forward TRP-2 | 5′-TTA TAT CCT TCG AAA CCA GGA-3′ |
| R0everse TRP-2 | 5′-GGG AAT GGA TAT TCC GTC TTA-3′ |
2.8. Western Blot Analysis
2.9. Brightening Assay with a Pigmented Human Epidermal 3D Skin Model, Melanoderm™
2.10. Statistical Analysis
3. Results
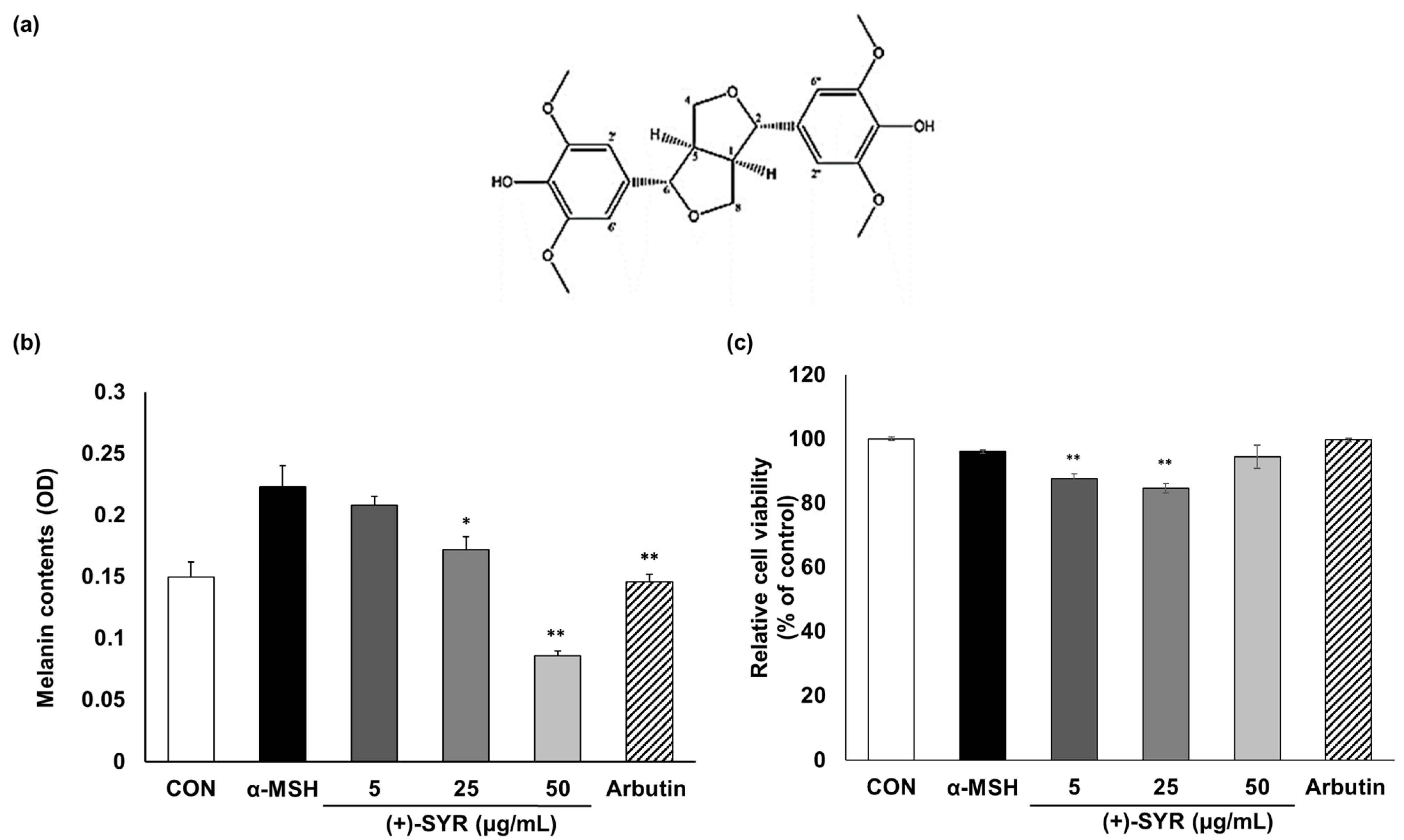
3.1. The Effect of (+)-SYR on Melanogenesis and Cell Viability of B16F10 Cells
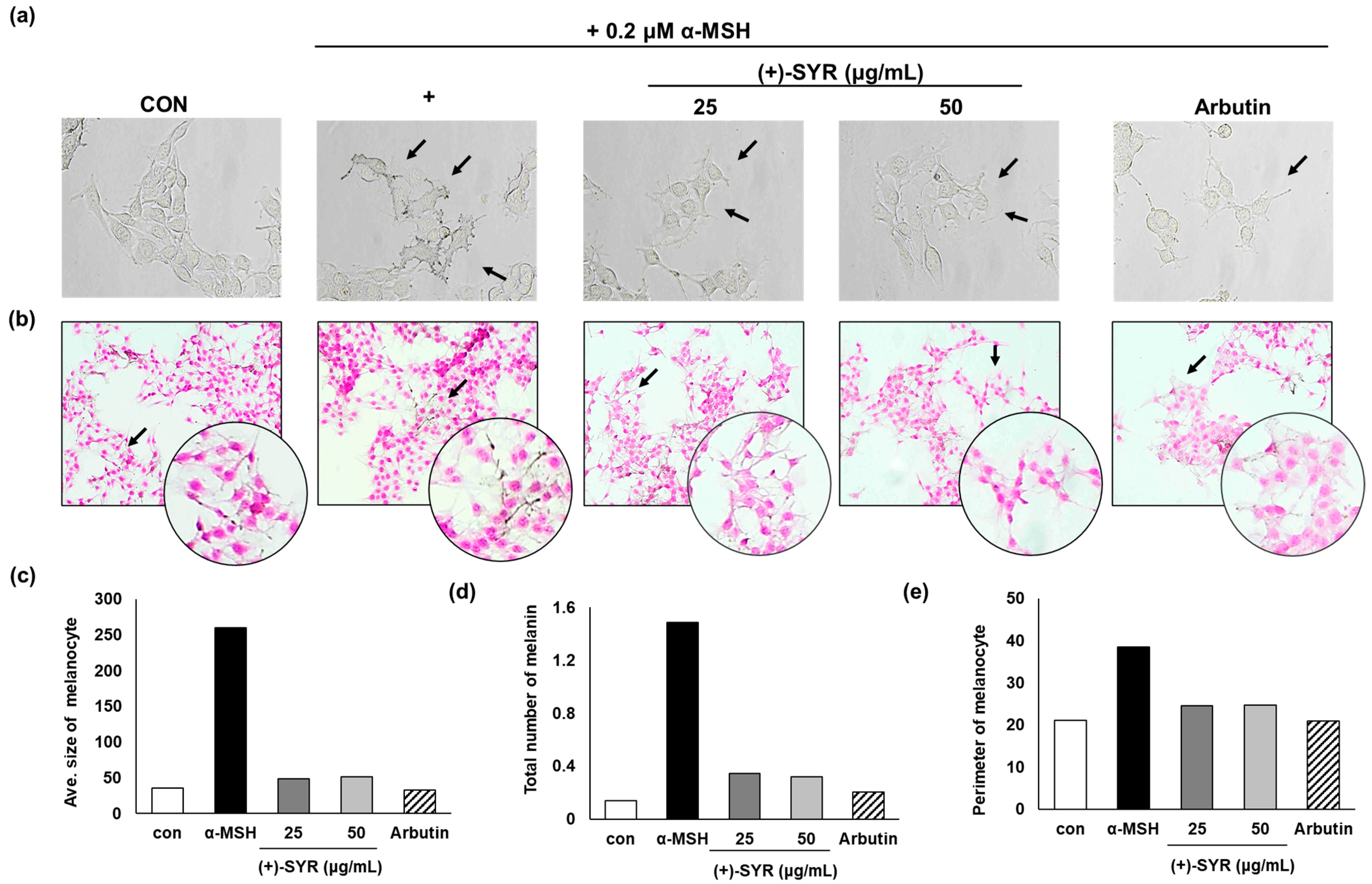
3.2. Effect of (+)-SYR on Cell Morphology of B16F10
3.3. (+)-SYR Downregulates the Expression of Melanogenic Genes without Affecting Enzymatic Activity
3.4. (+)-SYR Suppresses ROS Production in α-MSH-Stimulated B16F10
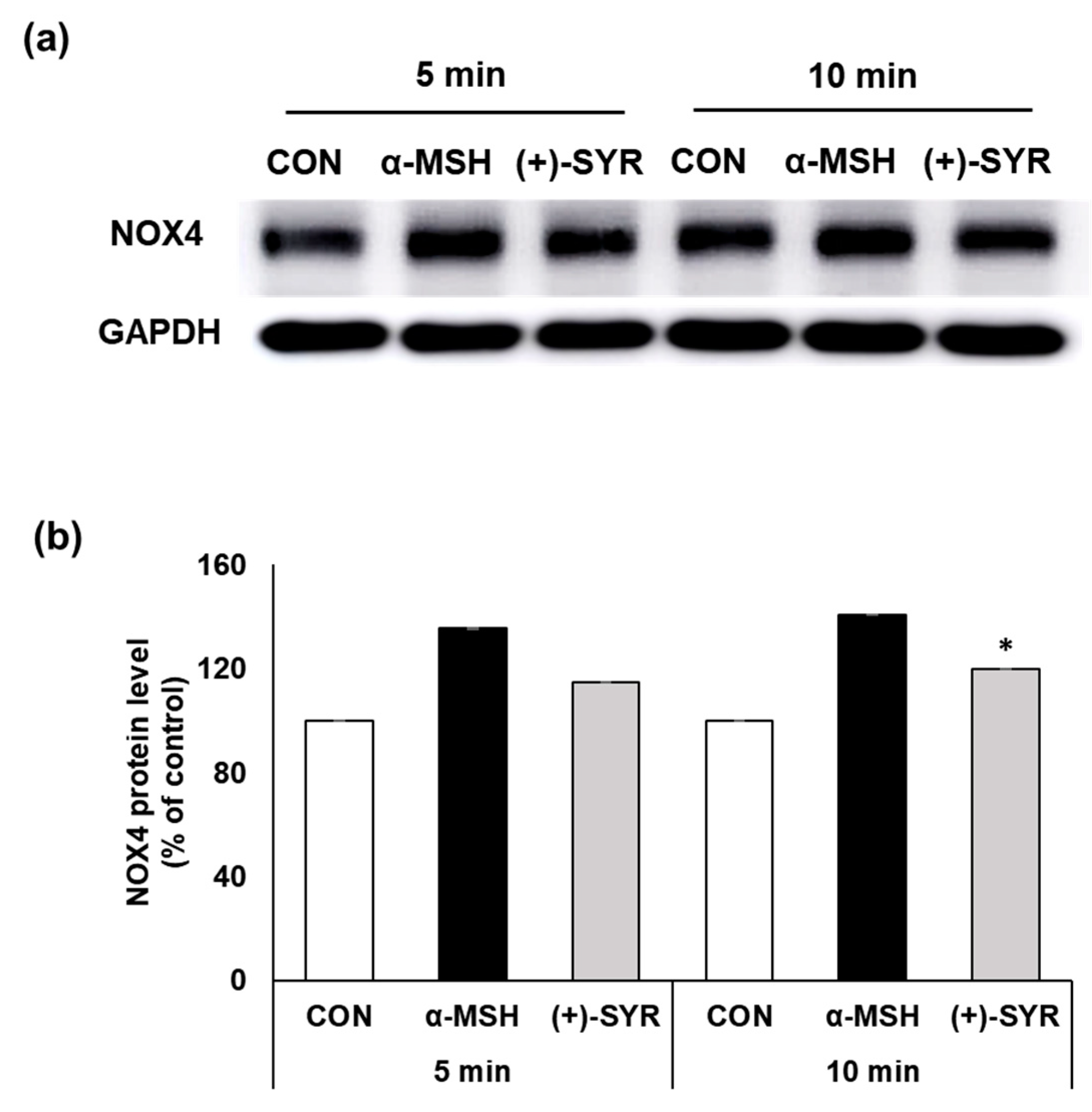
3.5. (+)-SYR Suppresses NOX4 in α-MSH Stimulated B16F10
3.6. Effects of (+)-SYR on the Pigmentation in a Pigmented Human Skin Model, Melanoderm™
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gillis, C.N. Panax ginseng pharmacology: A nitric oxide link? Biochem. Pharmacol. 1997, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-Y.; Kim, H.-M.; Kim, W.S.; Byun, E.-H.; Jang, B.-S.; Choi, D.S.; Byun, E.-B. Effect of gamma irradiation on the anti-oxidant and anti-melanogenic activity of black ginseng extract in B16F10 melanoma cells. Radiat. Phys. Chem. 2018, 149, 33–40. [Google Scholar] [CrossRef]
- Xie, J.-T.; Shao, Z.-H.; Hoek, T.L.V.; Chang, W.-T.; Li, J.; Mehendale, S.; Wang, C.-Z.; Hsu, C.-W.; Becker, L.B.; Yin, J.-J. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur. J. Pharmacol. 2006, 532, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Joo, Y.H.; Karadeniz, F.; Ko, J.; Kong, C.-S. Syringaresinol inhibits UVA-induced MMP-1 expression by suppression of MAPK/AP-1 signaling in HaCaT keratinocytes and human dermal fibroblasts. Int. J. Mol. Sci. 2020, 21, 3981. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-Y.; Oh, S.-R.; Ahn, K.-S.; Kwon, O.-K.; Lee, H.-K. (−)-Syringaresinol inhibits proliferation of human promyelocytic HL-60 leukemia cells via G1 arrest and apoptosis. Int. Immunopharmacol. 2008, 8, 967–973. [Google Scholar] [CrossRef]
- Chung, B.H.; Kim, S.; Kim, J.D.; Lee, J.J.; Baek, Y.Y.; Jeoung, D.; Lee, H.; Choe, J.; Ha, K.S.; Won, M.H.; et al. Syringaresinol causes vasorelaxation by elevating nitric oxide production through the phosphorylation and dimerization of endothelial nitric oxide synthase. Exp. Mol. Med. 2012, 44, 191–201. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Ju, M.-K.; Majumder, R.; Shukla, S.; Huh, Y.S.; Na, M.; Lee, S.H.; Han, Y.-K. Attenuation of inflammatory responses by (+)-syringaresinol via MAP-Kinase-mediated suppression of NF-κB signaling in vitro and in vivo. Sci. Rep. 2018, 8, 9216. [Google Scholar] [CrossRef]
- Kim, J.; Cho, S.Y.; Kim, S.H.; Cho, D.; Kim, S.; Park, C.-W.; Shimizu, T.; Cho, J.Y.; Seo, D.B.; Shin, S.S. Effects of Korean ginseng berry on skin antipigmentation and antiaging via FoxO3a activation. J. Ginseng Res. 2017, 41, 277–283. [Google Scholar] [CrossRef]
- Regad, T. Molecular and cellular pathogenesis of melanoma initiation and progression. Cell. Mol. Life Sci. 2013, 70, 4055–4065. [Google Scholar] [CrossRef]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef]
- Momtaz, S.; Lall, N.; Basson, A. Inhibitory activities of mushroom tyrosine and DOPA oxidation by plant extracts. S. Afr. J. Bot. 2008, 74, 577–582. [Google Scholar] [CrossRef]
- Hwang, K.-S.; Yang, J.Y.; Lee, J.; Lee, Y.-R.; Kim, S.S.; Kim, G.R.; Chae, J.S.; Ahn, J.H.; Shin, D.-S.; Choi, T.-Y. A novel anti-melanogenic agent, KDZ-001, inhibits tyrosinase enzymatic activity. J. Dermatol. Sci. 2018, 89, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.A.; Duperret, E.K.; Zhang, J.; Sadeghi, R.; Dahal, A.; O’Brien, K.T.; Cookson, R.; Winkler, J.D.; Ridky, T.W. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. eLife 2016, 5, e15104. [Google Scholar] [CrossRef]
- Dika, E.; Patrizi, A.; Lambertini, M.; Manuelpillai, N.; Fiorentino, M.; Altimari, A.; Ferracin, M.; Lauriola, M.; Fabbri, E.; Campione, E. Estrogen receptors and melanoma: A review. Cells 2019, 8, 1463. [Google Scholar] [CrossRef]
- Fernandez-Garcia, E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014, 5, 1994–2003. [Google Scholar] [CrossRef]
- Navabhatra, A.; Maniratanachote, R.; Yingngam, B. Antiphotoaging properties of Zingiber montanum essential oil isolated by solvent-free microwave extraction against ultraviolet B-irradiated human dermal fibroblasts. Toxicol. Res. 2022, 38, 235–248. [Google Scholar] [CrossRef]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.-R.; Kim, D.-S.; Park, K.-C. Topical hypopigmenting agents for pigmentary disorders and their mechanisms of action. Ann. Dermatol. 2012, 24, 1–6. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef]
- Yoshimura, M.; Watanabe, Y.; Kasai, K.; Yamakoshi, J.; Koga, T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci. Biotechnol. Biochem. 2005, 69, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- De Dormael, R.; Bastien, P.; Sextius, P.; Gueniche, A.; Ye, D.; Tran, C.; Chevalier, V.; Gomes, C.; Souverain, L.; Tricaud, C. Vitamin C prevents ultraviolet-induced pigmentation in healthy volunteers: Bayesian meta-analysis results from 31 randomized controlled versus vehicle clinical studies. J. Clin. Aesthetic Dermatol. 2019, 12, E53. [Google Scholar]
- Liu, Q.; Kim, C.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Synthesis and biological evaluation of resveratrol derivatives as melanogenesis inhibitors. Molecules 2015, 20, 16933–16945. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Lee, Y.I.; Almurayshid, A.; Jung, J.Y.; Lee, J.H. Effect of a topical antioxidant serum containing vitamin C, vitamin E, and ferulic acid after Q-switched 1064-nm Nd: YAG laser for treatment of environment-induced skin pigmentation. J. Cosmet. Dermatol. 2020, 19, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, C.-S.; Lim, K.-M. Rhododenol activates melanocytes and induces morphological alteration at sub-cytotoxic levels. Int. J. Mol. Sci. 2019, 20, 5665. [Google Scholar] [CrossRef]
- Seal, I.; Sil, S.; Das, A.; Roy, S. Assessment of toxicity and genotoxic safety profile of novel fisetin ruthenium-p-cymene complex in mice. Toxicol. Res. 2023, 39, 213–229. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Ariff, A.B. Discovery of new depigmenting compounds and their efficacy to treat hyperpigmentation: Evidence from in vitro study. J. Cosmet. Dermatol. 2019, 18, 703–727. [Google Scholar] [CrossRef]
- Han, E.; Chang, B.; Kim, D.; Cho, H.; Kim, S. Melanogenesis inhibitory effect of aerial part of Pueraria thunbergiana in vitro and in vivo. Arch. Dermatol. Res. 2015, 307, 57–72. [Google Scholar] [CrossRef]
- Panich, U.; Kongtaphan, K.; Onkoksoong, T.; Jaemsak, K.; Phadungrakwittaya, R.; Thaworn, A.; Akarasereenont, P.; Wongkajornsilp, A. Modulation of antioxidant defense by Alpinia galanga and Curcuma aromatica extracts correlates with their inhibition of UVA-induced melanogenesis. Cell Biol. Toxicol. 2010, 26, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Blumberg, J.B.; Chen, C.-Y.O. Extraction methods determine the antioxidant capacity and induction of quinone reductase by soy products in vitro. Food Chem. 2009, 116, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-based DPPH assay for antioxidant activity analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Garcia, A.M.G.; Meyskens, F.L., Jr. NADPH oxidase 1 overexpression enhances invasion via matrix metalloproteinase-2 and epithelial–mesenchymal transition in melanoma cells. J. Investig. Dermatol. 2012, 132, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-S.; Peshavariya, H.; Higuchi, M.; Brewer, A.C.; Chang, C.W.; Chan, E.C.; Dusting, G.J. Microphthalmia-associated transcription factor modulates expression of NADPH oxidase type 4: A negative regulator of melanogenesis. Free Radic. Biol. Med. 2012, 52, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Birch-Machin, M.A. Oxidative stress and ageing: The influence of environmental pollution, sunlight and diet on skin. Cosmetics 2017, 4, 4. [Google Scholar] [CrossRef]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sahu, R.; Matlam, M.; Deshmukh, V.; Dwivedi, J.; Jha, A. In vitro techniques to assess the proficiency of skin care cosmetic formulations. Pharmacogn. Rev. 2013, 7, 97. [Google Scholar]
- Lu, Y.; Tonissen, K.F.; Di Trapani, G. Modulating skin colour: Role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci. Rep. 2021, 41, BSR20210427. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.; Chung, H.-S.; Kang, M.; Lee, E.; Cho, C.; Kim, H.; Park, S.; Kim, H.-Y.; Hong, M.; Shin, M. Inhibition of production of reactive oxygen species and gene expression profile by treatment of ethanol extract of Moutan Cortex Radicis in oxidative stressed PC12 cells. Biol. Pharm. Bull. 2005, 28, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-H.; Jo, H.-G.; Yang, J.H.; Ki, S.H.; Shin, H.-J. Antioxidative and anti-melanogenic activities of bamboo stems (Phyllostachys nigra variety henosis) via PKA/CREB-mediated MITF downregulation in B16F10 melanoma cells. Int. J. Mol. Sci. 2018, 19, 409. [Google Scholar] [CrossRef]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic acid in skin health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Drummond, N.J.; Davies, N.O.; Lovett, J.E.; Miller, M.R.; Cook, G.; Becker, T.; Becker, C.G.; McPhail, D.B.; Kunath, T. A synthetic cell permeable antioxidant protects neurons against acute oxidative stress. Sci. Rep. 2017, 7, 11857. [Google Scholar] [CrossRef]
- Alam, M.B.; Park, N.H.; Song, B.-R.; Lee, S.-H. Antioxidant potential-rich betel leaves (Piper betle L.) exert depigmenting action by triggering autophagy and downregulating MITF/tyrosinase in vitro and in vivo. Antioxidants 2023, 12, 374. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol./Współczesna Onkol. 2022, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.D.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.; Raad, H.; Taieb, A.; Rezvani, H.R. NADPH oxidases and their roles in skin homeostasis and carcinogenesis. Antioxid. Redox Signal. 2018, 28, 1238–1261. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; McMartin, K.E. Inhibition of NADPH oxidase activity promotes differentiation of B16 melanoma cells. Oncol. Rep. 2008, 19, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-S.; Wu, J.-C.; Tsai, H.-E.; Dusting, G.J.; Chan, E.C.; Wu, C.-S.; Tai, M.-H. Proopiomelanocortin gene delivery induces apoptosis in melanoma through NADPH oxidase 4-mediated ROS generation. Free Radic. Biol. Med. 2014, 70, 14–22. [Google Scholar] [CrossRef]
- Cho, S.; Cho, M.; Kim, J.; Kaeberlein, M.; Lee, S.J.; Suh, Y. Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1alpha in a FOXO3-dependent mechanism. Oncotarget 2015, 6, 43–55. [Google Scholar] [CrossRef]
- Thach, T.T.; Lee, C.K.; Park, H.W.; Lee, S.J.; Lee, S.J. Syringaresinol induces mitochondrial biogenesis through activation of PPARbeta pathway in skeletal muscle cells. Bioorg. Med. Chem. Lett. 2016, 26, 3978–3983. [Google Scholar] [CrossRef]
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the use of kojic acid—A skin-lightening ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Chiaverini, C.; Beuret, L.; Flori, E.; Abbe, P.; Bille, K.; Bahadoran, P.; Ortonne, J.-P.; Bertolotto, C.; Ballotti, R. Microphthalmia-associated transcription factor regulates RAB27A gene expression and controls melanosome transport. J. Biol. Chem. 2008, 283, 12635–12642. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-Y.; Lin, C.-C.; Wang, H.-Y.; Shih, Y.; Chou, S.-T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef]
- Choi, W.; Kim, H.S.; Park, S.H.; Kim, D.; Hong, Y.D.; Kim, J.H.; Cho, J.Y. Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy. J. Ginseng Res. 2022, 46, 536–542. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Yoon, J.; Lim, K.-M. Syringaresinol Attenuates α-Melanocyte-Stimulating Hormone-Induced Reactive Oxygen Species Generation and Melanogenesis. Antioxidants 2024, 13, 876. https://doi.org/10.3390/antiox13070876
Kim K, Yoon J, Lim K-M. Syringaresinol Attenuates α-Melanocyte-Stimulating Hormone-Induced Reactive Oxygen Species Generation and Melanogenesis. Antioxidants. 2024; 13(7):876. https://doi.org/10.3390/antiox13070876
Chicago/Turabian StyleKim, Kyuri, Jihyun Yoon, and Kyung-Min Lim. 2024. "Syringaresinol Attenuates α-Melanocyte-Stimulating Hormone-Induced Reactive Oxygen Species Generation and Melanogenesis" Antioxidants 13, no. 7: 876. https://doi.org/10.3390/antiox13070876
APA StyleKim, K., Yoon, J., & Lim, K.-M. (2024). Syringaresinol Attenuates α-Melanocyte-Stimulating Hormone-Induced Reactive Oxygen Species Generation and Melanogenesis. Antioxidants, 13(7), 876. https://doi.org/10.3390/antiox13070876






