From Personal Care to Coastal Concerns: Investigating Polyethylene Glycol Impact on Mussel’s Antioxidant, Physiological, and Cellular Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Collection of Samples and Cell Viability Assay
2.3. Regulation Volume Decrease (RVD)
2.4. Biochemical Parameters
2.5. Oxidative Markers
2.5.1. Lipid Peroxidation
2.5.2. Protein Oxidation
2.5.3. SOD Activity
2.6. Data Analyses
3. Results
3.1. Cell Viability
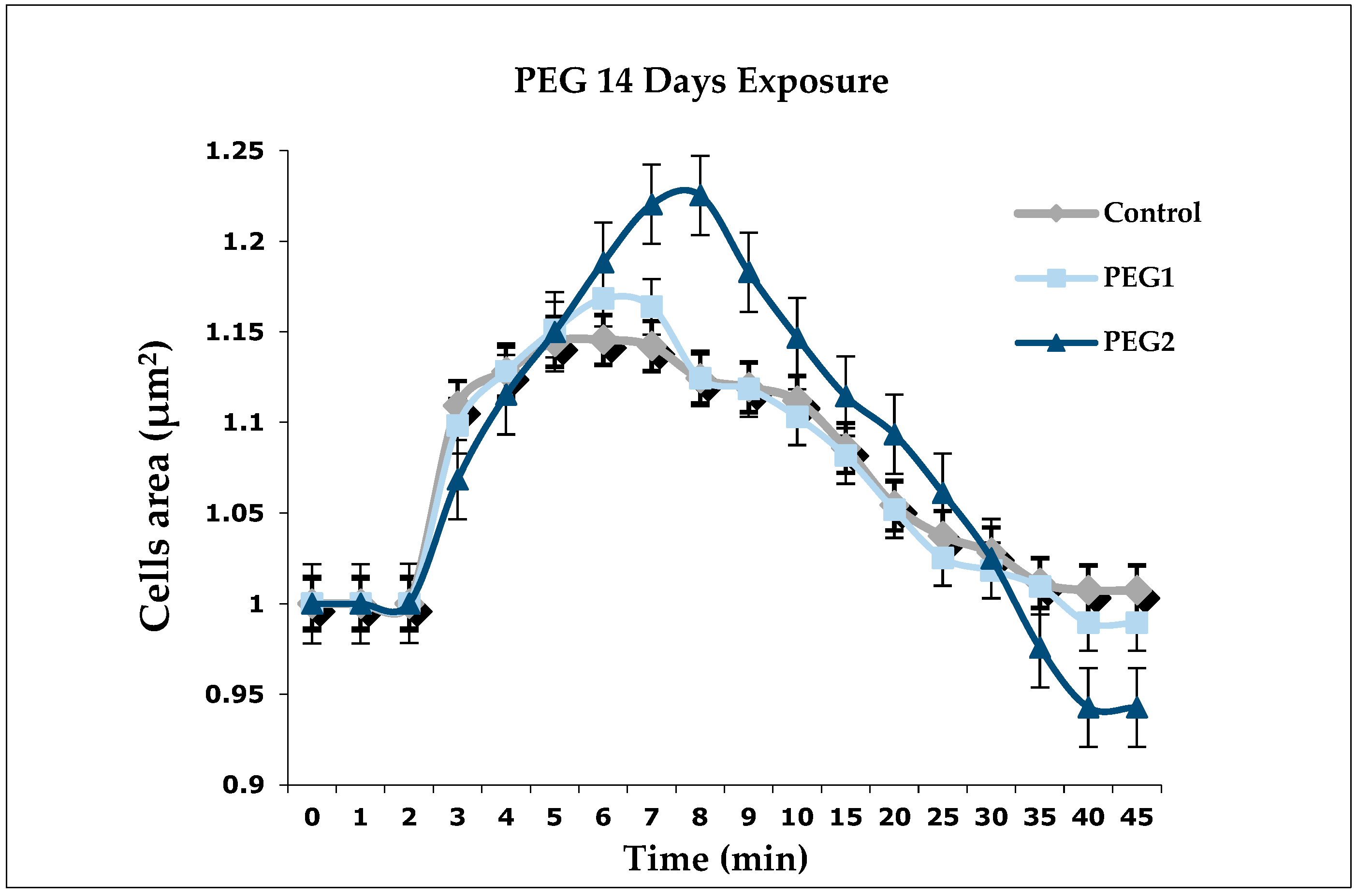
3.2. Regulation Volume Decrease
3.3. Biochemical Parameters
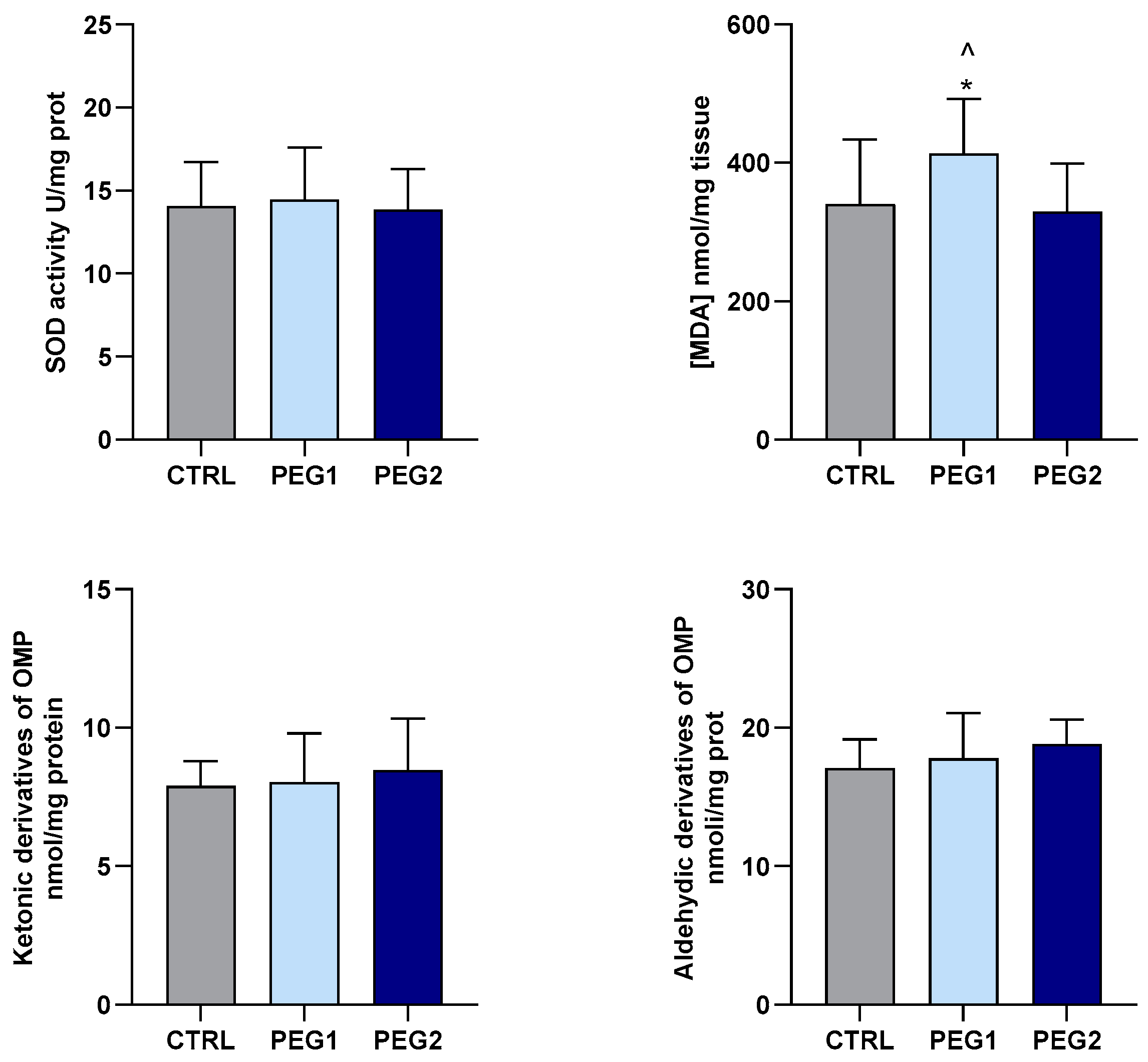
3.4. Oxidative Status Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.T.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Osuoha, J.O.; Anyanwu, B.O.; Ejileugha, C. Pharmaceuticals and personal care products as emerging contaminants: Need for combined treatment strategy. JHM Adv. 2023, 9, 100206. [Google Scholar] [CrossRef]
- Pinheiro, M.; Martins, I.; Raimundo, J.; Caetano, M.; Neuparth, T.; Santos, M.M. Stressors of emerging concern in deep-sea environments: Microplastics, pharmaceuticals, personal care products and deep-sea mining. Sci. Total Environ. 2023, 876, 162557. [Google Scholar] [CrossRef]
- Yusuf, A.; O’Flynn, D.; White, B.; Holland, L.; Parle-McDermott, A.; Lawler, J.; McCloughlin, T.; Harold, D.; Huerta, B.; Regan, F. Monitoring of emerging contaminants of concern in the aquatic environment: A review of studies showing the application of effect-based measures. Anal. Methods 2021, 13, 5120–5143. [Google Scholar] [CrossRef]
- Dewey, H.M.; Jones, J.M.; Keating, M.R.; Budhathoki-Uprety, J. Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on health and safety. ACS Chem. Health Saf. 2021, 29, 27–38. [Google Scholar] [CrossRef]
- Gerstell, E.; Marchessou, S.; Schmidt, J.; Spagnuolo, E. How COVID-19 Is Changing the World of Beauty; McKinsey & Company: New York, NY, USA, 2020; pp. 1–8. [Google Scholar]
- Picó, Y.; Barceló, D. Microplastics and other emerging contaminants in the environment after COVID-19 pandemic: The need of global reconnaissance studies. Curr. Opin. Environ. Sci. Health 2023, 33, 100468. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xi, H.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Sci. Total Enivron. 2021, 788, 147819. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Suresh Kumar, M. A global overview of endocrine disrupting chemicals in the environment: Occurrence, effects, and treatment methods. Int. J. Environ. Sci. Technol. 2022, 20, 12875–12902. [Google Scholar] [CrossRef]
- Merola, C.; Perugini, M.; Conte, A.; Angelozzi, G.; Bozzelli, M.; Amorena, M. Embryotoxicity of methylparaben to zebrafish (Danio rerio) early-life stages. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 236, 108792. [Google Scholar] [CrossRef]
- Quintaneiro, C.; Teixeira, B.; Benedé, J.L.; Chisvert, A.; Soares, A.M.; Monteiro, M.S. Toxicity effects of the organic UV-filter-4 Methylbenzylidene chamhor in zebrafish embryos. Chemosphere 2019, 218, 273–281. [Google Scholar] [CrossRef]
- Tumová, J.; Šauer, P.; Golovko, O.; Ucun, O.K.; Grabic, R.; Máchová, J.; Kroupová, H.K. Effect of polycyclic musk compound on aquatic organisms: A critical literature review supplemented by own data. Sci. Total Environ. 2019, 651, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Zicarelli, G.; Multisanti, C.R.; Falco, F.; Faggio, C. Evaluation of toxicity of Personal Care Products (PCPs) in freshwaters: Zebrafish as a model. Environ. Toxicol. Pharmacol. 2022, 94, 103923. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 2021, 51, 861. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.; Elliott, V.; Park, B.K.; Walker, D.; Hankin, M.; Taupin, P. PEG and PEG conjugates toxicity: Towards an understanding of the toxicity of PEG and its relevance to PEGylated biologicals. In PEGylated Protein Drugs: Basic Science and Clinical Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 127–146. [Google Scholar]
- Jang, H.J.; Shin, C.Y.; Kim, K.B. Safety evaluation of polyethylene glycol (PEG) compounds for cosmetic use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Traverso-Soto, J.M.; Rojas-Ojeda, P.; Sanz, J.L.; González-Mazo, E.; Lara-Martín, P.A. Anaerobic degradation of alcohol ethoxylates and polyethylene glycols in marine sediments. Sci. Total Environ. 2016, 544, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Imik, H.; Gunlu, A. Effects of sodium bicarbonate, polyethylene glycol and methionine added to rations with sorghum (Sorghum vulgare) in fattening lambs on growth performance, wool quality and some blood biochemical markers. Rev. Med. Vet. 2011, 162, 432–439. [Google Scholar]
- Shi, R. Polyethylene glycol repairs membrane damage and enhances functional recovery: A tissue engineering approach to spinal cord injury. Neurosci. Bull. 2013, 29, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Leth, P.M.; Gregersen, M. Ethylene glycol poisoning. Forensic Sci. Int. 2005, 155, 179–184. [Google Scholar] [CrossRef]
- Hatami, M.; Banaee, M.; Haghi, B.N. Sub-lethal toxicity of chlorpyrifos alone and in combination with polyethylene glycol to common carp (Cyprinus carpio). Chemosphere 2019, 219, 981–988. [Google Scholar] [CrossRef]
- Impellitteri, F.; Riolo, K.; Multisanti, C.R.; Zicarelli, G.; Piccione, G.; Faggio, C.; Giannetto, A. Evaluating quaternium-15 effects on Mytilus galloprovincialis: New insights on physiological and cellular responses. Sci. Total Environ. 2024, 918, 170568. [Google Scholar] [CrossRef] [PubMed]
- Miglioli, A.; Tredez, M.; Boosten, M.; Sant, C.; Carvalho, J.E.; Dru, P.; Canesi, L.; Schubert, M.; Dumollard, R. The Mediterranean mussel Mytilus galloprovincialis: A novel model for developmental studies in mollusks. Development 2024, 151, dev202256. [Google Scholar] [CrossRef]
- Świacka, K.; Maculewicz, J.; Smolarz, K.; Szaniawska, A.; Caban, M. Mytilidae as model organisms in the marine ecotoxicology of pharmaceuticals—A review. Environ. Pollut. 2019, 254, 113082. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, N.; Shah, I.M.; Galib, S.; Ahmad, F. Water Contamination through Xenobiotics and Their Toxic Effects on Aquatic Animals. In Xenobiotics in Aquatic Animals, 1st ed.; Rather, M.A., Amin, A., Hajam, Y.A., Jamwal, A., Ahmad, I., Eds.; Springer: Singapore, 2023; pp. 101–122. [Google Scholar]
- Campoy-Diaz, A.D.; Malanga, G.; Giraud-Billoud, M.; Vega, I.A. Changes in the oxidative status and damage by non-essential elements in the digestive gland of the gastropod Pomacea canaliculate. Front. Physiol. 2023, 14, 1123977. [Google Scholar] [CrossRef] [PubMed]
- Zicarelli, G.; Faggio, C.; Blahova, J.; Riesova, B.; Hesova, R.; Doubkova, V.; Svobodova, Z.; Lakdawala, P. Toxicity of water-soluble polymers polyethylene glycol and polyvinyl alcohol for fish and frog embryos. Sci. Total Environ. 2024, 933, 173154. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.N.; Readman, J.A.; Readman, J.W.; Lowe, D.M.; Frickers, P.E.; Beesley, A. Lysosomal cytotoxicity of carbon nanoparticles in cells of the molluscan immune system: An in vitro study. Nanotoxicology 2009, 3, 40–45. [Google Scholar] [CrossRef]
- Tresnakova, N.; Impellitteri, F.; Famulari, S.; Porretti, M.; Filice, M.; Caferro, A.; Savoca, S.; D’Iglio, C.; Imbrogno, S.; Albergamo, A.; et al. Fitness assessment of Mytilus galloprovincialis Lamarck, 1819 after exposure to herbicide metabolite propachlor ESA. Environ. Pollut. 2023, 331, 121878. [Google Scholar] [CrossRef]
- Filice, M.; Caferro, A.; Gattuso, A.; Sperone, E.; Agnisola, C.; Faggio, C.; Cerra, M.C.; Imbrogno, S. Effects of environmental hypoxia on the goldfish skeletal muscle: Focus on oxidative status and mitochondrial dynamics. J. Contam. Hydrol. 2024, 261, 104299. [Google Scholar] [CrossRef]
- Tkachenko, H.; Grudniewska, J. Evaluation of oxidative stress markers in the heart and liver of rainbow trout (Oncorhynchus mykiss Walbaum) exposed to the formalin. Fish Physiol. Biochem. 2016, 42, 1819–1832. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 246–357. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Filice, M.; Leo, S.; Mazza, R.; Amelio, D.; Garofalo, F.; Imbrogno, S.; Cerra, M.C.; Gattuso, A. The heart of the adult goldfish Carassius auratus as a target of Bisphenol A: A multifaceted analysis. Environ. Poll. 2021, 269, 116177. [Google Scholar] [CrossRef] [PubMed]
- Carrington, E.; Waite, J.H.; Sara, G.; Sebens, K.P. Mussels as a model system for integrative ecomechanics. Ann. Rev. Mar. Sci. 2015, 7, 443–469. [Google Scholar] [CrossRef] [PubMed]
- Curpan, A.S.; Impellitteri, F.; Plavan, G.; Ciobica, A.; Faggio, C. Mytilus galloprovincialis: An essential, low-cost model organism for the impact of xenobiotics on oxidative stress and public health. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 256, 109302. [Google Scholar] [CrossRef]
- Porretti, M.; Impellitteri, F.; Caferro, A.; Albergamo, A.; Litrenta, F.; Filice, M.; Imbrogno, S.; Di Bella, G.; Faggio, C. Assessment of the effects of non-phthalate plasticizer DEHT on the bivalve molluscs Mytilus galloprovincialis. Chemosphere 2023, 336, 139273. [Google Scholar] [CrossRef]
- Santovito, G.; Trentin, E.; Gobbi, I.; Bisaccia, P.; Tallandini, L.; Irato, P. Non-enzymatic antioxidant responses of Mytilus galloprovincialis: Insights into physiological role against metal-induced oxidative stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 240, 108909. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.; Cuccaro, A.; Pretti, C.; He, Y.; Soares, A.M.; Freitas, R. Comparative subcellular responses to pharmaceutical exposures in the mussel Mytilus galloprovincialis: An in vitro study. Environ. Toxicol. Pharmacol. 2023, 104, 104314. [Google Scholar] [CrossRef]
- Auguste, M.; Lasa, A.; Balbi, T.; Pallavicini, A.; Vezzulli, L.; Canesi, L. Impact of nanoplastics on hemolymph immune parameters and microbiota composition in Mytylus galloprovincialis. Mar. Envrion. Resear. 2020, 159, 105017. [Google Scholar] [CrossRef]
- Panebianco, A.; Rey-Campos, M.; Romero, A.; Diaz, A.P.; Novoa, B.; Figueras, A. Mytilus galloprovincialis releases immunologically functional haemocytes to the intervalvar space in response to tissue injury and infection. Fish Shellfish Immun. 2023, 138, 108806. [Google Scholar] [CrossRef]
- Wehner, F.; Olsen, H.; Tinel, H.; Kinne-Saffran, E.; Kinne, R.K. Cell volume regulation: Osmolytes, osmolyte transport, and signal transduction. Rev. Physiol. Biochem. Pharmacol. 2003, 148, 1–80. [Google Scholar]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef] [PubMed]
- Barmo, C.; Ciacci, C.; Canonico, B.; Fabbri, R.; Cortese, K.; Balbi, T.; Marcomini, A.; Pojana, G.; Gallo, G.; Canesi, L. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2013, 132, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Ann. Rev. Bioch. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Filice, M.; Reinero, F.R.; Cerra, M.C.; Faggio, C.; Leonetti, F.L.; Micarelli, P.; Giglio, G.; Sperone, E.; Barca, D.; Imbrogno, S. Contamination by trace elements and oxidative stress in the skeletal muscle of Scyliorhinus canicula from the Central Tyrrhenian Sea. Antioxidants 2023, 12, 524. [Google Scholar] [CrossRef]
- Vélez-Alavez, M.; Labrada-Martagón, V.; Méndez-Rodriguez, L.C.; Galván-Magaña, F.; Zenteno-Savín, T. Oxidative stress indicators and trace element concentrations in tissues of mako shark (Isurus oxyrinchus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 165, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, P.; Liu, H.; Yang, S.; Wang, L.; Wang, Z. Hepatic oxidative stress biomarker responses in freshwater fish Carassius auratus exposed to four benzophenone UV filters. Ecotoxicol. Environ. Saf. 2015, 119, 116–122. [Google Scholar] [CrossRef]
- Silva, D.C.; Serrano, L.; Oliveira, T.M.; Mansano, A.S.; Almeida, E.A.; Vieira, E.M. Effects of parabens on antioxidant system and oxidative damages in Nile tilapia (Oreochromis niloticus). Ecotoxicol. Environ. Saf. 2018, 162, 85–91. [Google Scholar] [CrossRef]
- Freitas, R.; Silvestro, S.; Coppola, F.; Costa, S.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Toxic impacts induced by Sodium lauryl sulfate in Mytilus galloprovincialis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 242, 110656. [Google Scholar] [CrossRef]

| Experimental Groups | Number of Specimens per Tank | Replicates | Total of Specimens per Group |
|---|---|---|---|
| Control (ctrl) | 25 | 2 | 50 |
| PEG1 (0.1 mg/L of PEG) | 25 | 2 | 50 |
| PEG2 (10 mg/L of PEG) | 25 | 2 | 50 |
| Haemocytes Viability | |||
|---|---|---|---|
| Ctrl | PEG1 | PEG2 | |
| Assays | |||
| TB | 99.0 ± 0.1 | 97.2 ± 1.0 | 94.3 ± 1.0 ** |
| NR | 99.9 ± 0.6 | 97.6 ± 1.1 | 97.4 ± 1.0 |
| DG Cells Viability | |||
|---|---|---|---|
| Ctrl | PEG1 | PEG2 | |
| Assays | |||
| TB | 98.7 ± 0.2 | 96.9 ± 1.0 | 93.3 ± 1.1 ** |
| NR | 99.4 ± 0.3 | 97.3 ± 0.5 * | 97.9 ± 0.7 |
| Haemolymph Biochemical Parameters | |||
|---|---|---|---|
| Ctrl | PEG1 | PEG2 | |
| Na+ | 476 ± 22 mmol/L | 503 ± 10 mmol/L | 524 ± 7.0 mmol/L |
| K+ | 12 ± 0.7 mmol/L | 12 ± 0.5 mmol/L | 12 ± 0.2 mmol/L |
| Cl− | 516 ± 21 mmol/L | 543 ± 19 mmol/L | 563 ± 0.5 mmol/L |
| P | 0.8 ± 0.1 mg/dL | 1.7 ± 0.1 mg/dL | 2 ± 0.2 mg/dL |
| Mg2+ | 116 ± 3.0 mg/dL | 114 ± 3.0 mg/dL | 117 ± 0.4 mg/dL |
| Ca2+ | 45 ± 4.0 mg/dL | 47 ± 0.6 mg/dL | 50 ± 0.1 mg/dL |
| LDH | 2.0 ± 0.0 U/L | 2.0 ± 0.0 U/L | 1.5 ± 0.5 U/L |
| Water Biochemical Parameters | |||
|---|---|---|---|
| Ctrl | PEG1 | PEG2 | |
| Na+ | 477 ± 65 mmol/L | 513 ± 18 mmol/L | 530 ± 26 mmol/L |
| K+ | 10 ± 1.3 mmol/L | 10 ± 0.3 mmol/L | 11 ± 5.4 mmol/L |
| Cl− | 510 ± 70 mmol/L | 560 ± 18 mmol/L | 567 ± 34 mmol/L |
| P | 5 ± 1.9 mg/dL | 7 ± 4.1 mg/dL | 1.5 ± 1.2 mg/dL |
| Mg2+ | 110 ± 9.0 mg/dL | 124 ± 3.0 mg/dL | 122 ± 31 mg/dL |
| Ca2+ | 41 ± 8.6 mg/dL | 47 ± 0.7 mg/dL | 50 ± 2.9 mg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Multisanti, C.R.; Zicarelli, G.; Caferro, A.; Filice, M.; Faggio, C.; Vazzana, I.; Blahova, J.; Lakdawala, P.; Cerra, M.C.; Imbrogno, S.; et al. From Personal Care to Coastal Concerns: Investigating Polyethylene Glycol Impact on Mussel’s Antioxidant, Physiological, and Cellular Responses. Antioxidants 2024, 13, 734. https://doi.org/10.3390/antiox13060734
Multisanti CR, Zicarelli G, Caferro A, Filice M, Faggio C, Vazzana I, Blahova J, Lakdawala P, Cerra MC, Imbrogno S, et al. From Personal Care to Coastal Concerns: Investigating Polyethylene Glycol Impact on Mussel’s Antioxidant, Physiological, and Cellular Responses. Antioxidants. 2024; 13(6):734. https://doi.org/10.3390/antiox13060734
Chicago/Turabian StyleMultisanti, Cristiana Roberta, Giorgia Zicarelli, Alessia Caferro, Mariacristina Filice, Caterina Faggio, Irene Vazzana, Jana Blahova, Pavla Lakdawala, Maria Carmela Cerra, Sandra Imbrogno, and et al. 2024. "From Personal Care to Coastal Concerns: Investigating Polyethylene Glycol Impact on Mussel’s Antioxidant, Physiological, and Cellular Responses" Antioxidants 13, no. 6: 734. https://doi.org/10.3390/antiox13060734








