The Survival of Human Intervertebral Disc Nucleus Pulposus Cells under Oxidative Stress Relies on the Autophagy Triggered by Delphinidin
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Nucleus Pulposus Cells Culture
2.2. Hydrogen Peroxide (H2O2) Treatment
2.3. Delphinidin Treatment
2.4. Establishment of Oxidative Stress-Induced Senescence (OSIS)
2.5. Cell Population Doubling Time (CPDT)
2.6. Cell Viability Assay
2.7. Crystal Violet Staining Assay
2.8. Clonogenic Survival Assay
2.9. Measurement of Reactive Oxygen Species (ROSs)
2.10. Senescence-Associated β-Galactosidase Staining (SA-β-Gal Staining)
2.11. Autophagy Flux Detection
2.12. Interleukin 1 Beta (IL-1β) Measurement
2.13. Western Blotting
2.14. Statistical Analysis
3. Results
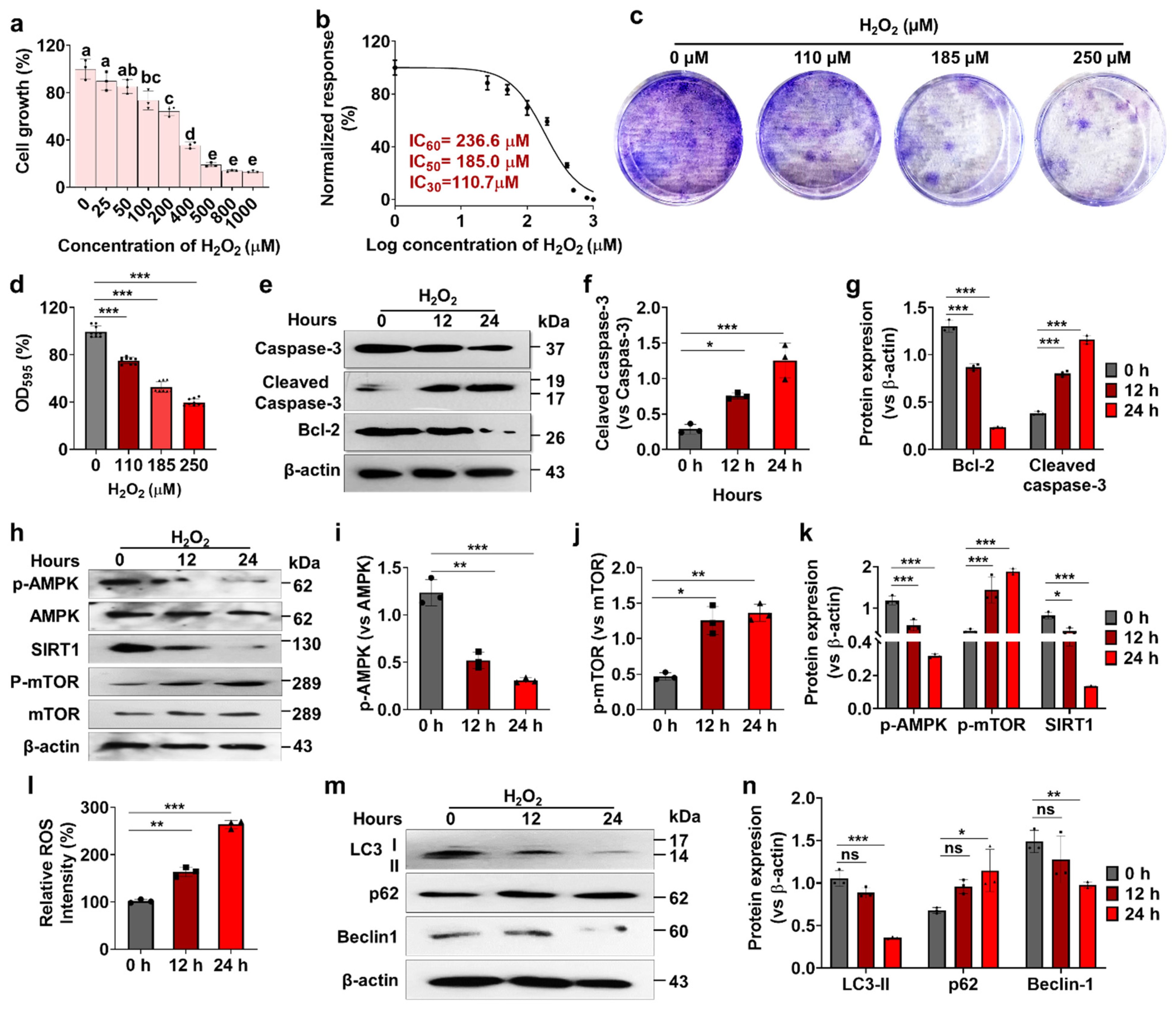
3.1. Effects of H2O2 on Human Nucleus Pulposus Cells (hNPCs) Growth, Stress-Responsive ROS, Autophagy, and Cell Death
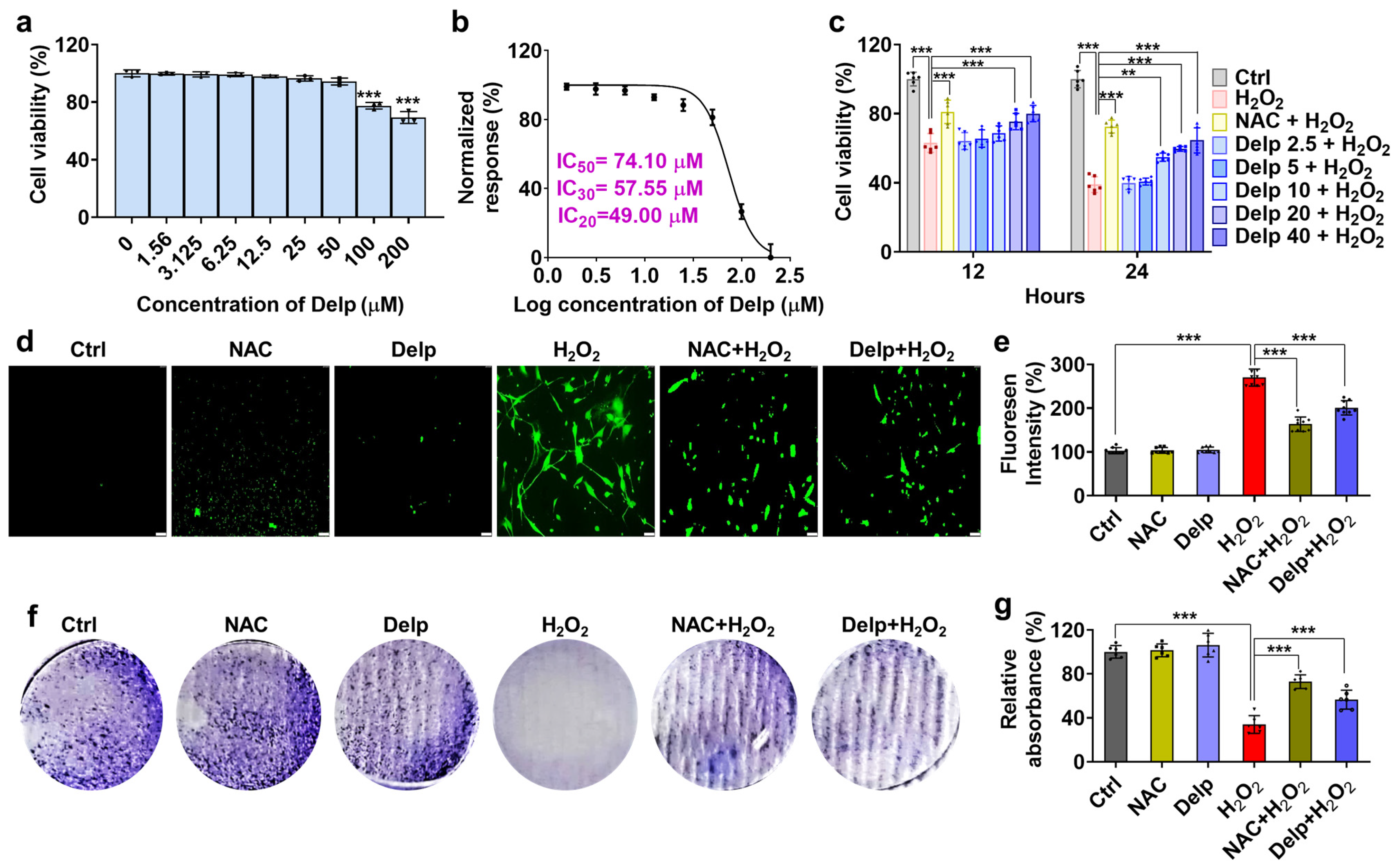
3.2. Effects of Delp on H2O2-Induced Oxidative Stress in hNPCs
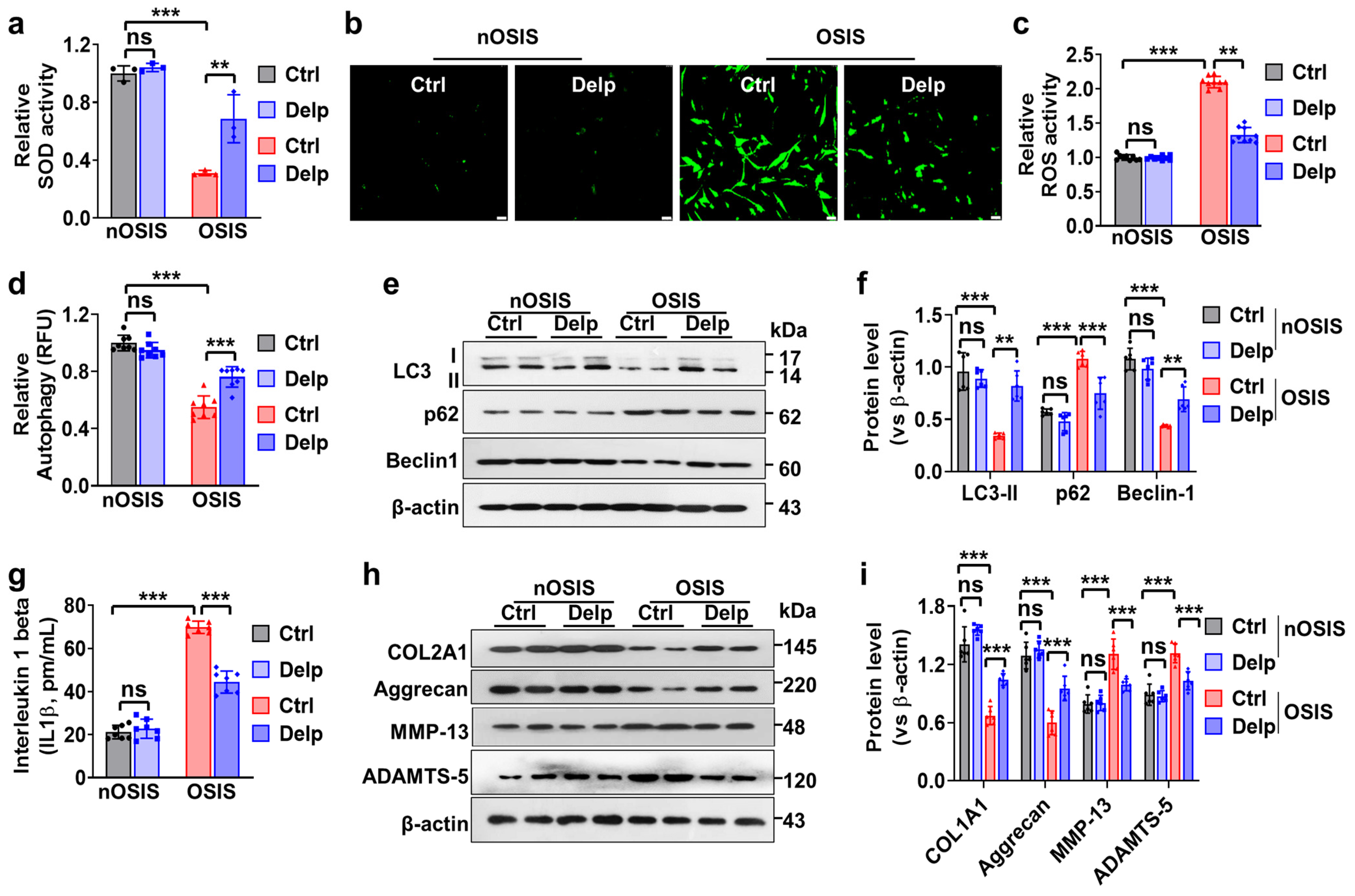
3.3. Confirmation of H2O2-Treated Oxidative Stress-Induced Senescence (OSIS) Model in hNPCs
3.4. Delp Protects hNPCs from OSIS via Controlling ROS Production, ECM Synthesis, and Autophagy
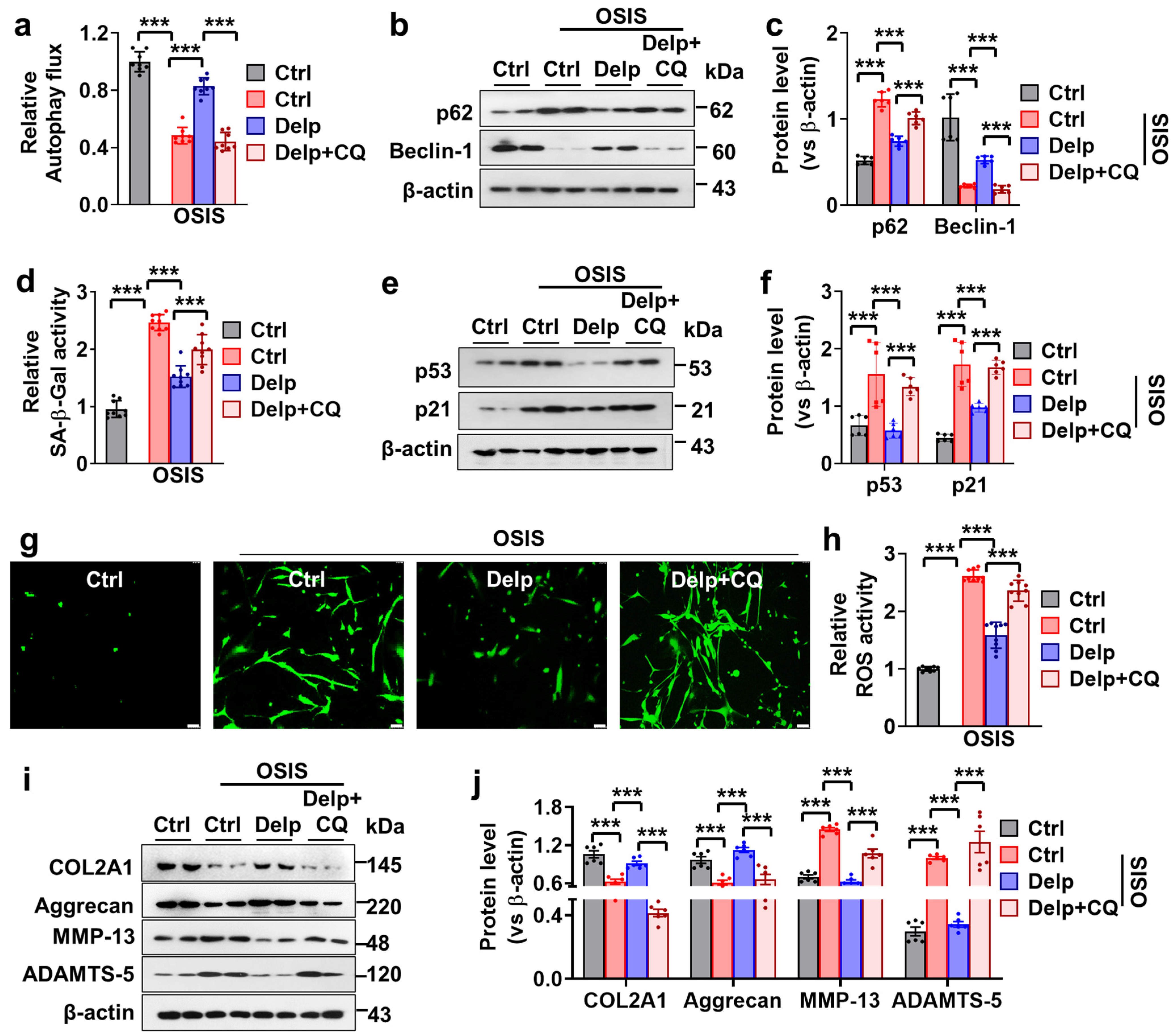
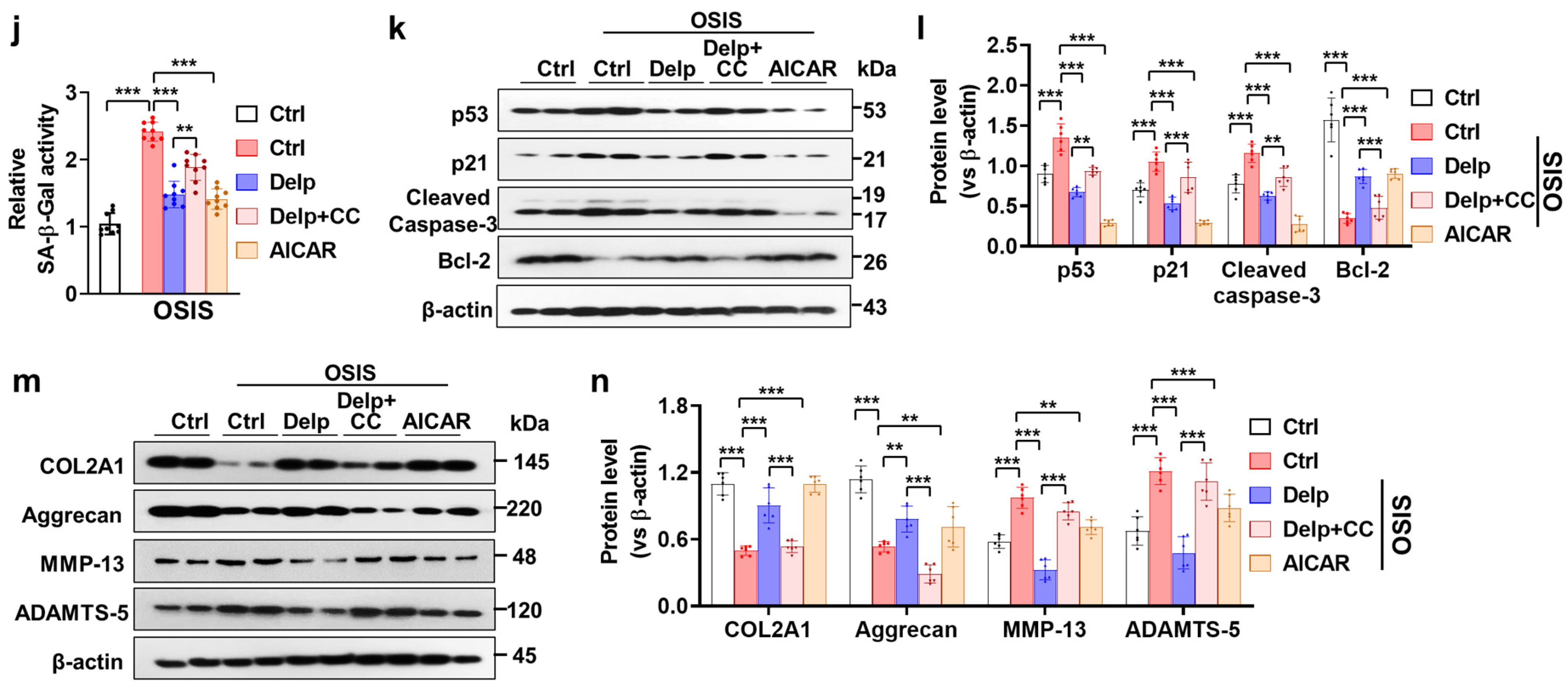
3.5. Autophagy Enhanced the Inhibitory Effect of Delp on the Senescence and ECM Degradation in OSIS hNPCs
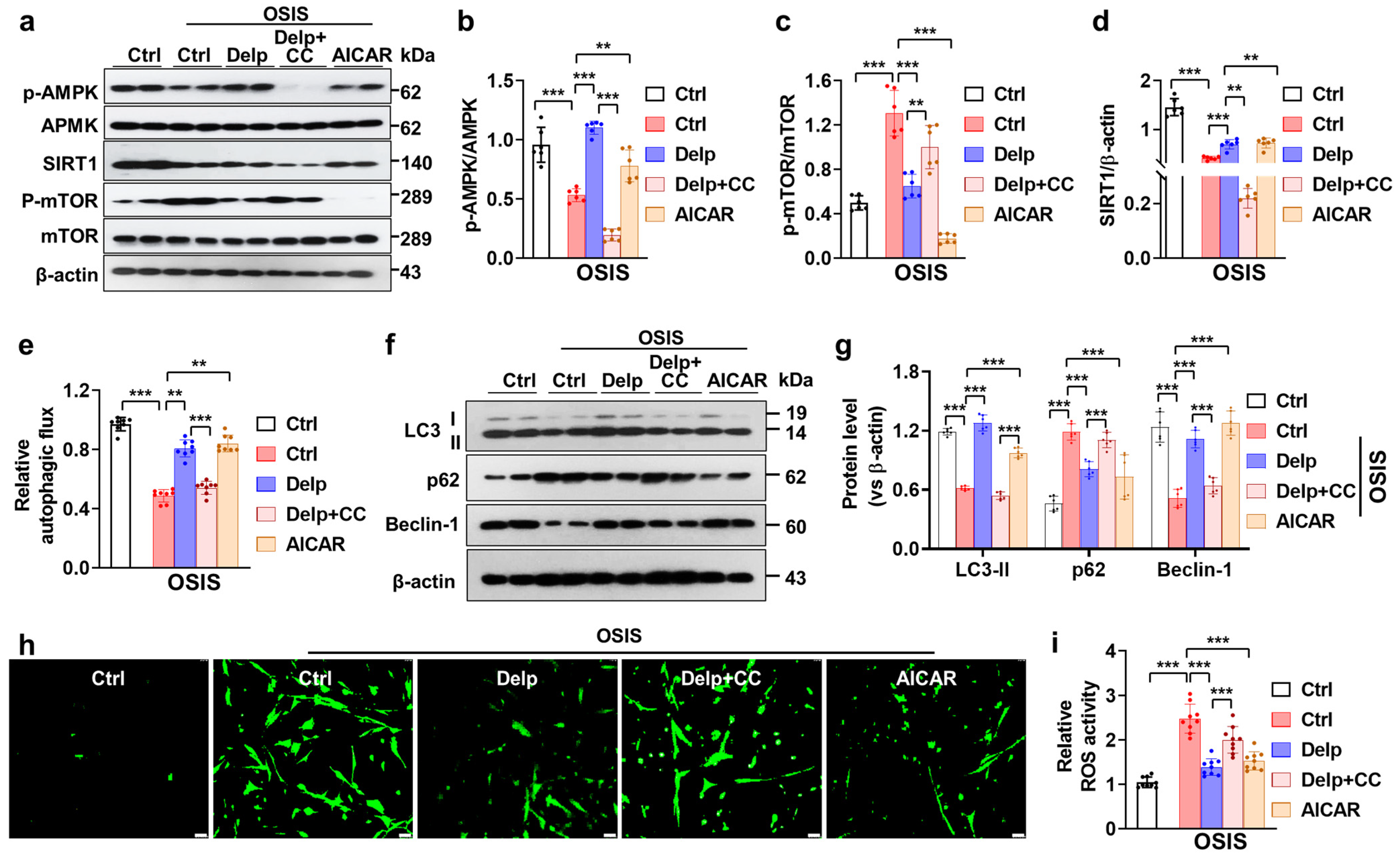
3.6. Activation of the AMPK Pathway Is Important for Delp-Induced Autophagy in the OSIS hNPCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, C.; Yang, M.; Lan, M.; Liu, C.; Zhang, Y.; Huang, B.; Liu, H.; Zhou, Y. ROS: Crucial Intermediators in the Pathogenesis of Intervertebral Disc Degeneration. Oxid. Med. Cell Longev. 2017, 2017, 5601593. [Google Scholar] [CrossRef]
- Cao, G.; Yang, S.; Cao, J.; Tan, Z.; Wu, L.; Dong, F.; Ding, W.; Zhang, F. The Role of Oxidative Stress in Intervertebral Disc Degeneration. Oxid. Med. Cell Longev. 2022, 2022, 2166817. [Google Scholar] [CrossRef]
- Kim, K.W.; Chung, H.N.; Ha, K.Y.; Lee, J.S.; Kim, Y.Y. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J. 2009, 9, 658–666. [Google Scholar] [CrossRef]
- Silwal, P.; Nguyen-Thai, A.M.; Mohammad, H.A.; Wang, Y.; Robbins, P.D.; Lee, J.Y.; Vo, N.V. Cellular Senescence in Intervertebral Disc Aging and Degeneration: Molecular Mechanisms and Potential Therapeutic Opportunities. Biomolecules 2023, 13, 686. [Google Scholar] [CrossRef]
- Dimozi, A.; Mavrogonatou, E.; Sklirou, A.; Kletsas, D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur. Cell Mater. 2015, 30, 89–102; discussion 103. [Google Scholar] [CrossRef]
- Krupkova, O.; Handa, J.; Hlavna, M.; Klasen, J.; Ospelt, C.; Ferguson, S.J.; Wuertz-Kozak, K. The Natural Polyphenol Epigallocatechin Gallate Protects Intervertebral Disc Cells from Oxidative Stress. Oxid. Med. Cell Longev. 2016, 2016, 7031397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Lin, X.; Dong, W.; Huang, W.; Jiang, W.; Lin, L.; Qiu, Q.; Zhang, X.; Shen, J.; Song, Z.; et al. SIRT1 alleviates senescence of degenerative human intervertebral disc cartilage endo-plate cells via the p53/p21 pathway. Sci. Rep. 2016, 6, 22628. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Yang, K.; Guo, S.; Wang, J.; Feng, C.; Chen, H. Hyper-osmolarity environment-induced oxidative stress injury promotes nucleus pulposus cell senescence in vitro. Biosci. Rep. 2019, 39, BSR20191711. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Licini, C.; Marzioni, D.; Mattioli-Belmonte, M. The multifaced role of HtrA1 in the development of joint and skeletal disorders. Bone 2022, 157, 116350. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Ashhotov, A.V.; Trefilova, V.V.; Novitsky, M.A.; Medvedev, G.V.; Petrova, M.M.; Narodova, E.A.; Kaskaeva, D.S.; Chumakova, G.A.; Garganeeva, N.P.; et al. High-Tech Methods of Cytokine Imbalance Correction in Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 3333. [Google Scholar] [CrossRef] [PubMed]
- Costachescu, B.; Niculescu, A.G.; Teleanu, R.I.; Iliescu, B.F.; Radulescu, M.; Grumezescu, A.M.; Dabija, M.G. Recent Advances in Managing Spinal Intervertebral Discs Degeneration. Int. J. Mol. Sci. 2022, 23, 6460. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shao, Z.; Chen, S.; Huang, D.; Peng, Y.; Chen, S.; Ma, K. TIGAR impedes compression-induced intervertebral disc degeneration by suppressing nucleus pulposus cell apoptosis and autophagy. J. Cell Physiol. 2020, 235, 1780–1794. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Bahar, M.E.; Kim, C.W.; Seo, M.S.; Song, M.G.; Song, S.Y.; Kim, S.Y.; Kim, D.R.; Kim, D.H. Autophagy in Osteoarthritis: A Double-Edged Sword in Cartilage Aging and Mechanical Stress Response: A Systematic Review. J. Clin. Med. 2024, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Zhang, J.; Chen, C.; Zhang, D.; Li, P.; Ma, F. Anthocyanin contributes more to hydrogen peroxide scavenging than other phenolics in apple peel. Food Chem. 2014, 152, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, O.M. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Jiang, M.; Wang, J.; Yang, S.; Liu, Z.; Zhang, H.; Zhu, X. Cyanidin attenuates the apoptosis of rat nucleus pulposus cells and the degeneration of intervertebral disc via the JAK2/STAT3 signal pathway in vitro and in vivo. Pharm. Biol. 2022, 60, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, Y.J.; Song, M.G.; Kim, D.R.; Zada, S.; Kim, D.H. Cytoprotective Effects of Delphinidin for Human Chondrocytes against Oxidative Stress through Activation of Autophagy. Antioxidants 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Chen, D.; Haqqi, T.M. Delphinidin inhibits IL-1beta-induced activation of NF-kappaB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology 2013, 52, 998–1008. [Google Scholar] [CrossRef]
- Maharajan, N.; Ganesan, C.D.; Moon, C.; Jang, C.H.; Oh, W.K.; Cho, G.W. Licochalcone D Ameliorates Oxidative Stress-Induced Senescence via AMPK Activation. Int. J. Mol. Sci. 2021, 22, 7324. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, D.; Xiao, H. Methods of cellular senescence induction using oxidative stress. Methods Mol. Biol. 2013, 1048, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Stoeber, K.; Kingsbury, S.; Ozanne, S.E.; Williams, G.H.; Hales, C.N. Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J. Biol. Chem. 2004, 279, 49439–49446. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Ritschel, W.A.; Agrawala, P.; Kappes, J.K.; Kraeling, M.; Hussain, S.A. Effect of age on the pharmacokinetics of coumarin. Arzneimittelforschung 1988, 38, 1466–1468. [Google Scholar]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yi, L.; Jin, X.; Mi, M.T.; Zhang, T.; Ling, W.H.; Yu, B. Delphinidin attenuates stress injury induced by oxidized low-density lipoprotein in human umbilical vein endothelial cells. Chem. Biol. Interact. 2010, 183, 105–112. [Google Scholar] [CrossRef]
- Jin, X.; Chen, M.; Yi, L.; Chang, H.; Zhang, T.; Wang, L.; Ma, W.; Peng, X.; Zhou, Y.; Mi, M. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Mol. Nutr. Food Res. 2014, 58, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Kritschil, R.; Scott, M.; Sowa, G.; Vo, N. Role of autophagy in intervertebral disc degeneration. J. Cell Physiol. 2022, 237, 1266–1284. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Y.; Wang, X.; Chang, X.; Fu, S. Role of Pyroptosis in Intervertebral Disc Degeneration and Its Therapeutic Implications. Biomolecules 2022, 12, 1804. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E.; Hwang, J.S.; Ahmed, M.; Lai, T.H.; Pham, T.M.; Elashkar, O.; Akter, K.M.; Kim, D.H.; Yang, J.; Kim, D.R. Targeting Autophagy for Developing New Therapeutic Strategy in Intervertebral Disc Degeneration. Antioxidants 2022, 11, 1571. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Pan, Z.; Zhan, Y.; Tang, Q.; Zheng, F.; Zhou, Y.; Wu, Y.; Zhou, Y.; Chen, D.; Chen, J.; et al. TFEB protects nucleus pulposus cells against apoptosis and senescence via restoring autophagic flux. Osteoarthr. Cartil. 2019, 27, 347–357. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Hao, J.; Shen, J.; Fang, J.; Dong, W.; Wang, D.; Zhang, X.; Shui, W.; Luo, Y.; et al. SIRT1 protects against apoptosis by promoting autophagy in degenerative human disc nucleus pulposus cells. Sci. Rep. 2014, 4, 7456. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.Y.; Zhang, H.H. Autophagy as a potential therapeutic target in intervertebral disc degeneration. Life Sci. 2021, 273, 119266. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, J.J.; Jin, M.Y.; Gu, Y.T.; Wu, C.C.; Guo, W.J.; Yan, Y.Z.; Zhang, Z.J.; Wang, J.L.; Zhang, X.L.; et al. Sirt6 overexpression suppresses senescence and apoptosis of nucleus pulposus cells by inducing autophagy in a model of intervertebral disc degeneration. Cell Death Dis. 2018, 9, 56. [Google Scholar] [CrossRef]
- Chen, J.W.; Ni, B.B.; Li, B.; Yang, Y.H.; Jiang, S.D.; Jiang, L.S. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: Implications for disc degeneration. Cell Physiol. Biochem. 2014, 34, 1175–1189. [Google Scholar] [CrossRef]
- Agostini, F.; Bisaglia, M.; Plotegher, N. Linking ROS Levels to Autophagy: The Key Role of AMPK. Antioxidants 2023, 12, 1406. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Shi, Y.; Chen, Z.; Zhou, X.; Luo, P.; Hong, C.; Tian, N.; Wu, Y.; Zhou, Y.; Lin, Y.; et al. Apigenin Alleviates Intervertebral Disc Degeneration via Restoring Autophagy Flux in Nucleus Pulposus Cells. Front. Cell Dev. Biol. 2021, 9, 787278. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhu, L.; Hong, X.; Wang, Y.T.; Wang, F.; Bao, J.P.; Xie, X.H.; Liu, L.; Wu, X.T. Resveratrol attenuated TNF-alpha-induced MMP-3 expression in human nucleus pulposus cells by activating autophagy via AMPK/SIRT1 signaling pathway. Exp. Biol. Med. 2016, 241, 848–853. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.; Wang, J.; Tang, C.; Khor, S.; Chen, J.; Chen, X.; Zhang, Z.; Tang, Q.; Wang, C.; et al. Berberine suppresses apoptosis and extracellular matrix (ECM) degradation in nucleus pulposus cells and ameliorates disc degeneration in a rodent model. Int. J. Biol. Sci. 2018, 14, 682–692. [Google Scholar] [CrossRef]
- Gao, S.; Li, N.; Chen, R.; Su, Y.; Song, Y.; Liang, S. Bushen Huoxue Formula Modulates Autophagic Flux and Inhibits Apoptosis to Protect Nucleus Pulposus Cells by Restoring the AMPK/SIRT1 Pathway. Biomed. Res. Int. 2022, 2022, 8929448. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahar, M.E.; Hwang, J.S.; Lai, T.H.; Byun, J.-H.; Kim, D.-H.; Kim, D.R. The Survival of Human Intervertebral Disc Nucleus Pulposus Cells under Oxidative Stress Relies on the Autophagy Triggered by Delphinidin. Antioxidants 2024, 13, 759. https://doi.org/10.3390/antiox13070759
Bahar ME, Hwang JS, Lai TH, Byun J-H, Kim D-H, Kim DR. The Survival of Human Intervertebral Disc Nucleus Pulposus Cells under Oxidative Stress Relies on the Autophagy Triggered by Delphinidin. Antioxidants. 2024; 13(7):759. https://doi.org/10.3390/antiox13070759
Chicago/Turabian StyleBahar, Md Entaz, Jin Seok Hwang, Trang Huyen Lai, June-Ho Byun, Dong-Hee Kim, and Deok Ryong Kim. 2024. "The Survival of Human Intervertebral Disc Nucleus Pulposus Cells under Oxidative Stress Relies on the Autophagy Triggered by Delphinidin" Antioxidants 13, no. 7: 759. https://doi.org/10.3390/antiox13070759
APA StyleBahar, M. E., Hwang, J. S., Lai, T. H., Byun, J.-H., Kim, D.-H., & Kim, D. R. (2024). The Survival of Human Intervertebral Disc Nucleus Pulposus Cells under Oxidative Stress Relies on the Autophagy Triggered by Delphinidin. Antioxidants, 13(7), 759. https://doi.org/10.3390/antiox13070759






