Green Light Mitigates Cyclic Chronic Heat-Stress-Induced Liver Oxidative Stress and Inflammation via NF-κB Pathway Inhibition in Geese
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
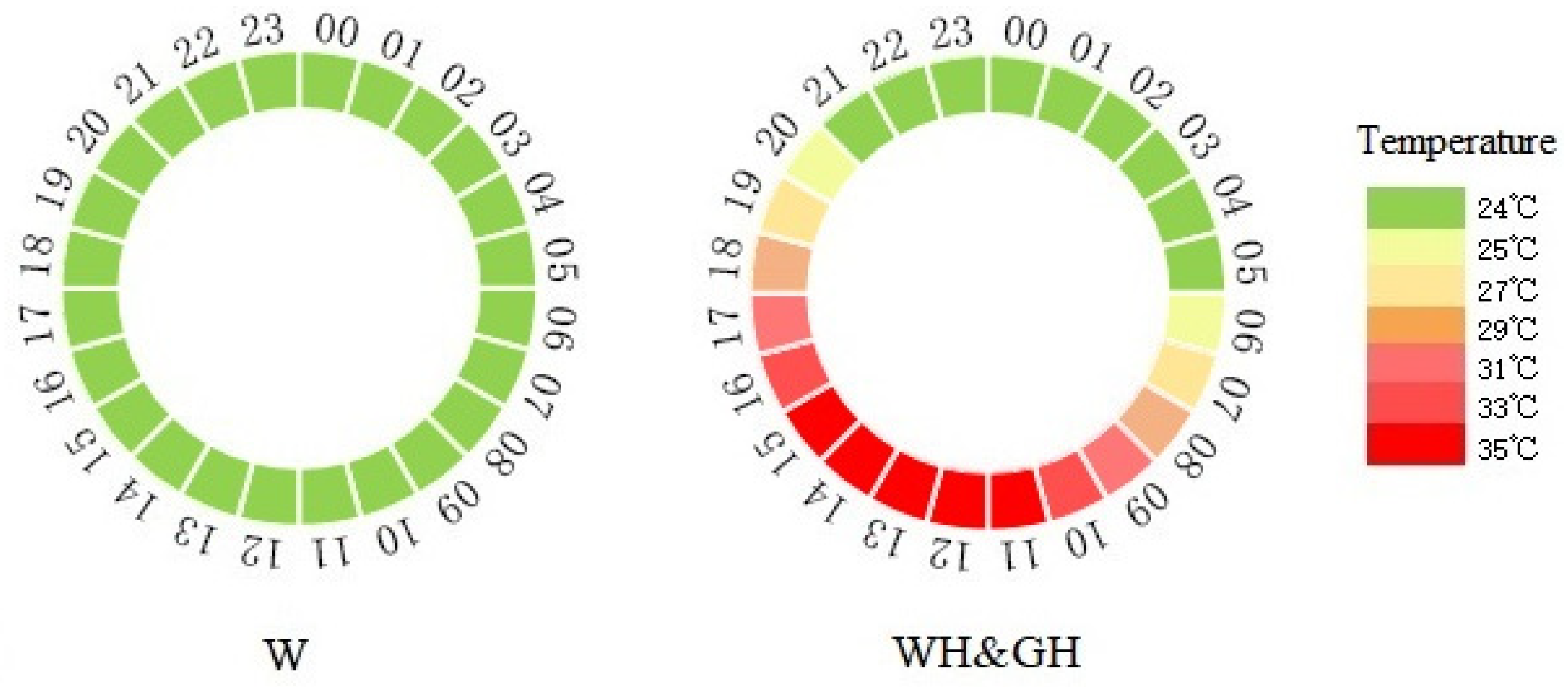
2.2. Animals and Treatment Design
2.3. Growth Performance and Tissue Weight
2.4. Blood Sampling and Analysis
2.5. Liver Sampling
2.6. Histomorphology and Histopathology Analyses of Liver
2.7. Liver Tissue Homogenate Preparation and Parameter Determination
2.8. Transcriptomic Analysis
2.9. qRT-PCR Analysis
2.10. Statistical Analysis
3. Results
3.1. Behaviour, Growth Performance, and Tissue Weight
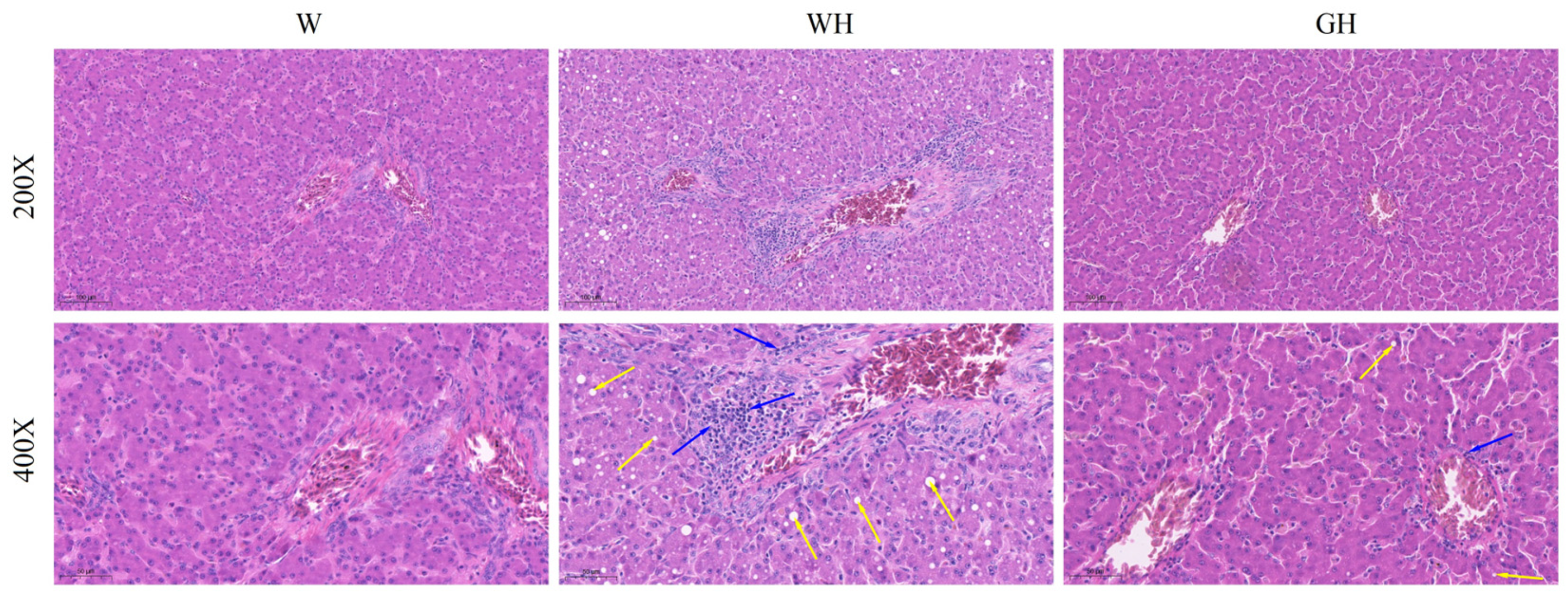
3.2. Liver Morphology and Histopathology
3.3. Serum Corticosterone and Liver Tests
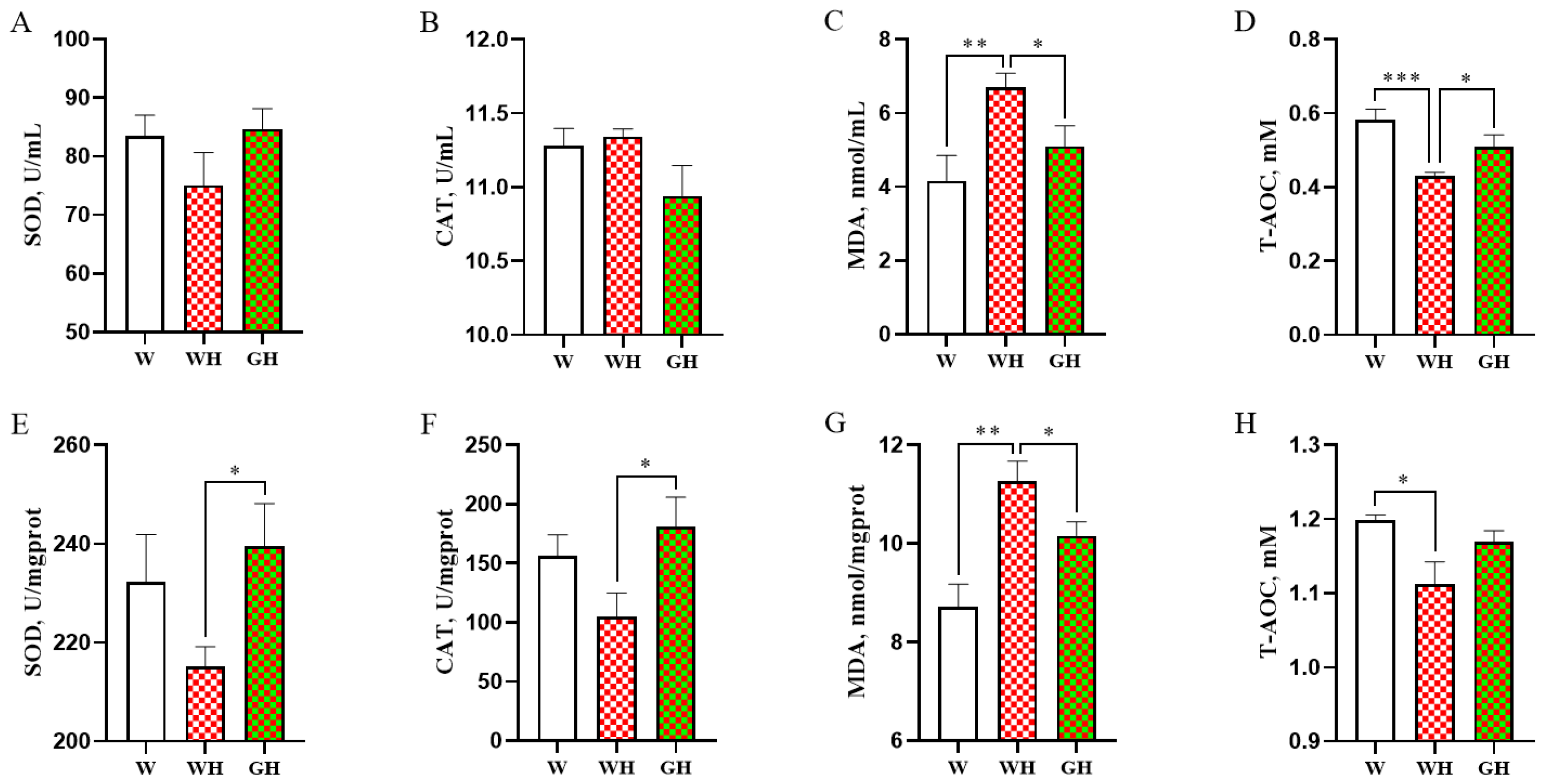
3.4. Antioxidant Parameters in Serum and Liver
3.5. Inflammatory Parameters in Serum and Liver
3.6. Liver Transcriptome Reveals the Potential Molecular Regulatory Mechanism of Monochromatic Green Light in Alleviating Heat Stress
3.6.1. mRNA Transcriptomes of Liver in W, WH, and GH Group
3.6.2. Enrichment Analysis of DEGs
3.6.3. Quantitative Real-Time PCR Validation of mRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arifwidodo, S.D.; Chandrasiri, O. Urban heat stress and human health in Bangkok, Thailand. Environ. Res. 2020, 185, 109398. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Increases in extreme heat stress in domesticated livestock species during the twenty-first century. Global Change Biol. 2021, 27, 5762–5772. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Deeb, N.; Cahaner, A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002, 81, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Biswal, J.; Vijayalakshmy, K.; Bhattacharya, T.K.; Rahman, H. Impact of heat stress on poultry production. World Poultry Sci. J. 2022, 78, 179–196. [Google Scholar] [CrossRef]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Cantet, J.M.; Yu, Z.; Rius, A.G. Heat stress-mediated activation of immune-inflammatory pathways. Antibiotics 2021, 10, 1285. [Google Scholar] [CrossRef]
- Kikusato, M.; Xue, G.; Pastor, A.; Niewold, T.A.; Toyomizu, M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2021, 100, 957–963. [Google Scholar] [CrossRef]
- Lian, P.; Braber, S.; Garssen, J.; Wichers, H.J.; Folkerts, G.; Fink-Gremmels, J.; Varasteh, S. Beyond heat stress: Intestinal integrity disruption and mechanism-based intervention strategies. Nutrients 2020, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, J.; Juliet Igbokwe, C.; Duan, Y.; Cai, M.; He, Y.; Zhang, H. Oligopeptide of RDPEER from watermelon seeds prevents heat stress-induced liver injury by suppressing oxidative stress and inflammation responses. J. Funct. Foods 2023, 105, 105563. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Zhi, S.Q.; Lan, W.K.; Huang, J.W.; Zhang, H.H. Effects of γ-aminobutyric acid and vitamin c supplementation on growth performance and serum biochemical indexes of Magang goose under heat stress. Anim. Husb. Feed. Sci. 2018, 39, 8–12. [Google Scholar]
- Chen, Y.P.; Cheng, Y.F.; Wen, C.; Zhou, Y.M. Protective effects of dietary mannan oligosaccharide on heat stress-induced hepatic damage in broilers. Environ. Sci. Pollut. Res. Int. 2020, 27, 29000–29008. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.D.; Morris, T.R. Poultry and coloured light. World Poult. Sci. J. 2000, 56, 189–207. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Cao, J.; Dong, Y.L.; Chen, Y.X. Role of monochromatic light on development of cecal tonsil in young broilers. Anat. Rec. 2014, 297, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Huth, J.C.; Archer, G.S. Comparison of two LED light bulbs to a dimmable CFL and their effects on broiler chicken growth, stress, and fear. Poult. Sci. 2015, 94, 2027–2036. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Q.; Wang, T.; Yang, Y.F.; Jado, A.; Elhadidi, Y.; Lin, W.F.; Li, J.; Pan, J.M. Effects of monochromatic blue light on reducing the adverse impact of induced cyclic chronic heat stress during the thermal manipulation of broiler embryos. Oxid. Med. Cell Longev. 2022, 2022, 9898311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Wang, Z.X.; Dong, Y.L.; Cao, J.; Chen, Y.X. Blue light alters the composition of the jejunal microbiota and promotes the development of the small intestine by reducing oxidative stress. Antioxidants 2022, 11, 274. [Google Scholar] [CrossRef]
- Archer, G.S.; Byrd, J.A. Effect of light spectrum on stress susceptibility and salmonella status of laying hens. Int. J. Poult. Sci. 2018, 17, 529–535. [Google Scholar] [CrossRef]
- Sultana, S.; Hassan, M.R.; Choe, H.S.; Ryu, K.S. The effect of monochromatic and mixed LED light colour on the behaviour and fear responses of broiler chicken. Avian Biol. Res. 2013, 6, 207–214. [Google Scholar] [CrossRef]
- Xie, D.; Wang, Z.X.; Dong, Y.L.; Cao, J.; Wang, J.F.; Chen, J.L.; Chen, Y.X. Effects of monochromatic light on immune response of broilers. Poult. Sci. 2008, 87, 1535–1539. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Cao, J.; Wang, Z.X.; Dong, Y.L.; Chen, Y.X. Effect of a combination of green and blue monochromatic light on broiler immune response. J. Photochem. Photobiol. B 2014, 138, 118–123. [Google Scholar] [CrossRef]
- Fernandes, A.M.; de Lucca Sartori, D.; de Oliveira Morais, F.J.; Salgado, D.D.; Pereira, D.F. Analysis of cluster and unrest behaviors of laying hens housed under different thermal conditions and light wave length. Animals 2021, 11, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kodaira, V.; Siriani, A.L.R.; Medeiros, H.P.; De Moura, D.J.; Pereira, D.F. Assessment of preference behavior of layer hens under different light colors and temperature environments in long-time footage using a computer vision system. Animals 2023, 13, 2426. [Google Scholar] [CrossRef]
- Rakibul, H.M.; Shabiha, S.; Seon, R.K. Effect of various monochromatic LED light colors on performance, blood properties, bone mineral density, and meat fatty acid composition of ducks. J. Poult. Sci. 2016, 54, 66–72. [Google Scholar]
- Rozenboim, I.; El Halawani, M.E.; Kashash, Y.; Piestun, Y.; Halevy, O. The effect of monochromatic photostimulation on growth and development of broiler birds. Gen. Comp. Endocrinol. 2013, 190, 214–219. [Google Scholar] [CrossRef]
- Jin, E.H.; Jia, L.J.; Li, J.; Yang, G.; Wang, Z.X.; Cao, J.; Chen, Y.X. Effect of monochromatic light on melatonin secretion and arylalkylamine N-acetyltransferase mRNA expression in the retina and pineal gland of broilers. Anat. Rec. 2011, 294, 1233–1241. [Google Scholar] [CrossRef]
- Cao, J.; Bian, J.; Wang, Z.; Dong, Y.; Chen, Y. Effect of monochromatic light on circadian rhythmic expression of clock genes and arylalkylamine N-acetyltransferase in chick retina. Chronobiol. Int. 2017, 34, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Jaadane, I.; Boulenguez, P.; Chahory, S.; Carre, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Retinal damage induced by commercial light emitting diodes (LEDs). Free Radic. Biol. Med. 2015, 84, 373–384. [Google Scholar] [CrossRef]
- Guo, B.B.; Dai, Z.C.; Chen, R.; Liu, J.; Shi, Z.D. Enhancing gosling growth and secretion of somatotrophic and thyrotrophic axis hormones through egg turning during incubation. Br. Poult. Sci. 2022, 64, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Cartoni Mancinelli, A.; Baldi, G.; Soglia, F.; Mattioli, S.; Sirri, F.; Petracci, M.; Castellini, C.; Zampiga, M. Impact of chronic heat stress on behavior, oxidative status and meat quality traits of fast-growing broiler chickens. Front. Physiol. 2023, 14, 1242094. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, K.W. An update on heat stress in laying hens. World Poult. Sci. J. 2023, 79, 689–712. [Google Scholar] [CrossRef]
- Çapar Akyüz, H.; Onbaşilar, E.E. Light wavelength on different poultry species. World Poult. Sci. J. 2019, 74, 79–88. [Google Scholar] [CrossRef]
- Prayitno, D.S.; Phillips, C.J.; Omed, H. The effects of color of lighting on the behavior and production of meat chickens. Poult. Sci. 1997, 76, 452–457. [Google Scholar] [CrossRef]
- Remonato Franco, B.; Shynkaruk, T.; Crowe, T.; Fancher, B.; French, N.; Gillingham, S.; Schwean-Lardner, K. Light color and the commercial broiler: Effect on behavior, fear, and stress. Poult. Sci. 2022, 101, 102052. [Google Scholar] [CrossRef]
- Xie, D.; Li, J.; Wang, Z.X.; Cao, J.; Li, T.T.; Chen, J.L.; Chen, Y.X. Effects of monochromatic light on mucosal mechanical and immunological barriers in the small intestine of broilers. Poult. Sci. 2011, 90, 2697–2704. [Google Scholar] [CrossRef]
- Mackie, A. The digestive tract: A complex system. In Interdisciplinary Approaches to Food Digestion; Gouseti, O., Bornhorst, G.M., Bakalis, S., Mackie, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 11–27. [Google Scholar]
- Liu, Y.H.; He, Y.K.; Fan, S.Q.; Gong, X.Y.; Zhou, Y.Q.; Jian, Y.W.; Ouyang, J.Y.; Jiang, Q.M.; Zhang, P.H. Effects of LED light colors on the growth performance, intestinal morphology, cecal short-chain fatty acid concentrations and microbiota in broilers. Animals 2023, 13, 3731. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Lu, Z.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018, 98, 4471–4478. [Google Scholar] [CrossRef] [PubMed]
- Hosseindoust, A.; Kang, H.K.; Kim, J.S. Quantifying heat stress; the roles on metabolic status and intestinal integrity in poultry, a review. Domest. Anim. Endocrinol. 2022, 81, 106745. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wen, J.; Zhang, H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult. Sci. 2007, 86, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.O. Parts yield of broilers reared under cycling high temperatures. Poult. sci 1993, 72, 1146–1150. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.F.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef]
- Emami, N.K.; Jung, U.; Voy, B.; Dridi, S. Radical response: Effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ding, K.N.; Shen, X.L.; Liu, H.X.; Zhang, Y.A.; Liu, Y.Q.; He, Y.M.; Tang, L.P. Chronic heat stress promotes liver inflammation in broilers via enhancing NF-κB and NLRP3 signaling pathway. BMC Vet. Res. 2022, 18, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.J.; Ahmed, I.; Liu, L.X.; Liu, Y.; Xu, Z.Q.; Duan, X.H.; Li, Q.H.; Dou, T.F.; Gu, D.H.; Rong, H.; et al. Selection for growth rate and body size have altered the expression profiles of somatotropic axis genes in chickens. PLoS ONE 2018, 13, e0195378. [Google Scholar] [CrossRef]
- Wang, T.J.; Wang, Z.X.; Cao, J.; Dong, Y.L.; Chen, Y.X. Monochromatic light affects the development of chick embryo liver via an anti-oxidation pathway involving melatonin and the melatonin receptor Mel1c. Can. J. Anim. Sci. 2014, 94, 391–400. [Google Scholar] [CrossRef]
- Guan, Q.Y.; Wang, Z.X.; Cao, J.; Dong, Y.L.; Chen, Y.X. Monochromatic blue light not green light exposure is associated with continuous light-induced hepatic steatosis in high fat diet fed-mice via oxidative stress. Ecotoxicol. Environ. Saf. 2022, 239, 113625. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Ali, S.; Sahin, N.; Hayirli, A. Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult. Sci. 2010, 89, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Smith, M.O.; Sahin, N. Molecular targets of dietary phytochemicals for the alleviation of heat stress in poultry. World Poult. Sci. J. 2019, 69, 113–124. [Google Scholar] [CrossRef]
- Kaminskyy, V.O.; Zhivotovsky, B. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Signal 2014, 21, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Xu, X.Q.; Leng, X.; He, M.H.; Wang, J.K.; Cheng, S.P.; Wu, H. Roles of reactive oxygen species in cell signaling pathways and immune responses to viral infections. Arch. Virol. 2017, 162, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.X.; Chang, Q.Q.; Wei, L.L.; Zhao, Z.H. The protect effects of chitosan oligosaccharides on intestinal integrity by regulating oxidative status and inflammation under oxidative stress. Mar. Drugs 2021, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.E.; El-Kassas, S.; El-Nahas, A.F.; Mahmoud, S. Modulatory effect of monochromatic blue light on heat stress response in commercial broilers. Oxid. Med. Cell Longev. 2017, 2017, 1351945. [Google Scholar] [CrossRef] [PubMed]
- Jastrebski, S.F.; Lamont, S.J.; Schmidt, C.J. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS ONE 2017, 12, e0181900. [Google Scholar] [CrossRef]
- Ma, B.B.; Zhang, L.; Li, J.L.; Xing, T.; Jiang, Y.; Gao, F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 2021, 100, 215–223. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Bouchama, A.; al-Sedairy, S.; Siddiqui, S.; Shail, E.; Rezeig, M. Elevated pyrogenic cytokines in heatstroke. Chest 1993, 10, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Q.; Zhang, Y.; Li, J.H.; Xia, H.F.; Zhang, D.Y.; Yao, S.L. The pathogenesis and therapeutic strategies of heat stroke-induced liver injury. Crit. Care 2022, 17, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Kato, N.; Ioi, H.; Nishimata, S.; Watanabe, C.; Kashiwagi, Y.; Takekuma, K.; Hoshika, A.; Szenborn, L.; Bergman, K. mRNA expression of T-helper 1, T-helper 2 cytokines in autoimmune hepatitis in childhood. Pediatr. Int. 2008, 50, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Castranova, V.; Shi, X.; Demers, L.M. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 1999, 45, 7–17. [Google Scholar] [CrossRef]
- Horodincu, L.; Solcan, C. Influence of different light spectra on melatonin synthesis by the pineal gland and influence on the immune system in chickens. Animals 2023, 13, 2095. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Reiter, R.J. Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur. J. Pharmacol. 2001, 426, 1–10. [Google Scholar] [CrossRef]
- Galano, A.; Tan Dx Fau—Reiter, R.J.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.F.; Zheng, Y.; Lv, Y.H.; Li, F.Y.; Su, L.H.; Qin, Y.W.; Zeng, W.X. Melatonin protects the mouse testis against heat-induced damage. Mol. Hum. Reprod. 2020, 26, 65–79. [Google Scholar] [CrossRef]
- Deng, C.C.; Zhang, J.P.; Huo, Y.N.; Xue, H.Y.; Wang, W.X.; Zhang, J.J.; Wang, X.Z. Melatonin alleviates the heat stress-induced impairment of Sertoli cells by reprogramming glucose metabolism. J. Pineal Res. 2022, 73, e12819. [Google Scholar] [CrossRef]
- Wang, H.; Wei, W.; Wang, N.P.; Gui, S.Y.; Wu, L.; Sun, W.Y.; Xu, S.Y. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005, 77, 1902–1915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Huang, Z.; Li, Q.J.; Zhong, G.Q.; Meng, J.J.; Wang, D.X.; Tu, R.H. Role of HSP90 in suppressing TLR4-mediated inflammation in ischemic postconditioning. Clin. Hemorheol. Microcirc. 2020, 76, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.A.-O.; Khanlarkhani, N. Melatonin application in targeting oxidative-induced liver injuries: A review. J. Cell Physiol. 2018, 233, 4015–4032. [Google Scholar] [CrossRef] [PubMed]



| Genes | Accession Number | Primer Sequence (5–3) | Fragment Size (bp) |
|---|---|---|---|
| β-Actin | XM_048058703.1 | TGACGCAGATCATGTTTGAGA | 159 |
| GCAGAGCGTAGCCCTCATAG | |||
| NFKB1 | XM_048066390.1 | TGATTGCTGCTGGAGTTAATGT | 172 |
| GCTGCTATGTGAAGAGGTGTT | |||
| NFKB2 | XM_048069036.1 | CTTCACCGCCTACCTTCGT | 172 |
| GCACAGCAAGTAGACCTCATC | |||
| NFKBIA | XM_048059840.1 | TCAGAAGCGTCAGCGTCCTCA | 139 |
| AGCAGGTACTCGACAACAGCCA | |||
| IL-6 | XM_048070285.1 | ATGTCGTCCGTCACTGTAGC | 170 |
| GCGTGGAAGTAGCCTGAGAA | |||
| IL-8 | XM_013190618.2 | AGGAAACTGGACTGCAGGGA | 188 |
| TTGTGCCTGACTTGTGTGCT | |||
| NOTCH1 | XM_048053605.1 | GCCTGTCCGAAGTGAACGAGTG | 189 |
| TGTAGCCGCTGGTCATGTCCTT | |||
| IL-1β | XM_048054159.1 | CCCACAAAAGAAGCTTCGCC | 172 |
| GAAGTCCTTGTGCGACGGC | |||
| HSP90α | XM_048069885.1 | GAGCGTCTTCGCAAACATGG | 106 |
| CAGAAACCAGGGTCTTGCCT | |||
| CAT | XM_048058871 | GCGTATGCTGATACACATAGACATCGT | 194 |
| CTCTCCTTCACAACAGGTTGATCTTCT | |||
| SOD1 | XM_048071453.1 | TTACTGGAAGAATCAGCGGCTTGTC | 136 |
| TCAGTTGGTCCACCGTGCTTCT |
| Items 1 | Group | p Value | ||
|---|---|---|---|---|
| W | WH | GH | ||
| BW (d28), g | 1775.1 ± 58.5 | 1790.8 ± 63.0 | 1803.4 ± 29.6 | 0.931 |
| BW (d70), g | 4038.3 ± 118.1 b | 3614.1 ± 132.1 a | 4013.3 ± 80.7 b | 0.031 |
| ADFI, g | 265.3 ± 5.0 b | 250.9 ± 3.1 a | 256.0 ± 1.3 ab | 0.049 |
| FCR | 4.90 ± 0.16 a | 5.76 ± 0.20 b | 4.84 ± 0.07 a | 0.029 |
| RWAF, % | 3.83 ± 0.23 b | 2.59 ± 0.30 a | 3.45 ± 0.17 b | 0.004 |
| LW, g | 71.57 ± 2.46 | 71.66 ± 3.19 | 67.64 ± 1.99 | 0.422 |
| Liver index 2, % | 1.70 ± 0.02 a | 1.94 ± 0.06 c | 1.69 ± 0.05 a | 0.006 |
| Items 1 | Group | p Value | ||
|---|---|---|---|---|
| W | WH | GH | ||
| CORT, ng/mL | 117.55 ± 9.18 a | 175.96 ± 4.82 c | 148.12 ± 7.45 b | <0.0001 |
| GPT, U/L | 68.32 ± 9.32 a | 103.8 ± 12.05 b | 72.88 ± 8.459 a | 0.049 |
| GOT, U/L | 80.55 ± 10.58 a | 133.70 ± 13.88 b | 112.10 ± 12.27 ab | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Yan, L.; Tang, Y.; Du, J.; Dai, Z.; Liu, J.; Lei, M.; Hou, Z.; Zhu, H. Green Light Mitigates Cyclic Chronic Heat-Stress-Induced Liver Oxidative Stress and Inflammation via NF-κB Pathway Inhibition in Geese. Antioxidants 2024, 13, 772. https://doi.org/10.3390/antiox13070772
Guo B, Yan L, Tang Y, Du J, Dai Z, Liu J, Lei M, Hou Z, Zhu H. Green Light Mitigates Cyclic Chronic Heat-Stress-Induced Liver Oxidative Stress and Inflammation via NF-κB Pathway Inhibition in Geese. Antioxidants. 2024; 13(7):772. https://doi.org/10.3390/antiox13070772
Chicago/Turabian StyleGuo, Binbin, Leyan Yan, Yi Tang, Jie Du, Zichun Dai, Jie Liu, Mingming Lei, Zhuocheng Hou, and Huanxi Zhu. 2024. "Green Light Mitigates Cyclic Chronic Heat-Stress-Induced Liver Oxidative Stress and Inflammation via NF-κB Pathway Inhibition in Geese" Antioxidants 13, no. 7: 772. https://doi.org/10.3390/antiox13070772
APA StyleGuo, B., Yan, L., Tang, Y., Du, J., Dai, Z., Liu, J., Lei, M., Hou, Z., & Zhu, H. (2024). Green Light Mitigates Cyclic Chronic Heat-Stress-Induced Liver Oxidative Stress and Inflammation via NF-κB Pathway Inhibition in Geese. Antioxidants, 13(7), 772. https://doi.org/10.3390/antiox13070772






