Therapeutic Potential of Plant-Derived Compounds and Plant Extracts in Rheumatoid Arthritis—Comprehensive Review
Abstract
:1. Introduction
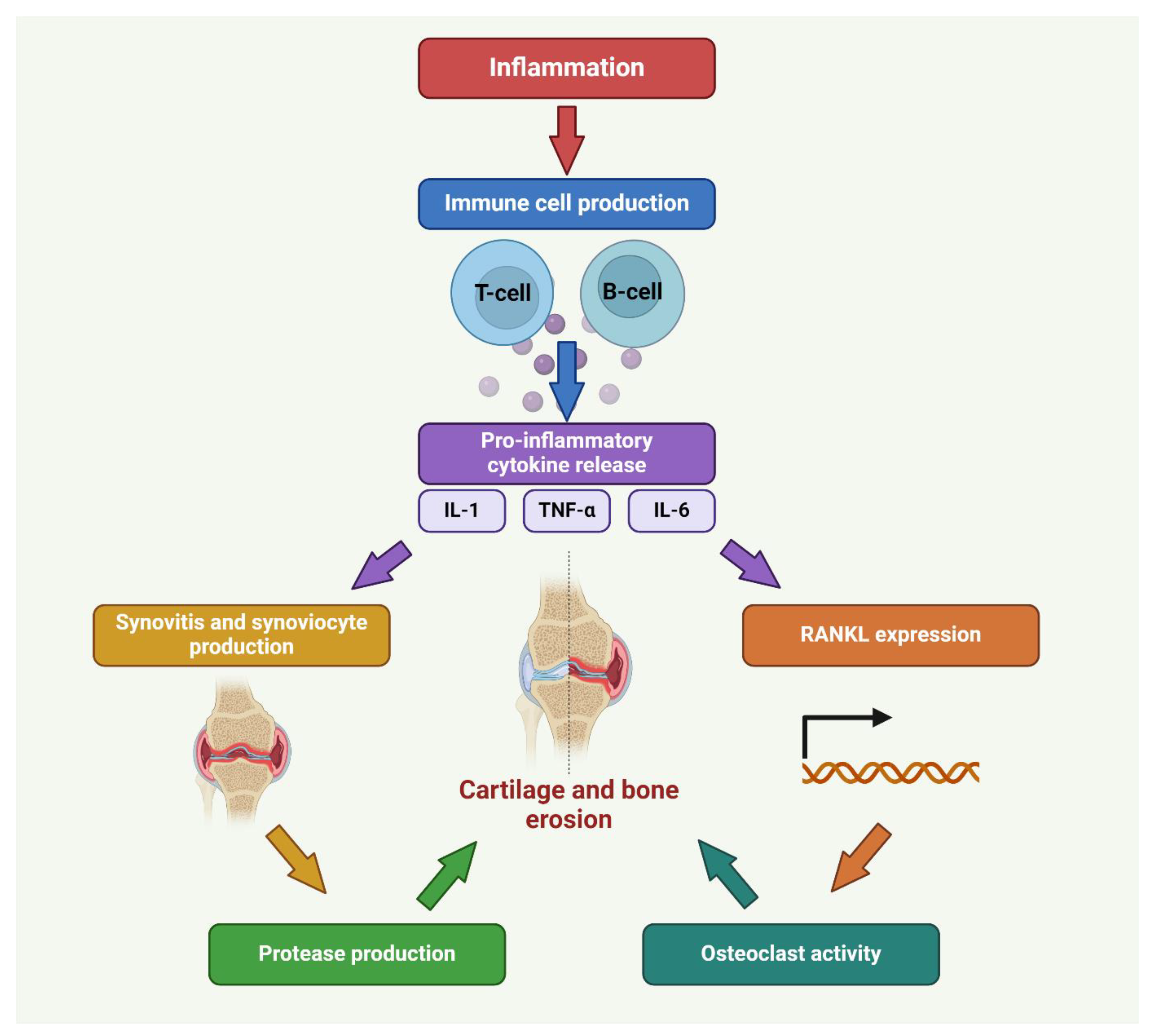
2. Pathophysiology of RA
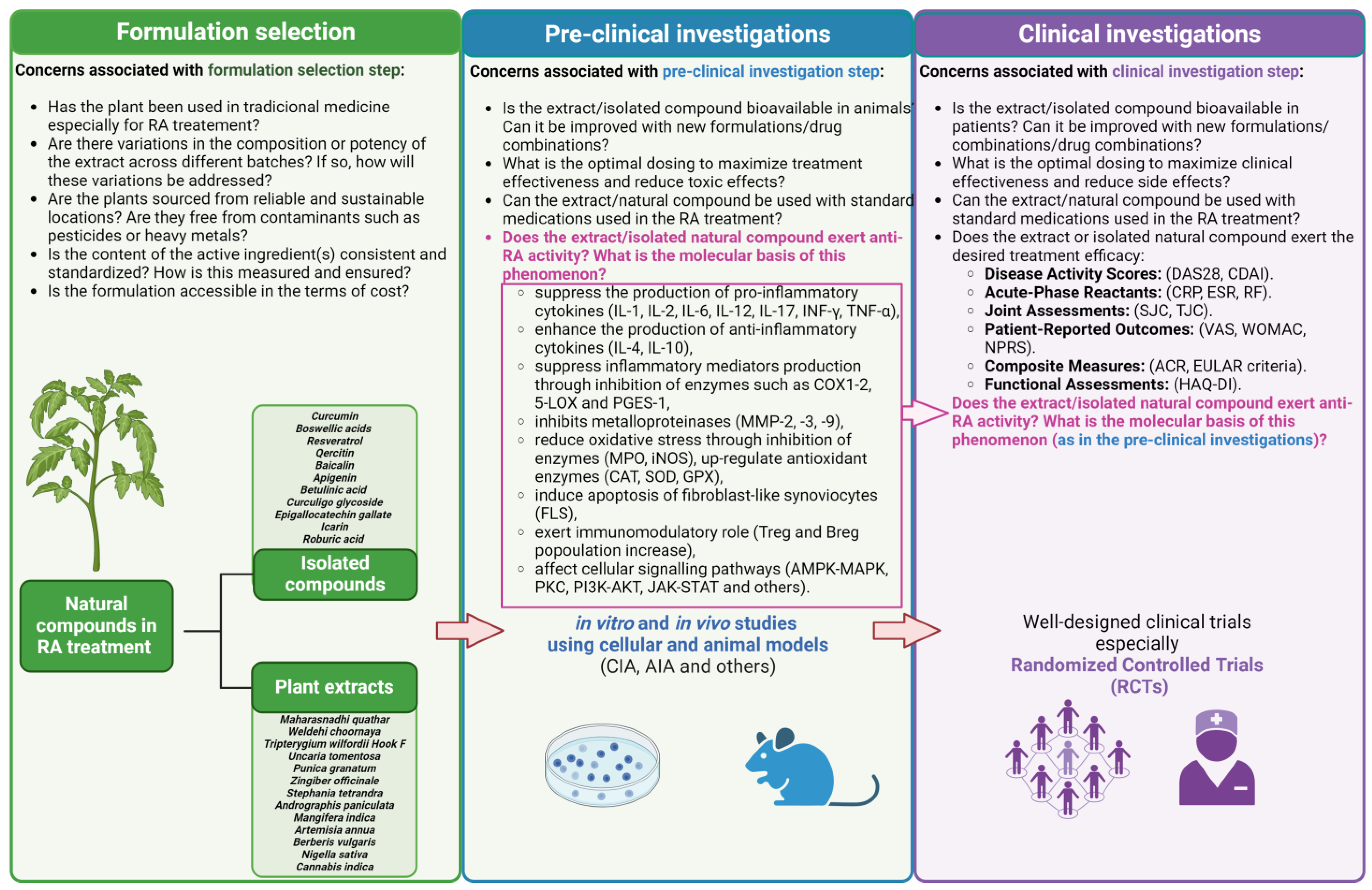
3. Natural Compounds as Anti-RA Agents—Overview
4. Plant-Derived Compounds Investigated in Clinical Trials
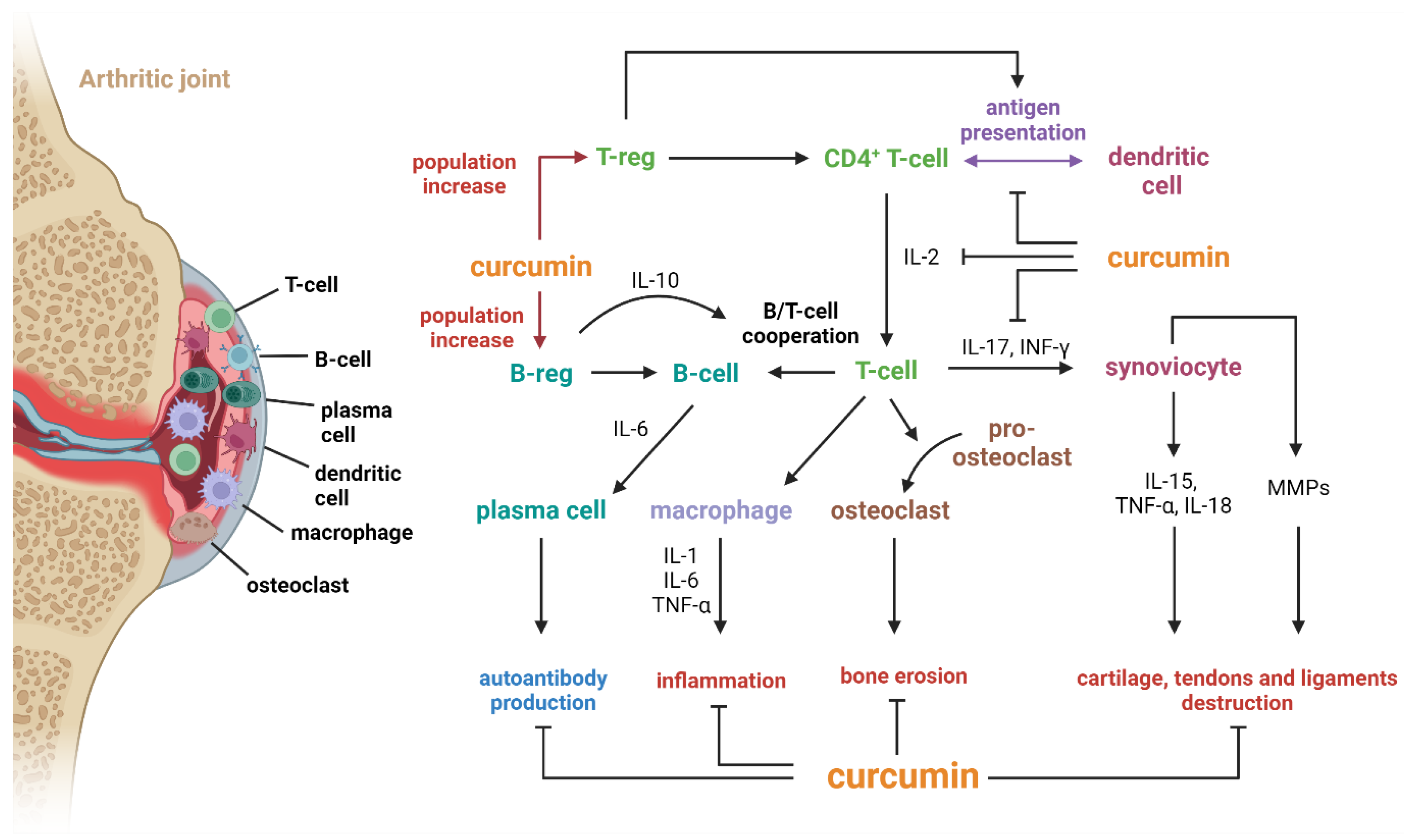
4.1. Curcumin
4.2. Boswellic Acids
4.3. Resveratrol
4.4. Quercetin
4.5. Baicalin
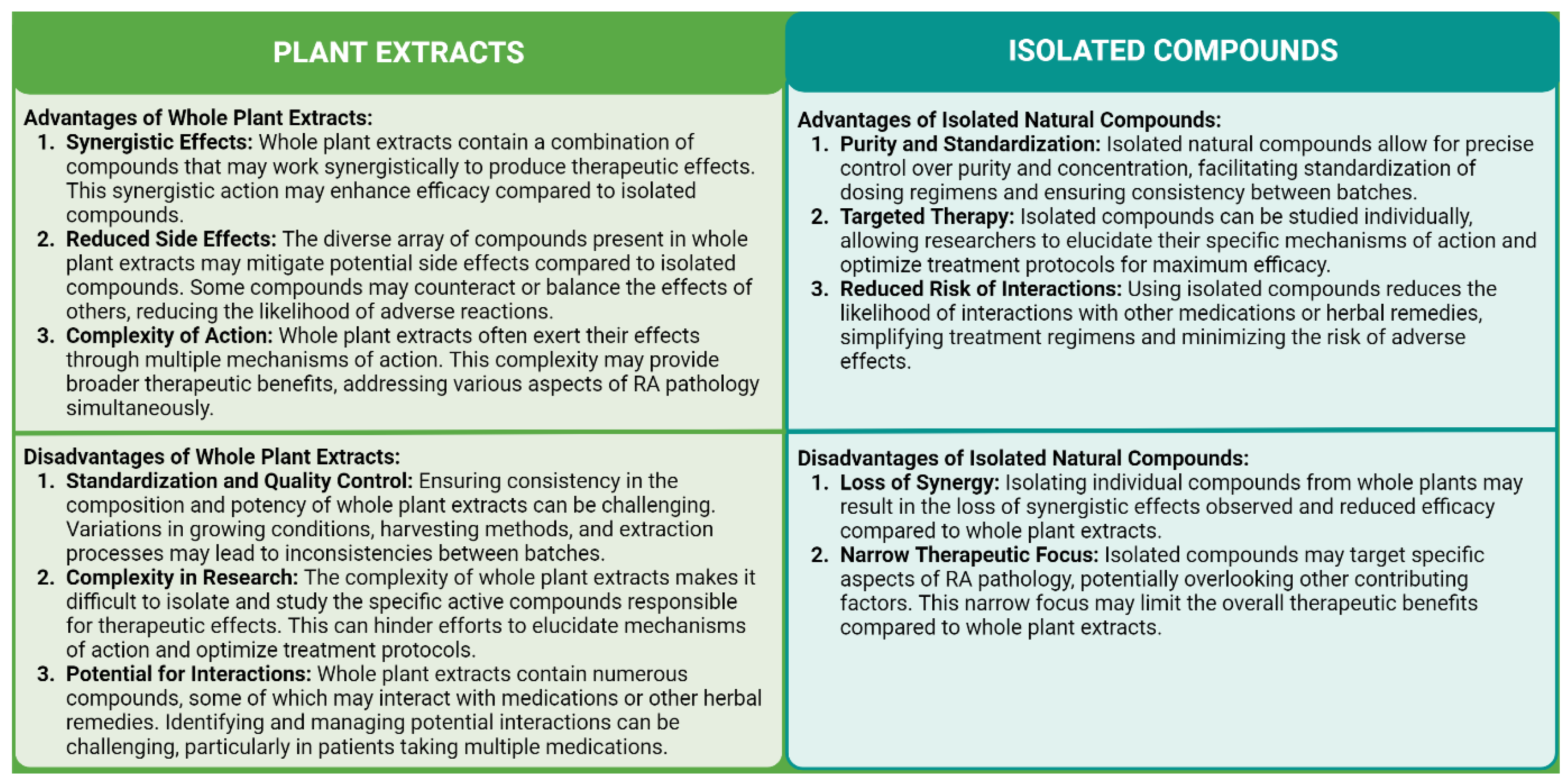
4.6. Plant Extracts
5. Other Compounds
5.1. Apigenin
5.2. Betulinic Acid
5.3. Curculigo Glycoside
5.4. Epigallocatechin Gallate
5.5. Icarin
5.6. Roburic Acid
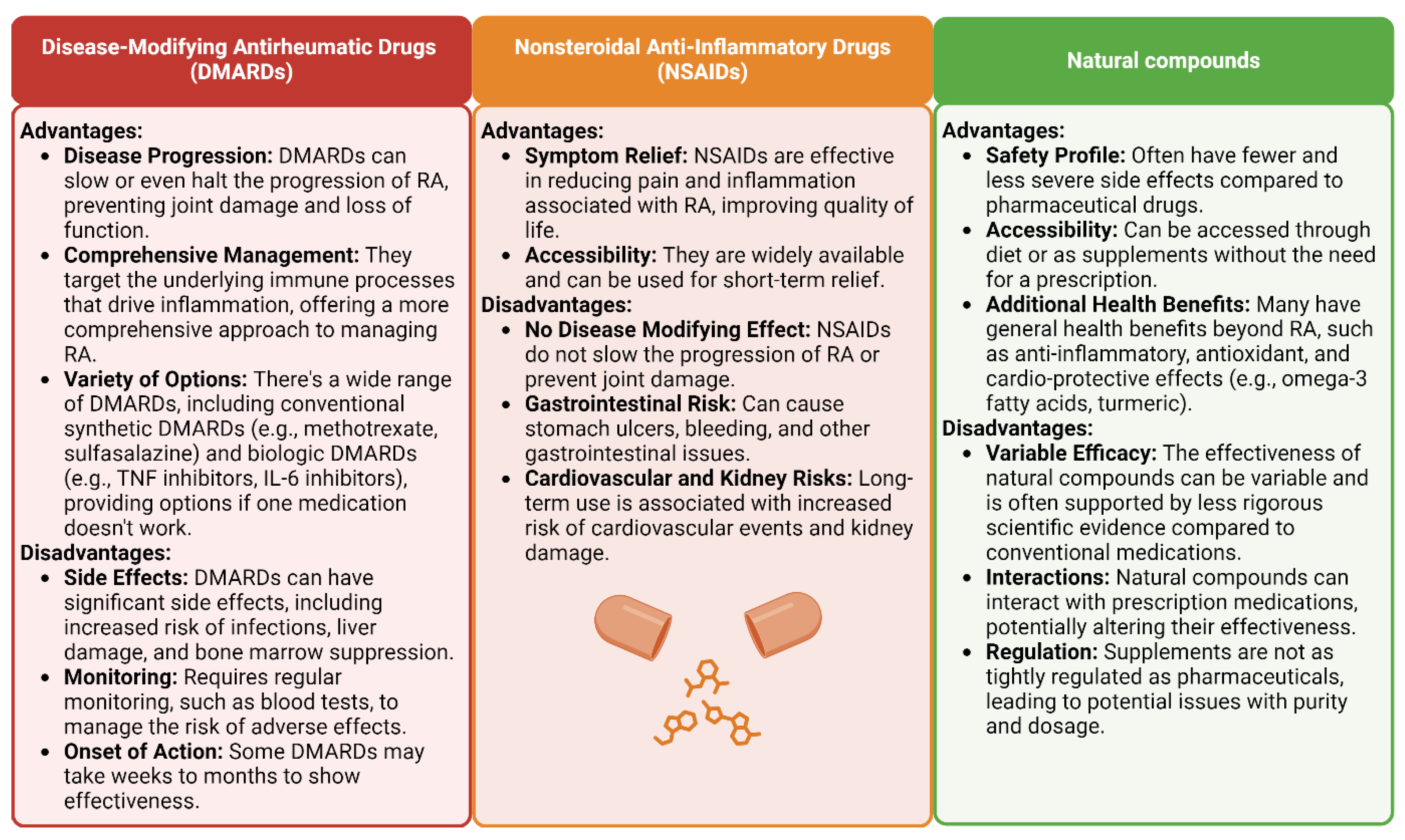
6. Marketed Formulations
7. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACPA | anti-citrullinated peptide/protein antibodies |
| ACR | American College of Rheumatology |
| ADA | anti-drug antibodies |
| AIA | adjuvant-induced arthritis |
| AIF | apoptosis-inducing factor |
| AKBA | acetyl keto boswellic acid |
| AKT | AKT serine/threonine kinase |
| AMPK | 5′-AMP-activated protein kinase catalytic subunit alpha-1 |
| AP-1 | adaptor protein 1 |
| APC | antigen-presenting cell |
| ARE | antioxidative response element |
| BAX | apoptosis regulator BAX |
| BCL-2 | apoptosis regulator Bcl-2 |
| Breg | B regulatory cells |
| CA | curculigoside A |
| CAT | catalase |
| CBD | cannabidiol |
| CCL-1 | monocyte chemoattractant protein-1 |
| CCR6 | chemokine receptor 6 |
| CDAI | Clinical Disease Activity Index |
| CIA | collagen-induced arthritis |
| COX | cyclooxygenase |
| CRP | C-reactive protein |
| CTLA4 | cytotoxic T-lymphocyte-associated protein 4 |
| CYP | cytochrome P450 |
| DAS | Disease Activity Score |
| DMARDs | disease-modifying antirheumatic drugs |
| EGCG | epigallocatechin gallate |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular signal-regulated kinase |
| ESR | erythrocyte sedimentation rate |
| EULAR | European League Against Rheumatism |
| FGFR | fibroblast growth factor receptor 1 |
| FLS | fibroblast-like synoviocytes |
| GCQG | glucosamine-chondroitin-quercetin glucoside |
| GPX | glutathione peroxidase |
| GSH | glutathione |
| GWAS | genome-wide association studies |
| HAQ-DI | Health Assessment Questionnaire Disability Index |
| HLA | human leukocyte antigen |
| HO-1 | heme oxygenase-1 |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| ICAM-1 | intercellular adhesion molecule 1 |
| IL | interleukin |
| IL-23R | interleukin-23 receptor |
| INF-γ | interferon-γ |
| iNOS | inducible nitric oxide synthase |
| IRF5 | interferon regulatory factor 5 |
| IκBα | NF-kappa-B inhibitor alpha |
| JAK | Janus kinase |
| LPO | lipid peroxidase |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MCL-1 | myeloid cell leukemia 1 |
| MDA | malondialdehyde |
| M-EC | membrane-camouflaged nanoparticles |
| MEK1 | dual specificity mitogen-activated protein kinase kinase 1 |
| MMP | matrix metalloproteinase |
| mPGES-1 | microsomal prostaglandin E2 synthase-1 |
| MPO | myeloperoxidase |
| MRQ | Maharasnadhi quathar |
| MTX | methotrexate |
| NFATc1 | nuclear factor of activated T cells 1 |
| NF-κB | nuclear factor kappa B |
| NO | nitric oxide |
| NOEL | no observed effect level |
| NPRS | Numeric Pain Rating Scale |
| NRF-2 | nuclear factor erythroid 2-related factor 2 |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| PADI4 | protein-arginine deiminase type-4 |
| PARP-1 | poly ADPribose polymerase |
| PBS | phosphate buffered saline |
| PGE-2 | prostaglandin E2 |
| PI3K | phosphatidylinositol 4,5-bisphosphate 3-kinase |
| PKC | protein kinase C |
| PROs | patient-reported outcomes |
| PTPN22 | phosphatase non-receptor type 22 |
| RA | rheumatoid arthritis |
| RANKL | receptor activator for nuclear factor κB ligand |
| RBA | roburic acid |
| RF | rheumatoid factor |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SE | shared epitope |
| SIRT1 | sirtuin 1 |
| SJC | swollen joint count |
| SOD | superoxide dismutase |
| STAT | signal transducer and activator of transcription |
| TBARS | thiobarbituric acid reacting substances |
| TGF-β | transforming growth factor β |
| THC | tetrahydrocannabinol |
| TJC | tender joint count |
| TNF-R1 | tumor necrosis factor receptor 1 |
| TNF-α | tumor necrosis factor-alpha |
| TRAF1/6 | TNF receptor-associated factor 1/6 |
| Tregs | regulatory T cells |
| UT | Uncaria tomentosa |
| VAS | Visual Analog Scale |
| VCAM-1 | vascular cell adhesion protein 1 |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| WC | Weldehi choornaya |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
References
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid Arthritis: Pathological Mechanisms and Modern Pharmacologic Therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Padyukov, L. Genetics of Rheumatoid Arthritis. Semin. Immunopathol. 2022, 44, 47–62. [Google Scholar] [CrossRef]
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maśliński, W. The Role of Anti-Citrullinated Protein Antibodies (ACPA) in the Pathogenesis of Rheumatoid Arthritis. Cent. Eur. J. Immunol. 2017, 42, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A. Treatment Guidelines in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2022, 48, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Recent Progress in Treatments of Rheumatoid Arthritis: An Overview of Developments in Biologics and Small Molecules, and Remaining Unmet Needs. Rheumatology 2021, 60, vi12–vi20. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.-F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2019, 27, 501–507. [Google Scholar] [CrossRef]
- Gravallese, E.M.; Firestein, G.S. Rheumatoid Arthritis—Common Origins, Divergent Mechanisms. N. Engl. J. Med. 2023, 388, 529–542. [Google Scholar] [CrossRef]
- Kerschbaumer, A.; Sepriano, A.; Bergstra, S.A.; Smolen, J.S.; van der Heijde, D.; Caporali, R.; Edwards, C.J.; Verschueren, P.; de Souza, S.; Pope, J.E.; et al. Efficacy of Synthetic and Biological DMARDs: A Systematic Literature Review Informing the 2022 Update of the EULAR Recommendations for the Management of Rheumatoid Arthritis. Ann. Rheum. Dis. 2023, 82, 95–106. [Google Scholar] [CrossRef]
- Kumar, A.; Nirma, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, K.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Kurkó, J.; Besenyei, T.; Laki, J.; Glant, T.T.; Mikecz, K.; Szekanecz, Z. Genetics of Rheumatoid Arthritis—A Comprehensive Review. Clin. Rev. Allergy Immunol. 2013, 45, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Dedmon, L.E. The Genetics of Rheumatoid Arthritis. Rheumatology 2020, 59, 2661–2670. [Google Scholar] [CrossRef]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and Environmental Risk Factors for Rheumatoid Arthritis. Best. Pract. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef]
- Arleevskaya, M.; Takha, E.; Petrov, S.; Kazarian, G.; Renaudineau, Y.; Brooks, W.; Larionova, R.; Korovina, M.; Valeeva, A.; Shuralev, E.; et al. Interplay of Environmental, Individual and Genetic Factors in Rheumatoid Arthritis Provocation. Int. J. Mol. Sci. 2022, 23, 8140. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.I.; Kravtsova, O.A.; Lemerle, J.; Renaudineau, Y.; Tsibulkin, A.P. How Rheumatoid Arthritis Can Result from Provocation of the Immune System by Microorganisms and Viruses. Front. Microbiol. 2016, 7, 1296. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kwon, E.-J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Yap, H.-Y.; Tee, S.Z.-Y.; Wong, M.M.-T.; Chow, S.-K.; Peh, S.-C.; Teow, S.-Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef]
- Cope, A.P.; Schulze-Koops, H.; Aringer, M. The Central Role of T Cells in Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2007, 25, S4–S11. [Google Scholar]
- Nandakumar, K.S.; Fang, Q.; Wingbro Ågren, I.; Bejmo, Z.F. Aberrant Activation of Immune and Non-Immune Cells Contributes to Joint Inflammation and Bone Degradation in Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 15883. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Kang, J.; Wang, X.; Niu, Q.; Liu, J.; Zhang, L. B Cells in Rheumatoid Arthritis: Pathogenic Mechanisms and Treatment Prospects. Front. Immunol. 2021, 12, 750753. [Google Scholar] [CrossRef]
- Volkov, M.; van Schie, K.A.; van der Woude, D. Autoantibodies and B Cells: The ABC of Rheumatoid Arthritis Pathophysiology. Immunol. Rev. 2020, 294, 148–163. [Google Scholar] [CrossRef]
- Sheng, S.; Wang, X.; Liu, X.; Hu, X.; Shao, Y.; Wang, G.; Mao, D.; Li, C.; Chen, B.; Chen, X. The Role of Resveratrol on Rheumatoid Arthritis: From Bench to Bedside. Front. Pharmacol. 2022, 13, 829677. [Google Scholar] [CrossRef] [PubMed]
- De Seny, D.; Baiwir, D.; Bianchi, E.; Cobraiville, G.; Deroyer, C.; Poulet, C.; Malaise, O.; Paulissen, G.; Kaiser, M.-J.; Hauzeur, J.-P.; et al. New Proteins Contributing to Immune Cell Infiltration and Pannus Formation of Synovial Membrane from Arthritis Diseases. Int. J. Mol. Sci. 2021, 23, 434. [Google Scholar] [CrossRef] [PubMed]
- Umbreen, H.; Zhang, X.; Tang, K.-T.; Lin, C.-C. Regulation of Myeloid Dendritic Cells by Synthetic and Natural Compounds for the Treatment of Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 24, 238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Chaubey, P.; Suvarna, V. Role of Natural Products in Alleviation of Rheumatoid Arthritis-A Review. J. Food Biochem. 2021, 45, e13673. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, S.; Mehta, H.C.; Sood, A.K.; Kaur, J. Antioxidant Status in Rheumatoid Arthritis and Role of Antioxidant Therapy. Clin. Chim. Acta 2003, 338, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Kolahi, S.; Aref-Hosseini, S.-R.; Mamegani, M.E.; Hekmatdoost, A. Beneficial Role of Antioxidants on Clinical Outcomes and Erythrocyte Antioxidant Parameters in Rheumatoid Arthritis Patients. Int. J. Prev. Med. 2014, 5, 835–840. [Google Scholar] [PubMed]
- Moradi, A.; Nezamoleslami, S.; Nezamoleslami, S.; Clark, C.C.T.; Sohouli, M.H.; Ghiasvand, R. The Association between Dietary Total Antioxidant Capacity with Risk of Rheumatoid Arthritis in Adults: A Case-Control Study. Clin. Nutr. ESPEN 2022, 51, 391–396. [Google Scholar] [CrossRef]
- Djordjevic, K.; Milojevic Samanovic, A.; Veselinovic, M.; Zivkovic, V.; Mikhaylovsky, V.; Mikerova, M.; Reshetnikov, V.; Jakovljevic, V.; Nikolic Turnic, T. Oxidative Stress Mediated Therapy in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 1938. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shang, W.; Zhao, Z.; Zhang, B.; Liu, C.; Cai, H. Curcumin Alleviates Rheumatoid Arthritis Progression through the Phosphatidylinositol 3-Kinase/Protein Kinase B Pathway: An in Vitro and in Vivo Study. Bioengineered 2022, 13, 12899–12911. [Google Scholar] [CrossRef] [PubMed]
- Więcek, K.; Kupczyk, P.; Chodaczek, G.; Woźniak, M. The Impact of Curcumin on the Inflammatory Profile of SW982 Cells in a Rheumatoid Arthritis Model. J. Immunol. Res. 2022, 2022, 1208970. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhou, D.; Xu, L.; Song, X. Curcumin Alleviates Rheumatoid Arthritis-Induced Inflammation and Synovial Hyperplasia by Targeting mTOR Pathway in Rats. Drug Des. Devel Ther. 2018, 12, 4095–4105. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An Orally Bioavailable Blocker of TNF and Other pro-Inflammatory Biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, W.; Liu, Y.; Hou, G.; Li, C. Treatment of Rheumatoid Arthritis with Curcumin Analog 3,5-Bis(Arylidene)-4-Piperidone. Future Med. Chem. 2023, 15, 2051–2064. [Google Scholar] [CrossRef]
- Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Curcumin Mediated Suppression of Nuclear Factor-κB Promotes Chondrogenic Differentiation of Mesenchymal Stem Cells in a High-Density Co-Culture Microenvironment. Arthritis Res. Ther. 2010, 12, R127. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Han, Z.; Tian, L.; Chen, K.; Fan, Y.; Ye, B.; Huang, W.; Wang, C.; Huang, Z. Curcumin Inhibits EMMPRIN and MMP-9 Expression through AMPK-MAPK and PKC Signaling in PMA Induced Macrophages. J. Transl. Med. 2014, 12, 266. [Google Scholar] [CrossRef]
- Xiao, J.; Cai, X.; Zhou, W.; Wang, R.; Ye, Z. Curcumin Relieved the Rheumatoid Arthritis Progression via Modulating the Linc00052/miR-126-5p/PIAS2 Axis. Bioengineered 2022, 13, 10973–10983. [Google Scholar] [CrossRef]
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-Inflammatory and Apoptotic Effects of the Polyphenol Curcumin on Human Fibroblast-like Synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405. [Google Scholar] [CrossRef]
- Akbari-Papkiadehi, F.; Saboor-Yaraghi, A.A.; Farhadi, E.; Tahmasebi, M.N.; Sharafat Vaziri, A.; Aghaghazvini, L.; Asgari, M.; Poursani, S.; Mansouri, F.; Jamshidi, A.; et al. Effect of Curcumin on the Expression of NOD2 Receptor and Pro-Inflammatory Cytokines in Fibroblast-like Synoviocytes (FLSs) of Rheumatoid Arthritis (RA) Patients. Adv. Rheumatol. 2023, 63, 27. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.K.; Higo, T.; Hunter, W.L.; Burt, H.M. The Antioxidants Curcumin and Quercetin Inhibit Inflammatory Processes Associated with Arthritis. Inflamm. Res. 2006, 55, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Saksena, A.K.; Khattri, S.; Kumar, S.; Dagur, R.S. Curcuma longa Extract Reduces Inflammatory and Oxidative Stress Biomarkers in Osteoarthritis of Knee: A Four-Month, Double-Blind, Randomized, Placebo-Controlled Trial. Inflammopharmacology 2016, 24, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian Haftcheshmeh, S.; Khosrojerdi, A.; Aliabadi, A.; Lotfi, S.; Mohammadi, A.; Momtazi-Borojeni, A.A. Immunomodulatory Effects of Curcumin in Rheumatoid Arthritis: Evidence from Molecular Mechanisms to Clinical Outcomes. In Reviews of Physiology, Biochemistry and Pharmacology. Reviews of Physiology, Biochemistry and Pharmacology; Pedersen, S.H.F., Ed.; Springer: Cham, Switzerland, 2021; Volume 179, pp. 1–29. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in Vitro Experiments to in Vivo and Clinical Studies; Pros and Cons. Curr. Drug Discov. Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef]
- Nickien, M.; Heuijerjans, A.; Ito, K.; van Donkelaar, C.C. Comparison between in Vitro and in Vivo Cartilage Overloading Studies Based on a Systematic Literature Review. J. Orthop. Res. 2018, 36, 2076–2086. [Google Scholar] [CrossRef]
- Damerau, A.; Gaber, T. Modeling Rheumatoid Arthritis In Vitro: From Experimental Feasibility to Physiological Proximity. Int. J. Mol. Sci. 2020, 21, 7916. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Ortmann, R.A.; Kimpel, D. Animal Models of Rheumatoid Arthritis and Their Relevance to Human Disease. Pathophysiology 2005, 12, 167–181. [Google Scholar] [CrossRef]
- Moudgil, K.D.; Kim, P.; Brahn, E. Advances in Rheumatoid Arthritis Animal Models. Curr. Rheumatol. Rep. 2011, 13, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Bevaart, L.; Vervoordeldonk, M.J.; Tak, P.P. Evaluation of Therapeutic Targets in Animal Models of Arthritis: How Does It Relate to Rheumatoid Arthritis? Arthritis Rheum. 2010, 62, 2192–2205. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.A.; McInnes, I.B.; Garside, P.; Brewer, J.M. Model Answers: Rational Application of Murine Models in Arthritis Research. Eur. J. Immunol. 2018, 48, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.S.; Suneetha, P.; Mohan, A.; Kumar, D.P.; Sarma, K.V.S. Comparison of Disease Activity Score in 28 Joints with ESR (DAS28), Clinical Disease Activity Index (CDAI), Health Assessment Questionnaire Disability Index (HAQ-DI) & Routine Assessment of Patient Index Data with 3 Measures (RAPID3) for Assessing Disease Activity in Patients with Rheumatoid Arthritis at Initial Presentation. Indian. J. Med. Res. 2017, 146, S57–S62. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, K.; Jayasinghe, C.; Wanigasekara, P.; Dissanayake, J.; Sominanda, A. Validity of Clinical Disease Activity Index (CDAI) to Evaluate the Disease Activity of Rheumatoid Arthritis Patients in Sri Lanka: A Prospective Follow up Study Based on Newly Diagnosed Patients. PLoS ONE 2022, 17, e0278285. [Google Scholar] [CrossRef] [PubMed]
- Hammer, H.B.; Michelsen, B.; Provan, S.A.; Sexton, J.; Lampa, J.; Uhlig, T.; Kvien, T.K. Tender Joint Count and Inflammatory Activity in Patients with Established Rheumatoid Arthritis: Results from a Longitudinal Study. Arthritis Care Res. 2020, 72, 27–35. [Google Scholar] [CrossRef]
- Kay, J.; Morgacheva, O.; Messing, S.P.; Kremer, J.M.; Greenberg, J.D.; Reed, G.W.; Gravallese, E.M.; Furst, D.E. Clinical Disease Activity and Acute Phase Reactant Levels Are Discordant among Patients with Active Rheumatoid Arthritis: Acute Phase Reactant Levels Contribute Separately to Predicting Outcome at One Year. Arthritis Res. Ther. 2014, 16, R40. [Google Scholar] [CrossRef]
- Lv, F.; Song, L.-J.; Li, X.-F. Combined Measurement of Multiple Acute Phase Reactants to Predict Relapse of Rheumatoid Arthritis. Int. J. Rheum. Dis. 2015, 18, 725–730. [Google Scholar] [CrossRef]
- Yildirim, K.; Karatay, S.; Melikoglu, M.A.; Gureser, G.; Ugur, M.; Senel, K. Associations between Acute Phase Reactant Levels and Disease Activity Score (DAS28) in Patients with Rheumatoid Arthritis. Ann. Clin. Lab. Sci. 2004, 34, 423–426. [Google Scholar]
- Bezuidenhout, J.A.; Pretorius, E. The Central Role of Acute Phase Proteins in Rheumatoid Arthritis: Involvement in Disease Autoimmunity, Inflammatory Responses, and the Heightened Risk of Cardiovascular Disease. Semin. Thromb. Hemost. 2020, 46, 465–483. [Google Scholar] [CrossRef]
- Gülfe, A.; Geborek, P.; Saxne, T. Response Criteria for Rheumatoid Arthritis in Clinical Practice: How Useful Are They? Ann. Rheum. Dis. 2005, 64, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.M.; Guthrie, L.C.; Alba, M.I. Brief Report: Rheumatoid Arthritis Response Criteria and Patient-Reported Improvement in Arthritis Activity: Is an American College of Rheumatology Twenty Percent Response Meaningful to Patients? Arthritis Rheumatol. 2014, 66, 2339–2343. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Brinks, R.; Dörner, T.; Daikh, D.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) SLE Classification Criteria Item Performance. Ann. Rheum. Dis. 2021, 80, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.H.; Makhmalbaf, H.; Birjandinejad, A.; Keshtan, F.G.; Hoseini, H.A.; Mazloumi, S.M. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) in Persian Speaking Patients with Knee Osteoarthritis. Arch. Bone Jt. Surg. 2014, 2, 57–62. [Google Scholar] [PubMed]
- Kim, M.-J.; Kang, B.-H.; Park, S.-H.; Kim, B.; Lee, G.-Y.; Seo, Y.-M.; Park, K.-S.; Yoo, J.-I. Association of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) with Muscle Strength in Community-Dwelling Elderly with Knee Osteoarthritis. Int. J. Environ. Res. Public. Health 2020, 17, 2260. [Google Scholar] [CrossRef]
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytother. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef]
- Javadi, M.; Khadem Haghighian, H.; Goodarzy, S.; Abbasi, M.; Nassiri-Asl, M. Effect of Curcumin Nanomicelle on the Clinical Symptoms of Patients with Rheumatoid Arthritis: A Randomized, Double-Blind, Controlled Trial. Int. J. Rheum. Dis. 2019, 22, 1857–1862. [Google Scholar] [CrossRef]
- Pourhabibi-Zarandi, F.; Rafraf, M.; Zayeni, H.; Asghari-Jafarabadi, M.; Ebrahimi, A.-A. Effects of Curcumin Supplementation on Metabolic Parameters, Inflammatory Factors and Obesity Values in Women with Rheumatoid Arthritis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytother. Res. 2022, 36, 1797–1806. [Google Scholar] [CrossRef]
- Khan, M.K.; Khan, I.A.; Liaquat, A. Therapeutic Potential of Curcumin with and without Strengthening Exercises in Improving Rheumatoid Arthritis. J. Coll. Physicians Surg. Pak. 2022, 32, 1640–1643. [Google Scholar] [CrossRef]
- Pourhabibi-Zarandi, F.; Shojaei-Zarghani, S.; Rafraf, M. Curcumin and Rheumatoid Arthritis: A Systematic Review of Literature. Int. J. Clin. Pract. 2021, 75, e14280. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.; Huang, L.; Jin, M.; He, Q.; Zhang, R.; Ma, J. Effect of Curcumin on Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front. Immunol. 2023, 14, 1121655. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, S.; Keyßer, G.; Feist, E. Curcumin: Useful Add-on for Rheumatic Diseases? J. Clin. Med. 2022, 11, 2908. [Google Scholar] [CrossRef]
- Matthewman, C.; Krishnakumar, I.M.; Swick, A.G. Review: Bioavailability and Efficacy of “free” Curcuminoids from Curcumagalactomannoside (CGM) Curcumin Formulation. Nutr. Res. Rev. 2024, 37, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Bučević Popović, V.; Karahmet Farhat, E.; Banjari, I.; Jeličić Kadić, A.; Puljak, L. Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study. Pharmaceuticals 2024, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.A.; Seifert, R. Clinical Trials on Curcumin in Relation to Its Bioavailability and Effect on Malignant Diseases: Critical Analysis. Naunyn Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3477–3491. [Google Scholar] [CrossRef]
- Jamwal, R. Bioavailable Curcumin Formulations: A Review of Pharmacokinetic Studies in Healthy Volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is Curcumin Bioavailability a Problem in Humans: Lessons from Clinical Trials. Expert. Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Lukefahr, A.L.; McEvoy, S.; Alfafara, C.; Funk, J.L. Drug-Induced Autoimmune Hepatitis Associated with Turmeric Dietary Supplement Use. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and Its Major Constituent (Curcumin) as Nontoxic and Safe Substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Ombredane, A.S.; Silva, V.R.P.; Andrade, L.R.; Pinheiro, W.O.; Simonelly, M.; Oliveira, J.V.; Pinheiro, A.C.; Gonçalves, G.F.; Felice, G.J.; Garcia, M.P.; et al. In Vivo Efficacy and Toxicity of Curcumin Nanoparticles in Breast Cancer Treatment: A Systematic Review. Front. Oncol. 2021, 11, 612903. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Frankincense: Systematic Review. BMJ 2008, 337, a2813. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T. Boswellic Acids in Chronic Inflammatory Diseases. Planta Med. 2006, 72, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T. Boswellic acids (components of frankincense) as the active principle in treatment of chronic inflammatory diseases. Wien. Med. Wochenschr. 2002, 152, 373–378. [Google Scholar] [CrossRef]
- Ammon, H.P.T. Modulation of the Immune System by Boswellia serrata Extracts and Boswellic Acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Umar, K.; Sarwar, A.H.M.G.; Khan, A.; Ahmad, N.; Ahmad, S.; Katiyar, C.K.; Husain, S.A.; Khan, H.A. Boswellia serrata Extract Attenuates Inflammatory Mediators and Oxidative Stress in Collagen Induced Arthritis. Phytomedicine 2014, 21, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its Role for Host Defense, Inflammation, and Neutrophil Function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Huang, J.-B.; Chen, Z.-R.; Yang, S.-L.; Hong, F.-F. Nitric Oxide Synthases in Rheumatoid Arthritis. Molecules 2023, 28, 4414. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Knaus, U.; Wagner, H. Effects of Boswellic Acid of Boswellia serrata and Other Triterpenic Acids on the Complement System. Phytomedicine 1996, 3, 77–80. [Google Scholar] [CrossRef]
- Banno, N.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Tabata, K.; Nakamura, Y.; Nishimura, R.; Kimura, Y.; Suzuki, T. Anti-Inflammatory Activities of the Triterpene Acids from the Resin of Boswellia Carteri. J. Ethnopharmacol. 2006, 107, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An Overall Assessment of in Vitro, Preclinical, Pharmacokinetic and Clinical Data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef]
- Nakhaei, K.; Bagheri-Hosseini, S.; Sabbaghzade, N.; Behmadi, J.; Boozari, M. Boswellic Acid Nanoparticles: Promising Strategies for Increasing Therapeutic Effects. Rev. Bras. Farmacogn. 2023, 33, 713–723. [Google Scholar] [CrossRef]
- Singh, G.B.; Bani, S.; Singh, S. Toxicity and Safety Evaluation of Boswellic Acids. Phytomedicine 1996, 3, 87–90. [Google Scholar] [CrossRef]
- Alluri, V.K.; Dodda, S.; Kilari, E.K.; Golakoti, T.; Sengupta, K. Toxicological Assessment of a Standardized Boswellia serrata Gum Resin Extract. Int. J. Toxicol. 2019, 38, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K. A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial to Assess the Safety and Efficacy of a Novel Boswellia serrata Extract in the Management of Osteoarthritis of the Knee. Phytother. Res. 2019, 33, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Banji, D.; Banji, O.J.F.; Rashida, S.; Alshahrani, S.; Alqahtani, S.S. Bioavailability, Anti-Inflammatory and Anti-Arthritic Effect of Acetyl Keto Boswellic Acid and Its Combination with Methotrexate in an Arthritic Animal Model. J. Ethnopharmacol. 2022, 292, 115200. [Google Scholar] [CrossRef]
- Choudhary, R.; Saroch, D.; Kumar, D.; Anjum, S.; Andrabi, N.I.; Akram, T.; Shah, B.A.; Shukla, S.K.; Bhagat, A.; Kour, G.; et al. Anti-Inflammatory and Anti-Arthritic Potential of Methotrexate in Combination with BA-25, an Amino Analogue of β-Boswellic Acid in the Treatment of Rheumatoid Arthritis. Cytokine 2023, 172, 156398. [Google Scholar] [CrossRef]
- Etzel, R. Special Extract of BOSWELLIA serrata (H 15) in the Treatment of Rheumatoid Arthritis. Phytomedicine 1996, 3, 91–94. [Google Scholar] [CrossRef]
- Sander, O.; Herborn, G.; Rau, R. Is H15 (resin extract of Boswellia serrata, “incense”) a useful supplement to established drug therapy of chronic polyarthritis? Results of a double-blind pilot study. Z. Rheumatol. 1998, 57, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.; Rezaei-Tavirani, M. Resveratrol: A Miraculous Natural Compound for Diseases Treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.F.; Lerner, A. Resveratrol in Rheumatological Diseases: A Systematic Review. Eur. J. Rheumatol. 2023, 10, 163–168. [Google Scholar] [CrossRef]
- Xuzhu, G.; Komai-Koma, M.; Leung, B.P.; Howe, H.S.; McSharry, C.; McInnes, I.B.; Xu, D. Resveratrol Modulates Murine Collagen-Induced Arthritis by Inhibiting Th17 and B-Cell Function. Ann. Rheum. Dis. 2012, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Cheon, Y.-H.; Kim, H.-O.; Suh, Y.-S.; Hur, J.H.; Jo, W.; Lim, H.-S.; Hah, Y.-S.; Sung, M.J.; Kwon, D.Y.; Lee, S.-I. Inhibitory Effects for Rheumatoid Arthritis of Dietary Supplementation with Resveratrol in Collagen-Induced Arthritis. J. Rheum. Dis. 2015, 22, 93–101. [Google Scholar] [CrossRef]
- Nakayama, H.; Yaguchi, T.; Yoshiya, S.; Nishizaki, T. Resveratrol Induces Apoptosis MH7A Human Rheumatoid Arthritis Synovial Cells in a Sirtuin 1-Dependent Manner. Rheumatol. Int. 2012, 32, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.S.; Song, J.K.; Kim, Y.-R.; Piao, L.; Won, M.; Park, K.A.; Choi, B.L.; Lee, H.; Hong, J.H.; Park, J.; et al. Caspase-8 Has an Essential Role in Resveratrol-Induced Apoptosis of Rheumatoid Fibroblast-like Synoviocytes. Rheumatology 2008, 47, 301–308. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an Effective Adjuvant Therapy in the Management of Rheumatoid Arthritis: A Clinical Study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients 2021, 13, 3095. [Google Scholar] [CrossRef] [PubMed]
- Detampel, P.; Beck, M.; Krähenbühl, S.; Huwyler, J. Drug Interaction Potential of Resveratrol. Drug Metab. Rev. 2012, 44, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, A.R.; Chow, H.S.; Martinez, J.A. Effects of Resveratrol on Drug- and Carcinogen-metabolizing Enzymes, Implications for Cancer Prevention. Pharmacol. Res. Perspect. 2017, 5, e00294. [Google Scholar] [CrossRef]
- Fares, S.; Omar, M.; Laurence, A.; Abu-Baker, S.; Shaza, A.; Fadi, H.; Jonathan, M.; Georges, K.; Koushik, S.; Elie, B.S.; et al. Over-the-Counter Anti-Inflammatory Supplements for Adjunctive Rheumatoid Arthritis Therapy: A Comprehensive Narrative Review. Aging Dis. 2024. [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Lin, J.; Liu, J.; O’Fee, A.; Pandey, C.; Benna-Doyle, S.; Maunder, A.; Rao, V.; Alesi, S.; Ng, B.; Ee, C. The Effectiveness and Safety of Lifestyle Medicine and Integrative Therapies in Inflammatory Arthritis: An Umbrella Review Using a Hierarchical Evidence Gathering Approach. Front. Med. 2024, 11, 1357914. [Google Scholar] [CrossRef]
- Guan, F.; Wang, Q.; Bao, Y.; Chao, Y. Anti-Rheumatic Effect of Quercetin and Recent Developments in Nano Formulation. RSC Adv. 2021, 11, 7280–7293. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A Critical Review of the Data Related to the Safety of Quercetin and Lack of Evidence of in Vivo Toxicity, Including Lack of Genotoxic/Carcinogenic Properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.A.; Iqbal, Z.; Ge, Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications—A Review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Cheng, M.; Zhang, X.; Chen, X. Quercetin Nanoformulations: A Promising Strategy for Tumor Therapy. Food Funct. 2021, 12, 6664–6681. [Google Scholar] [CrossRef] [PubMed]
- Tomou, E.-M.; Papakyriakopoulou, P.; Saitani, E.-M.; Valsami, G.; Pippa, N.; Skaltsa, H. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics 2023, 15, 1656. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Gupta, D.S.; Kaur, G.; Singh, T.; Ramniwas, S.; Sak, K.; Aggarwal, D.; Chhabra, R.S.; Gupta, M.; Saini, A.K.; et al. Nanoformulations of Quercetin for Controlled Delivery: A Review of Preclinical Anticancer Studies. Naunyn Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3443–3458. [Google Scholar] [CrossRef]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.J. Quercetin Loaded Nanoemulsion-Based Gel for Rheumatoid Arthritis: In Vivo and in Vitro Studies. Biomed. Pharmacother. 2019, 112, 108622. [Google Scholar] [CrossRef]
- Javadi, F.; Eghtesadi, S.; Ahmadzadeh, A.; Aryaeian, N.; Zabihiyeganeh, M.; Foroushani, A.R.; Jazayeri, S. The Effect of Quercetin on Plasma Oxidative Status, C-Reactive Protein and Blood Pressure in Women with Rheumatoid Arthritis. Int. J. Prev. Med. 2014, 5, 293–301. [Google Scholar] [PubMed]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef]
- Matsuno, H.; Nakamura, H.; Katayama, K.; Hayashi, S.; Kano, S.; Yudoh, K.; Kiso, Y. Effects of an Oral Administration of Glucosamine-Chondroitin-Quercetin Glucoside on the Synovial Fluid Properties in Patients with Osteoarthritis and Rheumatoid Arthritis. Biosci. Biotechnol. Biochem. 2009, 73, 288–292. [Google Scholar] [CrossRef]
- Bae, S.-C.; Jung, W.-J.; Lee, E.-J.; Yu, R.; Sung, M.-K. Effects of Antioxidant Supplements Intervention on the Level of Plasma Inflammatory Molecules and Disease Severity of Rheumatoid Arthritis Patients. J. Am. Coll. Nutr. 2009, 28, 56–62. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Zou, H. Baicalin Inhibits IL-17-Mediated Joint Inflammation in Murine Adjuvant-Induced Arthritis. Clin. Dev. Immunol. 2013, 2013, 268065. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Wang, H.-H.; Huang, S.-S.; Zhao, H.; Cao, Y.-G.; Wang, G.-Z.; Wang, D.; Wang, Z.-G.; Liu, Y.-H. Inhibitory Effect of Baicalin on Collagen-Induced Arthritis in Rats through the Nuclear Factor-κB Pathway. J. Pharmacol. Exp. Ther. 2014, 350, 435–443. [Google Scholar] [CrossRef]
- Wang, C.; Song, Y.; Wang, X.; Mao, R.; Song, L. Baicalin Ameliorates Collagen-Induced Arthritis Through the Suppression of Janus Kinase 1 (JAK1)/Signal Transducer and Activator of Transcription 3 (STAT3) Signaling in Mice. Med. Sci. Monit. 2018, 24, 9213–9222. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Cai, J.; Wang, S.; Cheng, Z.; Zhang, Z.; Zhang, C. Anti-Inflammatory Effect of Baicalin in Rats with Adjuvant Arthritis and Its Autophagy-Related Mechanism. Technol. Health Care 2022, 30, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Bai, Y.; Yang, Y.; Zhang, W.; Huang, L.; Ma, R.; Wang, L.; Duan, H.; Wan, Q. Baicalin Alleviates Collagen-induced Arthritis and Suppresses TLR2/MYD88/NF-κB P65 Signaling in Rats and HFLS-RAs. Mol. Med. Rep. 2020, 22, 2833–2841. [Google Scholar] [CrossRef]
- Fang, P.; Sun, Y.; Gu, X.; Shi, M.; Bo, P.; Zhang, Z.; Bu, L. Baicalin Ameliorates Hepatic Insulin Resistance and Gluconeogenic Activity through Inhibition of P38 MAPK/PGC-1α Pathway. Phytomedicine 2019, 64, 153074. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, A.; Pang, H.; Xue, W.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Li, K.; Xiao, W.; et al. Safety, Tolerability, and Pharmacokinetics of a Single Ascending Dose of Baicalein Chewable Tablets in Healthy Subjects. J. Ethnopharmacol. 2014, 156, 210–215. [Google Scholar] [CrossRef]
- Hang, Y.; Qin, X.; Ren, T.; Cao, J. Baicalin Reduces Blood Lipids and Inflammation in Patients with Coronary Artery Disease and Rheumatoid Arthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Lipids Health Dis. 2018, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Thabrew, M.I.; Senaratna, L.; Samarawickrema, N.; Munasinghe, C. Antioxidant Potential of Two Polyherbal. Preparations Used in Ayurveda for the Treatment of Rheumatoid Arthritis. J. Ethnopharmacol. 2001, 76, 285–291. [Google Scholar] [CrossRef]
- Tao, X.; Cush, J.J.; Garret, M.; Lipsky, P.E. A Phase I Study of Ethyl Acetate Extract of the Chinese Antirheumatic Herb Tripterygium wilfordii Hook F in Rheumatoid Arthritis. J. Rheumatol. 2001, 28, 2160–2167. [Google Scholar]
- Tao, X.; Younger, J.; Fan, F.Z.; Wang, B.; Lipsky, P.E. Benefit of an Extract of Tripterygium wilfordii Hook F in Patients with Rheumatoid Arthritis: A Double-Blind, Placebo-Controlled Study. Arthritis Rheum. 2002, 46, 1735–1743. [Google Scholar] [CrossRef]
- Goldbach-Mansky, R.; Wilson, M.; Fleischmann, R.; Olsen, N.; Silverfield, J.; Kempf, P.; Kivitz, A.; Sherrer, Y.; Pucino, F.; Csako, G.; et al. Comparison of Tripterygium wilfordii Hook F versus Sulfasalazine in the Treatment of Rheumatoid Arthritis: A Randomized Trial. Ann. Intern. Med. 2009, 151, 229–240, W49-51. [Google Scholar] [CrossRef]
- Lv, Q.-W.; Zhang, W.; Shi, Q.; Zheng, W.; Li, X.; Chen, H.; Wu, Q.; Jiang, W.; Li, H.; Gong, L.; et al. Comparison of Tripterygium wilfordii Hook F with Methotrexate in the Treatment of Active Rheumatoid Arthritis (TRIFRA): A Randomised, Controlled Clinical Trial. Ann. Rheum. Dis. 2015, 74, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Z.; Zhao, L.-D.; Chen, H.; Zhang, Y.; Wang, D.-F.; Huang, L.-F.; Lv, Q.-W.; Liu, B.; Li, Z.; Wei, W.; et al. Comparison of the Impact of Tripterygium wilfordii Hook F and Methotrexate Treatment on Radiological Progression in Active Rheumatoid Arthritis: 2-Year Follow up of a Randomized, Non-Blinded, Controlled Study. Arthritis Res. Ther. 2018, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Mur, E.; Hartig, F.; Eibl, G.; Schirmer, M. Randomized Double Blind Trial of an Extract from the Pentacyclic Alkaloid-Chemotype of Uncaria Tomentosa for the Treatment of Rheumatoid Arthritis. J. Rheumatol. 2002, 29, 678–681. [Google Scholar] [PubMed]
- Balbir-Gurman, A.; Fuhrman, B.; Braun-Moscovici, Y.; Markovits, D.; Aviram, M. Consumption of Pomegranate Decreases Serum Oxidative Stress and Reduces Disease Activity in Patients with Active Rheumatoid Arthritis: A Pilot Study. Isr. Med. Assoc. J. 2011, 13, 474–479. [Google Scholar]
- Ghavipour, M.; Sotoudeh, G.; Tavakoli, E.; Mowla, K.; Hasanzadeh, J.; Mazloom, Z. Pomegranate Extract Alleviates Disease Activity and Some Blood Biomarkers of Inflammation and Oxidative Stress in Rheumatoid Arthritis Patients. Eur. J. Clin. Nutr. 2017, 71, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Paknahad, Z.; Habibagahi, Z. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial, Evaluating the Garlic Supplement Effects on Some Serum Biomarkers of Oxidative Stress, and Quality of Life in Women with Rheumatoid Arthritis. Int. J. Clin. Pract. 2020, 74, e13498. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, N.; Shimada, Y.; Niizawa, A.; Kogure, T.; Mantani, N.; Sakai, S.; Hikiami, H.; Terasawa, K. Suppressive Effects of Stephania Tetrandra on the Neutrophil Function in Patients with Rheumatoid Arthritis. Phytother. Res. 2004, 18, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.R.; Robson, P.; Ho, M.; Jubb, R.W.; McCabe, C.S. Preliminary Assessment of the Efficacy, Tolerability and Safety of a Cannabis-Based Medicine (Sativex) in the Treatment of Pain Caused by Rheumatoid Arthritis. Rheumatology 2006, 45, 50–52. [Google Scholar] [CrossRef]
- Burgos, R.A.; Hancke, J.L.; Bertoglio, J.C.; Aguirre, V.; Arriagada, S.; Calvo, M.; Cáceres, D.D. Efficacy of an Andrographis Paniculata Composition for the Relief of Rheumatoid Arthritis Symptoms: A Prospective Randomized Placebo-Controlled Trial. Clin. Rheumatol. 2009, 28, 931–946. [Google Scholar] [CrossRef]
- López Mantecón, A.M.; Garrido, G.; Delgado-Hernández, R.; Garrido-Suárez, B.B. Combination of Mangifera indica L. Extract Supplementation plus Methotrexate in Rheumatoid Arthritis Patients: A Pilot Study. Phytother. Res. 2014, 28, 1163–1172. [Google Scholar] [CrossRef]
- Gao, X.; Lin, X.; Wang, Q.; Chen, J. Artemisinins: Promising Drug Candidates for the Treatment of Autoimmune Diseases. Med. Res. Rev. 2024, 44, 867–891. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ding, J.; Yang, C.; Gao, Y.; Li, X.; Chen, X.; Peng, Y.; Fang, J.; Xiao, S. Immunomodulatory and Anti-Inflammatory Properties of Artesunate in Experimental Colitis. Curr. Med. Chem. 2012, 19, 4541–4551. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Guo, M.-Y.; Luo, Y.; Yun, M.-D.; Yan, J.; Liu, T.; Xiao, C.-H. Effect of Artemisia Annua Extract on Treating Active Rheumatoid Arthritis: A Randomized Controlled Trial. Chin. J. Integr. Med. 2017, 23, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Stebbings, S.; Beattie, E.; McNamara, D.; Hunt, S. A Pilot Randomized, Placebo-Controlled Clinical Trial to Investigate the Efficacy and Safety of an Extract of Artemisia Annua Administered over 12 Weeks, for Managing Pain, Stiffness, and Functional Limitation Associated with Osteoarthritis of the Hip and Knee. Clin. Rheumatol. 2016, 35, 1829–1836. [Google Scholar] [CrossRef]
- Huang, D.-N.; Wu, F.-F.; Zhang, A.-H.; Sun, H.; Wang, X.-J. Efficacy of Berberine in Treatment of Rheumatoid Arthritis: From Multiple Targets to Therapeutic Potential. Pharmacol. Res. 2021, 169, 105667. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Hadidi, M.; Mahmoudi, M.; Asgari, M.; Hezaveh, Z.S.; Sadehi, S.K. The Effect of Black Barberry Hydroalcoholic Extract on Immune Mediators in Patients with Active Rheumatoid Arthritis: A Randomized, Double-Blind, Controlled Clinical Trial. Phytother. Res. 2021, 35, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, Pharmacology, and Therapeutic Uses of Black Seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Dereń, K.; Polak-Szczybyło, E.; Stępień, A.E. The Role of Bioactive Compounds of Nigella sativa in Rheumatoid Arthritis Therapy-Current Reports. Nutrients 2021, 13, 3369. [Google Scholar] [CrossRef]
- Gheita, T.A.; Kenawy, S.A. Effectiveness of Nigella Sativa Oil in the Management of Rheumatoid Arthritis Patients: A Placebo Controlled Study. Phytother. Res. 2012, 26, 1246–1248. [Google Scholar] [CrossRef]
- Kheirouri, S.; Hadi, V.; Alizadeh, M. Immunomodulatory Effect of Nigella Sativa Oil on T Lymphocytes in Patients with Rheumatoid Arthritis. Immunol. Investig. 2016, 45, 271–283. [Google Scholar] [CrossRef]
- Hadi, V.; Kheirouri, S.; Alizadeh, M.; Khabbazi, A.; Hosseini, H. Effects of Nigella Sativa Oil Extract on Inflammatory Cytokine Response and Oxidative Stress Status in Patients with Rheumatoid Arthritis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Avicenna J. Phytomed 2016, 6, 34–43. [Google Scholar] [PubMed]
- Biegert, C.; Wagner, I.; Lüdtke, R.; Kötter, I.; Lohmüller, C.; Günaydin, I.; Taxis, K.; Heide, L. Efficacy and Safety of Willow Bark Extract in the Treatment of Osteoarthritis and Rheumatoid Arthritis: Results of 2 Randomized Double-Blind Controlled Trials. J. Rheumatol. 2004, 31, 2121–2130. [Google Scholar]
- Zhang, C.-F.; Zhang, S.-L.; He, X.; Yang, X.-L.; Wu, H.-T.; Lin, B.-Q.; Jiang, C.-P.; Wang, J.; Yu, C.-H.; Yang, Z.-L.; et al. Antioxidant Effects of Genkwa Flos Flavonoids on Freund׳s Adjuvant-Induced Rheumatoid Arthritis in Rats. J. Ethnopharmacol. 2014, 153, 793–800. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Bai, J.-Y.; Xia, S.; Mao, M.; Li, X.; Li, N.; Chen, L. The Roles of Synovial Hyperplasia, Angiogenesis and Osteoclastogenesis in the Protective Effect of Apigenin on Collagen-Induced Arthritis. Int. Immunopharmacol. 2019, 73, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.-C.; Kim, C.; Lee, J.-M.; Cho, W.-S.; Lee, S.-G.; Jeong, M.; Cho, J.; Lee, K. Apigenin-Induced Apoptosis Is Mediated by Reactive Oxygen Species and Activation of ERK1/2 in Rheumatoid Fibroblast-like Synoviocytes. Chem. Biol. Interact. 2009, 182, 29–36. [Google Scholar] [CrossRef]
- Sun, Q.-W.; Jiang, S.-M.; Yang, K.; Zheng, J.-M.; Zhang, L.; Xu, W.-D. Apigenin Enhances the Cytotoxic Effects of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Human Rheumatoid Arthritis Fibroblast-like Synoviocytes. Mol. Biol. Rep. 2012, 39, 5529–5535. [Google Scholar] [CrossRef]
- Cao, D.; Fan, Q.; Li, Z.; Chen, M.; Jiang, Y.; Lin, R.; Li, J.; Zhao, C. Transcriptomic Profiling Revealed the Role of Apigenin-4′-O-α-L-Rhamnoside in Inhibiting the Activation of Rheumatoid Arthritis Fibroblast-like Synoviocytes via MAPK Signaling Pathway. Phytomedicine 2022, 102, 154201. [Google Scholar] [CrossRef]
- Kumazawa, Y.; Kawaguchi, K.; Takimoto, H. Immunomodulating Effects of Flavonoids on Acute and Chronic Inflammatory Responses Caused by Tumor Necrosis Factor Alpha. Curr. Pharm. Des. 2006, 12, 4271–4279. [Google Scholar] [CrossRef] [PubMed]
- Dion, C.; Haug, C.; Guan, H.; Ripoll, C.; Spiteller, P.; Coussaert, A.; Boulet, E.; Schmidt, D.; Wei, J.; Zhou, Y.; et al. Evaluation of the Anti-Inflammatory and Antioxidative Potential of Four Fern Species from China Intended for Use as Food Supplements. Nat. Prod. Commun. 2015, 10, 597–603. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, G.-H. Evaluation of Antioxidant and Inhibitory Activities for Different Subclasses Flavonoids on Enzymes for Rheumatoid Arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-Inflammatory Mechanisms of Apigenin: Inhibition of Cyclooxygenase-2 Expression, Adhesion of Monocytes to Human Umbilical Vein Endothelial Cells, and Expression of Cellular Adhesion Molecules. Arch. Pharm. Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Gielecińska, A.; Kciuk, M.; Mujwar, S.; Celik, I.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kontek, R. Substances of Natural Origin in Medicine: Plants vs. Cancer. Cells 2023, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Q. Betulinic Acid Inhibits Cell Proliferation, Migration, and Inflammatory Response in Rheumatoid Arthritis Fibroblast-like Synoviocytes. J. Cell Biochem. 2019, 120, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gong, Z.; Li, X.; Ma, Q.; Wu, M.; Liu, D.; Deng, L.; Pan, D.; Liu, Q.; Wei, Z.; et al. Betulinic Acid Inhibits the Migration and Invasion of Fibroblast-like Synoviocytes from Patients with Rheumatoid Arthritis. Int. Immunopharmacol. 2019, 67, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, J.-Y.; Zhang, L.; Pan, Y.-Y.; Chen, X.-Y.; Yuan, Y. Effects of Betulinic Acid on Synovial Inflammation in Rats with Collagen-Induced Arthritis. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420945078. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, N. Phytochemistry and Pharmacological Activity of Plants of Genus Curculigo: An Updated Review Since 2013. Molecules 2021, 26, 3396. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.S.; Sharma, V.; Thakur, M.; Dixit, V.K. Curculigo Orchioides: The Black Gold with Numerous Health Benefits. J. Chin. Integr. Med. 2010, 8, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Gao, G.; Zhang, L.; Shen, G.; Sun, W.; Gu, Z.; Fan, W. The Protective Effects of Curculigoside A on Adjuvant-Induced Arthritis by Inhibiting NF-ĸB/NLRP3 Activation in Rats. Int. Immunopharmacol. 2016, 30, 43–49. [Google Scholar] [CrossRef]
- Tan, S.; Xu, J.; Lai, A.; Cui, R.; Bai, R.; Li, S.; Liang, W.; Zhang, G.; Jiang, S.; Liu, S.; et al. Curculigoside Exerts Significant Anti-arthritic Effects in Vivo and in Vitro via Regulation of the JAK/STAT/NF-κB Signaling Pathway. Mol. Med. Rep. 2019, 19, 2057–2064. [Google Scholar] [CrossRef]
- Han, J.; Wan, M.; Ma, Z.; Hu, C.; Yi, H. Prediction of Targets of Curculigoside A in Osteoporosis and Rheumatoid Arthritis Using Network Pharmacology and Experimental Verification. Drug Des. Devel Ther. 2020, 14, 5235–5250. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, M.; Wang, L.; Li, J.; Wu, W.; Wang, Q. Network Pharmacology and Experimental Validation Methods to Reveal the Active Compounds and Hub Targets of Curculigo Orchioides Gaertn in Rheumatoid Arthritis. J. Orthop. Surg. Res. 2023, 18, 861. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.-J.; Yoo, W.-H.; Han, M.-K.; Lee, Y.-R.; Kim, J.-S.; Lee, S.-I. Epigallocatechin-3-Gallate Suppresses TNF-Alpha-Induced Production of MMP-1 and -3 in Rheumatoid Arthritis Synovial Fibroblasts. Rheumatol. Int. 2008, 29, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Marotte, H.; Kwan, K.; Ruth, J.H.; Campbell, P.L.; Rabquer, B.J.; Pakozdi, A.; Koch, A.E. Epigallocatechin-3-Gallate Inhibits IL-6 Synthesis and Suppresses Transsignaling by Enhancing Soluble Gp130 Production. Proc. Natl. Acad. Sci. USA 2008, 105, 14692–14697. [Google Scholar] [CrossRef] [PubMed]
- Riegsecker, S.; Wiczynski, D.; Kaplan, M.J.; Ahmed, S. Potential Benefits of Green Tea Polyphenol EGCG in the Prevention and Treatment of Vascular Inflammation in Rheumatoid Arthritis. Life Sci. 2013, 93, 307–312. [Google Scholar] [CrossRef]
- Fechtner, S.; Singh, A.; Chourasia, M.; Ahmed, S. Molecular Insights into the Differences in Anti-Inflammatory Activities of Green Tea Catechins on IL-1β Signaling in Rheumatoid Arthritis Synovial Fibroblasts. Toxicol. Appl. Pharmacol. 2017, 329, 112–120. [Google Scholar] [CrossRef]
- Karatas, A.; Dagli, A.F.; Orhan, C.; Gencoglu, H.; Ozgen, M.; Sahin, N.; Sahin, K.; Koca, S.S. Epigallocatechin 3-Gallate Attenuates Arthritis by Regulating Nrf2, HO-1, and Cytokine Levels in an Experimental Arthritis Model. Biotechnol. Appl. Biochem. 2020, 67, 317–322. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.; Pae, M.; Meydani, S.N. Green Tea EGCG, T Cells, and T Cell-Mediated Autoimmune Diseases. Mol. Aspects Med. 2012, 33, 107–118. [Google Scholar] [CrossRef]
- Min, S.-Y.; Yan, M.; Kim, S.B.; Ravikumar, S.; Kwon, S.-R.; Vanarsa, K.; Kim, H.-Y.; Davis, L.S.; Mohan, C. Green Tea Epigallocatechin-3-Gallate Suppresses Autoimmune Arthritis Through Indoleamine-2,3-Dioxygenase Expressing Dendritic Cells and the Nuclear Factor, Erythroid 2-Like 2 Antioxidant Pathway. J. Inflamm. 2015, 12, 53. [Google Scholar] [CrossRef]
- Ahmed, S.; Pakozdi, A.; Koch, A.E. Regulation of Interleukin-1beta-Induced Chemokine Production and Matrix Metalloproteinase 2 Activation by Epigallocatechin-3-Gallate in Rheumatoid Arthritis Synovial Fibroblasts. Arthritis Rheum. 2006, 54, 2393–2401. [Google Scholar] [CrossRef]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea Polyphenols Inhibit Rat Osteoclast Formation and Differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Morinobu, A.; Biao, W.; Tanaka, S.; Horiuchi, M.; Jun, L.; Tsuji, G.; Sakai, Y.; Kurosaka, M.; Kumagai, S. (−)-Epigallocatechin-3-Gallate Suppresses Osteoclast Differentiation and Ameliorates Experimental Arthritis in Mice. Arthritis Rheum. 2008, 58, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-K.; Chang, H.-H.; Chen, Y.-J.; Wang, C.-C.; Galson, D.L.; Hong, C.-Y.; Kok, S.-H. Epigallocatechin-3-Gallate Diminishes CCL2 Expression in Human Osteoblastic Cells via up-Regulation of Phosphatidylinositol 3-Kinase/Akt/Raf-1 Interaction: A Potential Therapeutic Benefit for Arthritis. Arthritis Rheum. 2008, 58, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Jung, Y.O.; Ryu, J.-G.; Oh, H.-J.; Son, H.-J.; Lee, S.H.; Kwon, J.-E.; Kim, E.-K.; Park, M.-K.; Park, S.-H.; et al. Epigallocatechin-3-Gallate Ameliorates Autoimmune Arthritis by Reciprocal Regulation of T Helper-17 Regulatory T Cells and Inhibition of Osteoclastogenesis by Inhibiting STAT3 Signaling. J. Leukoc. Biol. 2016, 100, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Silverman, M.D.; Marotte, H.; Kwan, K.; Matuszczak, N.; Koch, A.E. Down-Regulation of Myeloid Cell Leukemia 1 by Epigallocatechin-3-Gallate Sensitizes Rheumatoid Arthritis Synovial Fibroblasts to Tumor Necrosis Factor Alpha-Induced Apoptosis. Arthritis Rheum. 2009, 60, 1282–1293. [Google Scholar] [CrossRef]
- Reiter, M.P.; Ward, S.H.; Perry, B.; Mann, A.; Freeman, J.W.; Tiku, M.L. Intra-Articular Injection of Epigallocatechin (EGCG) Crosslinks and Alters Biomechanical Properties of Articular Cartilage, a Study via Nanoindentation. PLoS ONE 2022, 17, e0276626. [Google Scholar] [CrossRef]
- Song, X.; Zheng, Z.; Ouyang, S.; Chen, H.; Sun, M.; Lin, P.; Chen, Y.; You, Y.; Hao, W.; Tao, J.; et al. Biomimetic Epigallocatechin Gallate-Cerium Assemblies for the Treatment of Rheumatoid Arthritis. ACS Appl. Mater. Interfaces 2023, 15, 33239–33249. [Google Scholar] [CrossRef]
- Song, C.; Xu, S.; Chang, L.; Zhao, X.; Mei, X.; Ren, X.; Chen, Z. Preparation of EGCG Decorated, Injectable Extracellular Vesicles for Cartilage Repair in Rat Arthritis. Regen. Biomater. 2021, 8, rbab067. [Google Scholar] [CrossRef]
- Lee, F.; Bae, K.H.; Ng, S.; Yamashita, A.; Kurisawa, M. Hyaluronic Acid-Green Tea Catechin Conjugates as a Potential Therapeutic Agent for Rheumatoid Arthritis. RSC Adv. 2021, 11, 14285–14294. [Google Scholar] [CrossRef]
- Pradhan, A.; Sengupta, S.; Sengupta, R.; Chatterjee, M. Attenuation of Methotrexate Induced Hepatotoxicity by Epigallocatechin 3-Gallate. Drug Chem. Toxicol. 2023, 46, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, W.; Yan, X. Anti-Inflammatory and Immunoregulatory Effects of Icariin and Icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, Y.; Deng, X.; Yu, C.; Dai, N.; Yuan, X.; Chen, L.; Yu, S.; Si, W.; Wang, X.; et al. An Inhibitor of Cathepsin K, Icariin Suppresses Cartilage and Bone Degradation in Mice of Collagen-Induced Arthritis. Phytomedicine 2013, 20, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Ping, D.Q.; You, F.T.; Qiang, C.Y.; Tao, C. Icariin Prevents Cartilage and Bone Degradation in Experimental Models of Arthritis. Mediators Inflamm. 2016, 2016, 9529630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, Y.; Xiao, L.; Feng, E.; Wang, W.; Lin, L. The Effects of Icariine Concentration on Osteoclasts Bone Resorption Induced by Titanium Particles in Vitro. Regen. Biomater. 2015, 2, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Gao, W.; Shu, X.; Lu, X. A Natural Flavonoid Glucoside, Icariin, Regulates Th17 and Alleviates Rheumatoid Arthritis in a Murine Model. Mediators Inflamm. 2014, 2014, 392062. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, Q.; Cao, Z.; Li, H.; Zhou, Y.; Zhang, P. Icariin Decreases Cell Proliferation and Inflammation of Rheumatoid Arthritis-Fibroblast like Synoviocytes via GAREM1/MAPK Signaling Pathway. Immunopharmacol. Immunotoxicol. 2024, 46, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Meng, Q.; Wang, W.; Zhang, S.; Xiong, X.; Qin, S.; Zhang, J.; Li, A.; Liu, Z. Icariin Inhibits the Inflammation through Down-Regulating NF-κB/HIF-2α Signal Pathways in Chondrocytes. Biosci. Rep. 2020, 40, BSR20203107. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-M.; Luo, J.; Shi, X.-D.; Zhang, S.-X.; Zhu, X.-B.; Guo, J. Icariin Alleviates Rheumatoid Arthritis via Regulating miR-223-3p/NLRP3 Signalling Axis. Autoimmunity 2020, 53, 450–458. [Google Scholar] [CrossRef]
- Pu, L.; Meng, Q.; Li, S.; Liu, B.; Li, F. Icariin Arrests Cell Cycle Progression and Induces Cell Apoptosis through the Mitochondrial Pathway in Human Fibroblast-like Synoviocytes. Eur. J. Pharmacol. 2021, 912, 174585. [Google Scholar] [CrossRef]
- Verhoff, M.; Seitz, S.; Paul, M.; Noha, S.M.; Jauch, J.; Schuster, D.; Werz, O. Tetra- and Pentacyclic Triterpene Acids from the Ancient Anti-Inflammatory Remedy Frankincense as Inhibitors of Microsomal Prostaglandin E2 Synthase-1. J. Nat. Prod. 2014, 77, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yu, R.; Choi, Y.; Ma, Z.-Z.; Zhang, H.; Xiang, W.; Lee, D.Y.-W.; Berman, B.M.; Moudgil, K.D.; Fong, H.H.S.; et al. Discovery of Cyclooxygenase Inhibitors from Medicinal Plants Used to Treat Inflammation. Pharmacol. Res. 2010, 61, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, N.; Pan, S.; Zhang, Z.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Roburic Acid Suppresses NO and IL-6 Production via Targeting NF-κB and MAPK Pathway in RAW264.7 Cells. Inflammation 2017, 40, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, T.; Li, J.; Chen, F.; Xu, J.; Hu, L.; Jiang, L.; Xiang, Z.; Wang, X.; Sheng, J. Roburic Acid Targets TNF to Inhibit the NF-κB Signaling Pathway and Suppress Human Colorectal Cancer Cell Growth. Front. Immunol. 2022, 13, 853165. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, K.; Ma, C.; Wang, C.; Chen, D.; He, J.; Liu, Y.; Jiang, T.; Yuan, J.; Chen, L.; et al. Roburic Acid Attenuates Osteoclastogenesis and Bone Resorption by Targeting RANKL-Induced Intracellular Signaling Pathways. J. Cell Physiol. 2022, 237, 1790–1803. [Google Scholar] [CrossRef]
- Jia, N.; Gao, Y.; Li, M.; Liang, Y.; Li, Y.; Lin, Y.; Huang, S.; Lin, Q.; Sun, X.; He, Q.; et al. Metabolic Reprogramming of Proinflammatory Macrophages by Target Delivered Roburic Acid Effectively Ameliorates Rheumatoid Arthritis Symptoms. Signal Transduct. Target. Ther. 2023, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Corbi, G.; Costantino, M.; De Bellis, E.; Manzo, V.; Sellitto, C.; Stefanelli, B.; Colucci, F.; Filippelli, A. Biomarkers to Personalize the Treatment of Rheumatoid Arthritis: Focus on Autoantibodies and Pharmacogenetics. Biomolecules 2020, 10, 1672. [Google Scholar] [CrossRef]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.; den Heijer, M.; Kluijtmans, L.A.; van den Heuvel, L.P. A Candidate Genetic Risk Factor for Vascular Disease: A Common Mutation in Methylenetetrahydrofolate Reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef]
- Giletti, A.; Esperon, P. Genetic Markers in Methotrexate Treatments. Pharmacogenomics J. 2018, 18, 689–703. [Google Scholar] [CrossRef]
- Cutolo, M.; Capellino, S.; Montagna, P.; Villaggio, B.; Sulli, A.; Seriolo, B.; Straub, R.H. New Roles for Estrogens in Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2003, 21, 687–690. [Google Scholar]
- Mitra, A.; Kesisoglou, F. Impaired Drug Absorption Due to High Stomach pH: A Review of Strategies for Mitigation of Such Effect to Enable Pharmaceutical Product Development. Mol. Pharm. 2013, 10, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Reiss, S.N.; Buie, L.W.; Adel, N.; Goldman, D.A.; Devlin, S.M.; Douer, D. Hypoalbuminemia Is Significantly Associated with Increased Clearance Time of High Dose Methotrexate in Patients Being Treated for Lymphoma or Leukemia. Ann. Hematol. 2016, 95, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.-L.; Huang, H.-Y.; Lin, Y.-C.-D.; Cai, X.-X.; Kong, X.-J.; Luo, D.-L.; Zhou, Y.-H.; Huang, H.-D. Enzyme Activity of Natural Products on Cytochrome P450. Molecules 2022, 27, 515. [Google Scholar] [CrossRef] [PubMed]
- Wanwimolruk, S.; Prachayasittikul, V. Cytochrome P450 Enzyme Mediated Herbal Drug Interactions (Part 1). EXCLI J. 2014, 13, 347–391. [Google Scholar] [PubMed]
- Wanwimolruk, S.; Phopin, K.; Prachayasittikul, V. Cytochrome P450 Enzyme Mediated Herbal Drug Interactions (Part 2). EXCLI J. 2014, 13, 869–896. [Google Scholar] [PubMed]
- Ingegnoli, F.; Castelli, R.; Gualtierotti, R. Rheumatoid Factors: Clinical Applications. Dis. Markers 2013, 35, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, G.A.; Visser, H.; de Jong, B.A.; van den Hoogen, F.H.; Hazes, J.M.; Breedveld, F.C.; van Venrooij, W.J. The Diagnostic Properties of Rheumatoid Arthritis Antibodies Recognizing a Cyclic Citrullinated Peptide. Arthritis Rheum. 2000, 43, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Nielen, M.M.J.; van Schaardenburg, D.; Reesink, H.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; de Koning, M.H.M.T.; Habibuw, M.R.; Vandenbroucke, J.P.; Dijkmans, B.A.C. Specific Autoantibodies Precede the Symptoms of Rheumatoid Arthritis: A Study of Serial Measurements in Blood Donors. Arthritis Rheum. 2004, 50, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Bartelds, G.M.; Krieckaert, C.L.M.; Nurmohamed, M.T.; van Schouwenburg, P.A.; Lems, W.F.; Twisk, J.W.R.; Dijkmans, B.A.C.; Aarden, L.; Wolbink, G.J. Development of Antidrug Antibodies against Adalimumab and Association with Disease Activity and Treatment Failure during Long-Term Follow-Up. JAMA 2011, 305, 1460–1468. [Google Scholar] [CrossRef]
- Bendtzen, K.; Geborek, P.; Svenson, M.; Larsson, L.; Kapetanovic, M.C.; Saxne, T. Individualized Monitoring of Drug Bioavailability and Immunogenicity in Rheumatoid Arthritis Patients Treated with the Tumor Necrosis Factor Alpha Inhibitor Infliximab. Arthritis Rheum. 2006, 54, 3782–3789. [Google Scholar] [CrossRef] [PubMed]


| Natural Compound | Type of Study (Preclinical/Clinical) | Study Groups/Dosage Regimen | Biological/Clinical Effects | PMID |
|---|---|---|---|---|
| Curcumin | Clinical (Randomized Pilot Study) |
| The curcumin group exhibited the highest percentage of improvement in overall DAS and ACR (ACR 20, 50, and 70) scores. The administration of curcumin was determined to be safe and did not result in any adverse events. | 22407780 |
| Clinical (Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study) |
| Curcumin groups showed significant changes in their clinical symptoms at the end of the study, as evidenced by improvements in ESR, CRP, VAS, RF, DAS28, and ACR responses compared to the placebo group. Curcumin was determined to be safe and did not result in any adverse events. | 28850308 | |
| Clinical (Randomized, Double-Blind, Controlled Trial) |
| The administration of curcumin nanomicelles resulted in certain favorable alterations in DAS-28 and SJC, albeit without statistical significance. | 31482684 | |
| Clinical (Randomized, Double-Blind, Placebo-Controlled Clinical Trial) |
| Supplementing with curcumin resulted in a significant reduction in various health markers, including HOMA-IR, ESR, and CRP, as well as weight, BMI, and waist circumference, in the patients when compared to those who received a placebo. | 35178811 | |
| Clinical (Randomised Controlled Trial) |
| Curcumin supplementation with strengthening exercises led to a significantly higher reduction in RF, ESR, CRP, WOMAC pain, and stiffness. | 36474395 | |
| Boswellic acids | Pre-clinical (in vitro/in vivo) | 3-α-o-acetoxy-4β-amino-11-oxo-24-norurs-12-ene (BA-25) In vitro: 10 μM (BA-25) In vivo:
| In vitro: a significant decrease in NO, ROS, TNF-α, and IL-6 production with the combination treatment in comparison to MTX alone in LPS-stimulated RAW-264.7 cells. In vivo: combination therapy restored LPS-induced increases in pro-inflammatory cytokines. There were no notable alterations in the hematological and serum biochemical markers between the combination group and the vehicle group. Furthermore, BA-25 exhibited rapid absorption and a high volume of distribution. | 37820446 |
| Clinical (Double-Blind Pilot Study) | 9 tablets of
| There were no subjective, clinical, or laboratory parameters that exhibited a substantial or clinically meaningful change from the initial state, nor were there any differences seen between the two groups at any stage during the study. | 9566100 | |
| Resveratrol | Pre-clinical (in vitro/in vivo) | Mice with CIA were given resveratrol injections directly into their abdominal cavity every day for 10 days. The injections started either on day 10 to avoid the onset of arthritis, or on day 23 to treat the arthritis after it had already developed following the initial immunization. | Both preventive and curative treatments of resveratrol reduced clinical symptoms and bone degradation in mice with CIA. The protective benefits against arthritis were linked to significantly decreased levels of pro-inflammatory cytokines and collagen-specific IgG in the bloodstream. Additionally, there was a decrease in the number of Th17 cells and the generation of IL-17 in the draining lymph nodes. | 21953348 |
| In vitro | 100 μM | Resveratrol was found to induce RA synovial cell (MH7A) apoptosis. This was achieved by activating caspase-9 and caspase-3. Resveratrol also disrupted mitochondrial function, leading to a decrease in the production of BCL-X(L), a protein that promotes cell survival. This disruption allowed cytochrome c to be released from the mitochondria into the cytosol. These effects were dependent on the presence of SIRT1, a protein involved in various cellular processes. | 20697895 | |
| Pre-clinical (in vitro/in vivo) | FLSs isolated from RA patients treated with 50–200 μM of resveratrol | The activation of caspase-8 is necessary to initiate resveratrol-induced apoptotic signaling in RA FLS through the participation of the mitochondrial pathway. Exposure to resveratrol resulted in significant apoptotic cell death, characterized by the activation of caspase-9 and -3, cleavage of poly ADP-ribose polymerase (PARP-1), release of mitochondrial cytochrome c, and translocation of apoptosis-inducing factor (AIF) to the nucleus. These events occurred through both caspase-dependent and caspase-independent signaling pathways. It was observed that the activated caspase-8 initiated mitochondrial apoptotic events by causing BID cleavage without affecting the levels of the apoptosis regulator BAX (BAX) or BCL2. | 18276737 | |
| Clinical (Randomized Controlled Trial) |
| The resveratrol-treated group showed a substantial decrease in DAS28. In addition, the levels of specific biochemical markers, including CRP, ESR, undercarboxylated osteocalcin, MMP3, TNF-α, and IL-6, were considerably reduced in patients who had received resveratrol. | 29611086 | |
| Quercitin | Clinical (Randomized Double-Blind Clinical Trial) |
| No significant differences were observed between the quercetin and placebo groups in terms of markers of oxidative stress, such as total antioxidant capacity, oxidized low-density lipo-protein, MDA, and CRP, as well as blood pressure. | 24829713 |
| Clinical (Randomized Double-Blind Clinical Trial) |
| The quercitin group had a decrease in early morning stiffness, morning pain, and discomfort following activity. Additionally, there was a reduction in plasma TNF-α levels as well as DAS-28 and HAQ scores. | 27710596 | |
| Clinical | glucosamine-chondroitin-quercetin glucoside (GCQG) supplement treatment (consisting of glucosamine hydrochloride (1200 mg/day), shark cartilage powder (300 mg/day) containing 75–111 mg of chondroitin, and quercetin (45 mg/day) for three months | No improvements in pain symptoms, daily activities (e.g., walking), VAS, or changes in the synovial fluid properties concerning the protein concentration, the molecular size of hyaluronic acid, or chondroitin 6-sulphate concentration. | 19202302 | |
| Clinical (Randomized, Placebo-Controlled, Double-Blind, Three-treatment Cross-over Design Trial) | Quercetin + vitamin C (166 mg + 133 mg/capsule), alpha-lipoic acid (300 mg/capsule), or placebo for 4 weeks, 3 capsules/day. Each treatment period consisted of 4 weeks with two weeks of wash-out period before the subject started the next supplementation. | There were no notable variations observed in the levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) and CRP in the serum of patients across different therapies. There was no significant difference in illness severity assessments between treatments, while quercetin supplementation showed a tendency to decrease VAS. | 19571161 | |
| Baicalin | Pre-clinical (in vitro/in vivo) | Murine adjuvant-induced arthritis model: during postimmunization days 14 to 21, mice were injected intraperitoneally with either 100 μL of baicalin solution at a dosage of 100 mg/kg or an equal volume of phosphate buffered saline PBS as a control. In vitro: 20 μM | Baicalin suppressed the increase in splenic Th17 cell population in vivo and blocked the adherence of lymphocytes mediated by IL-17 to cultured synoviocytes. Baicalin suppressed the expression of ICAM-1, VCAM-1, IL-6, and TNF-α mRNA in cultured synoviocytes. Baicalin reduces joint inflammation induced by IL-17, which is likely produced by an increased number of splenic Th17 cells in experimental arthritis. | 23840239 |
| Pre-clinical (in vivo) | CIA rats were treated with baicalin (50, 100, or 200 mg/kg) once daily for 30 days. | Administration of baicalin through intraperitoneal injection for 30 days effectively inhibited the observable symptoms of CIA, including reduced functionality and inflammation in the paws. Furthermore, it reduced collagen-induced joint inflammatory damage and suppressed the release of TNF-α and IL-1β in both rat synovium and synoviocytes derived from RA patients. Additional mechanistic studies demonstrated that baicalin inhibits the production and phosphorylation of the NF-κB p65 protein in synovial tissue and synoviocytes isolated from RA patients. In addition, baicalin reduced the acetylation of NF-κB p65, which is inversely related to the increase in SIRT1 expression caused by baicalin under the same conditions. | 24893986 | |
| Pre-clinical (in vivo) |
| RA was generated in mice by intraperitoneally injecting them with a collagen II monoclonal antibody cocktail at a concentration of 5 mg/kg dissolved in PBS for 10 consecutive days. The mice were administered LPS intraperitoneally at a dosage of 100 μg per mouse in 200 μL sterile PBS either on day 1 or day 4. Baicalin exerted a notable reduction in disease activity in a mouse model, as evidenced by the decrease in pressure pain thresholds and clinical arthritis scores. The expression of TNF-α, IL-1β, IL-6, MMP-2, MMP-9, and inducible enzymes (iNOS, COX-2) was generally suppressed. Furthermore, the administration of baicalin resulted in the apoptosis of synovial fluid monocytes and significantly decreased the expression of JAK1/STAT3, but not MAPKs, in the synovium. | 30562763 | |
| Pre-clinical (in vivo) |
| Baicalin suppressed the expression of TNF-a, IL-6, IL-1, IL-17, COX2, and COX1 in the synovial tissue. Varying concentrations of baicalin decreased the levels of autophagy-related proteins while increasing the levels of BAX proteins in the synovial tissue of adjuvant arthritis model rats. | 35124596 | |
| Clinical (Randomized, Double-blind, Placebo-Controlled Trial) | 500 mg baicalin or placebo orally every day for 12 weeks | Baicalin demonstrated efficacy in lowering blood lipids and reducing inflammation in patients diagnosed with both coronary artery disease and RA, as evidenced by CRP and EULAR improvements. | 29935544 |
| Herb/Compound | Duration | Dosage/Form | Outcome Measures | Results | PMID |
|---|---|---|---|---|---|
| Maharasnadhi Quathar (MRQ) | 3 months | - | SOD, GPX, CAT, TBARS, serum iron, hemoglobin, TIBC | Increased SOD by 44.6%, GPX by 39.8%, CAT by 25.2%; Reduced TBARS by 34%; Improved serum iron by 26.8%, hemoglobin by 24.8%, TIBC by 16.1% | 11448551 |
| Weldehi Choornaya (WC) | 3 months | - | SOD, GPX, CAT, TBARS, serum iron, hemoglobin, TIBC | No notable enhancement in SOD, GPX, or CAT; Reduced TBARS by 21.8% | 11448551 |
| Tripterygium wilfordii Hook F (TWHF) | - | Varied doses | Safety, ACR20/70, DAS28, EULAR | High dose (360 mg/day) showed efficacy; 570 mg/day was well-tolerated; TWHF extract, administered at a dose of 60 mg three times daily, resulted in a significantly higher rate of achieving the ACR20 (20% improvement) compared to sulfasalazine over 24 weeks; TWHF monotherapy (20 mg three times a day) was comparable to that of MTX monotherapy (12.5 mg once a week) in managing disease activity in patients with active RA. Additionally, the combination of MTX and TWHF was found to be superior to MTX monotherapy for 12 or 24 weeks. | 11669150 12124856 19687490 24733191 29636089 |
| Uncaria tomentosa (UT) | 52 weeks | - | Sore joints, swollen joints, safety | Decrease in sore joints; No major adverse effects | 11950006 |
| Punica granatum (Pomegranate) | 8 weeks | 250 mg twice daily | DAS28, HAQ, CRP, MMP3, MDA, GPX, ESR | Decreased DAS28; improved blood biomarkers of inflammation, and oxidative stress | 27577177 |
| Zingiber officinale (Ginger) | 8 weeks | 500 mg twice daily | Total anti-oxidant capacity, MDA, HAQ | Improved oxidative stress markers and HAQ | 32159257 |
| Stephania tetrandra | 12 weeks | - | Granulocyte count, lipid peroxide, elastase | Reduced granulocyte count, lipid peroxide, and elastase activities; improved inflammation biomarkers | 15103675 |
| Cannabis (Sativex) | 5 weeks | Oromucosal spray | Pain, morning stiffness, sleep quality, DAS28 | Reduced pain and disease activity | 16282192 |
| Andrographis paniculata (Andrographis) | 14 weeks | 30% andrographolides extract | Pain, joint swelling, tenderness, ACR, EULAR, SF36 | Reduction in joint pain, swelling, and tenderness; not statistically significant | 19408036 |
| Mangifera indica (Vimang) | 180 days | 900 mg daily | DAS28, VAS, HAQ, ESR | Significant improvement in DAS28; reduced NSAID use | 24344049 |
| Artemisia annua | 12 weeks | 150 mg twice daily | Pain, stiffness, functional limitations | Reduced pain over 12 weeks | 26631103 |
| Berberis vulgaris (Barberry) | 12 weeks | 1000 mg/twice daily | IL-2, IL-4, IL-10, IL-17 | Decreased IL-17, increased IL-10; reduced RA intensity | 32914483 |
| Nigella sativa (Black Seed) | 2 months | 500 mg/twice daily | DAS28, hs-CRP, CD4+, CD8+, IL-10, TNF-α, MDA, NO | Decreased DAS28, CRP, increased CD4(+)/CD8(+) ratio | 27100726 |
| Willow Bark (Salix spp.) | - | Standardized extract | WOMAC pain score, VAS | No positive effects | 15517622 |
| Compound | Class | Mechanisms of Action | PMID | Pharmacokinetics/Toxicity | PMID |
|---|---|---|---|---|---|
| Apigenin | Flavonoid | Inhibits ERK1/2 pathway. Reduces production of IL-1β, IL-6, TNF-α. Inhibits the COX2 enzyme. Inhibits collagenase activity. Decreases NO production. Reduces expression of COX-2, VCAM-1, ICAM-1, E-selectin. Inhibits angiogenesis and osteoclastogenesis via the VEGF/VEGFR and RANKL/osteoprotegerin pathways | 24685587 31132731 19647729 22189539 35660352 21535545 18038911 | Currently, there is limited evidence indicating any harmful effects caused by apigenin. The pharmacokinetic behavior of apigenin influences its distribution in tissues and its bioactivity. The O-glycosylation or C-glycosylation of apigenin can have varying effects on its metabolism, which in turn might impact its antioxidant capability and biological advantages. The reason for this is likely because apigenin, despite its many beneficial benefits, has a very low solubility in water (1.35 μg/mL) and high permeability. Nevertheless, apigenin is widely regarded as safe, even when taken in large amounts, and there have been no reports of any harmful effects. | 30875872 |
| Betulinic Acid (BA) | Triterpenoid | Suppresses growth, motion, and infiltration of FLSs. Reduces TNF-α exposure effects, MMP expression, and inflammatory cytokines (IL-6, IL-8) production. Inhibits the AKT/NF-κB pathway. Inhibits migration, invasion, and actin cytoskeleton rearrangement in FLSs. Reduces mRNA levels of IL-1β, IL-6, IL-8, and IL-17A. Reduces NF-κB signal pathway activation. Lowers IL-1β, IL-6, and TNF-α levels. Suppresses VEGF and TGF-β transcription. Reduces NF-κB pathway protein expression. | 30367550 30553912 32718263 | Research has indicated that BA does not have any notable adverse effects. At a dosage of 50 μg/mL, BA did not impede the growth of normal peripheral blood cells. The limited water solubility of BA impedes its potential use as a medication. Modifications at carbon positions C-3, C-20, and C-28 of the molecule have the potential to enhance solubility while maintaining pharmacological action. | 34388528 |
| Curculigo glycoside | Steroidal saponin | Reduces inflammatory markers (TNF-α, IL-1β, IL-6, IL-12, IL-17A). Modulates the JAK/STAT/NF-κB signaling pathway. Reduces EGFR, MEK1, MMP2, FGFR1, and MCL1 expression. Inhibits inflammatory cell infiltration, pannus formation, and cartilage damage. Suppresses MMP9, JUN, and PTGS2 expression. | 26637957 30664158 33273808 37957674 | Some studies have shown that curculigo glycoside use in living organisms can be challenging, mainly due to low oral bioavailability. Curculigo glycoside passes through some metabolic changes, most of which are related to phase I and phase II biotransformation events. As a phenolic glycoside, curculigo glycoside can undergo phase I metabolic processes involving oxidation, hydrolysis, or reduction. This leads to the breakdown of ester and phenolic glycoside bonds. Furthermore, oxidation or reduction events may be initiated by phenolic hydroxyl groups and their derivatives, hydroxymethyl and benzoyloxy groups. Curculigoside demonstrated fast distribution, wide tissue uptake, and limited absorption into systemic circulation in rats. The absolute bioavailability values for oral dosages of 100, 200, and 400mg/kg were determined to be 0.38%, 0.22%, and 0.27%, respectively. According to the tissue distribution study, curculigoside was found in many organs, including the heart, lung, spleen, intestine, stomach, kidney, thymus, liver, brain, testis, and bone marrow, after being orally given at a dose of 150 mg/kg. Its broad distribution suggests that it can pharmacologically affect target tissues following pharmacokinetic optimization. Curculigoside specifically decreased the activity of CYP1A2, CYP2C8, and CYP3A4, but not that of other CYP isoforms. While extensive toxicological studies of curculigoside were not conducted, acute toxicity studies revealed that water extracts of Curculigo orchioides did not affect animal mortality, even at a dosage that was 1384 times higher than the recommended clinical dose (3–9 g daily). The LD50 of ethanol plant extracts was 215.9 g/kg, which was 1439 times higher than the recommended dose. Similarly, long-term toxicity experiments revealed that administering the ethanol extract of Curculigo orchioides to rats at a dose of 120 g/kg for 6 months resulted in damage to the liver, kidney, and reproductive organs. Administration of either 30 g/kg or 60 g/kg doses over a long time did not result in any toxicological consequences. | 27819189 23562803 34205154 25549926 |
| Epigallocatechin Gallate (EGCG) | Flavonoid (Catechin) | Reduces IL-1β, TNF-α, and IL-6 production. Neutralizes ROS. Modulates anti-oxidant processes and immune cell activity. Inhibits MMPs, cathepsins, and CCL2 production. Stimulates indoleamine-2,3-dioxygenase-producing dendritic cells and T regs. Activates ERK, NRF2, and HO-1. Suppresses STAT3 activation. Enhances PI3K and AKT activity. Reduces MCL-1 expression. | 18496696 18796608 23871988 28532672 31746064 26379475 16869002 22186621 18576345 18821707 26957211 19404960 | EGCG experiences challenges in its therapeutic application due to its limited oral bioavailability, which is attributed to its low absorption in the intestines and its short retention period or lack of stability. EGCG has been studied in doses ranging from 150 to 400 mg per day, with oral administration being the most commonly used method in animal models and clinical trials. On the other hand, it has been suggested that daily doses ranging from 140 mg to 1000 mg can cause damage to the liver. Lambert et al. examined the hepatotoxic effects of high dosages of EGCG in male CF-1 mice. An administration of a single dose of EGCG at a dosage of 1500 mg/kg, given orally, resulted in a 138-fold increase in plasma ALT levels and caused a decrease in mouse lifespan by 85%. Administration of EGCG once a day resulted in a hepatotoxic response. After administering two daily doses of 750 mg/kg EGCG, the levels of ALT in the plasma increased by 184 times. After receiving EGCG treatment, hepatic necrosis was observed. This was accompanied by increased levels of hepatic lipid peroxidation (5-fold change) and liver damage. The safe intake limit of 338 mg of EGCG/day for adults was determined based on the analysis of toxicological and human safety data for tea preparations ingested as a solid bolus dosage. According to evidence on adverse effects in humans, a recommended safe dose was estimated as 704 mg of EGCG per day (for tea preparations in a drinkable form). | 29580974 16387402 19883714 32140423 9311619 22016611 32438698 29799486 37446908 |
| Icarin and icaritin (metabolite) | Flavonoid glycoside | Reduces β3 integrin, cathepsin K, and RANKL expression. Suppresses the MAPK and NF-κB pathways. Alleviates bone degradation, suppresses proliferation, and promotes apoptosis of FLS cells. Reduces TNF-α, IL-1β, and IL-6 secretion. | 35676785 23746958 27199510 26816641 25374443 37647355 33155655 34678240 | Recent clinical trials suggest that icaritin has a reasonably good level of safety, however, it may lead to modest side effects such as skin rash and diarrhea. Icaritin suppresses the activity of UGT1A1, 1A3, 1A4, 1A7, 1A8, 1A10, 2B7, and 2B15 enzymes. This suggests that this chemical has the potential to interact with other medications that are processed by UGT enzymes. | 30922248 30522143 34873891 38886878 |
| Roburic Acid (RBA) | Tetracyclic triterpenoid | Inhibits mPGES-1 and COX1/2 enzymes. Suppresses the MAPK and NF-κB pathways. Direct association with TNF-α, prevents its binding to TNF-R1. Suppresses osteoclast development. Inhibits TRAF6 and NF-κB activity. Increases HO-1 and ARE activity. | 24844534 20188172 28761990 35222445 34796915 37500654 | No data. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kciuk, M.; Garg, A.; Rohilla, M.; Chaudhary, R.; Dhankhar, S.; Dhiman, S.; Bansal, S.; Saini, M.; Singh, T.G.; Chauhan, S.; et al. Therapeutic Potential of Plant-Derived Compounds and Plant Extracts in Rheumatoid Arthritis—Comprehensive Review. Antioxidants 2024, 13, 775. https://doi.org/10.3390/antiox13070775
Kciuk M, Garg A, Rohilla M, Chaudhary R, Dhankhar S, Dhiman S, Bansal S, Saini M, Singh TG, Chauhan S, et al. Therapeutic Potential of Plant-Derived Compounds and Plant Extracts in Rheumatoid Arthritis—Comprehensive Review. Antioxidants. 2024; 13(7):775. https://doi.org/10.3390/antiox13070775
Chicago/Turabian StyleKciuk, Mateusz, Anjali Garg, Manni Rohilla, Rishabh Chaudhary, Sanchit Dhankhar, Sachin Dhiman, Seema Bansal, Monika Saini, Thakur Gurjeet Singh, Samrat Chauhan, and et al. 2024. "Therapeutic Potential of Plant-Derived Compounds and Plant Extracts in Rheumatoid Arthritis—Comprehensive Review" Antioxidants 13, no. 7: 775. https://doi.org/10.3390/antiox13070775
APA StyleKciuk, M., Garg, A., Rohilla, M., Chaudhary, R., Dhankhar, S., Dhiman, S., Bansal, S., Saini, M., Singh, T. G., Chauhan, S., Mujwar, S., Gielecińska, A., & Kontek, R. (2024). Therapeutic Potential of Plant-Derived Compounds and Plant Extracts in Rheumatoid Arthritis—Comprehensive Review. Antioxidants, 13(7), 775. https://doi.org/10.3390/antiox13070775








