Myeloperoxidase as a Promising Therapeutic Target after Myocardial Infarction
Abstract
1. Introduction
2. Inflammation, Oxidative Stress, and Post-MI Ischemia-Reperfusion Injury
3. Biochemistry of MPO

4. MPO in Coronary Artery Disease
4.1. Clinical Evidence Correlating MPO with Worsened CAD Outcomes
4.2. Preclinical Evidence for MPO as a Driver of Atherosclerosis
4.3. Pro-Atherogenic Mechanisms of MPO
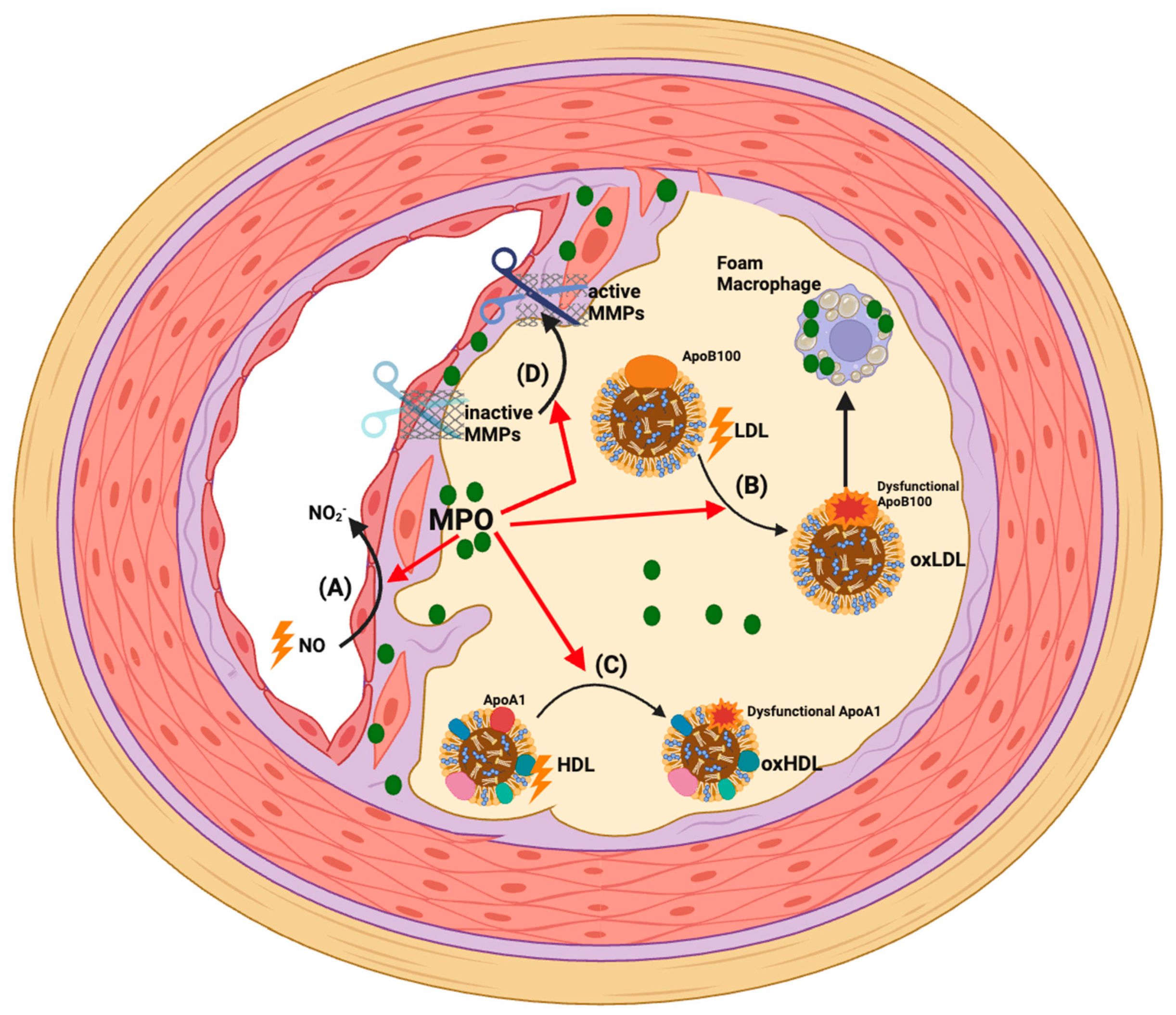
5. Actions of MPO Post-MI
5.1. Role of Neutrophils in MI
5.2. Clinical Evidence Correlating MPO with Post-MI Complications
5.3. Preclinical Evidence for MPO as a Driver of Cardiac Damage and Complications Post-MI
| Pre-Clinical Model of MI or Arrhythmia | MPO Intervention | Impact of Intervention on Study Outcome | Reference |
|---|---|---|---|
| Permanent LAD ligation in rats | Pre-treatment with anti-neutrophil antibody | Diminished α-chloro fatty acid aldehydes and neutrophil infiltration levels in the infarcted myocardium | Thukkani, A.K., Martinson, B.D., Albert, C.J., Vogler, G.A. & Ford, D.A., 2005 [181] |
| Temporary (ischemia-reperfusion model) LAD ligation in rats | MPO knockout genotype | Reduced LV dilation and improved LV function, although no difference in infarct size | Vasilyev et al., 2005 [182] |
| Permanent LAD ligation in mice | MPO knockout genotype | Decreased leukocyte infiltration, reduced LV dilation and preserved LV function | Askari et al., 2003 [183] |
| Both permanent and temporary (ischemia-reperfusion model) LAD ligation in mice | MPO knockout genotype | Decreased vulnerability for ventricular tachycardia and decreased heterogeneity of electrical conduction in the peri-infarct zone | Mollenhauer et al., 2017 [169] |
| Permanent LAD ligation in mice | Treatment with MPO inhibitor PF-1355 | Improved ejection fraction, decreased end diastolic volume and LV mass | Ali et al., 2016 [186] |
| Temporary (ischemia-reperfusion model) LAD ligation in mice | Treatment with MPO inhibitor AZM198 | Improved cardiac function and reduced structural remodelling | Guthoff et al., 2022 [187] |
| Right atrial electrophysical stimulation in mice | MPO knockout genotype | Protection from atrial fibrillation | Rudolph et al., 2010 [184] |
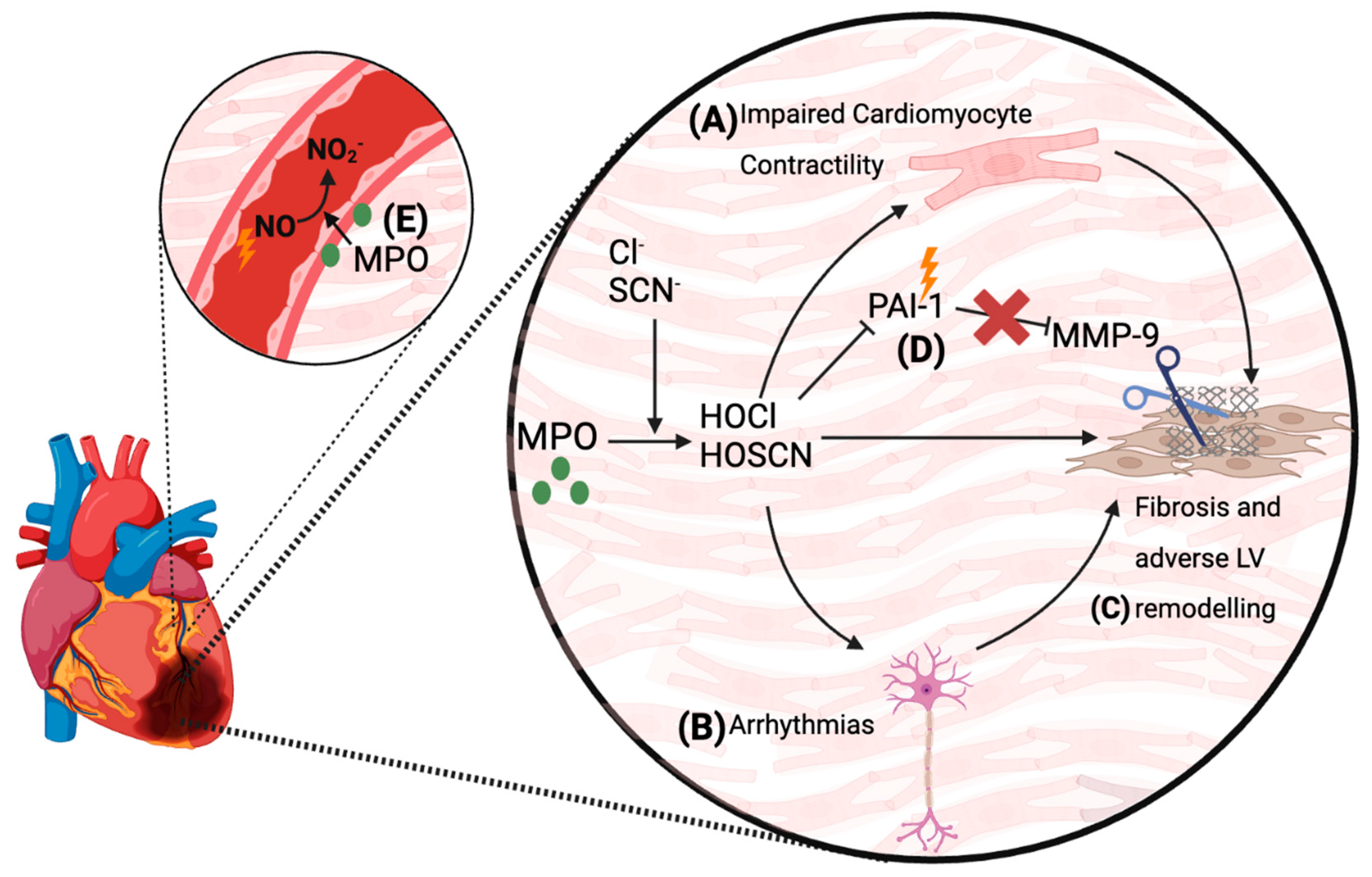
5.4. Proposed Mechanisms by Which MPO Mediates Cardiac Damage and Complications Post-MI
6. MPO as a Therapeutic Target for the Treatment of Cardiac Dysfunction Post-MI
7. Peroxidasin and MI
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Uppal, N.; Uppal, V.; Uppal, P. Progression of Coronary Artery Disease (CAD) from Stable Angina (SA) Towards Myocardial Infarction (MI): Role of Oxidative Stress. J. Clin. Diagn. Res. 2014, 8, 40–43. [Google Scholar] [CrossRef]
- Maddox, T.M.; Stanislawski, M.A.; Grunwald, G.K.; Bradley, S.M.; Ho, P.M.; Tsai, T.T.; Patel, M.R.; Sandhu, A.; Valle, J.; Magid, D.J.; et al. Nonobstructive Coronary Artery Disease and Risk of Myocardial Infarction. JAMA 2014, 312, 1754. [Google Scholar] [CrossRef]
- Dai, X. Genetics of coronary artery disease and myocardial infarction. World J. Cardiol. 2016, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Bochaton, T.; Claeys, M.J.; Garcia-Dorado, D.; Mewton, N.; Bergerot, C.; Jossan, C.; Amaz, C.; Boussaha, I.; Thibault, H.; Ovize, M. Importance of infarct size versus other variables for clinical outcomes after PPCI in STEMI patients. Basic Res. Cardiol. 2019, 115, 4. [Google Scholar] [CrossRef]
- Musiolik, J.; Van Caster, P.; Skyschally, A.; Boengler, K.; Gres, P.; Schulz, R.; Heusch, G. Reduction of infarct size by gentle reperfusion without activation of reperfusion injury salvage kinases in pigs. Cardiovasc. Res. 2010, 85, 110–117. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2016, 38, 774–784. [Google Scholar] [CrossRef]
- Vlodaver, Z.; Edwards, J.E. Rupture of ventricular septum or papillary muscle complicating myocardial infarction. Circulation 1977, 55, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Goldberg, S. Mechanical Complications of Myocardial Infarction. Am. J. Med. 2022, 135, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Burks, K.H.; Ko, J.M.; Filardo, G.; Guileyardo, J.M. Commonalities of cardiac rupture (left ventricular free wall or ventricular septum or papillary muscle) during acute myocardial infarction secondary to atherosclerotic coronary artery disease. Am. J. Cardiol. 2015, 115, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Camaj, A.; Fuster, V.; Giustino, G.; Bienstock, S.W.; Sternheim, D.; Mehran, R.; Dangas, G.D.; Kini, A.; Sharma, S.K.; Halperin, J.L.; et al. Left Ventricular Thrombus Following Acute Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial ischemia reperfusion injury: From basic science to clinical bedside. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- van der Weg, K.; Prinzen, F.W.; Gorgels, A.P. Editor’s Choice- Reperfusion cardiac arrhythmias and their relation to reperfusion-induced cell death. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 142–152. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar]
- Ibanez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef]
- Cung, T.-T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guérin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005, 434, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischaemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Andrienko, T.N.; Pasdois, P.; Pereira, G.C.; Ovens, M.J.; Halestrap, A.P. The role of succinate and ROS in reperfusion injury—A critical appraisal. J. Mol. Cell. Cardiol. 2017, 110, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, W.; Ao, X.; Chu, X.; Wan, Q.; Wang, Y.; Xiao, D.; Yu, W.; Li, M.; Yu, F.; et al. ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol. 2019, 20, 414–426. [Google Scholar] [CrossRef] [PubMed]
- El Kazzi, M.; Rayner, B.S.; Chami, B.; Dennis, J.M.; Thomas, S.R.; Witting, P.K. Neutrophil-Mediated Cardiac Damage After Acute Myocardial Infarction: Significance of Defining a New Target Cell Type for Developing Cardioprotective Drugs. Antioxid. Redox Signal. 2020, 33, 689–712. [Google Scholar] [CrossRef] [PubMed]
- Mauler, M.; Herr, N.; Schoenichen, C.; Witsch, T.; Marchini, T.; Härdtner, C.; Koentges, C.; Kienle, K.; Ollivier, V.; Schell, M.; et al. Platelet serotonin aggravates myocardial ischemia/reperfusion injury via neutrophil degranulation. Circulation 2019, 139, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Hoenderdos, K.; Lodge, K.M.; Hirst, R.A.; Chen, C.; Palazzo, S.G.C.; Emerenciana, A.; Summers, C.; Angyal, A.; Porter, L.; Juss, J.K.; et al. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax 2016, 71, 1030–1038. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef]
- Davies, M.; Hawkins, C.; Pattison, D.; Rees, M. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1199–1234. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J. Heme Peroxidases at Unperturbed and Inflamed Mucous Surfaces. Antioxidants 2021, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Loughran, N.B.; O’Connor, B.; Ó’Fágáin, C.; O’Connell, M.J. The phylogeny of the mammalian heme peroxidases and the evolution of their diverse functions. BMC Evol. Biol. 2008, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Péterfi, Z.; Tóth, Z.E.; Kovács, H.A.; Lázár, E.; Sum, A.; Donkó, Á.; Sirokmány, G.; Shah, A.M.; Geiszt, M. Peroxidasin-like protein: A novel peroxidase homologue in the human heart. Cardiovasc. Res. 2014, 101, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef]
- Oyenuga, A.O.; Couper, D.; Matsushita, K.; Boerwinkle, E.; Folsom, A.R. Association of monocyte myeloperoxidase with incident cardiovascular disease: The Atherosclerosis Risk in Communities Study. PLoS ONE 2018, 13, e0205310. [Google Scholar] [CrossRef]
- Sugiyama, S.; Okada, Y.; Sukhova, G.K.; Virmani, R.; Heinecke, J.W.; Libby, P. Macrophage Myeloperoxidase Regulation by Granulocyte Macrophage Colony-Stimulating Factor in Human Atherosclerosis and Implications in Acute Coronary Syndromes. Am. J. Pathol. 2001, 158, 879–891. [Google Scholar] [CrossRef]
- Thomas, S.R.; Witting, P.K.; Drummond, G.R. Redox control of endothelial function and dysfunction: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2008, 10, 1713–1765. [Google Scholar] [CrossRef]
- Maiocchi, S.L.; Ku, J.; Thai, T.; Chan, E.; Rees, M.D.; Thomas, S.R. Myeloperoxidase: A versatile mediator of endothelial dysfunction and therapeutic target during cardiovascular disease. Pharmacol. Ther. 2021, 221, 107711. [Google Scholar] [CrossRef]
- Galijasevic, S. The development of myeloperoxidase inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, J.C.; Whitehouse, W.M.; Winterbourn, C.C.; Kettle, J.A. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997, 327, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Davies, M.J.; Hawkins, C.L. Reactions and reactivity of myeloperoxidase-derived oxidants: Differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 2012, 46, 975–995. [Google Scholar] [CrossRef] [PubMed]
- Abu-Soud, H.M.; Hazen, S.L. Nitric Oxide Modulates the Catalytic Activity of Myeloperoxidase. J. Biol. Chem. 2000, 275, 5425–5430. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Mueller, D.M.; Fabjan, J.S.; Heinecke, J.W. Nitrogen dioxide radical generated by the myeloperoxidase-hydrogen peroxide-nitrite system promotes lipid peroxidation of low density lipoprotein. FEBS Lett. 1999, 455, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, C.J.; Winterbourn, C.C.; Senthilmohan, R.; Kettle, A.J. Nitrite as a Substrate and Inhibitor of Myeloperoxidase. J. Biol. Chem. 2000, 275, 11638–11644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Brennan, M.-L.; Shen, Z.; Macpherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase Functions as a Major Enzymatic Catalyst for Initiation of Lipid Peroxidation at Sites of Inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Heitzer, T.; Eiserich, J.P.; Lau, D.; Mollnau, H.; Ortak, M.; Petri, S.; Goldmann, B.; Duchstein, H.-J.; Berger, J.; et al. Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic. Biol. Med. 2004, 37, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.D.; Maiocchi, S.L.; Kettle, A.J.; Thomas, S.R. Mechanism and regulation of peroxidase-catalyzed nitric oxide consumption in physiological fluids: Critical protective actions of ascorbate and thiocyanate. Free Radic. Biol. Med. 2014, 72, 91–103. [Google Scholar] [CrossRef]
- Maiocchi, S.L.; Morris, J.C.; Rees, M.D.; Thomas, S.R. Regulation of the nitric oxide oxidase activity of myeloperoxidase by pharmacological agents. Biochem. Pharmacol. 2017, 135, 90–115. [Google Scholar] [CrossRef]
- Zhang, R. Association Between Myeloperoxidase Levels and Risk of Coronary Artery Disease. JAMA 2001, 286, 2136. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Braun, S.; Mehilli, J.; Von Beckerath, N.; Schömig, A.; Kastrati, A. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndromes. Eur. J. Clin. Investig. 2008, 38, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Heeschen, C.; Meinertz, T.; Zeiher, A.M.; Eiserich, J.P.; Münzel, T.; Simoons, M.L.; Hamm, C.W. Myeloperoxidase Serum Levels Predict Risk in Patients With Acute Coronary Syndromes. Circulation 2003, 108, 1440–1445. [Google Scholar] [CrossRef]
- Brennan, M.-L.; Penn, M.S.; Van Lente, F.; Nambi, V.; Shishehbor, M.H.; Aviles, R.J.; Goormastic, M.; Pepoy, M.L.; Mcerlean, E.S.; Topol, E.J.; et al. Prognostic Value of Myeloperoxidase in Patients with Chest Pain. N. Engl. J. Med. 2003, 349, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, E.; Ruwende, C.; Eng, C.; Chopra, V.; Yanamadala, S.; Clark, L.T.; Pinsky, D.J.; Marmur, J.D. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am. J. Cardiol. 2007, 99, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Heslop, C.L.; Frohlich, J.J.; Hill, J.S. Myeloperoxidase and C-reactive protein have combined utility for long-term prediction of cardiovascular mortality after coronary angiography. J. Am. Coll. Cardiol. 2010, 55, 1102–1109. [Google Scholar] [CrossRef]
- Mocatta, T.J.; Pilbrow, A.P.; Cameron, V.A.; Senthilmohan, R.; Frampton, C.M.; Richards, A.M.; Winterbourn, C.C. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J. Am. Coll. Cardiol. 2007, 49, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wu, Y.; Nicholls, S.J.; Hazen, S.L. Plasma myeloperoxidase predicts incident cardiovascular risks in stable patients undergoing medical management for coronary artery disease. Clin. Chem. 2011, 57, 33–39. [Google Scholar] [CrossRef]
- Düzgünçinar, O.; Yavuz, B.; Hazirolan, T.; Deniz, A.; Tokgözoglu, S.L.; Akata, D.; Demirpençe, E. Plasma myeloperoxidase is related to the severity of coronary artery disease. Acta Cardiol. 2008, 63, 147–152. [Google Scholar] [CrossRef]
- Janus, S.E.; Hajjari, J.; Chami, T.; Karnib, M.; Al-Kindi, S.G.; Rashid, I. Myeloperoxidase is Independently Associated with Incident Heart Failure in Patients with Coronary Artery Disease and Kidney Disease. Curr. Probl. Cardiol. 2022, 47, 101080. [Google Scholar] [CrossRef]
- Meuwese, M.C.; Stroes, E.S.G.; Hazen, S.L.; van Miert, J.N.; Kuivenhoven, J.A.; Schuab, R.G.; Wareham, N.J.; Luben, R.; Kastelein, J.J.P.; Khaw, K.; et al. Serum Myeloperoxidase Levels Are Associated with the Future Risk of Coronary Artery Disease in Apparently Healthy Individuals. J. Am. Coll. Cardiol. 2007, 50, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Yalcin, R.; Okyay, K.; Poyraz, F.; Bayraktar, N.; Pasaoglu, H.; Boyaci, B.; Cengel, A. Potential Role of Plasma Myeloperoxidase Level in Predicting Long-Term Outcome of Acute Myocardial Infarction. Tex. Heart Inst. J. 2012, 39, 500–506. [Google Scholar] [PubMed]
- Rebeiz, A.G.; Tamim, H.M.; Sleiman, R.M.; Abchee, A.G.; Ibrahim, Z.; Khoury, M.Y.; Youhanna, S.; Skouri, H.N.; Alam, S.E. Plasma myeloperoxidase concentration predicts the presence and severity of coronary disease in patients with chest pain and negative troponin-T. Coron. Artery Dis. 2011, 22, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Buffon, A.; Biasucci, L.M.; Liuzzo, G.; D’Onofrio, G.; Crea, F.; Maseri, A. Widespread Coronary Inflammation in Unstable Angina. N. Engl. J. Med. 2002, 347, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Kubala, L.; Lu, G.; Baldus, S.; Berglund, L.; Eiserich, J.P. Plasma levels of myeloperoxidase are not elevated in patients with stable coronary artery disease. Clin. Chim. Acta 2008, 394, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Scharnagl, H.; Kleber, M.E.; Genser, B.; Kickmaier, S.; Renner, W.; Weihrauch, G.; Grammer, T.; Rossmann, C.; Winkelmann, B.R.; Boehm, B.O.; et al. Association of myeloperoxidase with total and cardiovascular mortality in individuals undergoing coronary angiography—The LURIC study. Int. J. Cardiol. 2014, 174, 96–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ford, D.A. Lipid oxidation by hypochlorous acid: Chlorinated lipids in atherosclerosis and myocardial ischemia. Clin. Lipidol. 2010, 5, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Thukkani, A.K.; Mchowat, J.; Hsu, F.-F.; Brennan, M.-L.; Hazen, S.L.; Ford, D.A. Identification of α-Chloro Fatty Aldehydes and Unsaturated Lysophosphatidylcholine Molecular Species in Human Atherosclerotic Lesions. Circulation 2003, 108, 3128–3133. [Google Scholar] [CrossRef]
- Baldus, S.; Eiserich, J.P.; Brennan, M.L.; Jackson, R.M.; Alexander, C.B.; Freeman, B.A. Spatial mapping of pulmonary and vascular nitrotyrosine reveals the pivotal role of myeloperoxidase as a catalyst for tyrosine nitration in inflammatory diseases. Free Radic. Biol. Med. 2002, 33, 1010–1019. [Google Scholar] [CrossRef]
- Malle, E.; Waeg, G.; Schreiber, R.; Gröne, E.F.; Sattler, W.; Gröne, H.-J. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions. Eur. J. Biochem. 2000, 267, 4495–4503. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Kugiyama, K.; Aikawa, M.; Nakamura, S.; Ogawa, H.; Libby, P. Hypochlorous Acid, a Macrophage Product, Induces Endothelial Apoptosis and Tissue Factor Expression. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Eiserich, J.P.; Mani, A.; Castro, L.; Figueroa, M.; Chumley, P.; Ma, W.; Tousson, A.; White, C.R.; Bullard, D.C.; et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J. Clin. Investig. 2001, 108, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.K.; Wipper, S.; Reiter, B.; Rudolph, V.; Coym, A.; Detter, C.; Lau, D.; Klinke, A.; Friedrichs, K.; Rau, T.; et al. Myeloperoxidase deficiency preserves vasomotor function in humans. Eur. Heart J. 2012, 33, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Eiserich, J.P.; Baldus, S.; Brennan, M.L.; Ma, W.; Zhang, C.; Tousson, A.; Castro, L.; Lusis, A.J.; Nauseef, W.M.; Freeman, B.A. Myeloperoxidase, a Leukocyte-Derived Vascular NO Oxidase. Science 2002, 296, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Hazell, L.J.; Arnold, L.; Flowers, D.; Waeg, G.; Malle, E.; Stocker, R. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Investig. 1996, 97, 1535–1544. [Google Scholar] [CrossRef]
- Zheng, L.; Nukuna, B.; Brennan, M.-L.; Sun, M.; Goormastic, M.; Settle, M.; Schmitt, D.; Fu, X.; Thomson, L.; Fox, P.L.; et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Investig. 2004, 114, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Didonato, J.A.; Levison, B.S.; Schmitt, D.; Li, L.; Wu, Y.; Buffa, J.; Kim, T.; Gerstenecker, G.S.; Gu, X.; et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 2014, 20, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; Didonato, J.A.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184. [Google Scholar] [CrossRef]
- Hazell, L.J.; Baernthaler, G.; Stocker, R. Correlation between intima-to-media ratio, apolipoprotein B-100, myeloperoxidase, and hypochlorite-oxidized proteins in human atherosclerosis. Free Radic. Biol. Med. 2001, 31, 1254–1262. [Google Scholar] [CrossRef]
- Hazen, S.L.; Heinecke, J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Investig. 1997, 99, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Gauster, M.; Pfeifer, T.; Wadsack, C.; Fauler, G.; Stiegler, P.; Koefeler, H.; Beubler, E.; Schuligoi, R.; Heinemann, A.; et al. Protein Carbamylation Renders High-Density Lipoprotein Dysfunctional. Antioxid. Redox Signal. 2011, 14, 2337–2346. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Hardy, M.M.; Hazen, S.L.; Wagner, P.; Oh-ishi, S.; Steinbrecher, U.P.; Heinecke, J.W. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J. Biol. Chem. 1997, 272, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; Libby, P.; Soehnlein, O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2013, 3, 424. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Investig. 1994, 94, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.-L.; Anderson, M.M.; Shih, D.M.; Qu, X.-D.; Wang, X.; Mehta, A.C.; Lim, L.L.; Shi, W.; Hazen, S.L.; Jacob, J.S.; et al. Increased atherosclerosis in myeloperoxidase-deficient mice. J. Clin. Investig. 2001, 107, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Mcmillen, T.S.; Heinecke, J.W.; Leboeuf, R.C. Expression of Human Myeloperoxidase by Macrophages Promotes Atherosclerosis in Mice. Circulation 2005, 111, 2798–2804. [Google Scholar] [CrossRef] [PubMed]
- Castellani, L.W.; Chang, J.J.; Wang, X.; Lusis, A.J.; Reynolds, W.F. Transgenic mice express human MPO 2463G/A alleles at atherosclerotic lesions, developing hyperlipidemia and obesity in 2463G males. J. Lipid Res. 2006, 47, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Rashid, I.; Maghzal, G.J.; Chen, Y.-C.; Cheng, D.; Talib, J.; Newington, D.; Ren, M.; Vajandar, S.K.; Searle, A.; Maluenda, A.; et al. Myeloperoxidase is a potential molecular imaging and therapeutic target for the identification and stabilization of high-risk atherosclerotic plaque. Eur. Heart J. 2018, 39, 3301–3310. [Google Scholar] [CrossRef]
- Goldmann, B.U.; Rudolph, V.; Rudolph, T.K.; Holle, A.-K.; Hillebrandt, M.; Meinertz, T.; Baldus, S. Neutrophil activation precedes myocardial injury in patients with acute myocardial infarction. Free Radic. Biol. Med. 2009, 47, 79–83. [Google Scholar] [CrossRef]
- Rees, M.D.; Dang, L.; Thai, T.; Owen, D.M.; Malle, E.; Thomas, S.R. Targeted subendothelial matrix oxidation by myeloperoxidase triggers myosin II-dependent de-adhesion and alters signaling in endothelial cells. Free Radic. Biol. Med. 2012, 53, 2344–2356. [Google Scholar] [CrossRef]
- Rudolph, V.; Rudolph, T.K.; Schopfer, F.J.; Bonacci, G.; Lau, D.; Szöcs, K.; Klinke, A.; Meinertz, T.; Freeman, B.A.; Baldus, S. Bivalirudin Decreases NO Bioavailability by Vascular Immobilization of Myeloperoxidase. J. Pharmacol. Exp. Ther. 2008, 327, 324–331. [Google Scholar] [CrossRef]
- Klinke, A.; Nussbaum, C.; Kubala, L.; Friedrichs, K.; Rudolph, T.K.; Rudolph, V.; Paust, H.-J.; Schröder, C.; Benten, D.; Lau, D.; et al. Myeloperoxidase attracts neutrophils by physical forces. Blood 2011, 117, 1350–1358. [Google Scholar] [CrossRef]
- Kubala, L.; Kolářová, H.; Víteček, J.; Kremserová, S.; Klinke, A.; Lau, D.; Chapman, A.L.P.; Baldus, S.; Eiserich, J.P. The potentiation of myeloperoxidase activity by the glycosaminoglycan-dependent binding of myeloperoxidase to proteins of the extracellular matrix. Biochim. Biophys. Acta 2013, 1830, 4524–4536. [Google Scholar] [CrossRef]
- Rudolph, T.K.; Schaper, N.; Klinke, A.; Demir, C.; Goldmann, B.; Lau, D.; Köster, R.; Hellmich, M.; Meinertz, T.; Baldus, S.; et al. Liberation of vessel-adherent myeloperoxidase reflects plaque burden in patients with stable coronary artery disease. Atherosclerosis 2013, 231, 354–358. [Google Scholar] [CrossRef]
- Vita, J.A.; Brennan, M.-L.; Gokce, N.; Mann, S.A.; Goormastic, M.; Shishehbor, M.H.; Penn, M.S.; Keaney, J.F.; Hazen, S.L. Serum Myeloperoxidase Levels Independently Predict Endothelial Dysfunction in Humans. Circulation 2004, 110, 1134–1139. [Google Scholar] [CrossRef]
- Tiyerili, V.; Camara, B.; Becher, M.U.; Schrickel, J.W.; Lütjohann, D.; Mollenhauer, M.; Baldus, S.; Nickenig, G.; Andrié, R.P. Neutrophil-derived myeloperoxidase promotes atherogenesis and neointima formation in mice. Int. J. Cardiol. 2016, 204, 29–36. [Google Scholar] [CrossRef]
- Adam, M.; Gajdova, S.; Kolarova, H.; Kubala, L.; Lau, D.; Geisler, A.; Ravekes, T.; Rudolph, V.; Tsao, P.S.; Blankenberg, S.; et al. Red blood cells serve as intravascular carriers of myeloperoxidase. J. Mol. Cell. Cardiol. 2014, 74, 353–363. [Google Scholar] [CrossRef]
- Klinke, A.; Berghausen, E.; Friedrichs, K.; Molz, S.; Lau, D.; Remane, L.; Berlin, M.; Kaltwasser, C.; Adam, M.; Mehrkens, D.; et al. Myeloperoxidase aggravates pulmonary arterial hypertension by activation of vascular Rho-kinase. JCI Insight 2018, 3, e97530. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, A.R.; Abo-Aly, M.; Elsawalhy, E.; Campbell, C.; Ziada, K.M.; Abdel-Latif, A. Prognostic Role of Elevated Myeloperoxidase in Patients with Acute Coronary Syndrome: A Systemic Review and Meta-Analysis. Mediat. Inflamm. 2019, 2019, 2872607. [Google Scholar] [CrossRef]
- Hazell, L.J.; Stocker, R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem. J. 1993, 290, 165–172. [Google Scholar] [CrossRef]
- Podrez, E.A.; Schmitt, D.; Hoff, H.F.; Hazen, S.L. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J. Clin. Investig. 1999, 103, 1547–1560. [Google Scholar] [CrossRef]
- Podrez, E.A.; Febbraio, M.; Sheibani, N.; Schmitt, D.; Silverstein, R.L.; Hajjar, D.P.; Cohen, P.A.; Frazier, W.A.; Hoff, H.F.; Hazen, S.L. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Investig. 2000, 105, 1095–1108. [Google Scholar] [CrossRef]
- Frangie, C.; Daher, J. Role of myeloperoxidase in inflammation and atherosclerosis (Review). Biomed. Rep. 2022, 16, 53. [Google Scholar] [CrossRef]
- Battle, S.; Gogonea, V.; Willard, B.; Wang, Z.; Fu, X.; Huang, Y.; Graham, L.M.; Cameron, S.J.; Didonato, J.A.; Crabb, J.W.; et al. The pattern of apolipoprotein A-I lysine carbamylation reflects its lipidation state and the chemical environment within human atherosclerotic aorta. J. Biol. Chem. 2022, 298, 101832. [Google Scholar] [CrossRef]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 2001, 276, 41279–41287. [Google Scholar] [CrossRef]
- Rahilly-Tierney, C.R.; Spiro, A.; Vokonas, P.; Gaziano, J.M. Relation Between High-Density Lipoprotein Cholesterol and Survival to Age 85 Years in Men (from the VA Normative Aging Study). Am. J. Cardiol. 2011, 107, 1173–1177. [Google Scholar] [CrossRef]
- Newman, A.B.; Glynn, N.W.; Taylor, C.A.; Sebastiani, P.; Perls, T.T.; Mayeux, R.; Christensen, K.; Zmuda, J.M.; Barral, S.; Lee, J.H.; et al. Health and function of participants in the Long Life Family Study: A comparison with other cohorts. Aging 2011, 3, 63–76. [Google Scholar] [CrossRef]
- Park, J.S.; Cha, K.S.; Lee, H.W.; Oh, J.-H.; Choi, J.H.; Lee, H.C.; Hong, T.J.; Jeong, M.H.; Chae, S.C.; Kim, Y.J. Predictive and protective role of high-density lipoprotein cholesterol in acute myocardial infarction. Cardiol. J. 2013, 26, 176–185. [Google Scholar] [CrossRef]
- Sposito, A.C.; De Lima-Junior, J.C.; Moura, F.A.; Barreto, J.; Bonilha, I.; Santana, M.; Virginio, V.W.; Sun, L.; Carvalho, L.S.F.; Soares, A.A.S.; et al. Reciprocal Multifaceted Interaction Between HDL (High-Density Lipoprotein) and Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1550–1564. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell. Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef]
- Nagao, M.; Nakajima, H.; Toh, R.; Hirata, K.-I.; Ishida, T. Cardioprotective Effects of High-Density Lipoprotein Beyond its Anti-Atherogenic Action. J. Atheroscler. Thromb. 2018, 25, 985–993. [Google Scholar] [CrossRef]
- Tall, A.R. Plasma high density lipoproteins: Therapeutic targeting and links to atherogenic inflammation. Atherosclerosis 2018, 276, 39–43. [Google Scholar] [CrossRef]
- Kudinov, V.A.; Alekseeva, O.Y.; Torkhovskaya, T.I.; Baskaev, K.K.; Artyushev, R.I.; Saburina, I.N.; Markin, S.S. High-Density Lipoproteins as Homeostatic Nanoparticles of Blood Plasma. Int. J. Mol. Sci. 2020, 21, 8737. [Google Scholar] [CrossRef]
- Huang, R.; Silva, R.A.G.D.; Jerome, W.G.; Kontush, A.; Chapman, M.J.; Curtiss, L.K.; Hodges, T.J.; Davidson, W.S. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat. Struct. Mol. Biol. 2011, 18, 416–422. [Google Scholar] [CrossRef]
- Panzenboeck, U.; Raitmayer, S.; Reicher, H.; Lindner, H.; Glatter, O.; Malle, E.; Sattler, W. Effects of Reagent and Enzymatically Generated Hypochlorite on Physicochemical and Metabolic Properties of High Density Lipoproteins. J. Biol. Chem. 1997, 272, 29711–29720. [Google Scholar] [CrossRef]
- Marsche, G.; Hammer, A.; Oskolkova, O.; Kozarsky, K.F.; Sattler, W.; Malle, E. Hypochlorite-modified High Density Lipoprotein, a High Affinity Ligand to Scavenger Receptor Class B, Type I, Impairs High Density Lipoprotein-dependent Selective Lipid Uptake and Reverse Cholesterol Transport. J. Biol. Chem. 2002, 277, 32172–32179. [Google Scholar] [CrossRef]
- Bergt, C.; Pennathur, S.; Fu, X.; Byun, J.; O’Brien, K.; Mcdonald, T.O.; Singh, P.; Anantharamaiah, G.M.; Chait, A.; Brunzell, J.; et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA 2004, 101, 13032–13037. [Google Scholar] [CrossRef]
- Shao, B.; Pennathur, S.; Heinecke, J.W. Myeloperoxidase Targets Apolipoprotein A-I, the Major High Density Lipoprotein Protein, for Site-Specific Oxidation in Human Atherosclerotic Lesions. J. Biol. Chem. 2012, 287, 6375–6386. [Google Scholar] [CrossRef]
- Shao, B.; Tang, C.; Sinha, A.; Mayer, P.S.; Davenport, G.D.; Brot, N.; Oda, M.N.; Zhao, X.-Q.; Heinecke, J.W. Humans with Atherosclerosis Have Impaired ABCA1 Cholesterol Efflux and Enhanced High-Density Lipoprotein Oxidation by Myeloperoxidase. Circ. Res. 2014, 114, 1733–1742. [Google Scholar] [CrossRef]
- Shao, B.; Bergt, C.; Fu, X.; Green, P.; Voss, J.C.; Oda, M.N.; Oram, J.F.; Heinecke, J.W. Tyrosine 192 in Apolipoprotein A-I Is the Major Site of Nitration and Chlorination by Myeloperoxidase, but Only Chlorination Markedly Impairs ABCA1-dependent Cholesterol Transport. J. Biol. Chem. 2005, 280, 5983–5993. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Settle, M.; Brubaker, G.; Schmitt, D.; Hazen, S.L.; Smith, J.D.; Kinter, M. Localization of Nitration and Chlorination Sites on Apolipoprotein A-I Catalyzed by Myeloperoxidase in Human Atheroma and Associated Oxidative Impairment in ABCA1-dependent Cholesterol Efflux from Macrophages. J. Biol. Chem. 2005, 280, 38–47. [Google Scholar] [CrossRef]
- Undurti, A.; Huang, Y.; Lupica, J.A.; Smith, J.D.; DiDonato, J.A.; Hazen, S.L. Modification of High Density Lipoprotein by Myeloperoxidase Generates a Pro-inflammatory Particle. J. Biol. Chem. 2009, 284, 30825–30835. [Google Scholar] [CrossRef]
- Hewing, B.; Parathath, S.; Barrett, T.; Chung, W.K.K.; Astudillo, Y.M.; Hamada, T.; Ramkhelawon, B.; Tallant, T.C.; Yusufishaq, M.S.S.; Didonato, J.A.; et al. Effects of Native and Myeloperoxidase-Modified Apolipoprotein A-I on Reverse Cholesterol Transport and Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 779–789. [Google Scholar] [CrossRef]
- Kameda, T.; Ohkawa, R.; Yano, K.; Usami, Y.; Miyazaki, A.; Matsuda, K.; Kawasaki, K.; Sugano, M.; Kubota, T.; Tozuka, M. Effects of Myeloperoxidase-Induced Oxidation on Antiatherogenic Functions of High-Density Lipoprotein. J. Lipids 2015, 2015, 592594. [Google Scholar] [CrossRef]
- Zhou, B.; Zu, L.; Chen, Y.; Zheng, X.; Wang, Y.; Pan, B.; Dong, M.; Zhou, E.; Zhao, M.; Zhang, Y.; et al. Myeloperoxidase-oxidized high density lipoprotein impairs atherosclerotic plaque stability by inhibiting smooth muscle cell migration. Lipids Health Dis. 2017, 16, 3. [Google Scholar] [CrossRef]
- Zhang, R.Y.K.; Cochran, B.J.; Thomas, S.R.; Rye, K.A. Impact of Reperfusion on Temporal Immune Cell Dynamics after Myocardial Infarction. J. Am. Heart Assoc. 2023, 12, e027600. [Google Scholar] [CrossRef]
- Rudolph, V.; Steven, D.; Gehling, U.M.; Goldmann, B.; Rudolph, T.K.; Friedrichs, K.; Meinertz, T.; Heitzer, T.; Baldus, S. Coronary plaque injury triggers neutrophil activation in patients with coronary artery disease. Free Radic. Biol. Med. 2006, 42, 460–465. [Google Scholar] [CrossRef]
- Ferrante, G.; Nakano, M.; Prati, F.; Niccoli, G.; Mallus, M.T.; Ramazzotti, V.; Montone, R.A.; Kolodgie, F.D.; Virmani, R.; Crea, F. High Levels of Systemic Myeloperoxidase Are Associated with Coronary Plaque Erosion in Patients with Acute Coronary Syndromes. Circulation 2010, 122, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Noonan, J.; Bobik, A.; Peter, K. The tandem stenosis mouse model: Towards understanding, imaging, and preventing atherosclerotic plaque instability and rupture. Br. J. Pharmacol. 2022, 179, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, K.; Kolarova, H.; Kerkenpaß, C.; Mollenhauer, M.; Vitecek, J.; Rudolph, V.; Kubala, L.; Baldus, S.; Adam, M.; Klinke, A. MPO (Myeloperoxidase) Reduces Endothelial Glycocalyx Thickness Dependent on Its Cationic Charge. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1859–1867. [Google Scholar] [CrossRef]
- Teo, A.; Chan, L.L.Y.; Cheung, C.; Chia, P.Y.; Ong, S.W.X.; Fong, S.W.; Ng, L.F.P.; Renia, L.; Lye, D.C.; Young, B.E.; et al. Myeloperoxidase inhibition may protect against endothelial glycocalyx shedding induced by COVID-19 plasma. Commun. Med. 2023, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, D.L.; Roberts, E.; Grattendick, K.; Schwab, C.; Stuart, R.; Lincoln, J.; Allen, R.C.; Moguilevsky, N.; Bollen, A.; Lefkowitz, S.S. The Endothelium and Cytokine Secretion: The Role of Peroxidases as Immunoregulators. Cell. Immunol. 2000, 202, 23–30. [Google Scholar] [CrossRef]
- Etwebi, Z.; Landesberg, G.; Preston, K.; Eguchi, S.; Scalia, R. Mechanistic Role of the Calcium-Dependent Protease Calpain in the Endothelial Dysfunction Induced by MPO (Myeloperoxidase). Hypertension 2018, 71, 761–770. [Google Scholar] [CrossRef]
- Lau, D.; Mollnau, H.; Eiserich, J.P.; Freeman, B.A.; Daiber, A.; Gehling, U.M.; Brümmer, J.; Rudolph, V.; Münzel, T.; Heitzer, T.; et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. USA 2005, 102, 431–436. [Google Scholar] [CrossRef]
- El Kebir, D.; JόZsef, L.; Pan, W.; Filep, J.N.G. Myeloperoxidase Delays Neutrophil Apoptosis through CD11b/CD18 Integrins and Prolongs Inflammation. Circ. Res. 2008, 103, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, D.L.; Lincoln, J.; Lefkowitz, S.S.; Bollen, A.; Moguilevsky, N. Enhancement of macrophage-mediated bactericidal activity by macrophage-mannose receptor-ligand interaction. Immunol. Cell Biol. 1997, 75, 136–141. [Google Scholar] [CrossRef]
- Lefkowitz, D.L.; Mills, K.; Morgan, D.; Lefkowitz, S.S. Macrophage activation and immunomodulation by myeloperoxidase. Proc. Soc. Exp. Biol. Med. 1992, 199, 204–210. [Google Scholar] [CrossRef]
- Lefkowitz, D.L.; Mills, K.; Moguilevsky, N.; Bollen, A.; Vaz, A.; Lefkowitz, S.S. Regulation of macrophage function by human recombinant myeloperoxidase. Immunol. Lett. 1993, 36, 43–49. [Google Scholar] [CrossRef]
- Schloss, M.J.; Horckmans, M.; Nitz, K.; Duchene, J.; Drechsler, M.; Bidzhekov, K.; Scheiermann, C.; Weber, C.; Soehnlein, O.; Steffens, S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol. Med. 2016, 8, 937–948. [Google Scholar] [CrossRef]
- Kyne, L.; Hausdorff, J.M.; Knight, E.; Dukas, L.; Azhar, G.; Wei, J.Y. Neutrophilia and congestive heart failure after acute myocardial infarction. Am. Heart J. 2000, 139, 94–100. [Google Scholar] [CrossRef]
- Ma, Y. Role of Neutrophils in Cardiac Injury and Repair Following Myocardial Infarction. Cells 2021, 10, 1676. [Google Scholar] [CrossRef]
- Zhang, S.; Wan, Z.; Zhang, Y.; Fan, Y.; Gu, W.; Li, F.; Meng, L.; Zeng, X.; Han, D.; Li, X. Neutrophil count improves the GRACE risk score prediction of clinical outcomes in patients with ST-elevation myocardial infarction. Atherosclerosis 2015, 241, 723–728. [Google Scholar] [CrossRef]
- Dentali, F.; Nigro, O.; Squizzato, A.; Gianni, M.; Zuretti, F.; Grandi, A.M.; Guasti, L. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: A systematic review and meta-analysis of the literature. Int. J. Cardiol. 2018, 266, 31–37. [Google Scholar] [CrossRef]
- Praetner, M.; Zuchtriegel, G.; Holzer, M.; Uhl, B.; Schaubächer, J.; Mittmann, L.; Fabritius, M.; Fürst, R.; Zahler, S.; Funken, D.; et al. Plasminogen Activator Inhibitor-1 Promotes Neutrophil Infiltration and Tissue Injury on Ischemia–Reperfusion. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 829–842. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Weng, X.; Zeng, S.; Yu, L.; Guo, J.; Xu, Y. Brg1 deficiency in vascular endothelial cells blocks neutrophil recruitment and ameliorates cardiac ischemia-reperfusion injury in mice. Int. J. Cardiol. 2018, 269, 250–258. [Google Scholar] [CrossRef]
- Vajen, T.; Koenen, R.R.; Werner, I.; Staudt, M.; Projahn, D.; Curaj, A.; Sönmez, T.T.; Simsekyilmaz, S.; Schumacher, D.; Möllmann, J.; et al. Blocking CCL5-CXCL4 heteromerization preserves heart function after myocardial infarction by attenuating leukocyte recruitment and NETosis. Sci. Rep. 2018, 8, 10647. [Google Scholar] [CrossRef]
- Li, W.; Feng, G.; Gauthier, J.M.; Lokshina, I.; Higashikubo, R.; Evans, S.; Liu, X.; Hassan, A.; Tanaka, S.; Cicka, M.; et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J. Clin. Investig. 2019, 129, 2293–2304. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wen, S.; Jiao, J.; Tang, T.; Zhao, X.; Zhang, M.; Lv, B.; Lu, Y.; Zhou, X.; Li, J.; et al. IL-21 promotes myocardial ischaemia/reperfusion injury through the modulation of neutrophil infiltration. Br. J. Pharmacol. 2018, 175, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.H.C.; Souza, D.G.; Teixeira, M.M.; Amaral, F.A. Tissue Dependent Role of PTX3 During Ischemia-Reperfusion Injury. Front. Immunol. 2019, 10, 433658. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, H.; Yang, C.; Liu, J.; Zhang, H.; Wang, J.; Hu, S.; Sun, Z.; He, K.; Chen, G. Mesenchymal Stem Cells Promote the Resolution of Cardiac Inflammation after Ischemia Reperfusion via Enhancing Efferocytosis of Neutrophils. J. Am. Heart Assoc. 2020, 9, e014397. [Google Scholar] [CrossRef]
- Qin, X.; Peterson, M.R.; Haller, S.E.; Cao, L.; Thomas, D.P.; He, G. Caspase recruitment domain-containing protein 9 (CARD9) knockout reduces regional ischemia/reperfusion injury through an attenuated inflammatory response. PLoS ONE 2018, 13, e0199711. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, S.; Ding, S.; Abudupataer, M.; Zhang, Z.; Zhu, X.; Zhang, W.; Zou, Y.; Yang, X.; Ge, J.; et al. Excessive Neutrophil Extracellular Trap Formation Aggravates Acute Myocardial Infarction Injury in Apolipoprotein E Deficiency Mice via the ROS-Dependent Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 1209307. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Grune, J.; Lewis, A.J.M.; Yamazoe, M.; Hulsmans, M.; Rohde, D.; Xiao, L.; Zhang, S.; Ott, C.; Calcagno, D.M.; Zhou, Y.; et al. Neutrophils incite and macrophages avert electrical storm after myocardial infarction. Nat. Cardiovasc. Res. 2022, 1, 649–664. [Google Scholar] [CrossRef]
- Ma, Y.; Yabluchanskiy, A.; Iyer, R.P.; Cannon, P.L.; Flynn, E.R.; Jung, M.; Henry, J.; Cates, C.A.; Deleon-Pennell, K.Y.; Lindsey, M.L. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 2016, 110, 51–61. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Lee, C.N.; Moreno-Luna, R.; Neumeyer, J.; Piekarski, B.; Zhou, P.; Moses, M.A.; Sachdev, M.; Pu, W.T.; Emani, S.; et al. Host non-inflammatory neutrophils mediate the engraftment of bioengineered vascular networks. Nat. Biomed. Eng. 2017, 1, 81. [Google Scholar] [CrossRef]
- Hristov, M.; Zernecke, A.; Bidzhekov, K.; Liehn, E.A.; Shagdarsuren, E.; Ludwig, A.; Weber, C. Importance of CXC Chemokine Receptor 2 in the Homing of Human Peripheral Blood Endothelial Progenitor Cells to Sites of Arterial Injury. Circ. Res. 2007, 100, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Voisin, M.B.; Leoni, G.; Woodfin, A.; Loumagne, L.; Patel, N.S.; Di Paola, R.; Cuzzocrea, S.; Thiemermann, C.; Perretti, M.; Nourshargh, S. Neutrophil elastase plays a non-redundant role in remodeling the venular basement membrane and neutrophil diapedesis post-ischemia/reperfusion injury. J. Pathol. 2019, 248, 88–102. [Google Scholar] [CrossRef]
- Horckmans, M.; Ring, L.; Duchene, J.; Santovito, D.; Schloss, M.J.; Drechsler, M.; Weber, C.; Soehnlein, O.; Steffens, S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 2017, 38, 187–197. [Google Scholar] [CrossRef]
- Wang, X.S.; Kim, H.B.; Szuchman-Sapir, A.; McMahon, A.; Dennis, J.M.; Witting, P.K. Neutrophils recruited to the myocardium after acute experimental myocardial infarct generate hypochlorous acid that oxidizes cardiac myoglobin. Arch. Biochem. Biophys. 2016, 612, 103–114. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Östberg, N.K.; Tamassia, N.; Soehnlein, O. Biological Roles of Neutrophil-Derived Granule Proteins and Cytokines. Trends Immunol. 2019, 40, 648–664. [Google Scholar] [CrossRef]
- Grabowski, P.; Hesse, S.; Hollizeck, S.; Rohlfs, M.; Behrends, U.; Sherkat, R.; Tamary, H.; Ünal, E.; Somech, R.; Patıroğlu, T.; et al. Proteome Analysis of Human Neutrophil Granulocytes from Patients with Monogenic Disease Using Data-independent Acquisition. Mol. Cell. Proteom. 2019, 18, 760–772. [Google Scholar] [CrossRef]
- Rieckmann, J.C.; Geiger, R.; Hornburg, D.; Wolf, T.; Kveler, K.; Jarrossay, D.; Sallusto, F.; Shen-Orr, S.S.; Lanzavecchia, A.; Mann, M.; et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat. Immunol. 2017, 18, 583–593. [Google Scholar] [CrossRef]
- Rørvig, S.; Østergaard, O.; Heegaard, N.H.H.; Borregaard, N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: Correlation with transcriptome profiling of neutrophil precursors. J. Leukoc. Biol. 2013, 94, 711–721. [Google Scholar] [CrossRef]
- Stankovic, S.; Asanin, M.; Majkic-Singh, N.; Ignjatovic, S.; Mihailovic, M.; Nikolajevic, I.; Mrdovic, I.; Matic, D.; Savic, L.; Marinkovic, J.; et al. The usefulness of myeloperoxidase in prediction of in-hospital mortality in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Clin. Lab. 2012, 58, 125–131. [Google Scholar]
- Mollenhauer, M.; Friedrichs, K.; Lange, M.; Gesenberg, J.; Remane, L.; Kerkenpaß, C.; Krause, J.; Schneider, J.; Ravekes, T.; Maass, M.; et al. Myeloperoxidase Mediates Postischemic Arrhythmogenic Ventricular Remodeling. Circ. Res. 2017, 121, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Karadag, B.; Vatan, B.; Hacioglu, Y.; Duman, D.; Baskurt, M.; Keles, I.; Ongen, Z.; Vural, V.A. Serum myeloperoxidase level predicts reperfusion in patients with myocardial infarction receiving thrombolytic therapy. Heart Vessel. 2009, 24, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Samimi-Fard, S.; Abreu-Gonzalez, P.; Garcia-Gonzalez, M.J.; Kaski, J.C. Prognostic value of admission myeloperoxidase levels in patients with ST-segment elevation myocardial infarction and cardiogenic shock. Am. J. Cardiol. 2008, 101, 1537–1540. [Google Scholar] [CrossRef]
- Kacprzak, M.; Zielinska, M. Prognostic value of myeloperoxidase concentration in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Int. J. Cardiol. 2016, 223, 452–457. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.X.; Wu, X.Y.; Cui, Y.; Zou, Z.H.; Liu, Y.; Gao, J. Correlation Analysis of Plasma Myeloperoxidase Level with Global Registry of Acute Coronary Events Score and Prognosis in Patients with Acute Non-ST-Segment Elevation Myocardial Infarction. Front. Med. 2022, 9, 828174. [Google Scholar] [CrossRef]
- Koch, C.; Henrich, M.; Heidt, M.C. Sequential Analysis of Myeloperoxidase for Prediction of Adverse Events after Suspected Acute Coronary Ischemia. Clin. Cardiol. 2014, 37, 744–749. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Iqbal, N.; Wu, Y.; Hazen, S.L. Usefulness of Cardiac Biomarker Score for Risk Stratification in Stable Patients Undergoing Elective Cardiac Evaluation Across Glycemic Status. Am. J. Cardiol. 2013, 111, 465–470. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, T.; Ni, J.; Zhang, R. The expression of myeloperoxidase in thrombi is associated with reduced heme oxygenase-1 induction and worse left ventricular remodeling in patients with acute ST-elevation myocardial infarction. Clin. Cardiol. 2021, 44, 357–363. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Brennan, M.-L.; Philip, K.; Tong, W.; Mann, S.; Van Lente, F.; Hazen, S.L. Plasma Myeloperoxidase Levels in Patients with Chronic Heart Failure. Am. J. Cardiol. 2006, 98, 796–799. [Google Scholar] [CrossRef]
- Rudolph, V.; Rudolph, T.K.; Hennings, J.C.; Blankenberg, S.; Schnabel, R.B.; Steven, D.; Haddad, M.; Knittel, K.; Wende, S.; Wenzel, J.; et al. Activation of polymorphonuclear neutrophils in patients with impaired left ventricular function. Free Radic. Biol. Med. 2007, 43, 1189–1196. [Google Scholar] [CrossRef]
- Rudolph, V.; Rudolph, T.K.; Kubala, L.; Clauberg, N.; Maas, R.; Pekarova, M.; Klinke, A.; Lau, D.; Szöcs, K.; Meinertz, T.; et al. A myeloperoxidase promoter polymorphism is independently associated with mortality in patients with impaired left ventricular function. Free Radic. Biol. Med. 2009, 47, 1584–1590. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Thukkani, A.K.; Martinson, B.D.; Albert, C.J.; Vogler, G.A.; Ford, D.A. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, 2955–2964. [Google Scholar] [CrossRef]
- Vasilyev, N.; Williams, T.; Brennan, M.-L.; Unzek, S.; Zhou, X.; Heinecke, J.W.; Spitz, D.R.; Topol, E.J.; Hazen, S.L.; Penn, M.S. Myeloperoxidase-Generated Oxidants Modulate Left Ventricular Remodeling but Not Infarct Size after Myocardial Infarction. Circulation 2005, 112, 2812–2820. [Google Scholar] [CrossRef]
- Askari, A.T.; Brennan, M.-L.; Zhou, X.; Drinko, J.; Morehead, A.; Thomas, J.D.; Topol, E.J.; Hazen, S.L.; Penn, M.S. Myeloperoxidase and Plasminogen Activator Inhibitor 1 Play a Central Role in Ventricular Remodeling after Myocardial Infarction. J. Exp. Med. 2003, 197, 615–624. [Google Scholar] [CrossRef]
- Rudolph, V.; Andrié, R.P.; Rudolph, T.K.; Friedrichs, K.; Klinke, A.; Hirsch-Hoffmann, B.; Schwoerer, A.P.; Lau, D.; Fu, X.; Klingel, K.; et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 2010, 16, 470–474. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Pulli, B.; Courties, G.; Tricot, B.; Sebas, M.; Iwamoto, Y.; Hilgendorf, I.; Schob, S.; Dong, A.; Zheng, W.; et al. Myeloperoxidase Inhibition Improves Ventricular Function and Remodeling after Experimental Myocardial Infarction. JACC Basic Transl. Sci. 2016, 1, 633–643. [Google Scholar] [CrossRef]
- Guthoff, H.; Hof, A.; Klinke, A.; Maaß, M.; Konradi, J.; Mehrkens, D.; Geißen, S.; Nettersheim, F.S.; Braumann, S.; Michaelsson, E.; et al. Protective Effects of Therapeutic Neutrophil Depletion and Myeloperoxidase Inhibition on Left Ventricular Function and Remodeling in Myocardial Infarction. Antioxidants 2022, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Kalász, J.; Pásztor, E.T.; Fagyas, M.; Balogh, Á.; Tóth, A.; Csató, V.; Édes, I.; Papp, Z.; Borbély, A. Myeloperoxidase impairs the contractile function in isolated human cardiomyocytes. Free Radic. Biol. Med. 2015, 84, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Hawkins, C.L.; Rayner, B.S. Characterization of the cellular effects of myeloperoxidase-derived oxidants on H9c2 cardiac myoblasts. Arch. Biochem. Biophys. 2019, 665, 132–142. [Google Scholar] [CrossRef]
- Konijnenberg, L.S.F.; Damman, P.; Duncker, D.J.; Kloner, R.A.; Nijveldt, R.; Van Geuns, R.-J.M.; Berry, C.; Riksen, N.P.; Escaned, J.; van Royen, N. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc. Res. 2020, 116, 787–805. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; van Royen, N.; Schulz, R.; Heusch, G. The coronary circulation in acute myocardial ischaemia/reperfusion injury: A target for cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef]
- Lekstrom-Himes, J.A.; Gallin, J.I. Immunodeficiency Diseases Caused by Defects in Phagocytes. N. Engl. J. Med. 2000, 343, 1703–1714. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; Macfadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Soubhye, J.; Furtmüller, P.G.; Dufrasne, F.; Obinger, C. Inhibition of Myeloperoxidase. Handb. Exp. Pharmacol. 2020, 264, 261–285. [Google Scholar] [CrossRef]
- Soubhye, J.; Van Antwerpen, P.; Dufrasne, F. A patent review of myeloperoxidase inhibitors for treating chronic inflammatory syndromes (focus on cardiovascular diseases, 2013–2019). Expert Opin. Ther. Pat. 2020, 30, 595–608. [Google Scholar] [CrossRef]
- Roth Flach, R.J.; Su, C.; Bollinger, E.; Cortes, C.; Robertson, A.W.; Opsahl, A.C.; Coskran, T.M.; Maresca, K.P.; Keliher, E.J.; Yates, P.D.; et al. Myeloperoxidase inhibition in mice alters atherosclerotic lesion composition. PLoS ONE 2019, 14, e0214150. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tumanov, S.; Kong, S.M.Y.; Cheng, D.; Michaëlsson, E.; Bongers, A.; Power, C.; Ayer, A.; Stocker, R. Therapeutic inhibition of MPO stabilizes pre-existing high risk atherosclerotic plaque. Redox Biol. 2022, 58, 102532. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Talib, J.; Stanley, C.P.; Rashid, I.; Michaëlsson, E.; Lindstedt, E.-L.; Croft, K.D.; Kettle, A.J.; Maghzal, G.J.; Stocker, R. Inhibition of MPO (Myeloperoxidase) Attenuates Endothelial Dysfunction in Mouse Models of Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1448–1457. [Google Scholar] [CrossRef]
- Piek, A.; Koonen, D.P.Y.; Schouten, E.-M.; Lindtstedt, E.L.; Michaëlsson, E.; De Boer, R.A.; Silljé, H.H.W. Pharmacological myeloperoxidase (MPO) inhibition in an obese/hypertensive mouse model attenuates obesity and liver damage, but not cardiac remodeling. Sci. Rep. 2019, 9, 18765. [Google Scholar] [CrossRef]
- Gurm, Z.; Seth, M.; Daher, E.; Pielsticker, E.; Qureshi, M.I.; Zainea, M.; Tucciarone, M.; Hanzel, G.; Henke, P.K.; Sukul, D. Prevalence of coronary risk factors in contemporary practice among patients undergoing their first percutaneous coronary intervention: Implications for primary prevention. PLoS ONE 2021, 16, e0250801. [Google Scholar] [CrossRef]
- De Villiers, C.; Riley, P.R. Mouse models of myocardial infarction: Comparing permanent ligation and ischaemia-reperfusion. Dis. Models Mech. 2020, 13, dmm046565. [Google Scholar] [CrossRef]
- Lugrin, J.; Parapanov, R.; Krueger, T.; Liaudet, L. Murine Myocardial Infarction Model using Permanent Ligation of Left Anterior Descending Coronary Artery. J. Vis. Exp. 2019, e59591. [Google Scholar] [CrossRef]
- Morton, J.; Bao, S.; Vanags, L.Z.; Tsatralis, T.; Ridiandries, A.; Siu, C.; Ng, K.; Tan, J.T.M.; Celermajer, D.S.; Ng, M.K.C.; et al. Strikingly Different Atheroprotective Effects of Apolipoprotein A-I in Early- Versus Late-Stage Atherosclerosis. JACC Basic Transl. Sci. 2018, 3, 187–199. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Lund, L.H.; Shah, S.J.; Voors, A.A.; Erlinge, D.; Saraste, A.; Pirazzi, C.; Grove, E.L.; Barasa, A.; Schou, M.; et al. Myeloperoxidase Inhibition in Heart Failure With Preserved or Mildly Reduced Ejection Fraction: SATELLITE Trial Results. J. Card. Fail. 2023, 30, 104–110. [Google Scholar] [CrossRef]
- Lund, L.H.; Lam, C.S.P.; Pizzato, P.E.; Gabrielsen, A.; Michaëlsson, E.; Nelander, K.; Ericsson, H.; Holden, J.; Folkvaljon, F.; Mattsson, A.; et al. Rationale and design of ENDEAVOR: A sequential phase 2b-3 randomized clinical trial to evaluate the effect of myeloperoxidase inhibition on symptoms and exercise capacity in heart failure with preserved or mildly reduced ejection fraction. Eur. J. Heart Fail. 2023, 25, 1696–1707. [Google Scholar] [CrossRef]
- Michaëlsson, E.; Lund, L.H.; Hage, C.; Shah, S.J.; Voors, A.A.; Saraste, A.; Redfors, B.; Grove, E.L.; Barasa, A.; Richards, A.M.; et al. Myeloperoxidase Inhibition Reverses Biomarker Profiles Associated With Clinical Outcomes in HFpEF. JACC Heart Fail. 2023, 11, 775–787. [Google Scholar] [CrossRef]
- Hage, C.; Michaëlsson, E.; Kull, B.; Miliotis, T.; Svedlund, S.; Linde, C.; Donal, E.; Daubert, J.C.; Gan, L.M.; Lund, L.H. Myeloperoxidase and related biomarkers are suggestive footprints of endothelial microvascular inflammation in HFpEF patients. ESC Heart Fail. 2020, 7, 1534–1546. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Tong, W.; Troughton, R.W.; Martin, M.G.; Shrestha, K.; Borowski, A.; Jasper, S.; Hazen, S.L.; Klein, A.L. Prognostic Value and Echocardiographic Determinants of Plasma Myeloperoxidase Levels in Chronic Heart Failure. J. Am. Coll. Cardiol. 2007, 49, 2364–2370. [Google Scholar] [CrossRef]
- Gan, L.M.; Lagerstrom-Fermer, M.; Ericsson, H.; Nelander, K.; Lindstedt, E.-L.; Michaëlsson, E.; Kjaer, M.; Heijer, M.; Whatling, C.; Fuhr, R. Safety, tolerability, pharmacokinetics and effect on serum uric acid of the myeloperoxidase inhibitor AZD4831 in a randomized, placebo-controlled, phase I study in healthy volunteers. Br. J. Clin. Pharmacol. 2019, 85, 762–770. [Google Scholar] [CrossRef]
- Nelander, K.; Lagerstrom-Fermer, M.; Amilon, C.; Michaëlsson, E.; Heijer, M.; Kjaer, M.; Russell, M.; Han, D.; Lindstedt, E.L.; Whatling, C.; et al. Early Clinical Experience With AZD4831, A Novel Myeloperoxidase Inhibitor, Developed for Patients With Heart Failure With Preserved Ejection Fraction. Clin. Transl. Sci. 2021, 14, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Jurva, U.; Weidolf, L.; Sandinge, A.S.; Leandersson, C.; Ekdahl, A.; Li, X.Q.; Antonsson, T.; Sundell, J.; Westerlund, K.; Amilon, C.; et al. Biotransformation of the Novel Myeloperoxidase Inhibitor AZD4831 in Preclinical Species and Humans. Drug Metab. Dispos. 2023, 51, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Inghardt, T.; Antonsson, T.; Ericsson, C.; Hovdal, D.; Johannesson, P.; Johansson, C.; Jurva, U.; Kajanus, J.; Kull, B.; Michaëlsson, E.; et al. Discovery of AZD4831, a Mechanism-Based Irreversible Inhibitor of Myeloperoxidase, As a Potential Treatment for Heart Failure with Preserved Ejection Fraction. J. Med. Chem. 2022, 65, 11485–11496. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Kp, M.M.J.; Chua, J.; Hernandez-Resendiz, S.; Liehn, E.A.; Knöll, R.; Gan, L.M.; Michaëlsson, E.; Jonsson, M.K.B.; Ryden-Markinhuhta, K.; et al. Inhibiting cardiac myeloperoxidase alleviates the relaxation defect in hypertrophic cardiomyocytes. Cardiovasc. Res. 2022, 118, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-L.; Zhang, G.-G.; Peng, J. Vascular peroxidase 1: A novel enzyme in promoting oxidative stress in cardiovascular system. Trends Cardiovasc. Med. 2013, 23, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Salerno, J.C.; Cao, Z.; Pagano, P.J.; Lambeth, J.D. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic. Biol. Med. 2008, 45, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, Z.; Zhang, G.; Thannickal, V.J.; Cheng, G. Vascular peroxidase 1 catalyzes the formation of hypohalous acids: Characterization of its substrate specificity and enzymatic properties. Free Radic. Biol. Med. 2012, 53, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Péterfi, Z.; Geiszt, M. Peroxidasins: Novel players in tissue genesis. Trends Biochem. Sci. 2014, 39, 305–307. [Google Scholar] [CrossRef]
- Bhave, G.; Cummings, C.F.; Vanacore, R.M.; Kumagai-Cresse, C.; Ero-Tolliver, I.A.; Rafi, M.; Kang, J.-S.; Pedchenko, V.; Fessler, L.I.; Fessler, J.H.; et al. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat. Chem. Biol. 2012, 8, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, K.; Liu, Z.; Xu, Q.; You, B.; Li, C.; Cao, J.; Zhou, H.; Li, Z.; Chen, J.; et al. VPO1 Modulates Vascular Smooth Muscle Cell Phenotypic Switch by Activating Extracellular Signal-regulated Kinase 1/2 (ERK 1/2) in Abdominal Aortic Aneurysms. J. Am. Heart Assoc. 2018, 7, e010069. [Google Scholar] [CrossRef]
- Wierer, M.; Prestel, M.; Schiller, H.B.; Yan, G.; Schaab, C.; Azghandi, S.; Werner, J.; Kessler, T.; Malik, R.; Murgia, M.; et al. Compartment-resolved Proteomic Analysis of Mouse Aorta during Atherosclerotic Plaque Formation Reveals Osteoclast-specific Protein Expression. Mol. Cell. Proteom. 2018, 17, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhang, G.-G.; You, B.; Cheng, G.; Chen, L.; Shi, R.-Z. The role of losartan in preventing vascular remodeling in spontaneously hypertensive rats by inhibition of the H2O2/VPO1/HOCl/MMPs pathway. Biochem. Biophys. Res. Commun. 2017, 493, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.-Z.; Hu, C.-P.; Yuan, Q.; Yang, T.; Peng, J.; Li, Y.; Bai, Y.; Cao, Z.; Cheng, G.; Zhang, G. Involvement of vascular peroxidase 1 in angiotensin II-induced vascular smooth muscle cell proliferation. Cardiovasc. Res. 2011, 91, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Q.; Yang, Q.; Cao, J.; Wu, C.; Peng, H.; Zhang, X.; Chen, J.; Cheng, G.; Wu, Y.; et al. Vascular peroxidase 1 is a novel regulator of cardiac fibrosis after myocardial infarction. Redox Biol. 2019, 22, 101151. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, G.-G.; Liu, Z.; Xu, Q.; Li, C.; Cheng, G.; Shi, R.-Z. Peroxidasin promotes diabetic vascular endothelial dysfunction induced by advanced glycation end products via NOX2/HOCl/Akt/eNOS pathway. Redox Biol. 2021, 45, 102031. [Google Scholar]
- Yang, L.; Bai, Y.; Li, N.-S.; Hu, C.-P.; Peng, J.; Cheng, G.; Zhang, G.-G.; Shi, R.-Z. Vascular VPO1 expression is related to the endothelial dysfunction in spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 2013, 439, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Bathish, B.; Turner, R.; Paumann-Page, M.; Kettle, A.J.; Winterbourn, C.C. Characterisation of peroxidasin activity in isolated extracellular matrix and direct detection of hypobromous acid formation. Arch. Biochem. Biophys. 2018, 646, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Rudolph, V.; Roiss, M.; Ito, W.D.; Rudolph, T.K.; Eiserich, J.P.; Sydow, K.; Lau, D.; Szöcs, K.; Klinke, A.; et al. Heparins Increase Endothelial Nitric Oxide Bioavailability by Liberating Vessel-Immobilized Myeloperoxidase. Circulation 2006, 113, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Junior, R.F.R.; Dabkowski, E.R.; Shekar, K.C.; Connell, K.A.O.; Hecker, P.A.; Murphy, M.P. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic. Biol. Med. 2018, 117, 18–29. [Google Scholar] [CrossRef]
- Park, M.; Nishimura, T.; Baeza-Garza, C.D.; Caldwell, S.T.; Pun, P.B.L.; Prag, H.A.; Young, T.; Sauchanka, O.; Logan, A.; Forkink, M.; et al. Confirmation of the Cardioprotective Effect of MitoGamide in the Diabetic Heart. Cardiovasc. Drugs Ther. 2020, 34, 823–834. [Google Scholar] [CrossRef]
- Adlam, V.J.; Harrison, J.C.; Porteous, C.M.; James, A.M.; Smith, R.A.J.; Murphy, M.P.; Sammut, I.A. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005, 19, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinn, M.; Zhang, R.Y.K.; Bello, I.; Rye, K.-A.; Thomas, S.R. Myeloperoxidase as a Promising Therapeutic Target after Myocardial Infarction. Antioxidants 2024, 13, 788. https://doi.org/10.3390/antiox13070788
Quinn M, Zhang RYK, Bello I, Rye K-A, Thomas SR. Myeloperoxidase as a Promising Therapeutic Target after Myocardial Infarction. Antioxidants. 2024; 13(7):788. https://doi.org/10.3390/antiox13070788
Chicago/Turabian StyleQuinn, Maxwell, Richard Y. K. Zhang, Idris Bello, Kerry-Anne Rye, and Shane R. Thomas. 2024. "Myeloperoxidase as a Promising Therapeutic Target after Myocardial Infarction" Antioxidants 13, no. 7: 788. https://doi.org/10.3390/antiox13070788
APA StyleQuinn, M., Zhang, R. Y. K., Bello, I., Rye, K.-A., & Thomas, S. R. (2024). Myeloperoxidase as a Promising Therapeutic Target after Myocardial Infarction. Antioxidants, 13(7), 788. https://doi.org/10.3390/antiox13070788






