Application of Antioxidant Compounds in Bone Defect Repair
Abstract
1. Introduction
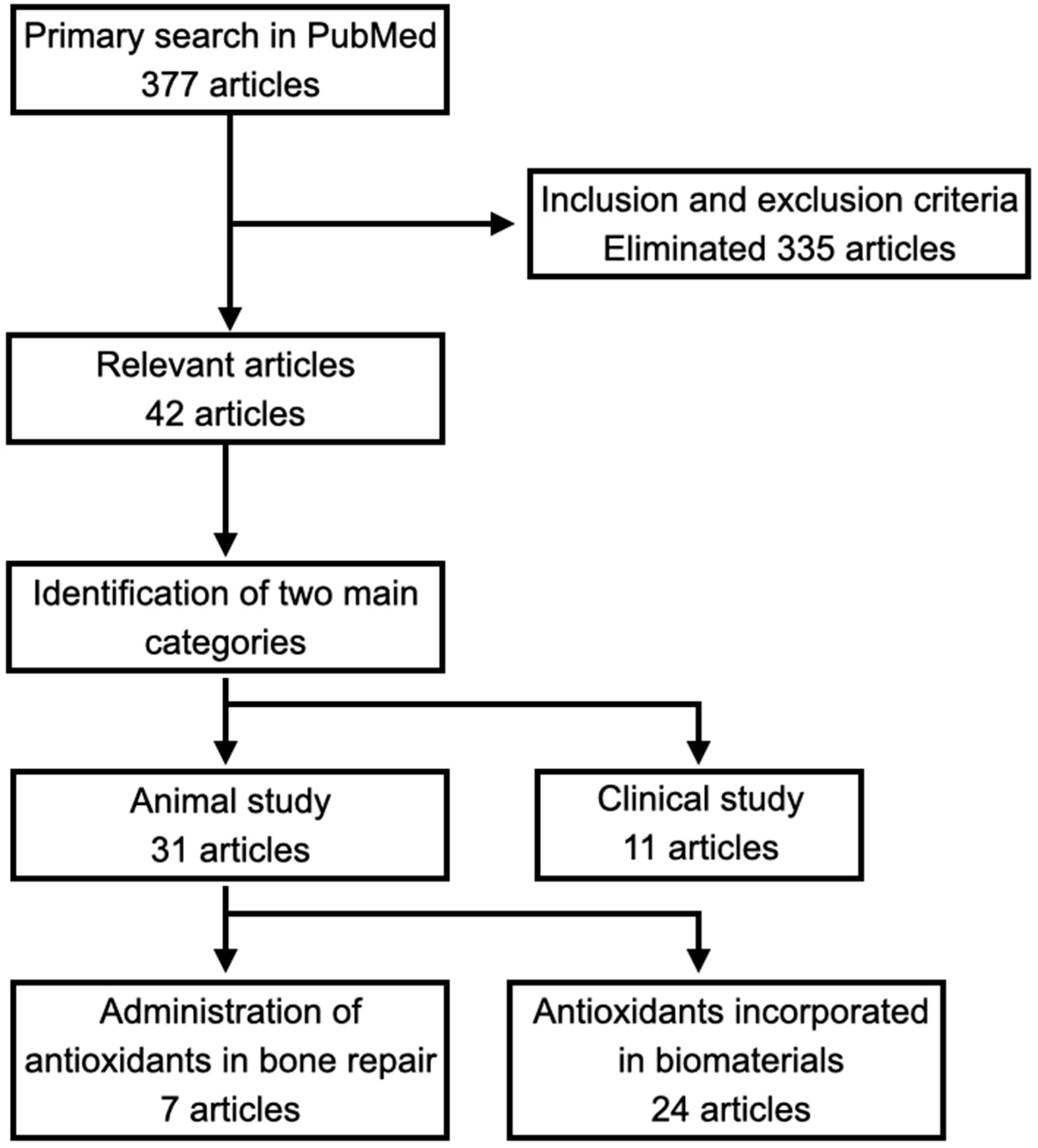
2. Methods
3. ROS and Oxidative Stress in Bone Repair Process
3.1. Inflammation Phase
3.2. Cartilage Callus Phase
3.3. Hard Bone Callus Phase
3.4. Remodeling Phase
3.5. Effects of ROS Induced by Systemic Inflammation on Bone Repair
3.6. Effects of NO (Nitric Oxide) and NO Synthase in Bone Repair
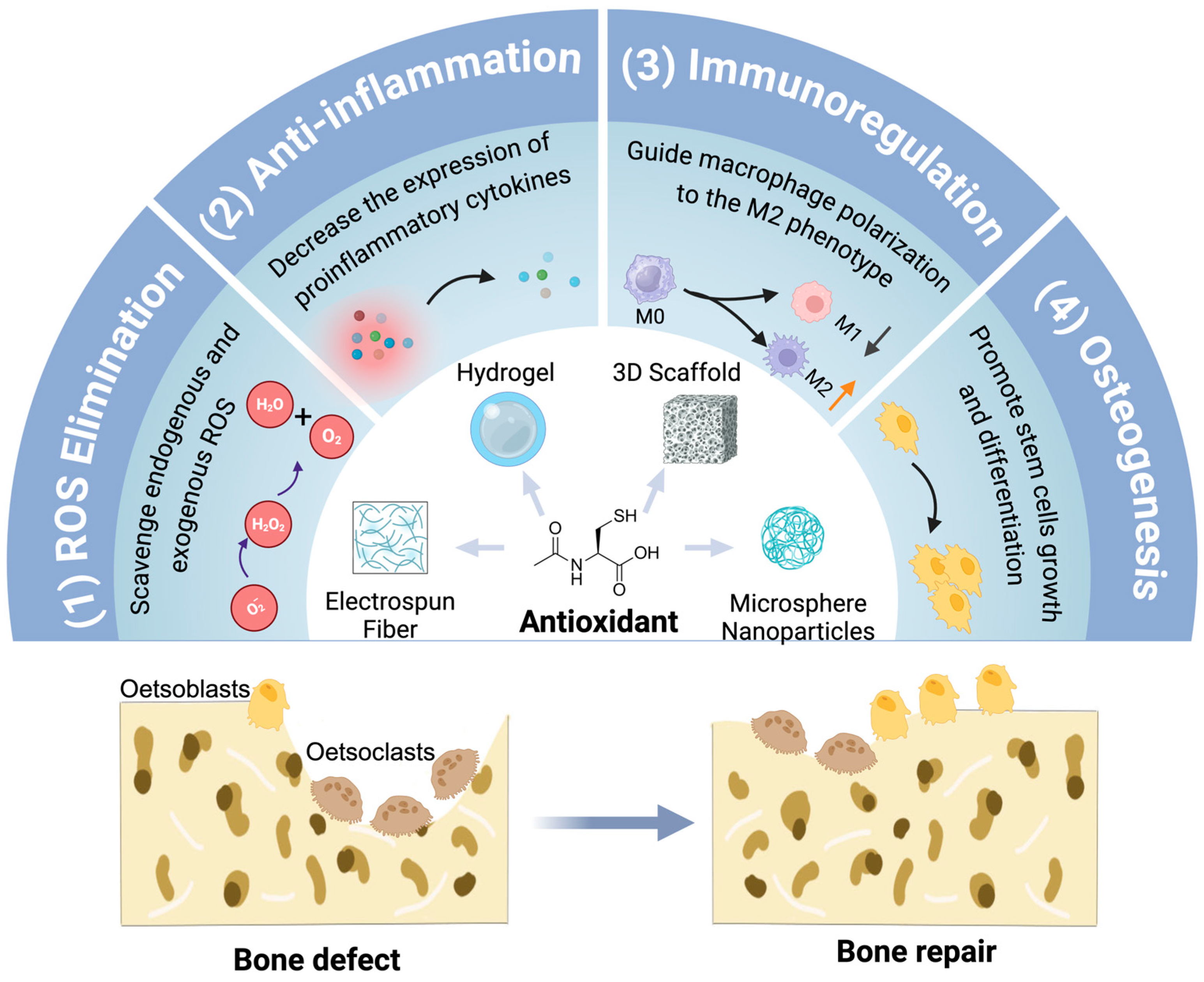
4. Classification of Antioxidants
4.1. Natural Antioxidants
4.2. Synthetic Antioxidants
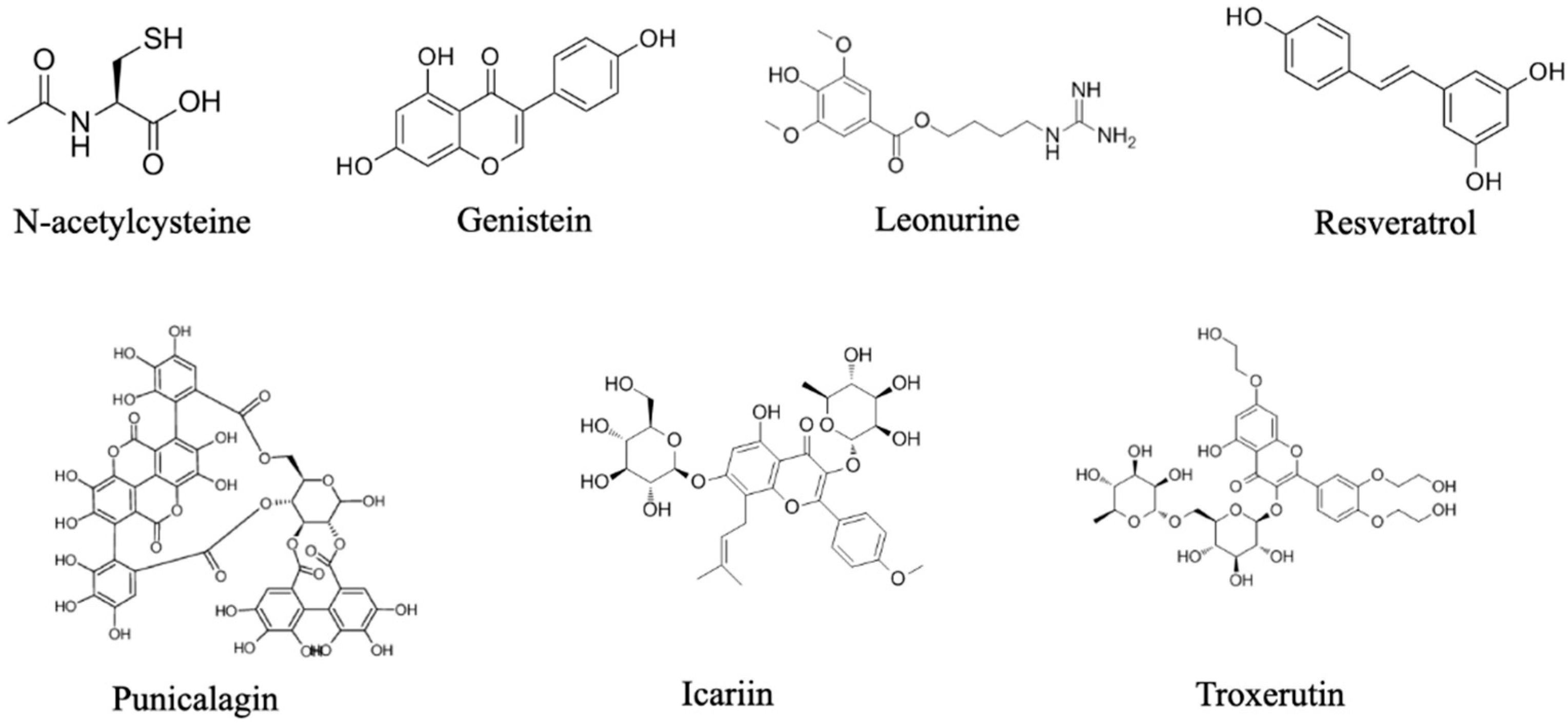
5. Administration of Antioxidants in Bone Repair
| Antioxidant Compound | Application Method | Animal Model | Biological Effects | Ref. |
|---|---|---|---|---|
| N-acetylcysteine | Topical injection | Alveolar defects in rats |
| [106] |
| Punicalagin | Topical injection | Femoral condyle defects in rats |
| [108] |
| Genistein | Intraperitoneal injection | Periodontitis bone defects in mice |
| [109] |
| Resveratrol | Subcutaneous injection | Periodontitis bone defects in rats |
| [112] |
| Leonurine | Intraperitoneal injection | Skull defects in rats |
| [113] |
| Troxerutin | Intramuscular injection | Femur defects in rats |
| [114] |
| Icariin | Intragastric administration | Fracture model in rats |
| [115] |
6. Antioxidants Incorporated in Biomaterials for Bone Repair
6.1. Hydrogels
6.2. 3D Scaffolds
6.3. Electrospun Fibers
6.4. Microspheres/Nanoparticles
7. The Role of ROS/Antioxidants in Human Skeletal System
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Huang, M.; Li, Z.; Shen, Z.; Feng, J.; Chen, H.; Wu, J.; Gao, J.; Wen, Z.; et al. Research hotspots and trends of bone defects based on Web of Science: A bibliometric analysis. J. Orthop. Surg. Res. 2020, 15, 463. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Int. J. Mol. Sci. 2024, 25, 3360. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Cerqueni, G.; Scalzone, A.; Licini, C.; Gentile, P.; Mattioli-Belmonte, M. Insights into oxidative stress in bone tissue and novel challenges for biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112433. [Google Scholar] [CrossRef]
- Huh, Y.J.; Kim, J.M.; Kim, H.; Song, H.; So, H.; Lee, S.Y.; Kwon, S.B.; Kim, H.J.; Kim, H.H.; Lee, S.H.; et al. Regulation of osteoclast differentiation by the redox-dependent modulation of nuclear import of transcription factors. Cell Death Differ. 2006, 13, 1138–1146. [Google Scholar] [CrossRef]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig. 2003, 112, 915–923. [Google Scholar] [CrossRef]
- Romagnoli, C.; Marcucci, G.; Favilli, F.; Zonefrati, R.; Mavilia, C.; Galli, G.; Tanini, A.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Role of GSH/GSSG redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like SaOS-2 cells. FEBS J. 2013, 280, 867–879. [Google Scholar] [CrossRef]
- Fontani, F.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: Involvement of JNK and ERK1/2 signalling. Calcif. Tissue Int. 2015, 96, 335–346. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Shi, J.; Yao, X.; Chen, W.; Wei, X.; Zhang, X.; Chu, P.K. Near-infrared light II—Assisted rapid biofilm elimination platform for bone implants at mild temperature. Biomaterials 2021, 269, 120634. [Google Scholar] [CrossRef]
- Guo, J.; Yang, X.; Chen, J.; Wang, C.; Kang, Y.; Jiang, T.; Chen, M.; Li, W.; Zhou, C.; Chen, Z. Accelerated Bone Regeneration by an Astaxanthin-Modified Antioxidant Aerogel through Relieving Oxidative Stress via the NRF2 Signaling Pathway. ACS Biomater. Sci. Eng. 2022, 8, 4524–4534. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, C.; Zhang, Z.; Zhang, Q.; Xu, Z.; Zhao, X.; Zhao, Y.; Cui, C.; Liu, W. Wet environment-induced adhesion and softening of coenzyme-based polymer elastic patch for treating periodontitis. Bioact. Mater. 2024, 35, 259–273. [Google Scholar] [CrossRef]
- Hagan, M.L.; Bahraini, A.; Pierce, J.L.; Bass, S.M.; Yu, K.; Elsayed, R.; Elsalanty, M.; Johnson, M.H.; McNeil, A.; McNeil, P.L.; et al. Inhibition of Osteocyte Membrane Repair Activity via Dietary Vitamin E Deprivation Impairs Osteocyte Survival. Calcif. Tissue Int. 2019, 104, 224–234. [Google Scholar] [CrossRef]
- Chen, M.; Li, M.; Wei, Y.; Xue, C.; Chen, M.; Fei, Y.; Tan, L.; Luo, Z.; Cai, K.; Hu, Y. ROS-activatable biomimetic interface mediates in-situ bioenergetic remodeling of osteogenic cells for osteoporotic bone repair. Biomaterials 2022, 291, 121878. [Google Scholar] [CrossRef]
- Agnes, J.P.; Santos, V.W.D.; das Neves, R.N.; Gonçalves, R.M.; Delgobo, M.; Girardi, C.S.; Lückemeyer, D.D.; Ferreira, M.A.; Macedo-Júnior, S.J.; Lopes, S.C.; et al. Antioxidants Improve Oxaliplatin-Induced Peripheral Neuropathy in Tumor-Bearing Mice Model: Role of Spinal Cord Oxidative Stress and Inflammation. J. Pain. 2021, 22, 996–1013. [Google Scholar] [CrossRef]
- Ye, Y.; Zhong, H.; Huang, S.; Lai, W.; Huang, Y.; Sun, C.; Zhang, Y.; Zheng, S. Reactive Oxygen Species Scavenging Hydrogel Regulates Stem Cell Behavior and Promotes Bone Healing in Osteoporosis. Tissue Eng. Regen. Med. 2023, 20, 981–992. [Google Scholar] [CrossRef]
- Tu, P.; Pan, Y.; Wang, L.; Li, B.; Sun, X.; Liang, Z.; Liu, M.; Zhao, Z.; Wu, C.; Wang, J.; et al. CD62E- and ROS-Responsive ETS Improves Cartilage Repair by Inhibiting Endothelial Cell Activation through OPA1-Mediated Mitochondrial Homeostasis. Biomater. Res. 2024, 28, 0006. [Google Scholar] [CrossRef]
- Lao, A.; Wu, J.; Li, D.; Shen, A.; Li, Y.; Zhuang, Y.; Lin, K.; Wu, J.; Liu, J. Functionalized Metal-Organic Framework-Modified Hydrogel That Breaks the Vicious Cycle of Inflammation and ROS for Repairing of Diabetic Bone Defects. Small 2023, 19, e2206919. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yang, S.; Ni, G.; Ji, J.; Luo, M.; Du, W. The Preparation and Effects of Organic-Inorganic Antioxidative Biomaterials for Bone Repair. Biomedicines 2023, 12, 70. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef]
- Tao, H.; Ge, G.; Liang, X.; Zhang, W.; Sun, H.; Li, M.; Geng, D. ROS signaling cascades: Dual regulations for osteoclast and osteoblast. Acta Biochim. Biophys. Sin. 2020, 52, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Toumi, H. Reactive oxygen species and exercise on bone metabolism: Friend or enemy? Jt. Bone Spine 2012, 79, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Ramos, A. In Sickness and in Health: The Oxygen Reactive Species and the Bone. Front. Bioeng. Biotechnol. 2021, 9, 745911. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef]
- Baht, G.S.; Vi, L.; Alman, B.A. The Role of the Immune Cells in Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 138–145. [Google Scholar] [CrossRef]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Tyurin, V.A.; Balasubramanian, K.; Winnica, D.; Tyurina, Y.Y.; Vikulina, A.S.; He, R.R.; Kapralov, A.A.; Macphee, C.H.; Kagan, V.E. Oxidatively modified phosphatidylserines on the surface of apoptotic cells are essential phagocytic ‘eat-me’ signals: Cleavage and inhibition of phagocytosis by Lp-PLA2. Cell Death Differ. 2014, 21, 825–835. [Google Scholar] [CrossRef]
- Fokam, D.; Hoskin, D. Instrumental role for reactive oxygen species in the inflammatory response. Front. Biosci. 2020, 25, 1110–1119. [Google Scholar] [CrossRef]
- Araźna, M.; Pruchniak, M.P.; Demkow, U. Reactive Oxygen Species, Granulocytes, and NETosis. Adv. Exp. Med. Biol. 2015, 836, 1–7. [Google Scholar] [CrossRef]
- Sônego, F.; Castanheira, F.V.; Ferreira, R.G.; Kanashiro, A.; Leite, C.A.; Nascimento, D.C.; Colón, D.F.; Borges Vde, F.; Alves-Filho, J.C.; Cunha, F.Q. Paradoxical Roles of the Neutrophil in Sepsis: Protective and Deleterious. Front. Immunol. 2016, 7, 155. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Xu, H.; Wang, C.; Liu, C.; Peng, Z.; Li, J.; Jin, Y.; Wang, Y.; Guo, J.; Zhu, L. Cotransplantation of mesenchymal stem cells and endothelial progenitor cells for treating steroid-induced osteonecrosis of the femoral head. Stem Cells Transl. Med. 2021, 10, 781–796. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, J.; Jiang, Q.; Zhao, Y.; Jiang, H.; Sun, P.; Feng, W. Identification and analysis of mitochondria-related central genes in steroid-induced osteonecrosis of the femoral head, along with drug prediction. Front. Endocrinol. 2024, 15, 1341366. [Google Scholar] [CrossRef]
- Chattopadhyay, R.; Raghavan, S.; Rao, G.N. Resolvin D1 via prevention of ROS-mediated SHP2 inactivation protects endothelial adherens junction integrity and barrier function. Redox Biol. 2017, 12, 438–455. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef]
- Aghajanian, P.; Mohan, S. The art of building bone: Emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018, 6, 19. [Google Scholar] [CrossRef]
- Portal-Núñez, S.; Esbrit, P.; Alcaraz, M.J.; Largo, R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem. Pharmacol. 2016, 108, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Dong, S. The Signaling Pathways Involved in Chondrocyte Differentiation and Hypertrophic Differentiation. Stem Cells Int. 2016, 2016, 2470351. [Google Scholar] [CrossRef]
- Jing, W.; Liu, C.; Su, C.; Liu, L.; Chen, P.; Li, X.; Zhang, X.; Yuan, B.; Wang, H.; Du, X. Role of reactive oxygen species and mitochondrial damage in rheumatoid arthritis and targeted drugs. Front. Immunol. 2023, 14, 1107670. [Google Scholar] [CrossRef]
- Zhang, S.; Li, T.; Feng, Y.; Zhang, K.; Zou, J.; Weng, X.; Yuan, Y.; Zhang, L. Exercise improves subchondral bone microenvironment through regulating bone-cartilage crosstalk. Front. Endocrinol. 2023, 14, 1159393. [Google Scholar] [CrossRef]
- Goldring, M.B. Integrin-dependent recruitment of Src to ROS-producing endosomes in osteoarthritic cartilage. Sci. Signal. 2023, 16, eadj9760. [Google Scholar] [CrossRef]
- Sánchez-de-Diego, C.; Valer, J.A.; Pimenta-Lopes, C.; Rosa, J.L.; Ventura, F. Interplay between BMPs and Reactive Oxygen Species in Cell Signaling and Pathology. Biomolecules 2019, 9, 534. [Google Scholar] [CrossRef]
- Nugud, A.; Sandeep, D.; El-Serafi, A.T. Two faces of the coin: Minireview for dissecting the role of reactive oxygen species in stem cell potency and lineage commitment. J. Adv. Res. 2018, 14, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Scammell, B.E.; Roach, H.I. A new role for the chondrocyte in fracture repair: Endochondral ossification includes direct bone formation by former chondrocytes. J. Bone Miner. Res. 1996, 11, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.L.; Morgan, E.F. Measurement of fracture callus material properties via nanoindentation. Acta Biomater. 2008, 4, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Turrubiates-Hernández, F.J.; Márquez-Sandoval, Y.F.; González-Estevez, G.; Reyes-Castillo, Z.; Muñoz-Valle, J.F. The Relevance of Selenium Status in Rheumatoid Arthritis. Nutrients 2020, 12, 3007. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.; Zhou, X.; Guo, J.; Zheng, C. Advances in the study of traditional Chinese medicine affecting bone metabolism through modulation of oxidative stress. Front. Pharmacol. 2023, 14, 1235854. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Sun, Y.; Tan, X.; Wang, C.; Wang, Z.; Ye, L. Multiscale design of stiffening and ROS scavenging hydrogels for the augmentation of mandibular bone regeneration. Bioact. Mater. 2023, 20, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Kanjwal, M.A.; Ghaferi, A.A. Graphene Incorporated Electrospun Nanofiber for Electrochemical Sensing and Biomedical Applications: A Critical Review. Sensors 2022, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Yahara, Y.; Nguyen, T.; Ishikawa, K.; Kamei, K.; Alman, B.A. The origins and roles of osteoclasts in bone development, homeostasis and repair. Development 2022, 149, dev199908. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Chen, Y.; Hashim, R.; He, C.; Mo, X.; Zhou, X. Reactive Oxygen Species-Based Biomaterials for Regenerative Medicine and Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2021, 9, 821288. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Mei, J.; Shao, D.; Zhou, F.; Qiao, H.; Liang, Y.; Li, K.; Tang, T. Cerium Oxide Nanoparticles Regulate Osteoclast Differentiation Bidirectionally by Modulating the Cellular Production of Reactive Oxygen Species. Int. J. Nanomed. 2020, 15, 6355–6372. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yuan, K.; Zhang, Q.; Guo, J.J.; Yang, H.; Zhou, F. Antioxidant PDA-PEG nanoparticles alleviate early osteoarthritis by inhibiting osteoclastogenesis and angiogenesis in subchondral bone. J. Nanobiotechnol. 2022, 20, 479. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Chen, Z.; Han, X.; Zhou, L.; Pang, F.; Wu, R.; Shen, Y.; He, X.; Hong, Z.; Li, Z.; et al. A novel RANKL-targeted flavonoid glycoside prevents osteoporosis through inhibiting NFATc1 and reactive oxygen species. Clin. Transl. Med. 2021, 11, e392. [Google Scholar] [CrossRef] [PubMed]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xiao, Q.; Li, X.; Chen, J. Advanced oxidation protein products aggravate age-related bone loss by increasing sclerostin expression in osteocytes via ROS-dependent downregulation of Sirt1. Int. J. Mol. Med. 2021, 47, 108. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, A.; Zhang, H.; Liu, B.; Geng, Y.; Xu, Y.; Zuo, G.; Jia, P. Iron overload induced osteocytes apoptosis and led to bone loss in Hepcidin(−/−) mice through increasing sclerostin and RANKL/OPG. Bone 2022, 164, 116511. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M.L. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants 2023, 12, 373. [Google Scholar] [CrossRef]
- Ru, J.Y.; Wang, Y.F. Osteocyte apoptosis: The roles and key molecular mechanisms in resorption-related bone diseases. Cell Death Dis. 2020, 11, 846. [Google Scholar] [CrossRef]
- Hu, X.F.; Wang, L.; Xiang, G.; Lei, W.; Feng, Y.F. Angiogenesis impairment by the NADPH oxidase-triggered oxidative stress at the bone-implant interface: Critical mechanisms and therapeutic targets for implant failure under hyperglycemic conditions in diabetes. Acta Biomater. 2018, 73, 470–487. [Google Scholar] [CrossRef]
- Sheng, N.; Xing, F.; Wang, J.; Zhang, Q.Y.; Nie, R.; Li-Ling, J.; Duan, X.; Xie, H.Q. Recent progress in bone-repair strategies in diabetic conditions. Mater. Today Bio 2023, 23, 100835. [Google Scholar] [CrossRef]
- Vrtačnik, P.; Zupan, J.; Mlakar, V.; Kranjc, T.; Marc, J.; Kern, B.; Ostanek, B. Epigenetic enzymes influenced by oxidative stress and hypoxia mimetic in osteoblasts are differentially expressed in patients with osteoporosis and osteoarthritis. Sci. Rep. 2018, 8, 16215. [Google Scholar] [CrossRef]
- Lee, K.E.; Mo, S.; Lee, H.S.; Jeon, M.; Song, J.S.; Choi, H.J.; Cho, H.; Kang, C.M. Deferoxamine Reduces Inflammation and Osteoclastogenesis in Avulsed Teeth. Int. J. Mol. Sci. 2021, 22, 8225. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.; Xie, S.; Liang, D.; Shi, W.; Chen, S.; Li, G.; Tang, W.; Liu, C.; He, Q. Local H(2) release remodels senescence microenvironment for improved repair of injured bone. Nat. Commun. 2023, 14, 7783. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Matteo, M.; Rollo, T.; De Rosario, F.; Greco, P.; Vendemiale, G.; Serviddio, G. Sex hormones modulate circulating antioxidant enzymes: Impact of estrogen therapy. Redox Biol. 2013, 1, 340–346. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Min. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial dysfunction in aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, A.S.; Torres, S.; Truong, T.; Moyer, E.L.; Kumar, A.; Tahimic, C.G.T.; Alwood, J.S.; Globus, R.K. Skeletal tissue regulation by catalase overexpression in mitochondria. Am. J. Physiol. Cell Physiol. 2020, 319, C734–C745. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Yu, Y.; Sharma, D.; Pruett-Miller, S.M.; Ren, Y.; Zhang, G.F.; Karner, C.M. Glutathione limits RUNX2 oxidation and degradation to regulate bone formation. JCI Insight 2023, 8, e166888. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- van’t Hof, R.J.; Ralston, S.H. Nitric oxide and bone. Immunology 2001, 103, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Yao, C.; Sun, J.; Zhang, B.; Chen, H.; Miao, J.; Zhang, Y. Alamandine attenuates ovariectomy-induced osteoporosis by promoting osteogenic differentiation via AMPK/eNOS axis. BMC Musculoskelet. Disord. 2024, 25, 45. [Google Scholar] [CrossRef] [PubMed]
- Afzal, F.; Polak, J.; Buttery, L. Endothelial nitric oxide synthase in the control of osteoblastic mineralizing activity and bone integrity. J. Pathol. 2004, 202, 503–510. [Google Scholar] [CrossRef]
- van’t Hof, R.J.; Armour, K.J.; Smith, L.M.; Armour, K.E.; Wei, X.Q.; Liew, F.Y.; Ralston, S.H. Requirement of the inducible nitric oxide synthase pathway for IL-1-induced osteoclastic bone resorption. Proc. Natl. Acad. Sci. USA 2000, 97, 7993–7998. [Google Scholar] [CrossRef]
- Watanuki, M.; Sakai, A.; Sakata, T.; Tsurukami, H.; Miwa, M.; Uchida, Y.; Watanabe, K.; Ikeda, K.; Nakamura, T. Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J. Bone Miner. Res. 2002, 17, 1015–1025. [Google Scholar] [CrossRef]
- van’t Hof, R.J.; Macphee, J.; Libouban, H.; Helfrich, M.H.; Ralston, S.H. Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology 2004, 145, 5068–5074. [Google Scholar] [CrossRef]
- Anastasio, A.T.; Paniagua, A.; Diamond, C.; Ferlauto, H.R.; Fernandez-Moure, J.S. Nanomaterial Nitric Oxide Delivery in Traumatic Orthopedic Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 592008. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Bansal, S.; Choudhary, S.; Sharma, M.; Kumar, S.S.; Lohan, S.; Bhardwaj, V.; Syan, N.; Jyoti, S. Tea: A native source of antimicrobial agents. Food Res. Int. 2013, 53, 568–584. [Google Scholar] [CrossRef]
- Ricardo, V.; Sousa, L.G.; Regalo, I.H.; Pitol, D.L.; Bombonato-Prado, K.F.; Regalo, S.C.H.; Siessere, S. Lycopene enhances bone neoformation in calvaria bone defects of ovariectomized rats. Braz. Dent. J. 2023, 34, 50–56. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sundquist, A.R. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann. N. Y. Acad. Sci. 1992, 669, 7–20. [Google Scholar] [CrossRef]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Moini, H.; Packer, L.; Saris, N.-E.L. Antioxidant and Prooxidant Activities of α-Lipoic Acid and Dihydrolipoic Acid. Toxicol. Appl. Pharmacol. 2002, 182, 84–90. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Naganuma, T.; Traversa, E. Stability of the Ce3+ valence state in cerium oxide nanoparticle layers. Nanoscale 2012, 4, 4950–4953. [Google Scholar] [CrossRef]
- Powell, S.R. The antioxidant properties of zinc. J. Nutr. 2000, 130, 1447s–1454s. [Google Scholar] [CrossRef]
- Bray, T.M.; Bettger, W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, J.; Feng, Z.; Guo, S.; Wang, M.; Wang, Z.; Li, Z.; Li, H.; Sui, L. N-acetylcysteine regulates dental follicle stem cell osteogenesis and alveolar bone repair via ROS scavenging. Stem Cell Res. Ther. 2022, 13, 466. [Google Scholar] [CrossRef]
- Tangtrongsup, S.; Kisiday, J.D. Differential Effects of the Antioxidants N-Acetylcysteine and Pyrrolidine Dithiocarbamate on Mesenchymal Stem Cell Chondrogenesis. Cell Mol. Bioeng. 2019, 12, 153–163. [Google Scholar] [CrossRef]
- Huang, L.; Lu, S.; Bian, M.; Wang, J.; Yu, J.; Ge, J.; Zhang, J.; Xu, Q. Punicalagin attenuates TNF-alpha-induced oxidative damage and promotes osteogenic differentiation of bone mesenchymal stem cells by activating the Nrf2/HO-1 pathway. Exp. Cell Res. 2023, 430, 113717. [Google Scholar] [CrossRef]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Anti-inflammatory, anti-osteoclastic, and antioxidant activities of genistein protect against alveolar bone loss and periodontal tissue degradation in a mouse model of periodontitis. J. Biomed. Mater. Res. A 2017, 105, 2510–2521. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Clemente-Napimoga, J.T.; Macedo, C.G.; Freitas, F.F.; Stipp, R.N.; Pinho-Ribeiro, F.A.; Casagrande, R.; Verri, W.A., Jr. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J. Nat. Prod. 2013, 76, 2316–2321. [Google Scholar] [CrossRef]
- Balli, U.; Cetinkaya, B.O.; Keles, G.C.; Keles, Z.P.; Guler, S.; Sogut, M.U.; Erisgin, Z. Assessment of MMP-1, MMP-8 and TIMP-2 in experimental periodontitis treated with kaempferol. J. Periodontal Implant. Sci. 2016, 46, 84–95. [Google Scholar] [CrossRef]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef]
- Zhao, B.; Peng, Q.; Wang, D.; Zhou, R.; Wang, R.; Zhu, Y.; Qi, S. Leonurine Protects Bone Mesenchymal Stem Cells from Oxidative Stress by Activating Mitophagy through PI3K/Akt/mTOR Pathway. Cells 2022, 11, 1724. [Google Scholar] [CrossRef]
- Yang, X.; Shao, J.; Wu, X.M.; Pan, F.F.; Yang, S.A.; Pan, X.H.; Jin, A.M. Troxerutin Stimulates Osteoblast Differentiation of Mesenchymal Stem Cell and Facilitates Bone Fracture Healing. Front. Pharmacol. 2021, 12, 723145. [Google Scholar] [CrossRef]
- Xiang, S.; Zhao, L.; Tang, C.; Ling, L.; Xie, C.; Shi, Y.; Liu, W.; Li, X.; Cao, Y. Icariin inhibits osteoblast ferroptosis via Nrf2/HO-1 signaling and enhances healing of osteoporotic fractures. Eur. J. Pharmacol. 2024, 965, 176244. [Google Scholar] [CrossRef]
- Willson, T.M.; CharifsonPaul, S.; Baxter, A.D.; Geddie, N.G. Bone targeted drugs 1. Identification of heterocycles with hydroxyapatite affinity. Bioorg. Med. Chem. Lett. 1996, 6, 1043–1046. [Google Scholar] [CrossRef]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.; Wang, Y.; Zhao, B.; Zhang, Y.; Han, F.; Zheng, Z.; Hu, D. Curcumin pretreatment prevents hydrogen peroxide-induced oxidative stress through enhanced mitochondrial function and deactivation of Akt/Erk signaling pathways in rat bone marrow mesenchymal stem cells. Mol. Cell Biochem. 2018, 443, 37–45. [Google Scholar] [CrossRef]
- Tan, L.; Cao, Z.; Chen, H.; Xie, Y.; Yu, L.; Fu, C.; Zhao, W.; Wang, Y. Curcumin reduces apoptosis and promotes osteogenesis of human periodontal ligament stem cells under oxidative stress in vitro and in vivo. Life Sci. 2021, 270, 119125. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhou, Q.; Lu, Y.; Wei, Q.; Tang, H.; Zhang, D.; Liu, Z.; Wang, G.; Wu, D. ROS-scavenging hydrogel as protective carrier to regulate stem cells activity and promote osteointegration of 3D printed porous titanium prosthesis in osteoporosis. Front. Bioeng. Biotechnol. 2023, 11, 1103611. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Wu, T.; Shi, K.; Bei, Z.; Wang, M.; Chu, B.; Xu, K.; Pan, M.; Li, Y.; et al. An Injectable Thermosensitive Hydrogel Containing Resveratrol and Dexamethasone-Loaded Carbonated Hydroxyapatite Microspheres for the Regeneration of Osteoporotic Bone Defects. Small Methods 2024, 8, e2300843. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, G.; Liu, S.; Li, X.; Xu, K.; Liu, P.; Cai, K. A Multifunction Hydrogel-Coating Engineered Implant for Rescuing Biofilm Infection and Boosting Osseointegration by Macrophage-Related Immunomodulation. Adv. Healthc. Mater. 2023, 12, e2300722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xia, X.; Liu, J.; Hou, M.; Liu, Y.; Zhou, Z.; Xu, Y.; He, F.; Yang, H.; Zhang, Y.; et al. Cartilage-inspired self-assembly glycopeptide hydrogels for cartilage regeneration via ROS scavenging. Bioact. Mater. 2024, 32, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Niu, Y.; Zhang, H.; Ouyang, H.; Zhang, G.; Fu, Y. Baicalin Nanocomplexes with an In Situ-Forming Biomimetic Gel Implant for Repair of Calvarial Bone Defects via Localized Sclerostin Inhibition. ACS Appl. Mater. Interfaces 2023, 15, 9044–9057. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Huang, Z.; Liu, W.; Liu, Y.; He, X. Functional beta-TCP/MnO2/PCL artificial periosteum promoting osteogenic differentiation of BMSCs by reducing locally reactive oxygen species level. J. Biomed. Mater. Res. A 2023, 111, 1678–1691. [Google Scholar] [CrossRef]
- Wang, L.; Shen, M.; Hou, Q.; Wu, Z.; Xu, J.; Wang, L. 3D printing of reduced glutathione grafted gelatine methacrylate hydrogel scaffold promotes diabetic bone regeneration by activating PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2022, 222, 1175–1191. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Liu, L.; Wang, Y.; Ju, Y.; Zeng, C.; Lu, Z.; Xie, D.; Guo, J. Zinc-Based Tannin-Modified Composite Microparticulate Scaffolds with Balanced Antimicrobial Activity and Osteogenesis for Infected Bone Defect Repair. Adv. Healthc. Mater. 2023, 12, e2300303. [Google Scholar] [CrossRef]
- Wu, X.; Ding, J.; Xu, P.; Feng, X.; Wang, Z.; Zhou, T.; Tu, C.; Cao, W.; Xie, J.; Deng, L.; et al. A cell-free ROS-responsive hydrogel/oriented poly(lactide-co-glycolide) hybrid scaffold for reducing inflammation and restoring full-thickness cartilage defects in vivo. Biomed. Mater. 2021, 16, 064101. [Google Scholar] [CrossRef]
- Xie, X.; Pei, F.; Wang, H.; Tan, Z.; Yang, Z.; Kang, P. Icariin: A promising osteoinductive compound for repairing bone defect and osteonecrosis. J. Biomater. Appl. 2015, 30, 290–299. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Luo, Y.; Huang, Y.; Wu, G. Slowly Delivered Icariin/Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells to Promote the Healing of Calvarial Critical-Size Bone Defects. Stem Cells Int. 2016, 2016, 1416047. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gu, Q.; Chen, M.; Zhang, C.; Chen, S.; Zhao, J. Controlled delivery of icariin on small intestine submucosa for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Tian, J.; Kook, Y.J.; Thangavelu, M.; Choi, J.H.; Khang, G. A BMSCs-laden quercetin/duck’s feet collagen/hydroxyapatite sponge for enhanced bone regeneration. J. Biomed. Mater. Res. A 2020, 108, 784–794. [Google Scholar] [CrossRef]
- Song, J.E.; Tripathy, N.; Lee, D.H.; Park, J.H.; Khang, G. Quercetin Inlaid Silk Fibroin/Hydroxyapatite Scaffold Promotes Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2018, 10, 32955–32964. [Google Scholar] [CrossRef] [PubMed]
- Nadaf, A.; Gupta, A.; Hasan, N.; Fauziya; Ahmad, S.; Kesharwani, P.; Ahmad, F.J. Recent update on electrospinning and electrospun nanofibers: Current trends and their applications. RSC Adv. 2022, 12, 23808–23828. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Wang, Z.; Zhang, H.; Yuan, X.; Kong, D. Controlled Release of PDGF-bb by Coaxial Electrospun Dextran/Poly(L-lactide-co-ε-caprolactone) Fibers with an Ultrafine Core/Shell Structure. J. Biomater. Sci. Polym. Ed. 2010, 21, 803–819. [Google Scholar] [CrossRef]
- Gao, X.; Al-Baadani, M.A.; Wu, M.; Tong, N.; Shen, X.; Ding, X.; Liu, J. Study on the Local Anti-Osteoporosis Effect of Polaprezinc-Loaded Antioxidant Electrospun Membrane. Int. J. Nanomed. 2022, 17, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, J.S.; Lee, M.S.; An, S.; Yang, K.; Lee, K.; Yang, H.S.; Lee, H.; Cho, S.-W. Plant Flavonoid-Mediated Multifunctional Surface Modification Chemistry: Catechin Coating for Enhanced Osteogenesis of Human Stem Cells. Chem. Mater. 2017, 29, 4375–4384. [Google Scholar] [CrossRef]
- Raja, I.S.; Preeth, D.R.; Vedhanayagam, M.; Hyon, S.-H.; Lim, D.; Kim, B.; Rajalakshmi, S.; Han, D.-W. Polyphenols-loaded electrospun nanofibers in bone tissue engineering and regeneration. Biomater. Res. 2021, 25, 29. [Google Scholar] [CrossRef]
- Yin, L.; Wang, K.; Lv, X.; Sun, R.; Yang, S.; Yang, Y.; Liu, Y.; Liu, J.; Zhou, J.; Yu, Z. The fabrication of an ICA-SF/PLCL nanofibrous membrane by coaxial electrospinning and its effect on bone regeneration in vitro and in vivo. Sci. Rep. 2017, 7, 8616. [Google Scholar] [CrossRef]
- Pignatello, R.; Pecora, T.M.G.; Cutuli, G.G.; Catalfo, A.; De Guidi, G.; Ruozi, B.; Tosi, G.; Cianciolo, S.; Musumeci, T. Antioxidant activity and photostability assessment of trans-resveratrol acrylate microspheres. Pharm. Dev. Technol. 2019, 24, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qiu, J.; Zheng, L.; Ren, N.; Li, J.; Liu, H.; Miao, J. Sustained delivery of BMP-2 enhanced osteoblastic differentiation of BMSCs based on surface hydroxyapatite nanostructure in chitosan–HAp scaffold. J. Biomater. Sci. Polym. Ed. 2014, 25, 1813–1827. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Ma, S.; Fu, M.; Wu, M.; Li, J.; Wu, K.; Zhuang, X.; Lu, Z.; Guo, J. Injective Programmable Proanthocyanidin-Coordinated Zinc-Based Composite Hydrogel for Infected Bone Repair. Adv. Healthc. Mater. 2024, 13, e2302690. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, D.; Huangfu, H.; Chen, S.; Qin, Q.; Ren, S.; Zhang, Y.; Fu, L.; Zhou, Y. Highly efficient photothermal branched Au-Ag nanoparticles containing procyanidins for synergistic antibacterial and anti-inflammatory immunotherapy. Biomater. Sci. 2023, 11, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Yu, Y.; Liu, H.; Li, X.; Sun, W.; Wu, W.; Liu, C.; Miao, L. Remodeling the periodontitis microenvironment for osteogenesis by using a reactive oxygen species-cleavable nanoplatform. Acta Biomater. 2021, 135, 593–605. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, L.; Zhou, Y.; Ma, W.; Zhang, N.; Chang, J.; Lin, K.; Xu, Y.; Jiang, X. Evaluation of osteogenesis and angiogenesis of icariin loaded on micro/nano hybrid structured hydroxyapatite granules as a local drug delivery system for femoral defect repair. J. Mater. Chem. B 2015, 3, 4871–4883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, Y.; Ma, W.; Jiang, X.; Takemra, A.; Uemura, M.; Xia, L.; Lin, K.; Xu, Y. The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. J. Mater. Chem. B 2017, 5, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Gonde, N.P.; Rathod, S.R.; Kolte, A.P. Comparative evaluation of 1% melatonin gel in the treatment of intrabony defect: A randomized controlled clinical trial. J. Periodontol. 2022, 93, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S.; et al. Lycopene and bone: An in vitro investigation and a pilot prospective clinical study. J. Transl. Med. 2020, 18, 43. [Google Scholar] [CrossRef]
- Mackinnon, E.S.; Rao, A.V.; Josse, R.G.; Rao, L.G. Supplementation with the antioxidant lycopene significantly decreases oxidative stress parameters and the bone resorption marker N-telopeptide of type I collagen in postmenopausal women. Osteoporos. Int. 2011, 22, 1091–1101. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Hossain, S.; Wakatsuki, H.; Tanabe, Y.; Ohno, M.; Kato, S.; Shido, O.; Hashimoto, M. Perilla seed oil improves bone health by inhibiting bone resorption in healthy Japanese adults: A 12-month, randomized, double-blind, placebo-controlled trial. Phytother. Res. 2023, 37, 2230–2241. [Google Scholar] [CrossRef]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation With Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Corbi, G.; Nobile, V.; Conti, V.; Cannavo, A.; Sorrenti, V.; Medoro, A.; Scapagnini, G.; Davinelli, S. Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial. Int. J. Mol. Sci. 2023, 24, 2063. [Google Scholar] [CrossRef]
- Ruiz-Ramos, M.; Vargas, L.A.; Fortoul Van der Goes, T.I.; Cervantes-Sandoval, A.; Mendoza-Nunez, V.M. Supplementation of ascorbic acid and alpha-tocopherol is useful to preventing bone loss linked to oxidative stress in elderly. J. Nutr. Health Aging 2010, 14, 467–472. [Google Scholar] [CrossRef]
- Vallibhakara, S.A.; Nakpalat, K.; Sophonsritsuk, A.; Tantitham, C.; Vallibhakara, O. Effect of Vitamin E Supplement on Bone Turnover Markers in Postmenopausal Osteopenic Women: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients 2021, 13, 4226. [Google Scholar] [CrossRef]
- Sanders, K.M.; Kotowicz, M.A.; Nicholson, G.C. Potential role of the antioxidant N-acetylcysteine in slowing bone resorption in early post-menopausal women: A pilot study. Transl. Res. 2007, 150, 215. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.J.; Kim, B.J.; Kim, S.R.; Chun, S.; Ryu, J.S.; Kim, G.S.; Lee, M.C.; Koh, J.M.; Chung, S.J. Homocysteine-lowering therapy or antioxidant therapy for bone loss in Parkinson’s disease. Mov. Disord. 2010, 25, 332–340. [Google Scholar] [CrossRef]
- Majidi, Z.; Ansari, M.; Maghbooli, Z.; Ghasemi, A.; Ebrahimi, S.S.S.; Hossein-Nezhad, A.; Emamgholipour, S. Oligopin® Supplementation Mitigates Oxidative Stress in Postmenopausal Women with Osteopenia: A Randomized, Double-blind, Placebo-Controlled Trial. Phytomedicine 2021, 81, 153417. [Google Scholar] [CrossRef]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef]
- Bai, L.; Tao, G.; Feng, M.; Xie, Y.; Cai, S.; Peng, S.; Xiao, J. Hydrogel Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2023, 15, 1334. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Akçan, R.; Aydogan, H.C.; Yildirim, M.; Taştekin, B.; Sağlam, N. Nanotoxicity: A challenge for future medicine. Turk. J. Med. Sci. 2020, 50, 1180–1196. [Google Scholar] [CrossRef]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.Z.; Zhang, Y.L.; Sun, W.J.; Zhu, Y.Q.; Cui, Y.; Qi, L. LC, a novel estrone-rhein hybrid compound, promotes proliferation and differentiation and protects against cell death in human osteoblastic MG-63 cells. Mol. Cell Endocrinol. 2011, 344, 59–68. [Google Scholar] [CrossRef]
- Carbone, E.J.; Rajpura, K.; Allen, B.N.; Cheng, E.; Ulery, B.D.; Lo, K.W. Osteotropic nanoscale drug delivery systems based on small molecule bone-targeting moieties. Nanomedicine 2017, 13, 37–47. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Sun, H.; Yu, M.; Wang, H.; Meng, Q.; Li, Z.; Liu, D.; Bai, J.; Liu, G.; et al. Transformation of arginine into zero-dimensional nanomaterial endows the material with antibacterial and osteoinductive activity. Sci. Adv. 2023, 9, eadf8645. [Google Scholar] [CrossRef]
- Su, Z.; Burchfield, J.G.; Yang, P.; Humphrey, S.J.; Yang, G.; Francis, D.; Yasmin, S.; Shin, S.Y.; Norris, D.M.; Kearney, A.L.; et al. Global redox proteome and phosphoproteome analysis reveals redox switch in Akt. Nat. Commun. 2019, 10, 5486. [Google Scholar] [CrossRef]
- Fu, G.; Xu, Q.; Qiu, Y.; Jin, X.; Xu, T.; Dong, S.; Wang, J.; Ke, Y.; Hu, H.; Cao, X.; et al. Suppression of Th17 cell differentiation by misshapen/NIK-related kinase MINK1. J. Exp. Med. 2017, 214, 1453–1469. [Google Scholar] [CrossRef]

| Biomaterials | Antioxidants | Animal Model | Biological Effects | Ref. |
|---|---|---|---|---|
| Hydrogel | Epigallocatechin-3-gallate (EGCG) | Bone defects in rabbits |
| [121] |
| Hydrogel | Resveratrol | Femoral defects in rats |
| [122] |
| Hydrogel | Baicalin | Calvarial defects in rats |
| [125] |
| Hydrogel | Tannic Acid | Implant-associated infection model in rats |
| [123] |
| Hydrogel | Fucoidan | Cartilage defects in rabbits |
| [124] |
| Hydrogel | Hyaluronic acid | Segmental bone defect models |
| [18] |
| 3D Scaffold | Glutathione | Calvarial defects in mice |
| [127] |
| 3D Scaffold | Tannic acid | Femoral condyle defects in rats |
| [128] |
| 3D Scaffold | Icariin | Calvarial defects in mice |
| [133] |
| 3D Scaffold | Quercetin | Calvarial defect in rats |
| [134] |
| 3D Scaffold | Icariin | Osteonecrosis of the femoral head in rabbits |
| [130] |
| 3D Scaffold | Icariin | Calvarial bone defects |
| [131] |
| 3D Scaffold | Icariin | Steroid-associated osteonecrosis in rabbits |
| [132] |
| 3D Scaffold | Quercetin | Calvarial defects in rats |
| [135] |
| Electrospun fiber | Polaprezinc | Calvarial bone defects |
| [138] |
| Electrospun fiber | Icariin | Calvarial defect in rats |
| [141] |
| Electrospun fiber | Catechin | Calvarial defects in mice |
| [139] |
| Electrospun fiber | Curcumin | Calvarial defects in mice |
| [140] |
| Microsphere | Proanthocyanidin | Femoral condyle defects in rats |
| [144] |
| Nanoparticle | Procyanidins | Periodontitis bone defects in rats |
| [145] |
| Nanoparticle | N-acetylcysteine | Periodontitis bone defects in rats |
| [146] |
| Microsphere | Resveratrol | Femoral defects in rats |
| [122] |
| Micro/nano hybrid structured granules | Icariin | Femoral plug defects in rats |
| [147] |
| Microsphere | Quercetin | Femur defects in rats |
| [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Y.; Tang, Q.; Zhang, Y.; Yin, Y.; Chen, L. Application of Antioxidant Compounds in Bone Defect Repair. Antioxidants 2024, 13, 789. https://doi.org/10.3390/antiox13070789
Wang J, Zhang Y, Tang Q, Zhang Y, Yin Y, Chen L. Application of Antioxidant Compounds in Bone Defect Repair. Antioxidants. 2024; 13(7):789. https://doi.org/10.3390/antiox13070789
Chicago/Turabian StyleWang, Jiajia, Yubing Zhang, Qingming Tang, Yinan Zhang, Ying Yin, and Lili Chen. 2024. "Application of Antioxidant Compounds in Bone Defect Repair" Antioxidants 13, no. 7: 789. https://doi.org/10.3390/antiox13070789
APA StyleWang, J., Zhang, Y., Tang, Q., Zhang, Y., Yin, Y., & Chen, L. (2024). Application of Antioxidant Compounds in Bone Defect Repair. Antioxidants, 13(7), 789. https://doi.org/10.3390/antiox13070789







