Growth, Antioxidant Capacity, and Liver Health in Largemouth Bass (Micropterus salmoides) Fed Multi-Strain Yeast-Based Paraprobiotic: A Lab-to-Pilot Scale Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diets
2.2. Experimental Conditions and Fish
2.3. Sampling
2.4. Hepatic Histology and Immunofluorescence
2.5. Hematological Assays and Antioxidative Activity Assays
2.6. Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Morphometrics
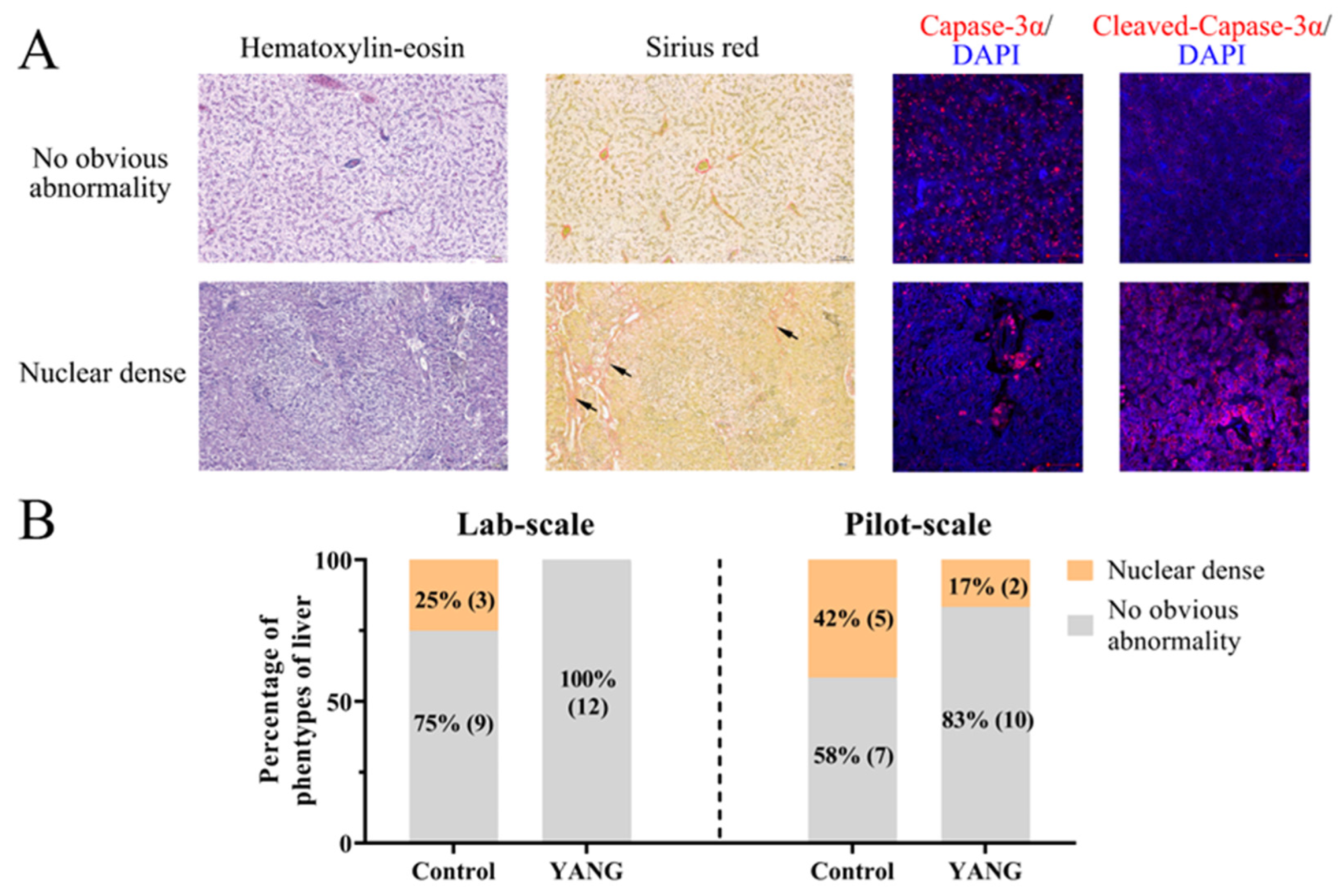
3.2. Liver Histological Examination
3.3. Plasma Liver Functions
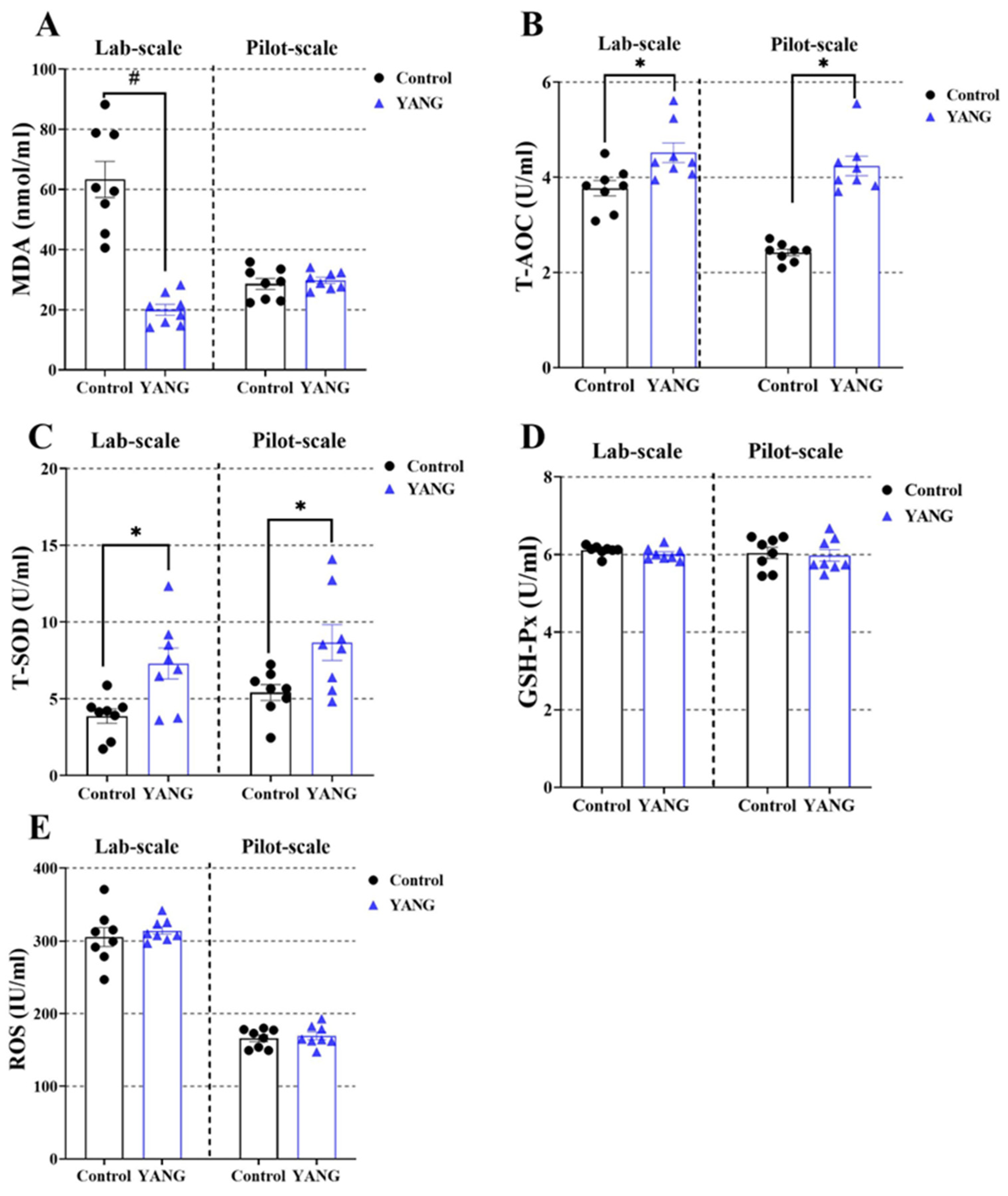
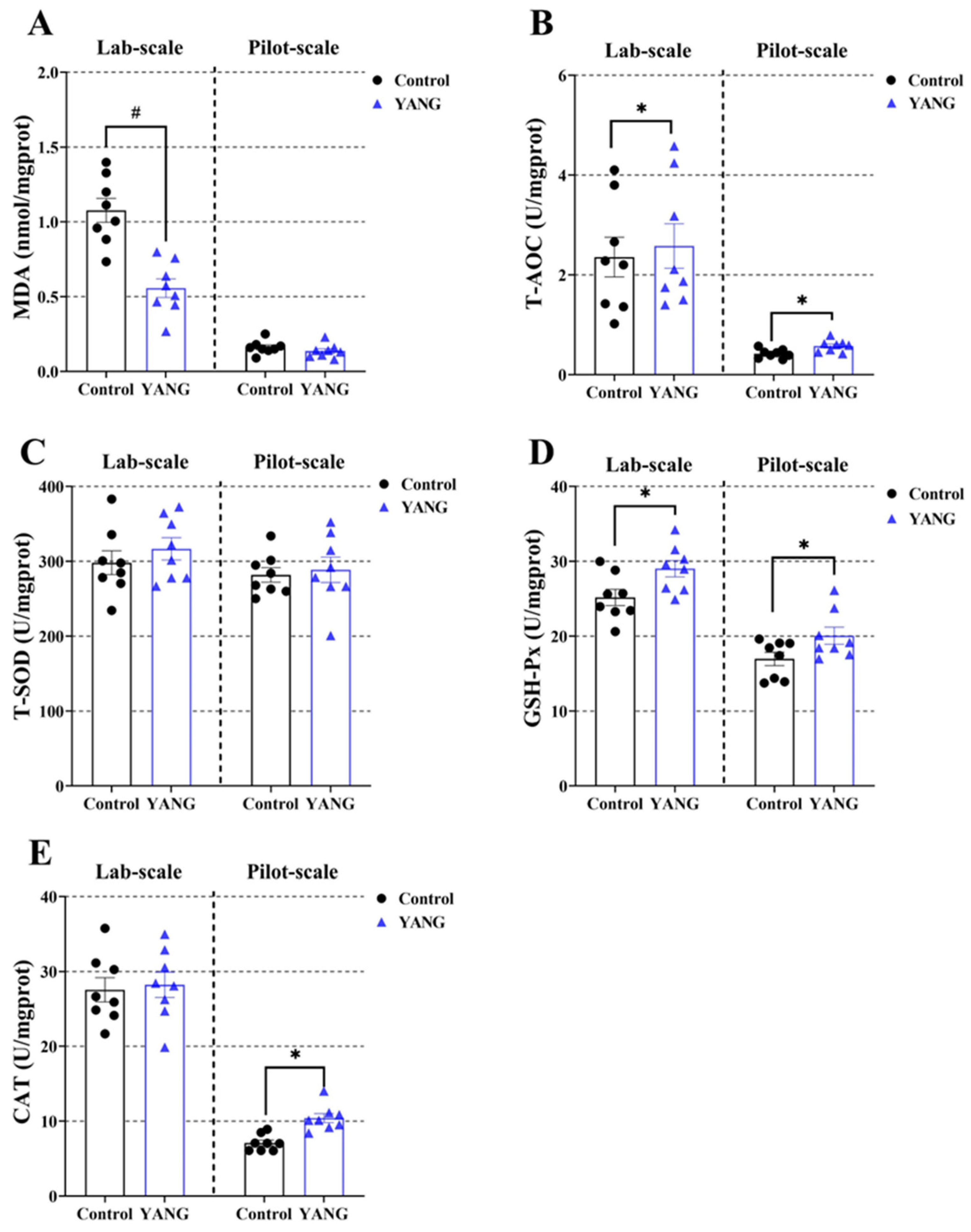
3.4. Plasma and Liver Antioxidation Parameters
3.5. Hepatic Antioxidation Relevant Genes Expression
3.6. Hepatic Immune Relevant Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Yearbook Fishery and Aquaculture Statistics 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; p. 10. [Google Scholar]
- Loc, V.T.T.; Bush, S.R.; Sinh, L.X.; Khiem, N.T. High and low value fish chains in the Mekong Delta: Challenges for livelihoods and governance. Environ. Dev. Sustain. 2010, 12, 889–908. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Ringø, E.; Shenavar Masouleh, A.; Esteban, M.Á. Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: A review. Rev. Aquac. 2016, 8, 89–102. [Google Scholar] [CrossRef]
- Liu, A.; Santigosa, E.; Dumas, A.; Hernandez, J.M. Vitamin nutrition in salmonid aquaculture: From avoiding deficiencies to enhancing functionalities. Aquaculture 2022, 561, 738654. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef]
- Li, S.; Tran, N.T. Paraprobiotics in Aquaculture. In Probiotics in Aquaculture; Springer: Cham, Switzerland, 2022; pp. 131–164. [Google Scholar]
- Gyan, W.R.; Ayiku, S.; Yang, J.; Asumah, J. Effects of yeast antimicrobial peptide in aquaculture. J. Fish. Aquac. Dev. 2019, 6, 1048. [Google Scholar]
- Petit, J.; Wiegertjes, G.F. Long-lived effects of administering beta-glucans: Indications for trained immunity in fish. Dev. Comp. Immunol. 2016, 64, 93–102. [Google Scholar] [CrossRef]
- Petit, J.; Bailey, E.C.; Wheeler, R.T.; De Oliveira, C.A.; Forlenza, M.; Wiegertjes, G.F. Studies into β-glucan recognition in fish suggests a key role for the C-type lectin pathway. Front. Immunol. 2019, 10, 280. [Google Scholar] [CrossRef]
- Korcova, J.; Machova, E.; Filip, J.; Bystricky, S. Biophysical properties of carboxymethyl derivatives of mannan and dextran. Carbohydr. Polym. 2015, 134, 6–11. [Google Scholar] [CrossRef]
- Cummings, R.D. The mannose receptor ligands and the macrophage glycome. Curr. Opin. Struct. Biol. 2022, 75, 102394. [Google Scholar] [CrossRef]
- Wang, J.; Lei, P.; Gamil, A.A.A.; Lagos, L.; Yue, Y.; Schirmer, K.; Mydland, L.T.; Overland, M.; Krogdahl, Å.; Kortner, T.M. Rainbow Trout (Oncorhynchus Mykiss) Intestinal Epithelial Cells as a Model for Studying Gut Immune Function and Effects of Functional Feed Ingredients. Front. Immunol. 2019, 10, 152. [Google Scholar] [CrossRef]
- Divya, M.; Gopi, N.; Iswarya, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Vaseeharan, B. beta-glucan extracted from eukaryotic single-celled microorganism Saccharomyces cerevisiae: Dietary supplementation and enhanced ammonia stress tolerance on Oreochromis mossambicus. Microb. Pathog. 2020, 139, 103917. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Moate, R.; Davies, S.J.; Spring, P.; Sweetman, J.; Bradley, G. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 2009, 87, 3226–3234. [Google Scholar] [CrossRef]
- Faustino, M.; Durao, J.; Pereira, C.F.; Pintado, M.E.; Carvalho, A.P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae-A sustainable source of functional ingredients. Carbohydr. Polym. 2021, 272, 118467. [Google Scholar] [CrossRef]
- Villumsen, K.R.; Ohtani, M.; Forberg, T.; Aasum, E.; Tinsley, J.; Bojesen, A.M. Synbiotic feed supplementation significantly improves lipid utilization and shows discrete effects on disease resistance in rainbow trout (Oncorhynchus mykiss). Sci. Rep. 2020, 10, 16993. [Google Scholar] [CrossRef]
- Wu, X.; Hao, Q.; Teame, T.; Ding, Q.; Liu, H.; Ran, C.; Yang, Y.; Xia, L.; Wei, S.; Zhou, Z.; et al. Gut microbiota induced by dietary GWF® contributes to growth promotion, immune regulation and disease resistance in hybrid sturgeon (Acipenserbaerii x Acipenserschrenckii): Insights from a germ-free zebrafish model. Aquaculture 2020, 520, 734966. [Google Scholar] [CrossRef]
- Meng, D.; Hao, Q.; Zhang, Q.; Yu, Z.; Liu, S.; Yang, Y.; Ran, C.; Zhang, Z.; Zhou, Z. A compound of paraprobiotic and postbiotic derived from autochthonous microorganisms improved growth performance, epidermal mucus, liver and gut health and gut microbiota of common carp (Cyprinus carpio). Aquaculture 2023, 570, 739378. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, X.; Hu, W.; Tang, T.; Bai, J.; Zhao, S.; Ao, Z.; Wei, Z.; Gao, W.; Zhang, W. Effects of dietary nucleotide and yeast cell wall on growth performance, feed utilization, anti-oxidative and immune response of grass carp (Ctenopharyngodon idella). Fish Shellfish. Immunol. 2023, 134, 108574. [Google Scholar] [CrossRef]
- Rawling, M.D.; Pontefract, N.; Rodiles, A.; Anagnostara, I.; Leclercq, E.; Schiavone, M.; Castex, M.; Merrifield, D.L. The effect of feeding a novel multistrain yeast fraction on European seabass (Dicentrachus labrax) intestinal health and growth performance. J. World Aquac. Soc. 2019, 50, 1108–1122. [Google Scholar] [CrossRef]
- Xie, X.; Wang, J.; Guan, Y.; Xing, S.; Liang, X.; Xue, M.; Wang, J.; Chang, Y.; Leclercq, E. Cottonseed protein concentrate as fishmeal alternative for largemouth bass (Micropterus salmoides) supplemented a yeast-based paraprobiotic: Effects on growth performance, gut health and microbiome. Aquaculture 2022, 551, 737898. [Google Scholar] [CrossRef]
- Rawling, M.; Leclercq, E.; Foey, A.; Castex, M.; Merrifield, D. A novel dietary multi-strain yeast fraction modulates intestinal toll-like-receptor signalling and mucosal responses of rainbow trout (Oncorhynchus mykiss). PLoS ONE 2021, 16, e0245021. [Google Scholar]
- Xie, X.; Liang, X.; Wang, H.; Zhu, Q.; Wang, J.; Chang, Y.; Leclercq, E.; Xue, M.; Wang, J. Effects of paraprobiotics on bile acid metabolism and liver health in largemouth bass (Micropterus salmoides) fed a cottonseed protein concentrate-based diet. Anim. Nutr. 2023, 13, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H. Metabolic Liver Disease Induced by a High Carbohydrate Diet in Largemouth Bass (Micropterus salmoides), and the Alleviating Mechanism of Bile Acid Supplementation. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- Ma, D.M.; Fan, J.J.; Zhu, H.P.; Su, H.H.; Jiang, P.; Yu, L.Y.; Liao, G.L.; Bai, J.J. Histologic examination and transcriptome analysis uncovered liver damage in largemouth bass from formulated diets. Aquaculture 2020, 526, 735329. [Google Scholar] [CrossRef]
- Amoah, A.; Coyle, S.D.; Webster, C.D.; Durborow, R.M.; Bright, L.A.; Tidwell, J.H. Effects of graded levels of carbohydrate on growth and survival of largemouth bass, Micropterus salmoides. J. World Aquac. Soc. 2008, 39, 397–405. [Google Scholar] [CrossRef]
- Xu, X.; Chen, N.; Liu, Z.; Gou, S.; Yin, J. Effects of dietary starch sources and levels on liver histology in largemouth bass, Micropterus salmoides. J. Shanghai Ocean. Univ. 2016, 25, 61–70. [Google Scholar]
- Yu, H.H.; Liang, X.F.; Chen, P.; Wu, X.F.; Zheng, Y.H.; Luo, L.; Qin, Y.C.; Long, X.C.; Xue, M. Dietary supplementation of Grobiotic®-A increases short-term inflammatory responses and improves long-term growth performance and liver health in largemouth bass (Micropterus salmoides). Aquaculture 2019, 500, 327–337. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds—A review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Xv, Z.; Zhong, Y.; Wei, Y.; Zhang, T.; Zhou, W.; Jiang, Y.; Chen, Y.; Lin, S. Yeast culture supplementation alters the performance and health status of juvenile largemouth bass (Micropterus salmoides) fed a high-plant protein diet. Aquac. Nutr. 2021, 27, 2637–2650. [Google Scholar] [CrossRef]
- Liu, Y.; Han, S.-L.; Luo, Y.; Li, L.-Y.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y. Impaired peroxisomal fat oxidation induces hepatic lipid accumulation and oxidative damage in Nile tilapia. Fish Physiol. Biochem. 2020, 46, 1229–1242. [Google Scholar] [CrossRef]
- Morita, M.; Ishida, N.; Uchiyama, K.; Yamaguchi, K.; Itoh, Y.; Shichiri, M.; Yoshida, Y.; Hagihara, Y.; Naito, Y.; Yoshikawa, T.; et al. Fatty liver induced by free radicals and lipid peroxidation. Free Radic. Res. 2012, 46, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.B.; Tian, K.; Cao, Y.W.; Bao, J.L.; Wang, M.; He, C.W.; Hu, Y.J.; Su, H.X.; Wan, J.B. Protective Effect of Panax notoginseng Saponins on Acute Ethanol-Induced Liver Injury Is Associated with Ameliorating Hepatic Lipid Accumulation and Reducing Ethanol-Mediated Oxidative Stress. J. Agric. Food Chem. 2015, 63, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-P.; Shin, J.-H.; Seo, S.-H.; Kim, S.-G.; Lee, S.H.; Shin, E.-H. Effects of antioxidants in reducing accumulation of fat in hepatocyte. Int. J. Mol. Sci. 2018, 19, 2563. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, M.; Morais, S.; He, M.; Wang, H.; Wang, J.; Pastor, J.J.; Gonçalves, R.A.; Liang, X. Effects of a Phytogenic Supplement Containing Olive By-Product and Green Tea Extracts on Growth Performance, Lipid Metabolism, and Hepatic Antioxidant Capacity in Largemouth Bass (Micropterus salmoides) Fed a High Soybean Meal Diet. Antioxidants 2022, 11, 2415. [Google Scholar] [CrossRef]
- Cardozo, L.F.; Pedruzzi, L.M.; Stenvinkel, P.; Stockler-Pinto, M.B.; Daleprane, J.B.; Leite, M., Jr.; Mafra, D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 2013, 95, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xu, M.; Jeong, S.; Qian, Y.; Wu, H.; Xia, Q.; Kong, X. The Role of Nrf2 in Liver Disease: Novel Molecular Mechanisms and Therapeutic Approaches. Front. Pharmacol. 2018, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 signaling. React. Oxyg. Species 2019, 8, 312. [Google Scholar] [CrossRef]
- Zhang, D.D.; Lo, S.C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004, 24, 10941–10953. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]



| Diets | Control | YANG |
|---|---|---|
| Ingredients | ||
| Fishmeal 1 | 30 | 30 |
| Cottonseed protein concentrate 2 | 23.5 | 23.5 |
| Clostridium autoethanogenum protein 3 | 4 | 4 |
| Wheat flour | 9 | 9 |
| Tapioca starch | 5 | 5 |
| α-cellulose | 4.6 | 4.6 |
| Spray-dried blood cell powder | 4 | 4 |
| Wheat gluten | 4 | 4 |
| Soybean meal 4 | 2 | 2 |
| Monocalcium phosphate Ca(H2PO4)2 | 1.7 | 1.7 |
| Kelp powder | 1.5 | 1.5 |
| Fish oil | 3.52 | 3.52 |
| Plant oil mix | 5.5 | 5.5 |
| Vitamin and mineral premix 5 | 1.38 | 1.38 |
| DL-Methionine | 0.2 | 0.2 |
| L-Threonine | 0.1 | 0.1 |
| MsYbP 6 | 0 | 0.08 |
| Proximate composition (DM, %) | ||
| Dry matter | 93.9 | 92.6 |
| Crude protein | 50.8 | 51.1 |
| Crude lipid | 12.1 | 12.3 |
| Crude ash | 10.1 | 10.0 |
| Gross energy (MJ/kg) | 22.1 | 21.9 |
| Gene | Primer | Sequence 5′–3′ | Amplicon Size (bp) | Amplification Efficiency (%) | Tm (°C) |
|---|---|---|---|---|---|
| ef-1α | F | TGCTGCTGGTGTTGGTGAGTT | 147 | 102.8 | 60.4 |
| R | TTCTGGCTGTAAGGGGGCTC | ||||
| nrf2 | F | CAGACGGGGAAACAAACAATG | 170 | 96.8 | 55.0 |
| R | GGGGTAAAATACGCCACAATAAC | ||||
| keap1 | F | TCATTGGGGAATCACATCTTTG | 199 | 99.6 | 56.5 |
| R | TGTCCAGAAAAGTGTTGCCATC | ||||
| cu/zn-sod | F | ACCACAGAAACTTACGCGACA | |||
| R | TTCACAGGGTCTGAATCGCC | ||||
| mn-sod | F | TGCTTTGCAGAGTTGGTCAG | 183 | 97.0 | 58.0 |
| R | CATAAGTGGCATGGTGCTTG | ||||
| cat | F | GTCCTTCATCCACTCCCAGA | 162 | 90.0 | 55.0 |
| R | CCATAGCCATTCATGTGACG | ||||
| gsh-px | F | CTCCTCAACCAGGCAAAC | |||
| R | ATACCCCCCTCACAACAA | ||||
| tnfα | F | CTTCGTCTACAGCCAGGCATCG | 161 | 105.7 | 63.0 |
| R | TTTGGCACACCGACCTCACC | ||||
| il-1β | F | CGTGACTGACAGCAAAAAGAGG | 166 | 101.3 | 59.4 |
| R | GATGCCCAGAGCCACAGTTC | ||||
| il-10 | F | CGGCACAGAAATCCCAGAGC | 119 | 113.6 | 62.1 |
| R | CAGCAGGCTCACAAAATAAACATCT | ||||
| tgf-β1 | F | GCTCAAAGAGAGCGAGGATG | 118 | 95.6 | 59.0 |
| R | TCCTCTACCATTCGCAATCC |
| Lab-Scale | Pilot-Scale | |||||
|---|---|---|---|---|---|---|
| Control | YANG | p Value | Control | YANG | p Value | |
| Growth (lab-scale: n = 4 tank; pilot-scale: n = 12 fish; mean ± SEM) | ||||||
| FBW (g) a | 102.4 ± 1.6 | 116.8 ± 1.3 | 0.001 | 323.7 ± 10.8 | 367.7 ± 7.0 | 0.04 |
| WGR (%) b | 213.9 ± 3.3 | 272.7 ± 5.0 | 0.001 | 243.7 ± 10.8 | 287.7 ± 7.0 | 0.04 |
| Survival (%) c | 92.5 ± 3.3 | 100.0 ± 0.0 | 0.06 | - | - | - |
| Feed consumption (g) c | 1443 ± 11 | 1698 ± 24 | 0.001 | - | - | - |
| Morphometry (lab-scale: n = 13–16 fish; pilot-scale: n = 12 fish; mean ± SEM) | ||||||
| CF d | 2.57 ± 0.06 | 2.60 ± 0.06 | 0.66 | 1.73 ± 0.05 | 1.81 ± 0.04 | 0.19 |
| VSI (%) e | 6.82 ± 0.18 | 6.92 ± 0.28 | 0.73 | 10.47 ± 0.38 | 10.17 ± 0.38 | 0.59 |
| HSI (%) f | 1.55 ± 0.07 | 1.50 ± 0.08 | 0.61 | 2.47 ± 0.098 | 2.58 ± 0.09 | 0.43 |
| VAI (%) g | 1.59 ± 0.18 | 2.07 ± 0.19 | 0.04 | 2.09 ± 0.12 | 2.63 ± 0.21 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xie, X.; Liu, Y.; Liu, J.; Liang, X.; Wang, H.; Li, G.; Xue, M. Growth, Antioxidant Capacity, and Liver Health in Largemouth Bass (Micropterus salmoides) Fed Multi-Strain Yeast-Based Paraprobiotic: A Lab-to-Pilot Scale Evaluation. Antioxidants 2024, 13, 792. https://doi.org/10.3390/antiox13070792
Wang J, Xie X, Liu Y, Liu J, Liang X, Wang H, Li G, Xue M. Growth, Antioxidant Capacity, and Liver Health in Largemouth Bass (Micropterus salmoides) Fed Multi-Strain Yeast-Based Paraprobiotic: A Lab-to-Pilot Scale Evaluation. Antioxidants. 2024; 13(7):792. https://doi.org/10.3390/antiox13070792
Chicago/Turabian StyleWang, Jie, Xiaoze Xie, Yangyang Liu, Jiacheng Liu, Xiaofang Liang, Hao Wang, Gang Li, and Min Xue. 2024. "Growth, Antioxidant Capacity, and Liver Health in Largemouth Bass (Micropterus salmoides) Fed Multi-Strain Yeast-Based Paraprobiotic: A Lab-to-Pilot Scale Evaluation" Antioxidants 13, no. 7: 792. https://doi.org/10.3390/antiox13070792
APA StyleWang, J., Xie, X., Liu, Y., Liu, J., Liang, X., Wang, H., Li, G., & Xue, M. (2024). Growth, Antioxidant Capacity, and Liver Health in Largemouth Bass (Micropterus salmoides) Fed Multi-Strain Yeast-Based Paraprobiotic: A Lab-to-Pilot Scale Evaluation. Antioxidants, 13(7), 792. https://doi.org/10.3390/antiox13070792








