Design and Synthesis of Novel Antioxidant 2-Substituted-5,7,8-Trimethyl-1,4-Benzoxazine Hybrids: Effects on Young and Senescent Fibroblasts
Abstract
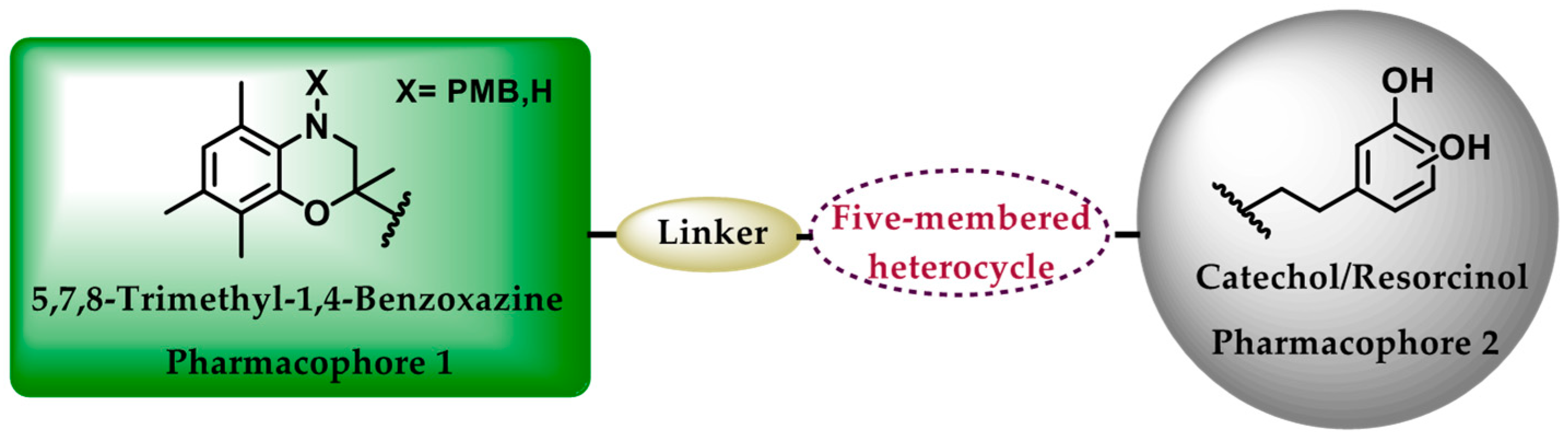
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General
2.1.2. Synthetic Procedures
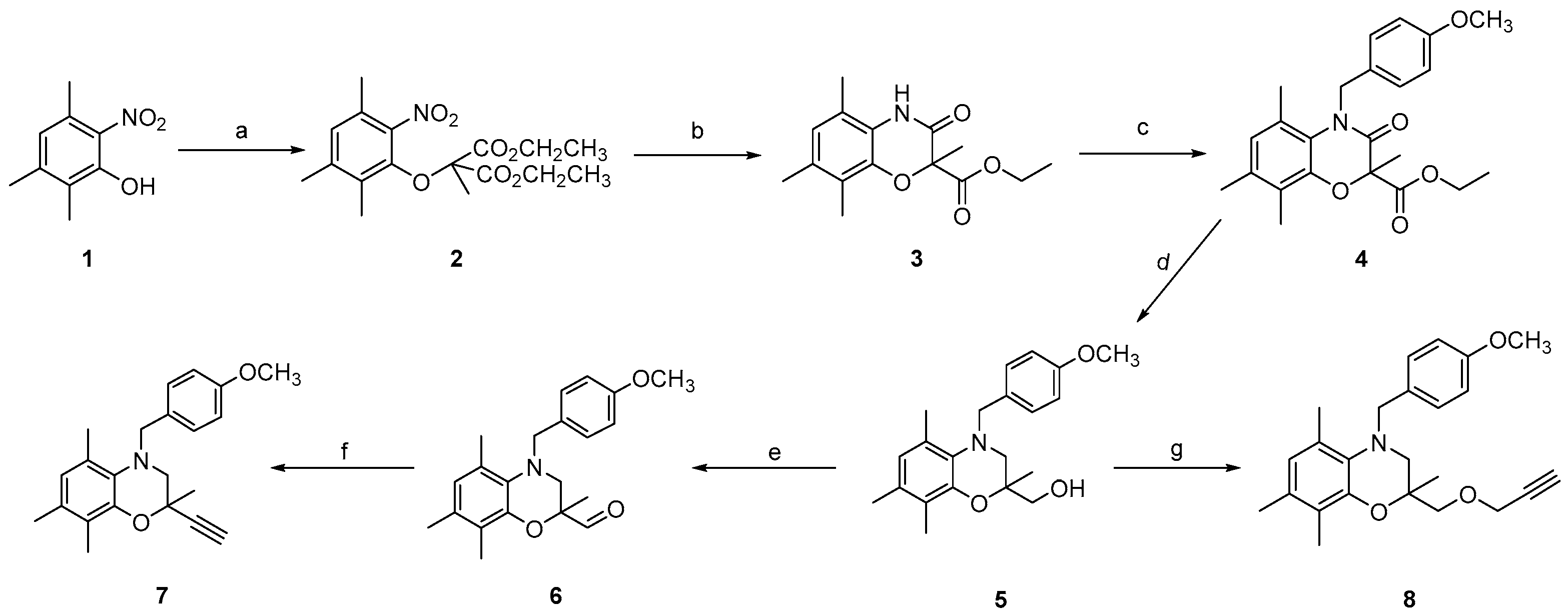
Ethyl 4-(4-methoxybenzyl)-2,5,7,8-tetramethyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-2-carboxylate (4)
(4-(4-Methoxybenzyl)-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[b][1,4]oxazin-2-yl)methanol (5)
4-(4-Methoxybenzyl)-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[b][1,4]oxazine-2-carbaldehyde (6)
2-Ethynyl-4-(4-methoxybenzyl)-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[b][1,4]oxazine (7)
4-(4-Μethoxybenzyl)-2,5,7,8-tetramethyl-2-((prop-2-yn-1-yloxy)methyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (8)
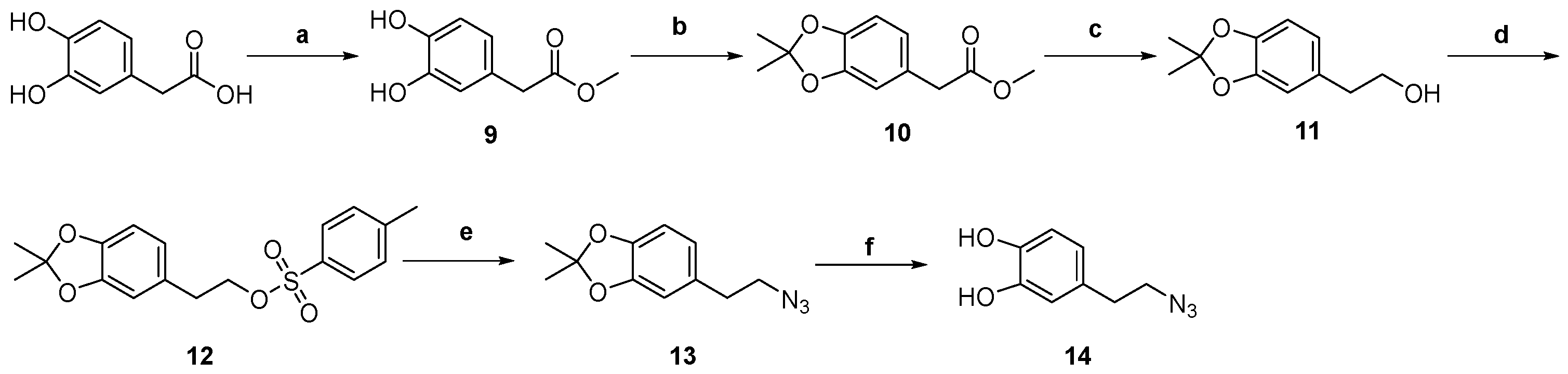
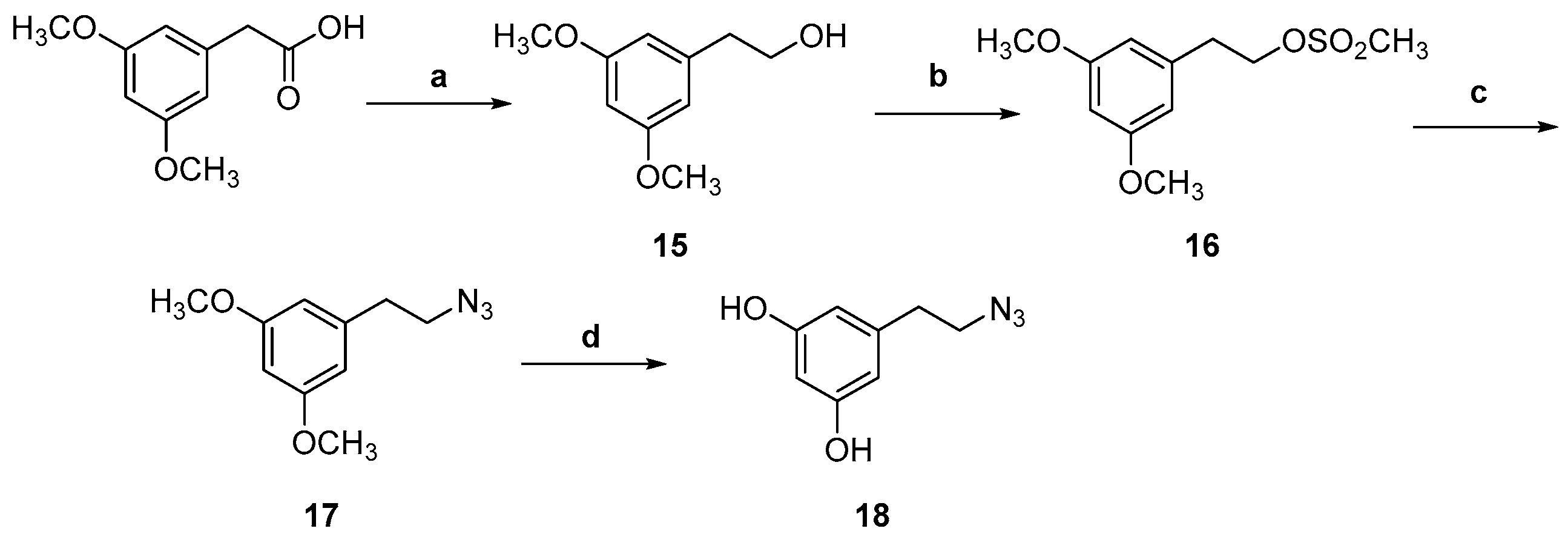
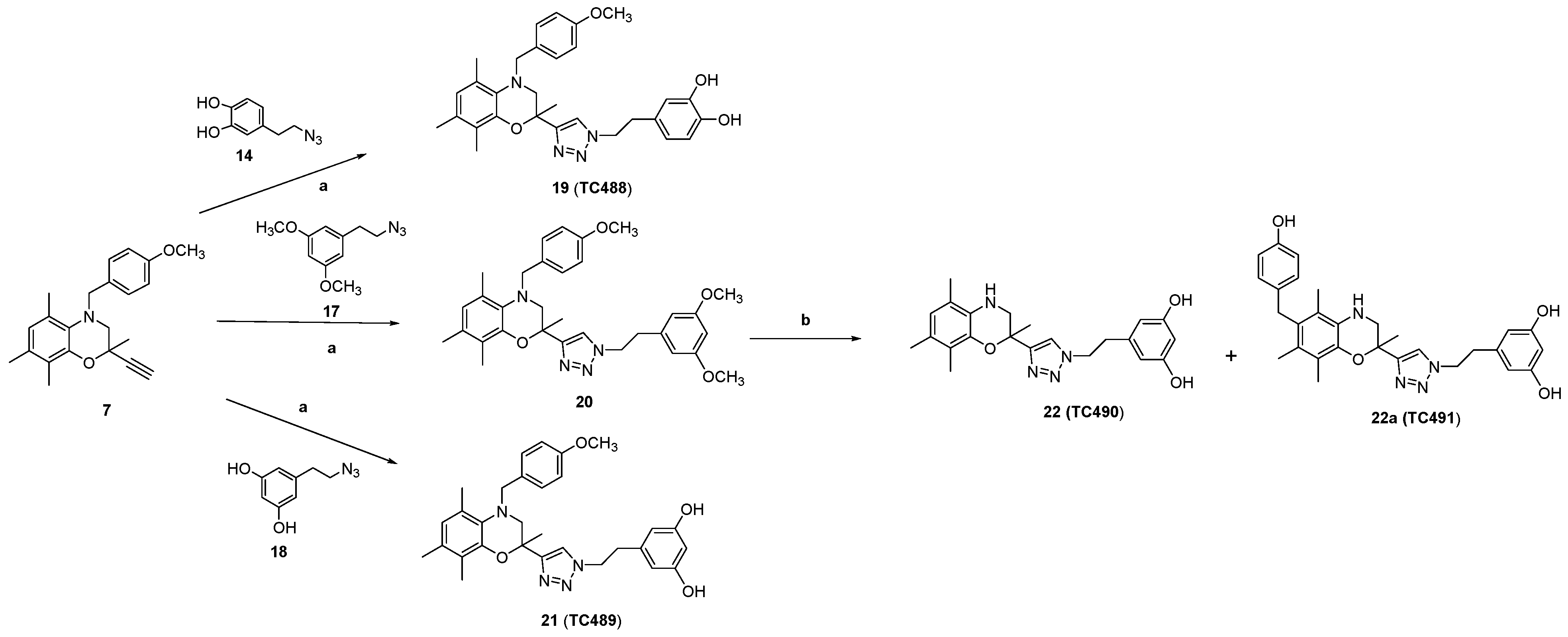
Synthesis of 1,4-disubstituted 1,2,3-triazoles (19, 20, 21, 23, 25)
4-(2-(4-(((4-(4-Methoxybenzyl)-2,5,7,8-tetramethyl-3,4-dihydro-2H-benzo[b][1,4]oxazin-2-yl)methoxy)methyl)-1H-1,2,3-triazol-1-yl)ethyl)benzene-1,2-diol (24, TC483)
General Procedure for the Deprotection of Methoxy Groups of Compounds 20 and 25
2.2. Cells and Culture Conditions
2.3. Radical Scavenging Assay
2.4. Cytotoxicity Assay
2.5. Intracellular Reactive Oxygen Species (ROS) Levels
2.6. Cellular Glutathions (GSH) Levels
2.7. RNA Extraction and Gene Expression Analysis
3. Results
3.1. Chemical Synthesis
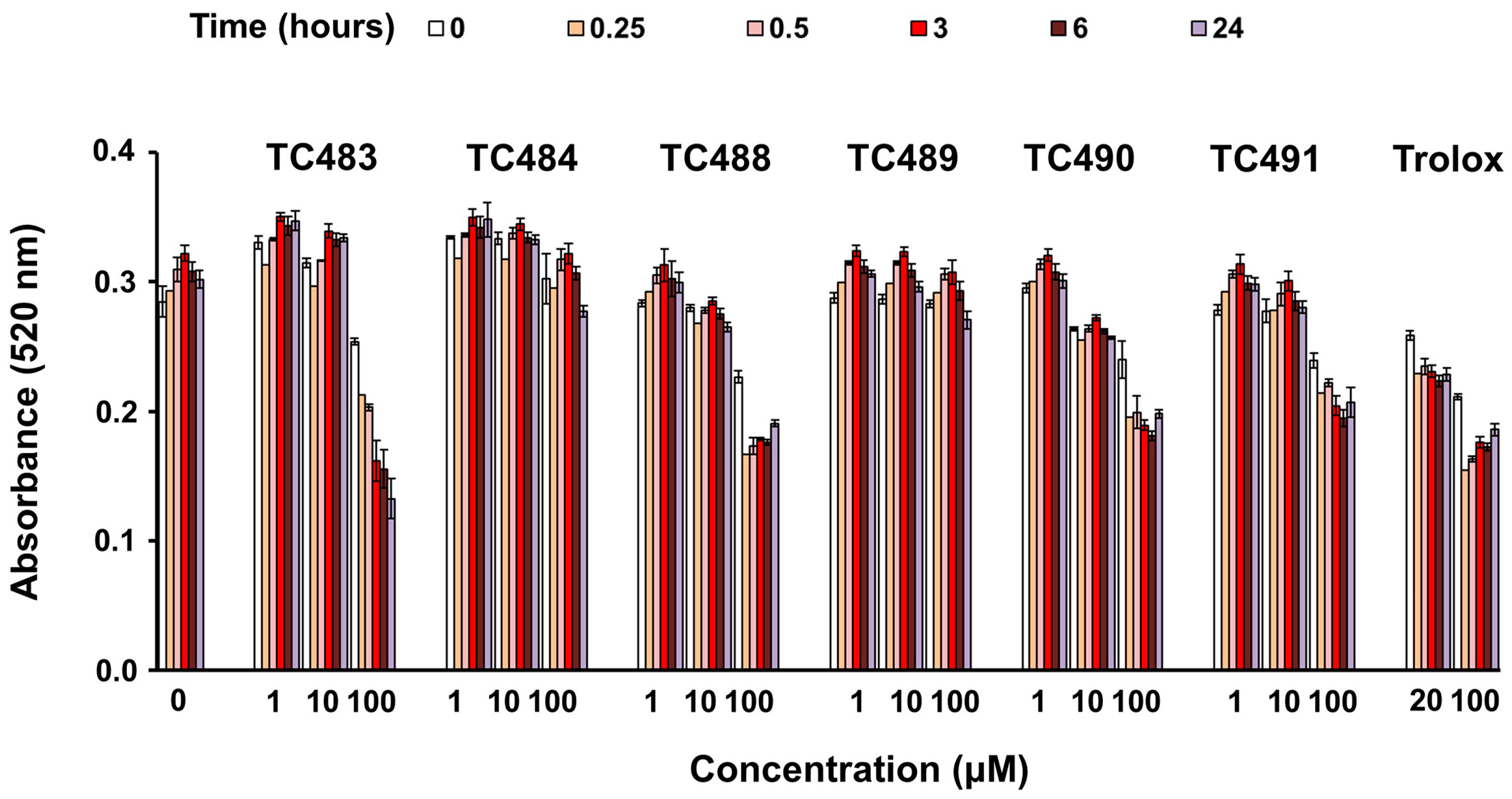
3.2. Free-Radical Scavenging Activity of the Test Compounds
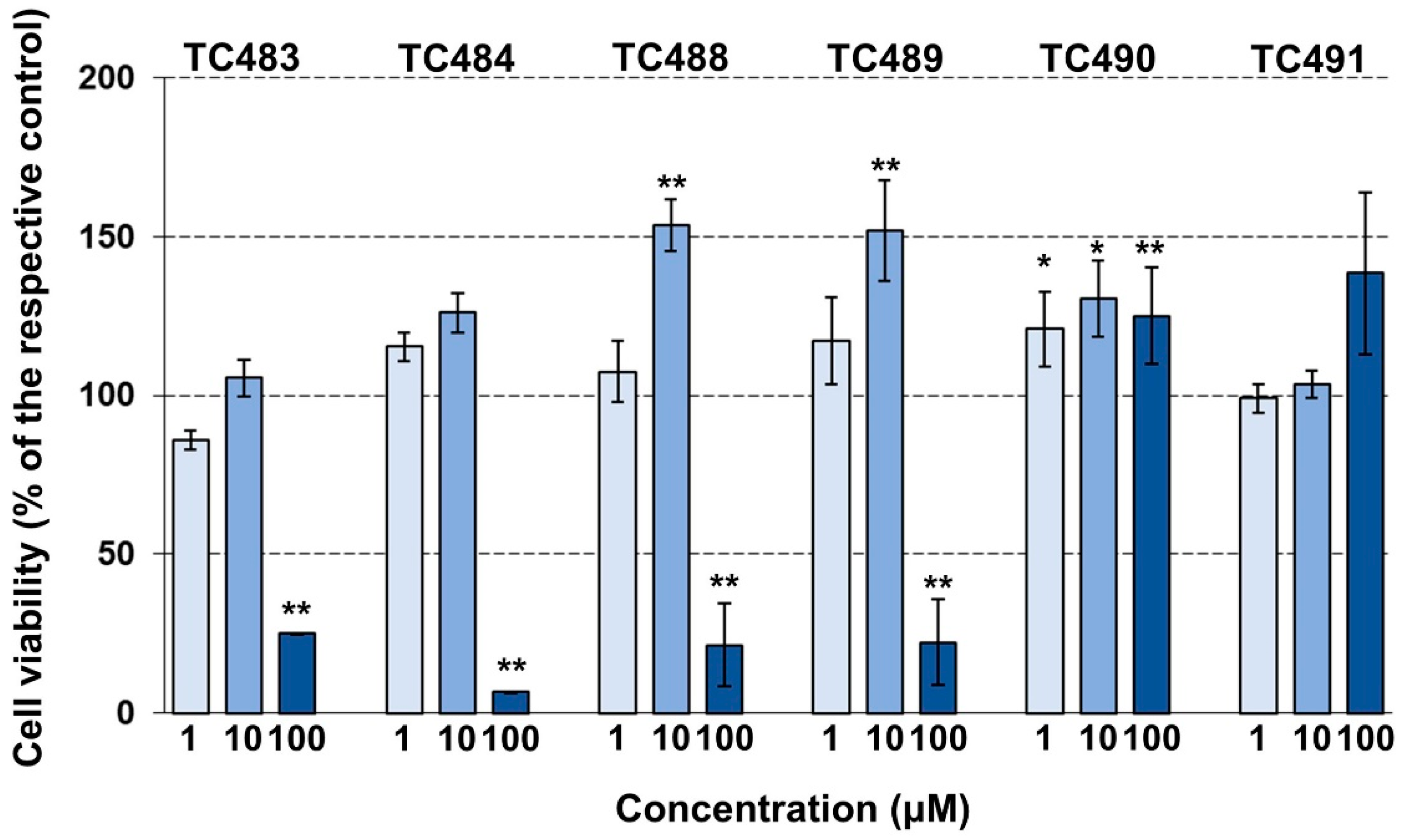
3.3. Cytotoxic Activity of the Test Compounds
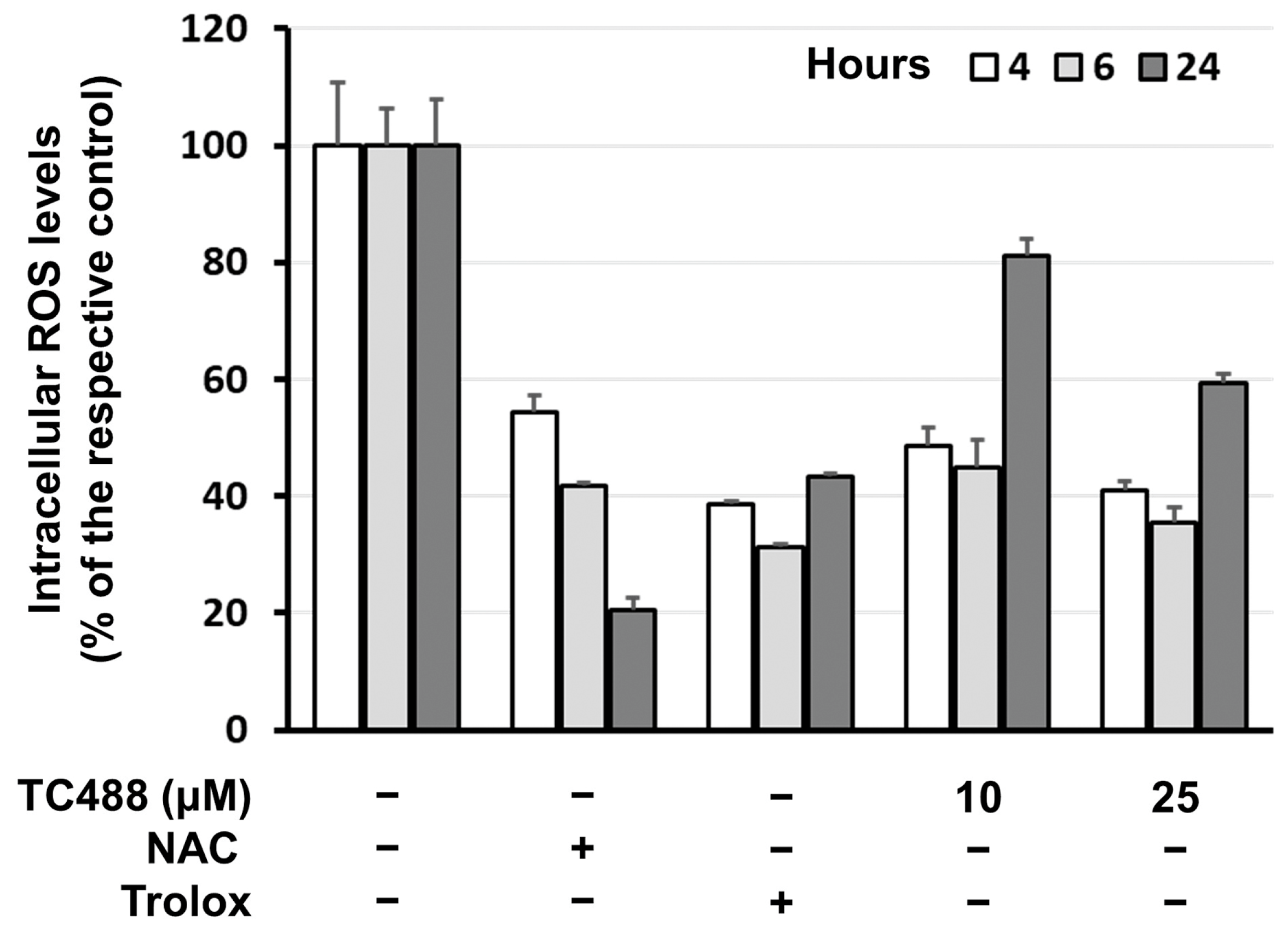
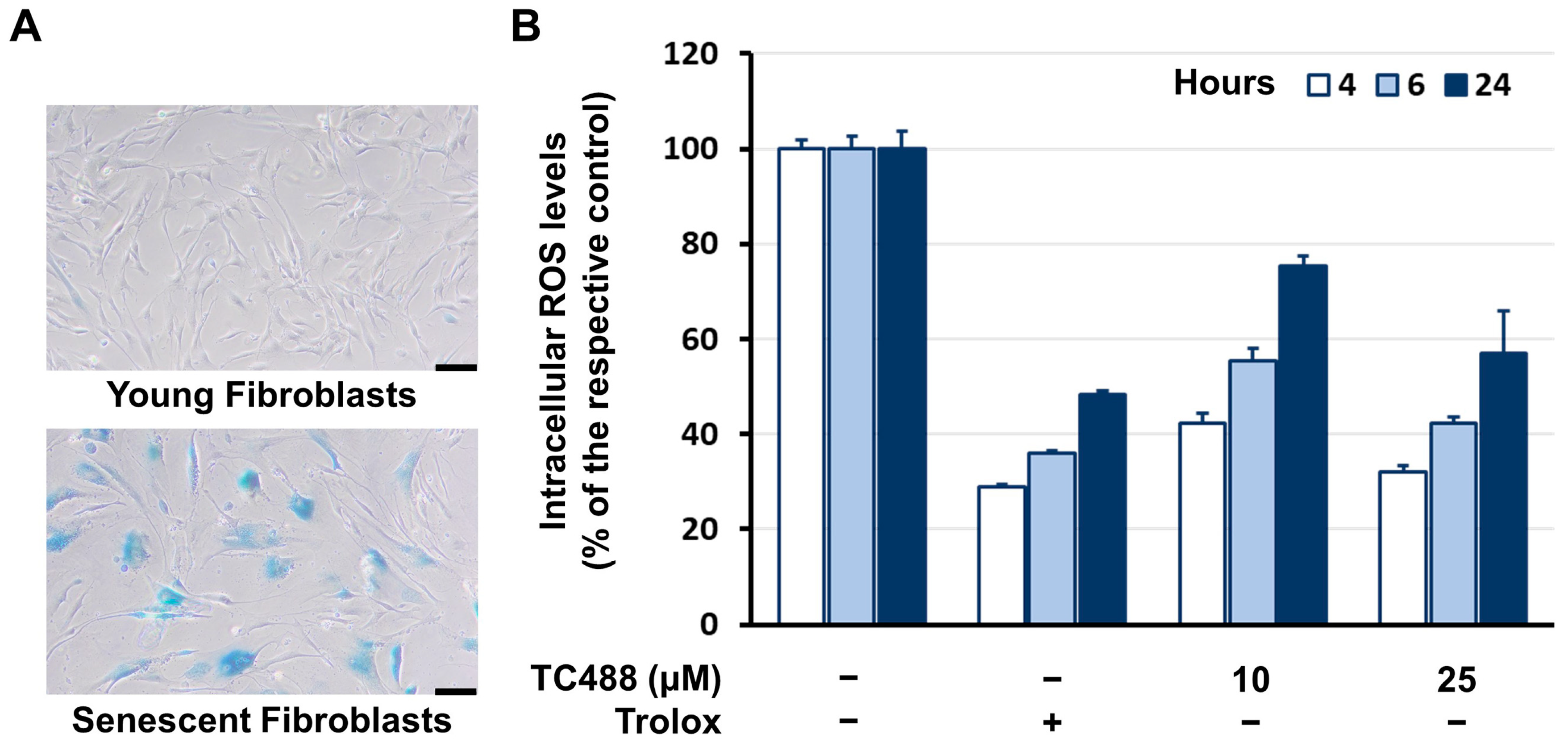
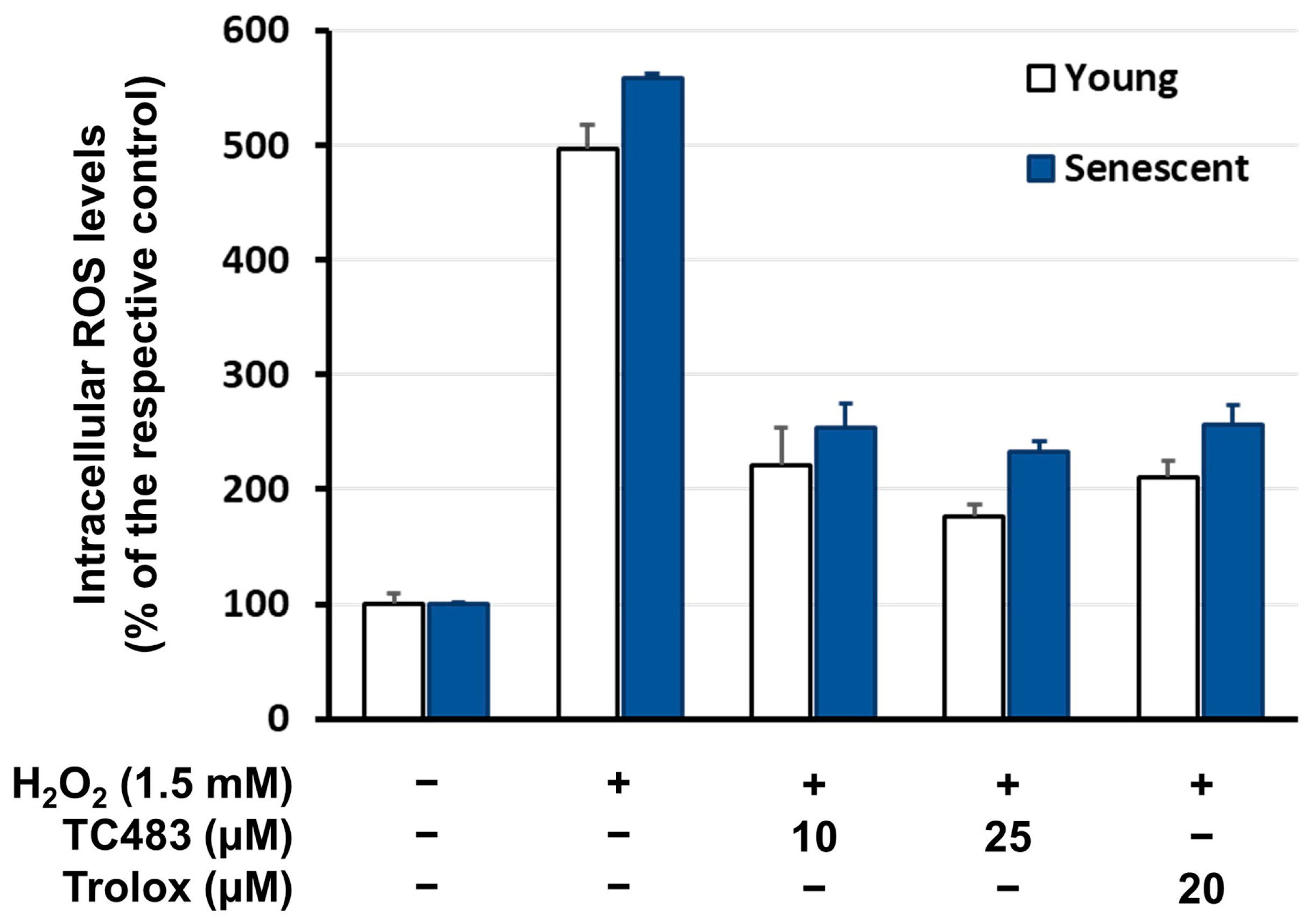
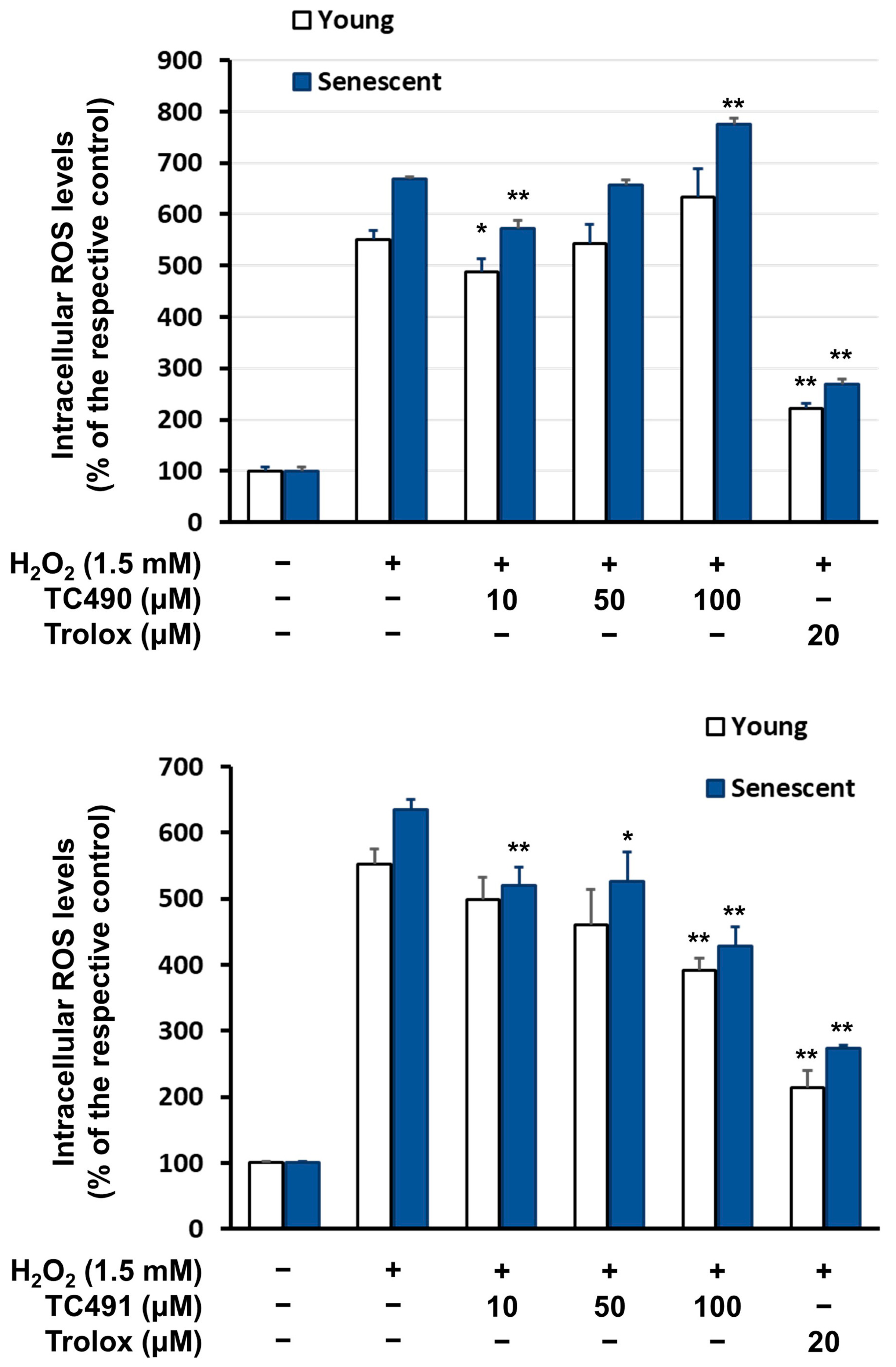
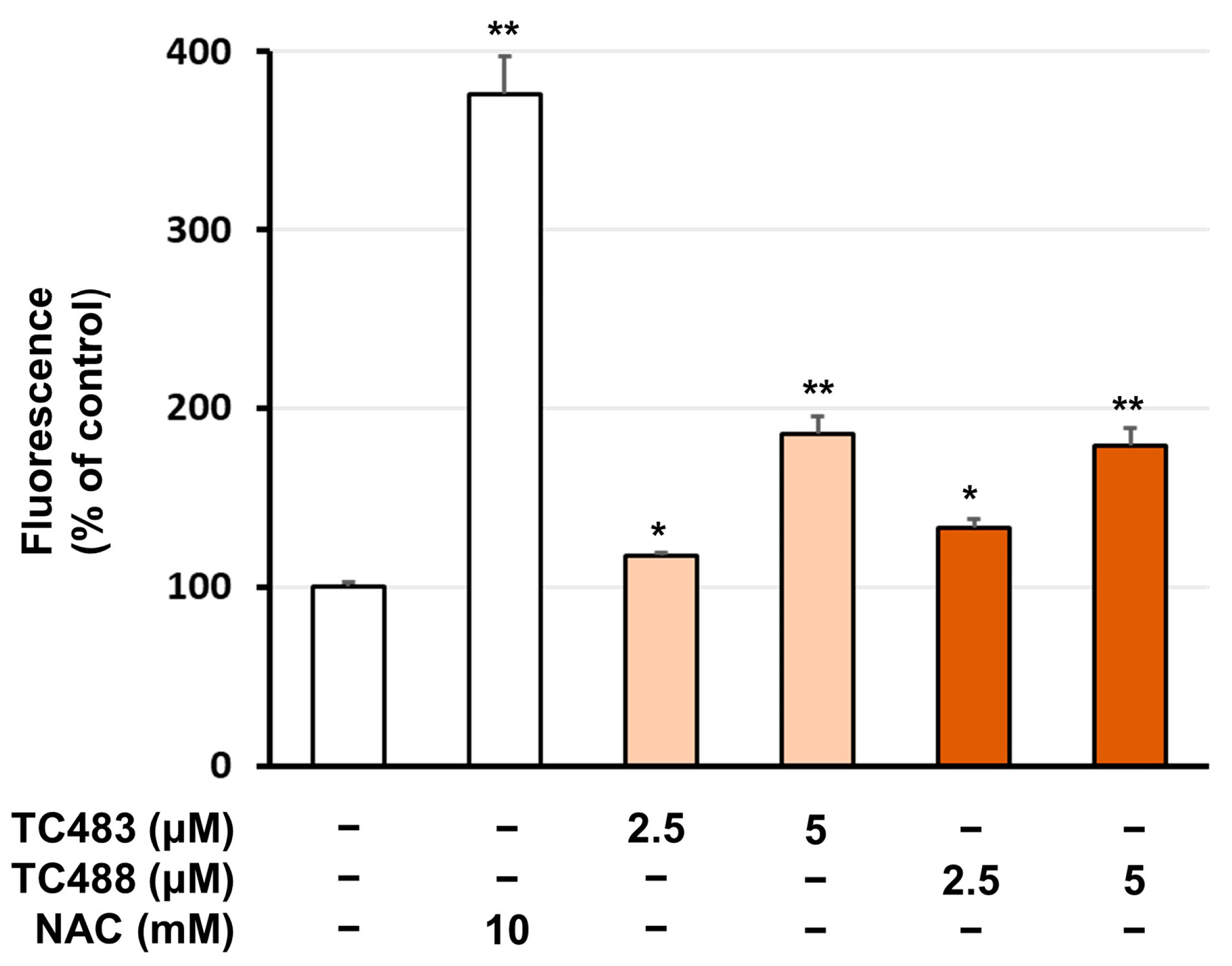
3.4. Intracellular Reactive Oxygen Species (ROS) Levels
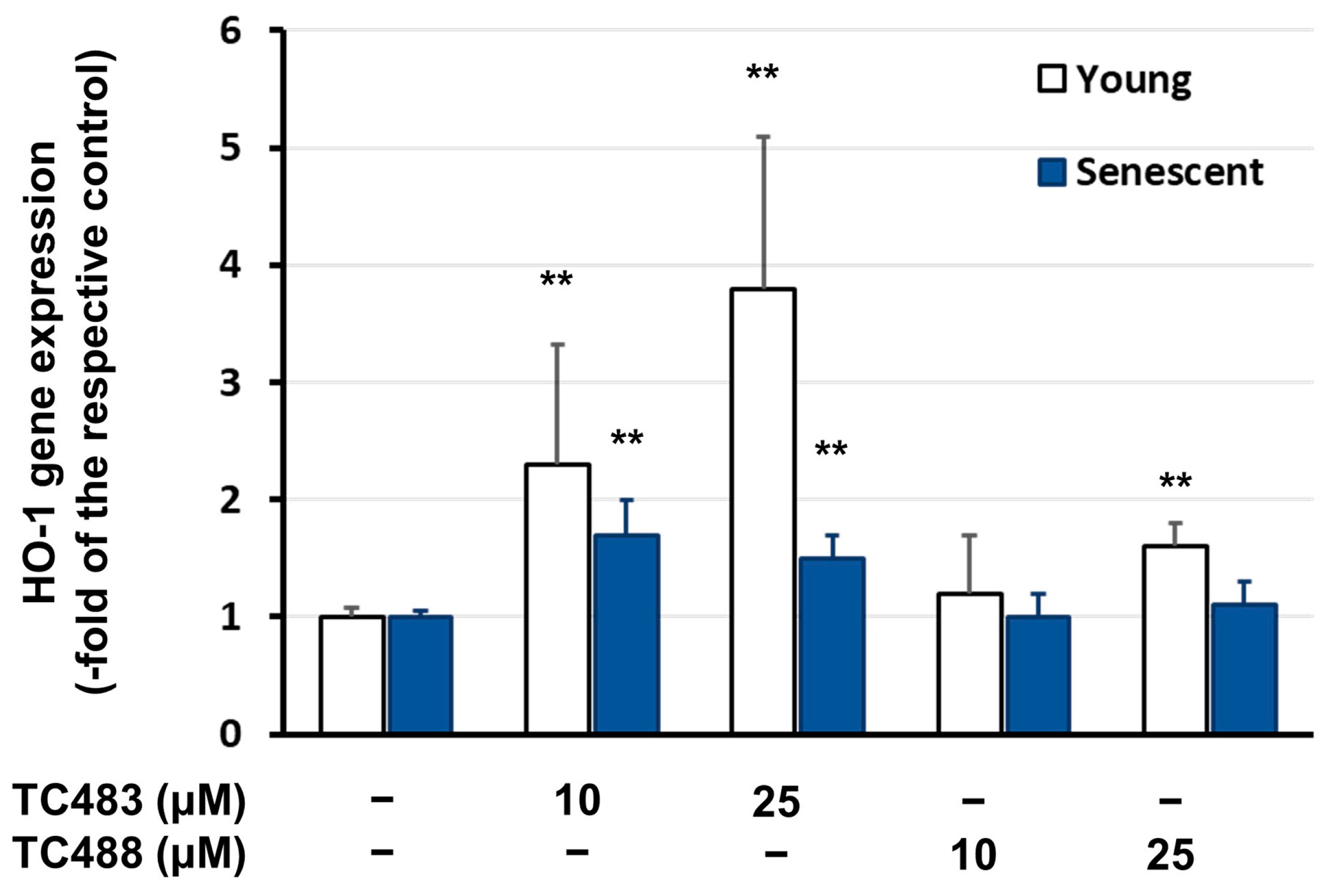
3.5. Effect of the Test Compounds on Gene Expression
3.6. Effect of the Test Compounds on Glutathione (GSH) Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ageing. Available online: http://www.un.org/development/desa/pd/content/ageing-1 (accessed on 24 May 2024).
- de Magalhães, J.P.; Stevens, M.; Thornton, D. The Business of Anti-Aging Science. Trends Biotechnol. 2017, 35, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Ziada, A.S.; Smith, M.-S.R.; Côté, H.C.F. Updating the Free Radical Theory of Aging. Front. Cell Dev. Biol. 2020, 8, 575645. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, O.; Medrano, E.F.; von Zglinicki, T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000, 35, 927–945. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Finkel, T. Free radicals and senescence. Exp. Cell Res. 2008, 314, 1918–1922. [Google Scholar] [CrossRef] [PubMed]
- Dimozi, A.; Mavrogonatou, E.; Sklirou, A.; Kletsas, D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur. Cell Mater. 2015, 30, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Pratsinis, H.; Papadopoulou, A.; Karamanos, N.K.; Kletsas, D. Extracellular matrix alterations in senescent cells and their significance in tissue homeostasis. Matrix Biol. 2019, 75–76, 27–42. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Tan, Y.; Chen, H.; Wan, Y. Benzoxazine: A Privileged Scaffold in Medicinal Chemistry. Curr. Med. Chem. 2023, 30, 372–389. [Google Scholar] [CrossRef] [PubMed]
- Zinad, D.S.; Mahal, A.; Mohapatra, R.K.; Sarangi, A.K.; Pratama, R.M.F. Medicinal chemistry of oxazines as promising agents in drug discovery. Chem. Biol. Drug Des. 2020, 95, 16–47. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Min, L.-J.; Han, L.; Liu, X.H. Recent Advances on Synthesis of 1,4-Benzoxazines and its Derivatives. Curr. Org. Chem. 2021, 25, 2840–2855. [Google Scholar] [CrossRef]
- Ilas, J.; Anderluh, P.S.; Dolenc, M.S.; Kikelj, D. Recent advances in the synthesis of 2H-1,4-benzoxazin-3-(4H)-ones and 3,4-dihydro-2H-1,4-benzoxazines. Tetrahedron 2005, 61, 7325–7348. [Google Scholar] [CrossRef]
- Tang, Z.; Xia, Z.; Chang, S.; Wang, Z. Synthesis and fungicidal activity of novel 2-aryl-3-(1,3,4-thiadiazolyl)-6(8)-methyl-1,3-benzoxazines. Bioorg. Med. Chem. Lett. 2015, 25, 3378–3381. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zuo, H.; Li, Z.; He, X.-Y.; Wang, L.-Y.; Tian, X.; Zhao, B.-X.; Miao, J.-Y.; Shin, D.-S. Synthesis of benzo[b][1,4]oxazin-3(4H)-ones via smiles rearrangement for antimicrobial activity. Med. Chem. Res. 2011, 20, 670–677. [Google Scholar] [CrossRef]
- Pamerla, M.; Reddy, D.R.S.; Rao, B.S.; Bodipati, N.; Murthy, Y.L.N. Antimicrobial evaluation of 1,4-benzoxazine derivatives. Med. Chem. Res. 2015, 24, 611–615. [Google Scholar] [CrossRef]
- Zampieri, D.; Mamolo, M.G.; Filingeri, J.; Fortuna, S.; De Logu, A.; Sanna, A.; Zanon, D. Design, synthesis and antimycobacterial activity of benzoxazinone derivatives and open-ring analogues: Preliminary data and computational analysis. Bioorg. Med. Chem. Lett. 2019, 29, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Yadav, L.; Lal, J.; Jaiswal, P.K.; Mathur, M.; Swami, A.K.; Chaudhary, S. Synthesis, antimicrobial activity, structure-activity relationship and cytotoxic studies of a new series of functionalized (Z)-3-(2-oxo-2-substituted ethylidene)-3,4-dihydro-2H-benzo[b][1,4]oxazin-2-ones. Bioorg. Med. Chem. Lett. 2017, 27, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Sangani, S.S.; Dabhi, R.C.; Kawad, M.; Parmar, J.; Arya, P.S.; Chauhan, R.J.; Muddassir, M.; Christy, M.; Ameta, R.K. Buchwald coupling promoted benign synthesis of benzoxazine derivatives supported Cu complexes with their multipurpose potential in antimicrobial and catalytical fields. J. Mol. Struct. 2023, 1285, 135380. [Google Scholar] [CrossRef]
- Patil, V.P.; Markad, V.L.; Kodam, K.M.; Waghmode, S.B. Facile preparation of tetrahydro-5H-pyrido[1,2,3-de]-1,4-benzoxazines via reductive cyclization of 2-(8-quinolinyloxy)ethanones and their antioxidant activity. Bioorg. Med. Chem. Lett. 2013, 23, 6259–6263. [Google Scholar] [CrossRef]
- Largeron, M.; Lockhart, B.; Pfeiffer, B.; Fleury, M.-B. Synthesis and in vitro evaluation of new 8-amino-1,4-benzoxazine derivatives as neuroprotective antioxidants. J. Med. Chem. 1999, 42, 5043–5052. [Google Scholar] [CrossRef] [PubMed]
- Gein, V.L.; Rassudikhina, N.A.; Shepelina, N.V.; Vakhrin, M.I.; Babushkina, E.B.; Voronina, E.V. Reaction of substituted o-aminophenols with acylpyruvic acid esters and α-ketoglutaric acid. Antibacterial activity of the products. Pharm. Chem. J. 2008, 42, 529–532. [Google Scholar] [CrossRef]
- Li, X.K.; Liu, N.; Zhang, H.N.; Knudson, S.E.; Slayden, R.A.; Tonge, P.J. Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: Novel antibacterial agents against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2010, 20, 6306–6309. [Google Scholar] [CrossRef] [PubMed]
- Kundu, T.; Bhattacharjee, B.; Hazra, S.; Ghosh, A.K.; Bandyopadhyay, D.; Pramanik, A. Synthesis and Biological Assessment of Pyrrolobenzoxazine Scaffold as a Potent Antioxidant. J. Med. Chem. 2019, 62, 6315–6329. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Jaiswal, P.K.; Yadav, D.K.; Saran, M.; Prikhodko, J.; Mathur, M.; Swami, A.K.; Mashevskaya, I.V.; Chaudhary, S. Microwave-assisted one-pot efficient synthesis of functionalized 2-oxo-2-phenylethylidenes-linked 2-oxo-benzo[1,4]oxazines and 2-oxo-quino[1,4]oxalines: Synthetic Applications, Antioxidant activity, SAR and Cytotoxic Studies. Acta Chim. Slov. 2017, 64, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Koini, E.N.; Avlonitis, N.; Martins-Duarte, E.S.; de Souza, W.; Vommaro, R.C.; Calogeropoulou, T. Divergent synthesis of 2,6-diaryl-substituted 5,7,8-trimethyl-1,4-benzoxazines via microwave-promoted palladium-catalyzed Suzuki Miyaura cross coupling and biological evaluation. Tetrahedron 2012, 68, 10302–10309. [Google Scholar] [CrossRef]
- Tricarico, D.; Rolland, J.-F.; Cannone, G.; Mele, A.; Cippone, V.; Laghezza, A.; Carbonara, G.; Fracchiolla, G.; Tortorella, P.; Loiodice, F.; et al. Structural Nucleotide Analogs Are Potent Activators/Inhibitors of Pancreatic beta Cell KATP Channels: An Emerging Mechanism Supporting Their Use as Antidiabetic Drugs. J. Pharmacol. Exp. Ther. 2012, 340, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, I.; Ali, K.; Panda, G.A. Synthetic Overview of Benzoxazines and Benzoxazepines as Anticancer Agents. ChemMedChem 2023, 18, e202200617. [Google Scholar] [CrossRef] [PubMed]
- Bollu, R.; Palem, J.D.; Bantu, R.; Guguloth, V.; Nagarapu, L.; Polepalli, S.; Jain, N. Rational design, synthesis and anti-proliferative evaluation of novel 1,4-benzoxazine-[1,2,3]triazole hybrids. Eur. J. Med. Chem. 2015, 89, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Narsimha, S.; Battula, K.S.; Nukala, S.K.; Gondru, R.; Reddy, Y.N.; Nagavelli, V.R. One-pot synthesis of fused benzoxazino[1,2,3]triazolyl[4,5-c]quinolinone derivatives and their anticancer activity. RSC Adv. 2016, 6, 74332–74339. [Google Scholar] [CrossRef]
- Nagaraju, A.; Kumar Nukala, S.; Narasimha Swamy Thirukovela, T.; Manchal, R. In Vitro Anticancer and In Silico Studies of Some 1,4-Benzoxazine-1,2,4-oxadiazole Hybrids. ChemistrySelect 2021, 6, 3318–3321. [Google Scholar] [CrossRef]
- Benarjee, V.; Saritha, B.; Hari Gangadhar, K.; Sailaja, B.B.V. Synthesis of some new 1,4-benzoxazine-pyrazoles in water as EGFR targeting anticancer agents. J. Mol. Struct. 2022, 1265, 133188. [Google Scholar] [CrossRef]
- Das, B.C.; Madhukumar, A.V.; Anguiano, J.; Mani, S. Design, synthesis and biological evaluation of 2H-benzo[b][1,4]oxazine derivatives as hypoxia targeted compounds for cancer therapeutics. Bioorg. Med. Chem. Lett. 2009, 19, 4204–4206. [Google Scholar] [CrossRef] [PubMed]
- Aicher, T.D.; Van Huis, C.A.; Hurd, A.R.; Skalitzky, D.J.; Taylor, C.B.; Beleh, O.M.; Glick, G.; Toogood, P.L.; Yang, B.; Zheng, T.; et al. Discovery of LYC-55716: A Potent, Selective, and Orally Bioavailable Retinoic Acid Receptor-Related Orphan Receptor-γ (RORγ) Agonist for Use in Treating Cancer. J. Med. Chem. 2021, 64, 13410–13428. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.M.; Schwendimann, L.; Gressens, P.; Largeron, M. Regiospecific synthesis of neuroprotective 1,4-benzoxazine derivatives through a tandem oxidation–Diels–Alder reaction. Org. Biomol. Chem. 2015, 13, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Touzeau, F.; Arrault, A.; Guillaumet, G.; Scalbert, E.; Pfeiffer, B.; Rettori, M.-C.; Renard, P.; Mérour, J.-Y. Synthesis and biological evaluation of new 2-(4,5-dihydro-1H-imidazol-2-yl)-3,4-dihydro-2H-1,4-benzoxazine derivatives. J. Med. Chem. 2003, 46, 1962–1979. [Google Scholar] [CrossRef] [PubMed]
- Bourlot, A.S.; Sanchez, I.; Dureng, G.; Guillaumet, G.; Massingham, R.; Monteil, A.; Winslow, E.; Pujol, M.D.; Merour, J.Y. New Substituted 1,4-Benzoxazine Derivatives with Potential Intracellular Calcium Activity. J. Med. Chem. 1998, 41, 3142–3158. [Google Scholar] [CrossRef] [PubMed]
- Pirotte, B.; Florence, X.; Goffina, E.; Lebrunb, P. 2,2-Dimethyl-3,4-dihydro-2H-1,4-benzoxazines as isosteres of 2,2-dimethylchromans acting as inhibitors of insulin release and vascular smooth muscle relaxants. Med. Chem. Commun. 2019, 10, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Applications of Bioisosteres in the Design of Biologically Active Compounds. J. Agric. Food Chem. 2023, 71, 18087–18644. [Google Scholar] [CrossRef] [PubMed]
- Koini, E.N.; Papazafiri, P.; Vassilopoulos, A.; Koufaki, M.; Horvàth, Z.; Koncz, I.; Virág, L.; Papp, G.J.; Varró, A.; Calogeropoulou, T. 5,7,8-Trimethyl-benzopyran and 5,7,8-Trimethyl-1,4-benzoxazine Aminoamide Derivatives as Novel Antiarrhythmics against Ischemia-Reperfusion Injury. J. Med. Chem. 2009, 52, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Koukouli, F.; Paspaltsis, I.; Salta, E.; Xanthopoulos, K.; Koini, E.N.; Calogeropoulou, T.; Sklaviadis, T. Inhibition of PrPSc formation in scrapie infected N2a cells by 5,7,8-trimethyl-3,4-dihydro-2H-1,4- benzoxazine derivatives. Prion 2012, 6, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Filippou, P.S.; Koini, E.N.; Calogeropoulou, T.; Kalliakmani, P.; Panagiotidis, C.A.; Kyriakidis, D.A. Regulation of the Escherichia coli AtoSC two component system by synthetic biologically active 5;7;8-trimethyl 1;4-benzoxazine analogues. Bioorg. Med. Chem. 2011, 19, 5061–5070. [Google Scholar] [CrossRef] [PubMed]
- de Sena Murteira Pinheiro, P.; Franco, L.S.; Montagnoli, T.L.; Fraga, C.A.M. Molecular hybridization: A powerful tool for multitarget drug discovery. Expert Opin. Drug. Discov. 2024, 19, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Vaishnani, M.J.; Bijani, S.; Rahamathulla, M.; Baldaniya, L.; Jain, V.; Thajudeen, K.Y.; Ahmed, M.M.; Farhana, S.A.; Pasha, I. Biological importance and synthesis of 1,2,3-triazole derivatives: A review. Green Chem. Lett. Rev. 2024, 17, 2307989. [Google Scholar] [CrossRef]
- Bender, C.; Candi, I.; Rogel, E. Efficacy of Hydroxytyrosol-Rich Food Supplements on Reducing Lipid Oxidation in Humans. Int. J. Mol. Sci. 2023, 24, 5521. [Google Scholar] [CrossRef] [PubMed]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxyhtyrosol alleviate intestinal inflammation, oxidative stress and apoptosis re-sulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Z.; Wei, F.; Hou, G.; You, Y.; Wang, X.; Cao, S.; Yang, X.; Liu, W.; Zhang, S.; et al. Hydroxytyrosol Ameliorates Intervertebral Disc Degeneration and Neuropathic Pain by Reducing Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2022, 2022, 2240894. [Google Scholar] [CrossRef]
- Utami, N.D.; Nordin, A.; Katas, H.; Bt Hj Idrus, R.; Fauzi, M.B. Molecular Action of Hydroxytyrosol in Wound Healing: An In Vitro Evidence-Based Review. Biomolecules 2020, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Tabanez, M.; Santos, I.R.; Ikebara, J.M.; Camargo, M.L.M.; Dos Santos, B.A.; Freire, B.M.; Batista, B.L.; Takada, S.H.; Squitti, R.; Kihara, A.H.; et al. The Impact of Hydroxytyrosol on the Metallomic-Profile in an Animal Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14950. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, I.; Borriello, M.; Vilasi, S.; Iannuzzi, C. Hydroxytyrosol Inhibits Protein Oligomerization and Amyloid Aggregation in Human Insulin. Int. J. Mol. Sci. 2020, 21, 4636. [Google Scholar] [CrossRef]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immunemediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Velotti, F.; Bernini, R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients 2023, 15, 1774. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Navarro, C.; Montoro-García, S.; Orenes-Piñero, E. Hydroxytyrosol: Its role in the prevention of cardiovascular diseases. Heliyon 2023, 9, e12963. [Google Scholar] [CrossRef] [PubMed]
- Vijakumaran, U.; Shanmugam, J.; Heng, J.W.; Azman, S.S.; Yazid, M.D.; Haizum Abdullah, N.A.; Sulaiman, N. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules 2023, 28, 1861. [Google Scholar] [CrossRef] [PubMed]
- Vachiramon, V.; Kositkuljorn, C.; Leerunyakul, K.; Chanprapaph, K. Isobutylamido thiazolyl resorcinol for prevention of UVB induced hyperpigmentation. J. Cosmet. Dermatol. 2021, 20, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, W.; Nihei, K. Chemical synthesis and tyrosinase inhibitory activity of resorcinol alkyl glucosides, hydroxyalkyl resorcinols, and alkyl resorcinols. J. Mol. Struct. 2022, 1268, 133668. [Google Scholar] [CrossRef]
- Tanaka, Y.; Suzuki, M.; Kodachi, Y.; Nihei, K. Molecular design of potent, hydrophilic tyrosinase inhibitors based on the natural dihydrooxyresveratrol skeleton. Carbohydr. Res. 2019, 472, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Vargas, M.A.; Rosales-Hernández, M.C.; Martínez-Fonseca, N.; Padilla-Martínez, I.; Fonseca-Sabater, Y.; Martínez-Ramos, F. 2-Acetyl-4-aminoresorcinol derivatives: Synthesis, antioxidant activity and molecular docking studies. Med. Chem. Res. 2018, 27, 1186–1197. [Google Scholar] [CrossRef]
- Skrzypek, A.; Matysiak, J.; Karpi’nska, M.; Czarnecka, K.; Kręcisz, P.; Stary, D.; Kukułowicz, J.; Paw, B.; Bajda, M.; Szymánski, P.; et al. Biological evaluation and molecular docking of novel 1,3,4-thiadiazole-resorcinol conjugates as multifunctional cholinesterases inhibitors. Bioorg. Chem. 2021, 107, 104617. [Google Scholar] [CrossRef] [PubMed]
- Borlon, C.; Debacq-Chainiaux, F.; Hinrichs, C.; Scharffetter-Kochanek, K.; Toussaint, O.; Wlaschek, M. The gene expression profile of psoralen plus UVA-induced premature senescence in skin fibroblasts resembles a combined DNA-damage and stress-induced cellular senescence response phenotype. Exp. Gerontol. 2007, 42, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Papadopoulou, A.; Fotopoulou, A.; Tsimelis, S.; Bassiony, H.; Yiacoumettis, A.M.; Panagiotou, P.N.; Pratsinis, H.; Kletsas, D. Down-Regulation of the Proteoglycan Decorin Fills in the Tumor-Promoting Phenotype of Ionizing Radiation-Induced Senescent Human Breast Stromal Fibroblasts. Cancers 2021, 13, 1987. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, H.; Armatas, A.; Dimozi, A.; Lefaki, M.; Vassiliu, P.; Kletsas, D. Paracrine anti-fibrotic effects of neonatal cells and living cell constructs on young and senescent human dermal fibroblasts. Wound Repair Regen. 2013, 21, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Pratsinis, H.; Haroutounian, S. Synthesis and antioxidant activity of 3-substituted guaiazulene derivatives. Nat. Prod. Lett. 2002, 16, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Konstantinou, A.; Kletsas, D. Long-term exposure to TNF-alpha leads human skin fibroblasts to a p38 MAPK- and ROS-mediated premature senescence. Biogerontology 2018, 19, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Ishkaeva, R.A.; Zoughaib, M.; Laikov, A.V.; Angelova, P.R.; Abdullin, T.I. Probing Cell Redox State and Glutathione-Modulating Factors Using a Monochlorobimane-Based Microplate Assay. Antioxidants 2022, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Liu, X.; Wang, H.; Kohyama, T.; Kobayashi, T.; Wen, F.Q.; Romberger, D.J.; Abe, S.; MacNee, W.; Rahman, I.; et al. Glutathione prevents inhibition of fibroblast-mediated collagen gel contraction by cigarette smoke. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L409–L417. [Google Scholar] [CrossRef] [PubMed]
- Khamrai, U.; Ronsheim, M.; Karak, S.K. Novel Benzodioxane and Benzoxazine Derivatives Useful as CC Chemokine Receptor Ligans. U.S. Patent 2010/0152160A1, 17 July 2010. [Google Scholar]
- Koufaki, M.; Kiziridi, C.; Alexi, X.; Alexis, M.N. Design and synthesis of novel neuroprotective 1,2-dithiolane/chroman hybrids. Bioorg. Med. Chem. 2009, 17, 6432–6441. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S. Methanolysis of Dimethyl (1-Diazo-2-oxopropyl) Phosphonate: Generation of Dimethyl (Diazomethyl) Phosphonate and Reaction with Carbonyl Compounds. Synth. Commun. 1989, 19, 561–564. [Google Scholar] [CrossRef]
- Muller, S.; Liepold, B.; Roth, G.J.; Bestmann, H.J. An Improved One-pot Procedure for the Synthesis of Alkynes from Aldehydes. Synlett 1996, 1996, 521–522. [Google Scholar] [CrossRef]
- Pietruszka, J.; Witt, A. Synthesis of the Bestmran–Ohgira Reagent. Synthesis 2006, 24, 4266–4268. [Google Scholar] [CrossRef]
- Koufaki, M.; Calogeropoulou, T.; Chondrogianni, N.; Papahatjis, D.; Gonos, E.; Fotopoulou, T.; Proussis, K.; Chazapi, E. Bioinspired Proteasome Activators with Antiageing Activity. EP3761950A1. Available online: https://worldwide.espacenet.com/patent/search/family/064901007/publication/EP3761950A1?q=EP3761950A1 (accessed on 28 June 2024).
- Tassano, E.; Alama, A.; Basso, A.; Dondo, G.; Galatini, A.; Riva, R.; Banfi, L. Conjugation of Hydroxytyrosol with Other Natural Phenolic Fragments: From Waste to Antioxidants and Antitumour Compounds. Eur. J. Org. Chem. 2015, 2015, 6710–6726. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, S.; Li, C.; Cui, L.; Ma, J.; Hui, Y. The non-canonical effects of heme oxygenase-1, a classical fighter against oxidative stress. Redox Biol. 2021, 47, 102170. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. Functions of NQO1 in Cellular Protection and CoQ(10) Metabolism and its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol. 2017, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Bruggisser, R.; von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002, 68, 445. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.; Cohen, I.; Campbell, M. Reduction of MTT by aqueous herbal extracts in the absence of cells. J. Ethnopharmacol. 2004, 93, 381. [Google Scholar] [CrossRef] [PubMed]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J. Biol. Chem. 2014, 289, 26882. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotopoulou, T.; Papadopoulou, A.; Tzani, A.; Mamais, M.; Mavrogonatou, E.; Pratsinis, H.; Koufaki, M.; Kletsas, D.; Calogeropoulou, T. Design and Synthesis of Novel Antioxidant 2-Substituted-5,7,8-Trimethyl-1,4-Benzoxazine Hybrids: Effects on Young and Senescent Fibroblasts. Antioxidants 2024, 13, 798. https://doi.org/10.3390/antiox13070798
Fotopoulou T, Papadopoulou A, Tzani A, Mamais M, Mavrogonatou E, Pratsinis H, Koufaki M, Kletsas D, Calogeropoulou T. Design and Synthesis of Novel Antioxidant 2-Substituted-5,7,8-Trimethyl-1,4-Benzoxazine Hybrids: Effects on Young and Senescent Fibroblasts. Antioxidants. 2024; 13(7):798. https://doi.org/10.3390/antiox13070798
Chicago/Turabian StyleFotopoulou, Theano, Adamantia Papadopoulou, Andromachi Tzani, Michail Mamais, Eleni Mavrogonatou, Harris Pratsinis, Maria Koufaki, Dimitris Kletsas, and Theodora Calogeropoulou. 2024. "Design and Synthesis of Novel Antioxidant 2-Substituted-5,7,8-Trimethyl-1,4-Benzoxazine Hybrids: Effects on Young and Senescent Fibroblasts" Antioxidants 13, no. 7: 798. https://doi.org/10.3390/antiox13070798







