Trehalose Protects against Superoxide Dismutase 1 Proteinopathy in an Amyotrophic Lateral Sclerosis Model
Abstract
:1. Introduction
2. Material and Methods
2.1. Yeast Strains and Growth Conditions
2.2. Induction of Oxidative Stress and Treatment with Trehalose
2.3. Quantification of hSod1 Inclusions
2.4. hSOD1 Activity
2.5. Cell Longevity
2.6. Intracellular Oxidation
3. Results and Discussion
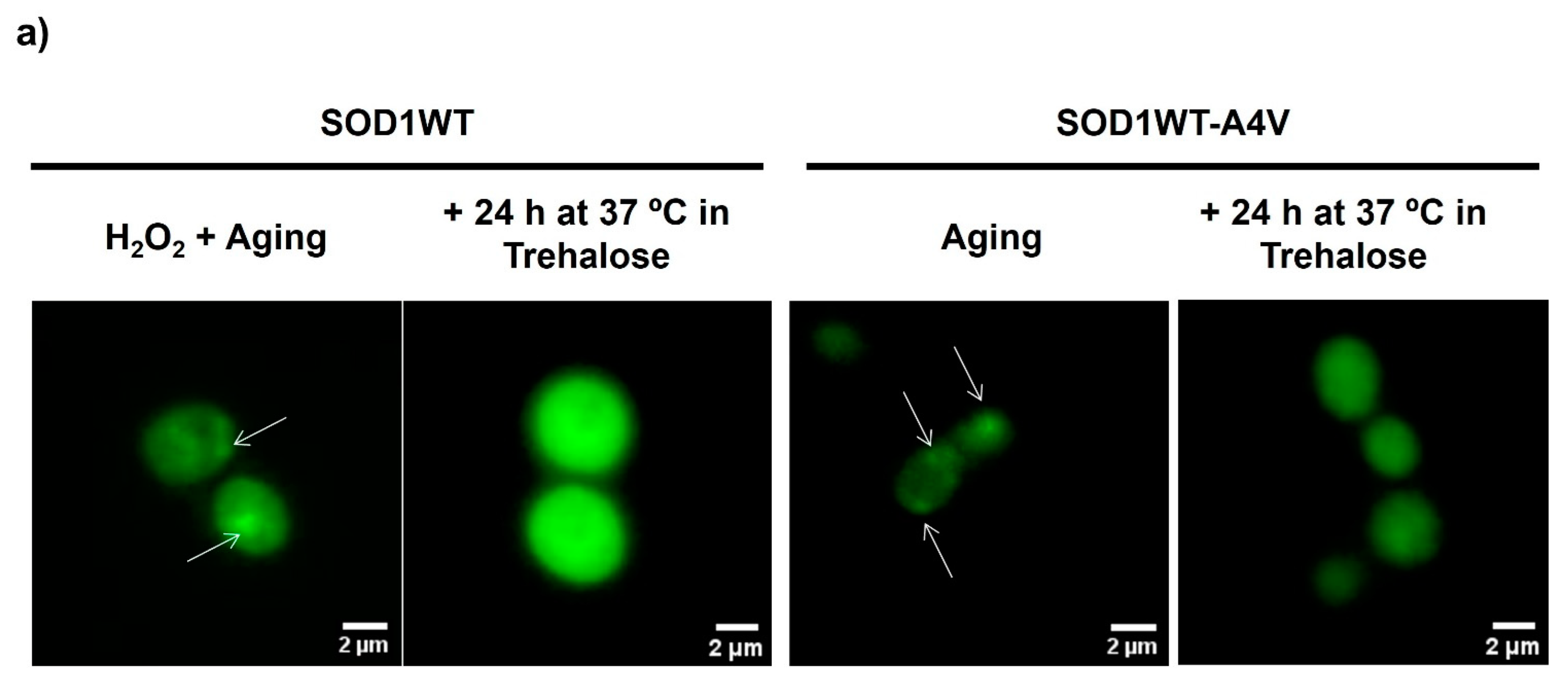
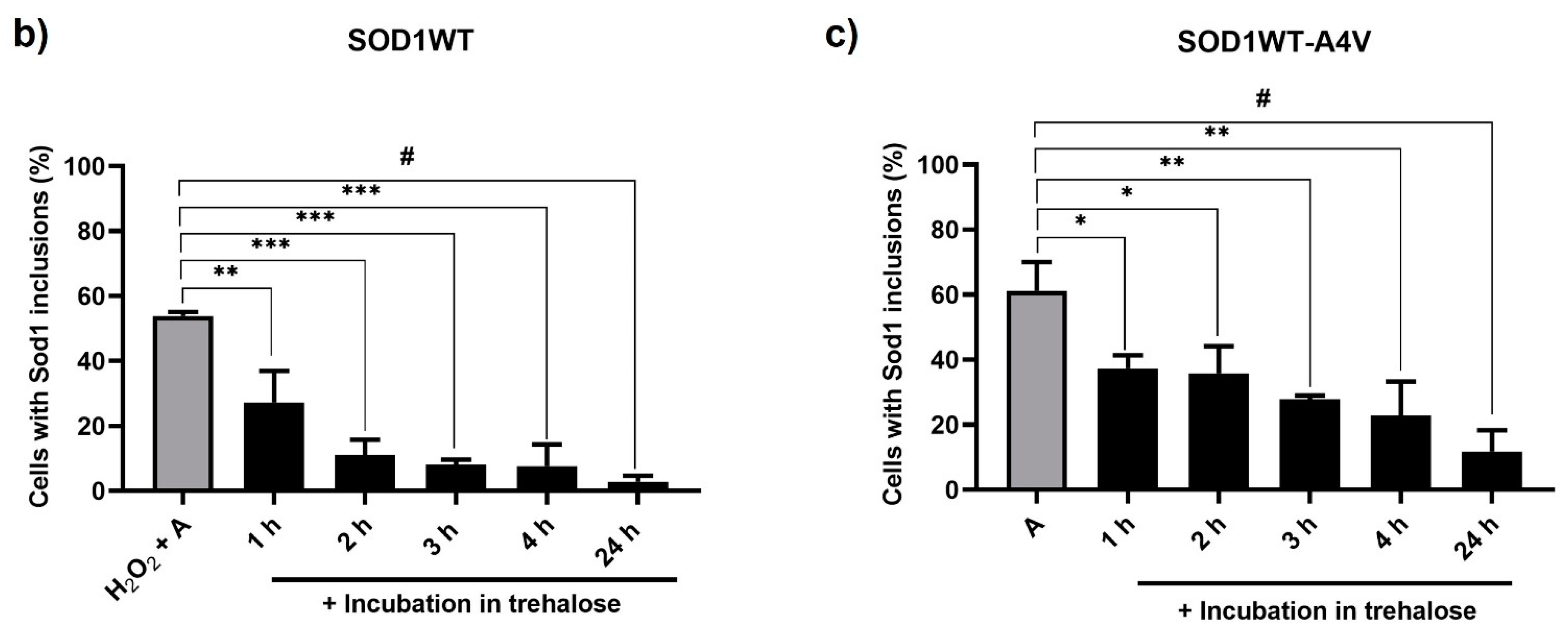
3.1. Treatment with Trehalose Decreased hSod1 Inclusions
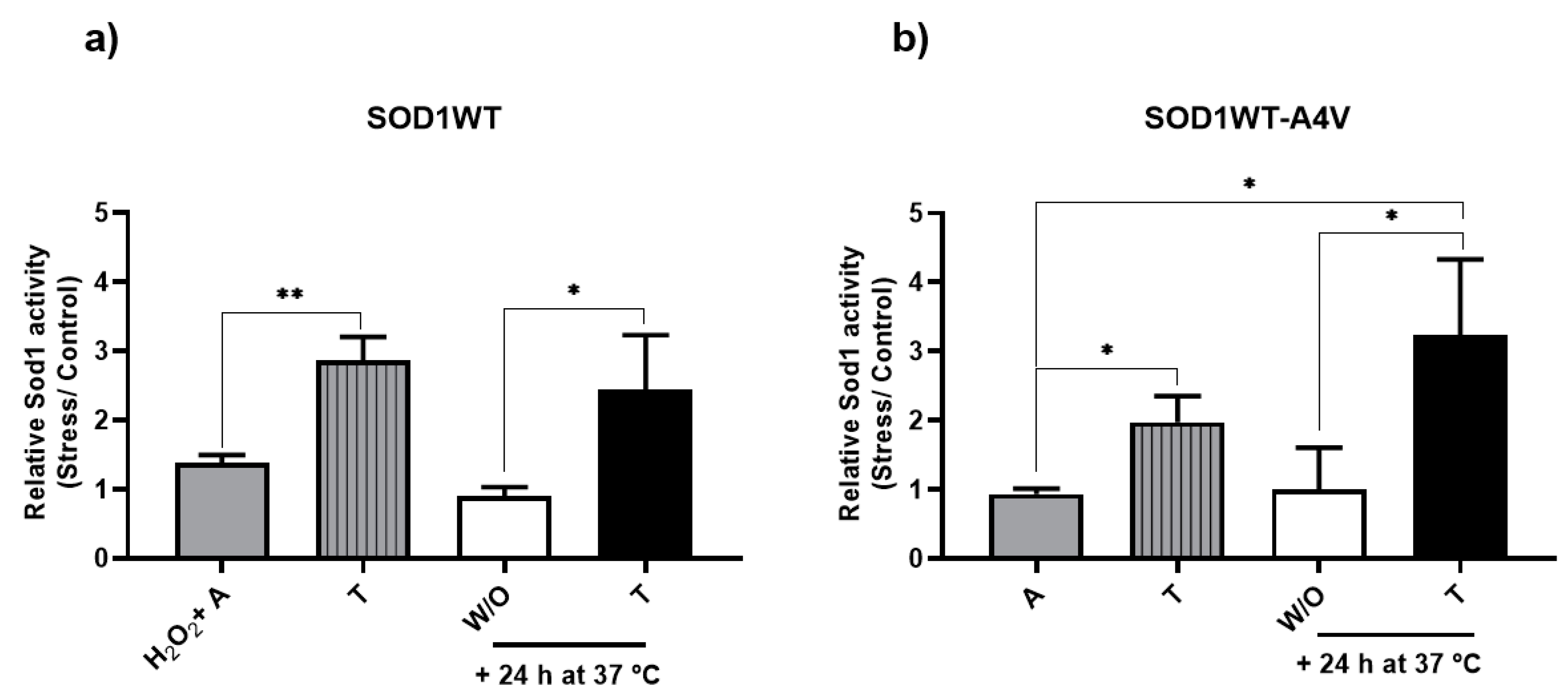
3.2. Treatment with Trehalose Increased Sod1 Activity
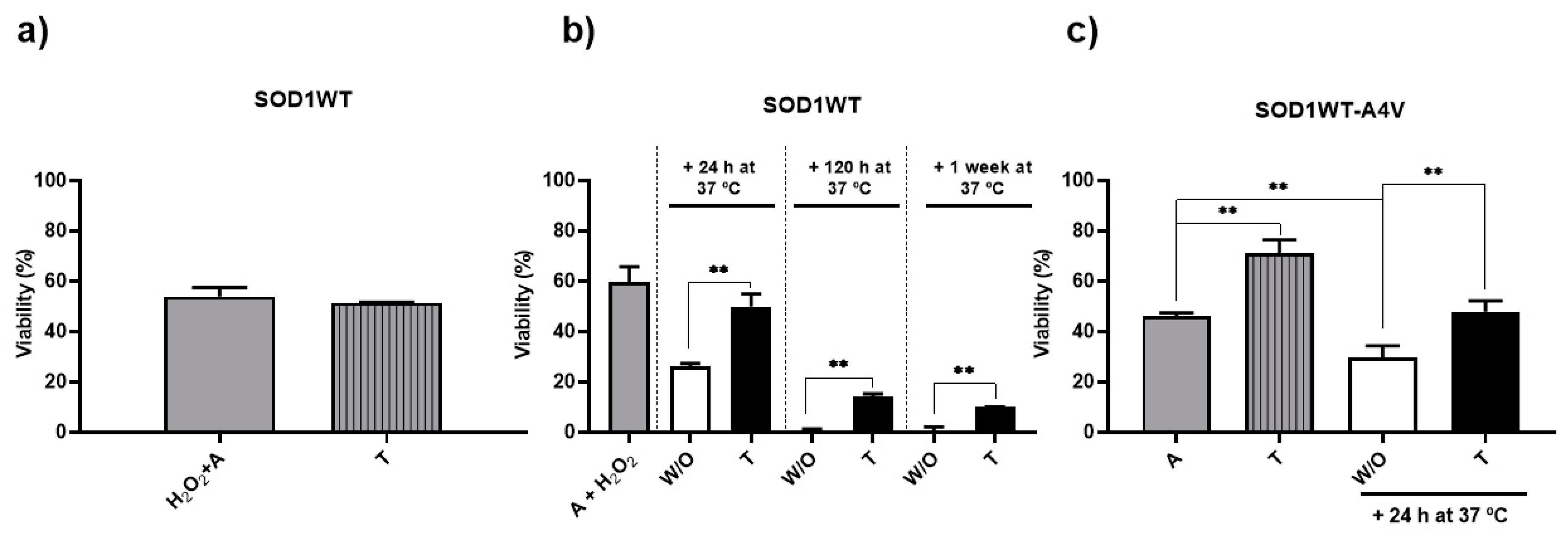
3.3. Trehalose Reduced Intracellular Oxidation and Increased Longevity
4. Final Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Liu, T.; Liu, L.; Yao, X.; Chen, L.; Fan, D.; Zhan, S.; Wang, S. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 2020, 267, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yang, J.; Wu, L.; Liu, Y.; Wang, X.; Zheng, Q.; Li, L. Accelerating drug development for amyotrophic lateral sclerosis: Construction and application of a disease course model using historical placebo group data. Orphanet J. Rare Dis. 2024, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Berdyński, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Żekanowski, C.; Kuźma-Kozakiewicz, M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, A.M.; Groen, E.J.; Koppers, M.; van den Berg, L.H.; Pasterkamp, R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013, 125, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Renton, A.E.; Chiò, A.; Traynor, B.J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 2014, 17, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Genoud, S.; Roudeau, S.; Rookyard, A.; Abdeen, A.; Cottam, V.; Hare, D.J.; White, M.; Altvater, J.; Fifita, J.A.; et al. Altered SOD1 maturation and post-translational modification in amyotrophic lateral sclerosis spinal cord. Brain 2022, 145, 3108–3130. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.C.A.; Silva Magalhães, R.S.; de Araújo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef] [PubMed]
- Brasil, A.A.; Magalhães, R.S.S.; De Carvalho, M.D.C.; Paiva, I.; Gerhardt, E.; Pereira, M.D.; Outeiro, T.F.; Eleutherio, E.C.A. Implications of fALS Mutations on Sod1 Function and Oligomerization in Cell Models. Mol. Neurobiol. 2018, 55, 5269–5281. [Google Scholar] [CrossRef] [PubMed]
- Brasil, A.A.; de Carvalho, M.D.C.; Gerhardt, E.; Queiroz, D.D.; Pereira, M.D.; Outeiro, T.F.; Eleutherio, E.C.A. Characterization of the activity, aggregation, and toxicity of heterodimers of WT and ALS-associated mutant Sod1. Proc. Natl. Acad. Sci. USA 2019, 116, 25991. [Google Scholar] [CrossRef]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. 2021, 60, 9215–9246. [Google Scholar] [CrossRef]
- Saccon, R.A.; Bunton-Stasyshyn, R.K.; Fisher, E.M.; Fratta, P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain 2013, 136, 2342–2358. [Google Scholar] [CrossRef] [PubMed]
- Onwe, R.O.; Onwosi, C.O.; Ezugworie, F.N.; Ekwealor, C.C.; Okonkwo, C.C. Microbial trehalose boosts the ecological fitness of biocontrol agents, the viability of probiotics during long-term storage and plants tolerance to environmental-driven abiotic stress. Sci. Total Environ. 2022, 806, 150432. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.D.; de Holanda Paranhos, L.; Eleutherio, E.C. Trehalose promotes biological fitness of fungi. Fungal Biol. 2024, in press. [Google Scholar] [CrossRef]
- Singer, M.A.; Lindquist, S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1998, 1, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yoon, Y.S.; Lee, S.J. Mechanism of neuroprotection by trehalose: Controversy surrounding autophagy induction. Cell Death Dis. 2018, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Pupyshev, A.B.; Klyushnik, T.P.; Akopyan, A.A.; Singh, S.K.; Tikhonova, M.A. Disaccharide trehalose in experimental therapies for neurodegenerative disorders: Molecular targets and translational potential. Pharmacol. Res. 2022, 183, 106373. [Google Scholar] [CrossRef] [PubMed]
- Castillo, K.; Nassif, M.; Valenzuela, V.; Rojas, F.; Matus, S.; Mercado, G.; Court, F.A.; van Zundert, B.; Hetz, C. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy 2013, 9, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.V.; Taylor, A.B.; Holloway, S.; Hart, P.J. Structures of mouse SOD1 and human/mouse SOD1 chimeras. Arch. Biochem. Biophys. 2010, 503, 183–190. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Wang, X.; Yu, X.; Duan, W.; Hong, K.; Wang, J.; Han, H.; Li, C. Trehalose decreases mutant SOD1 expression and alleviates motor deficiency in early but not end-stage amyotrophic lateral sclerosis in a SOD1-G93A mouse model. Neuroscience 2015, 298, 12–25. [Google Scholar] [CrossRef]
- Mannarino, S.C.; Amorim, M.A.; Pereira, M.D.; Moradas-Ferreira, P.; Panek, A.D.; Costa, V.; Eleutherio, E.C. Glutathione is necessary to ensure benefits of calorie restriction during ageing in Saccharomyces cerevisiae. Mech. Ageing. Dev. 2008, 129, 700–705. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Neto, J.R.; Ribeiro, G.D.; Magalhães, R.S.S.; Follmer, C.; Outeiro, T.F.; Eleutherio, E.C.A. Glycation modulates superoxide dismutase 1 aggregation and toxicity in models of sporadic amyotrophic lateral sclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166835. [Google Scholar] [CrossRef]
- Boyd, S.D.; Ullrich, M.S.; Calvo, J.S.; Behnia, F.; Meloni, G.; Winkler, D.D. Mutations in Superoxide Dismutase 1 (Sod1) Linked to familial amyotrophic lateral sclerosis can disrupt high-affinity zinc-binding promoted by the copper chaperone for Sod1 (Ccs). Molecules 2020, 25, 1086. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.; Brasil, A.A.; França, M.B.; de Almeida, D.S.G.; Rona, G.B.; Magalhães, R.S.S. Oxidative stress and aging: Learning from yeast lessons. Fungal Biol. 2018, 122, 514–525. [Google Scholar] [CrossRef]
- Pinmanee, P.; Sompinit, K.; Arnthong, J.; Suwannarangsee, S.; Jantimaporn, A.; Khongkow, M.; Nimchua, T.; Sukyai, P. Enhancing the Productivity and Stability of Superoxide Dismutase from Saccharomyces cerevisiae TBRC657 and Its Application as a Free Radical Scavenger. Fermentation 2022, 8, 169. [Google Scholar] [CrossRef]
- Petrov, D.; Daura, X.; Zagrovic, B. Effect of Oxidative Damage on the Stability and Dimerization of Superoxide Dismutase 1. Biophys. J. 2016, 110, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Nordström, U.; Tsiakas, K.; Keskin, I.; Elpers, C.; Mannil, M.; Heller, R.; Nolan, M.; Alburaiky, S.; Zetterström, P.; et al. The motor system is exceptionally vulnerable to absence of the ubiquitously expressed superoxide dismutase-1. Brain Commun. 2023, 5, fcad017. [Google Scholar] [CrossRef] [PubMed]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef]
- Herdeiro, R.S.; Pereira, M.D.; Panek, A.D.; Eleutherio, E.C. Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim. Biophys. Acta 2006, 1760, 340–346. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, X.; Yang, M.; Chen, L.; Tang, Z.; Wang, C.; Cui, Y. Trehalose Attenuates Oxidative Stress and Endoplasmic Reticulum Stress-Mediated Apoptosis in IPEC-J2 Cells Subjected to Heat Stress. Animals 2022, 12, 2093. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, R.S.S.; Monteiro Neto, J.R.; Ribeiro, G.D.; Paranhos, L.H.; Eleutherio, E.C.A. Trehalose Protects against Superoxide Dismutase 1 Proteinopathy in an Amyotrophic Lateral Sclerosis Model. Antioxidants 2024, 13, 807. https://doi.org/10.3390/antiox13070807
Magalhães RSS, Monteiro Neto JR, Ribeiro GD, Paranhos LH, Eleutherio ECA. Trehalose Protects against Superoxide Dismutase 1 Proteinopathy in an Amyotrophic Lateral Sclerosis Model. Antioxidants. 2024; 13(7):807. https://doi.org/10.3390/antiox13070807
Chicago/Turabian StyleMagalhães, Rayne S. S., José R. Monteiro Neto, Gabriela D. Ribeiro, Luan H. Paranhos, and Elis C. A. Eleutherio. 2024. "Trehalose Protects against Superoxide Dismutase 1 Proteinopathy in an Amyotrophic Lateral Sclerosis Model" Antioxidants 13, no. 7: 807. https://doi.org/10.3390/antiox13070807




