OxInflammatory Responses in the Wound Healing Process: A Systematic Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Guiding Question
2.2. Literary Research
2.3. Selection of Studies
2.4. Data Extraction
2.5. Bias Analysis
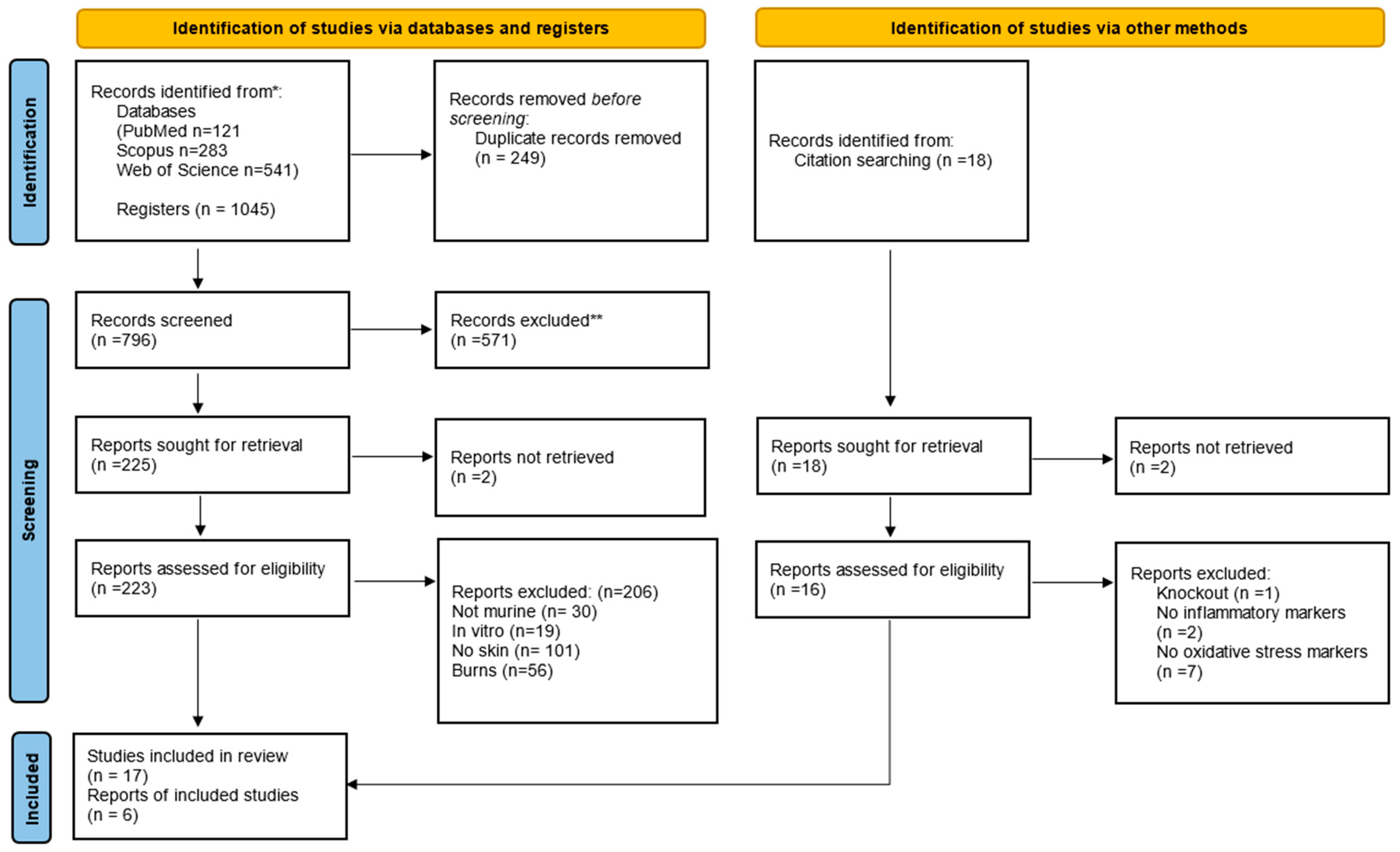
3. Results
3.1. Publication Characteristics
3.2. Characteristics of the Animal Models
3.3. Excisional Wound Characteristics
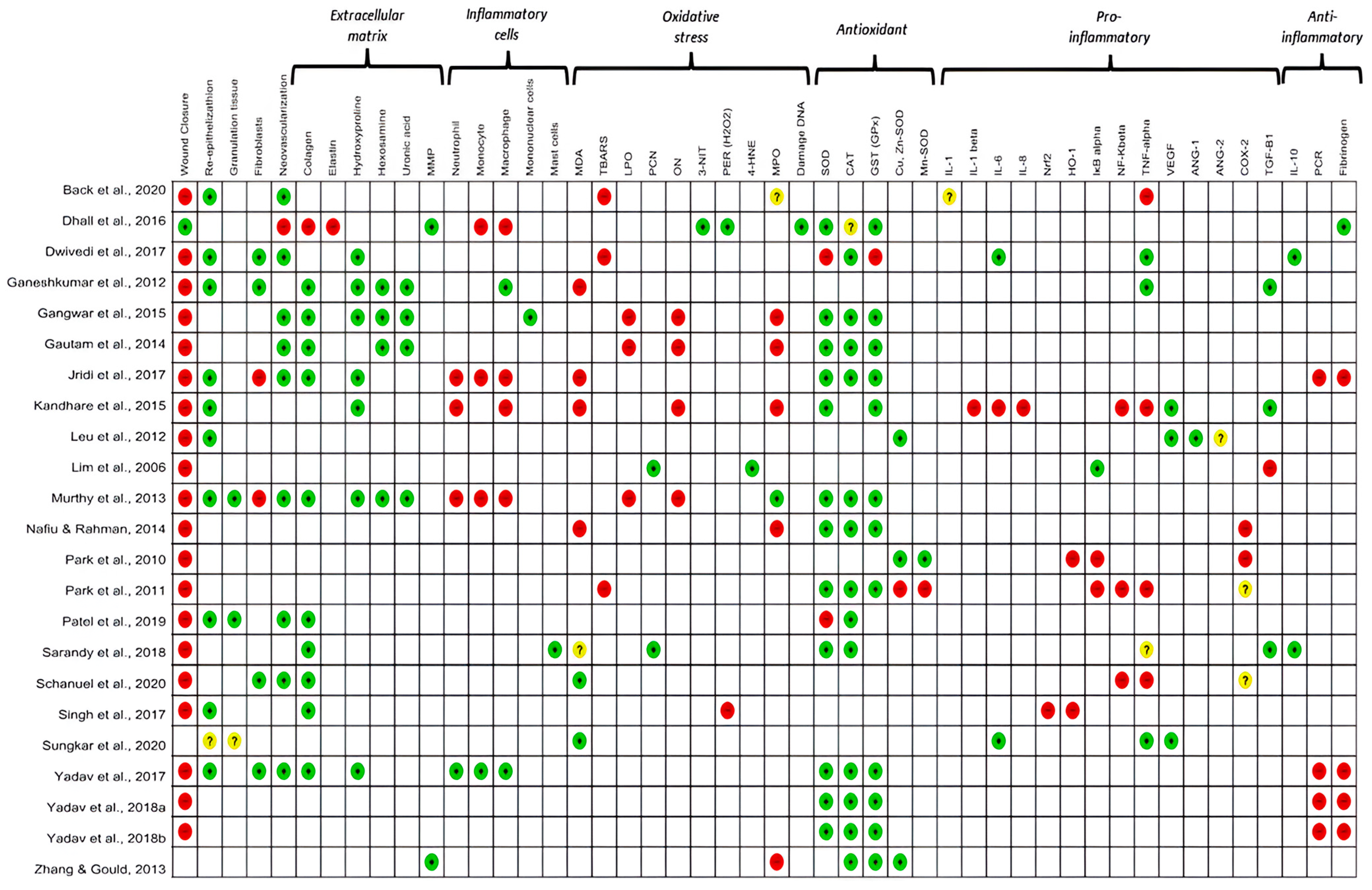
3.4. Main Biological Results
3.4.1. Oxidative Stress
3.4.2. Inflammation
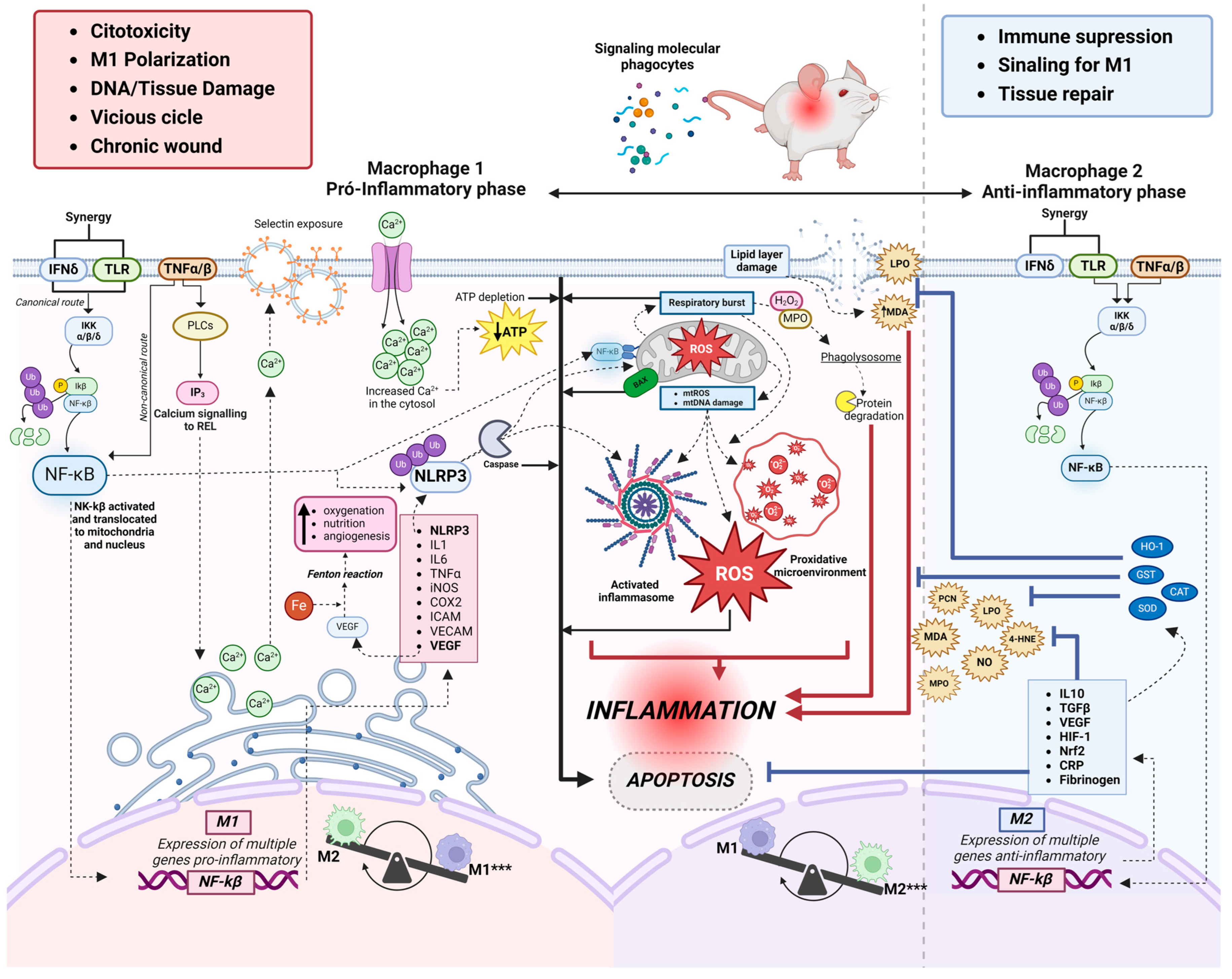
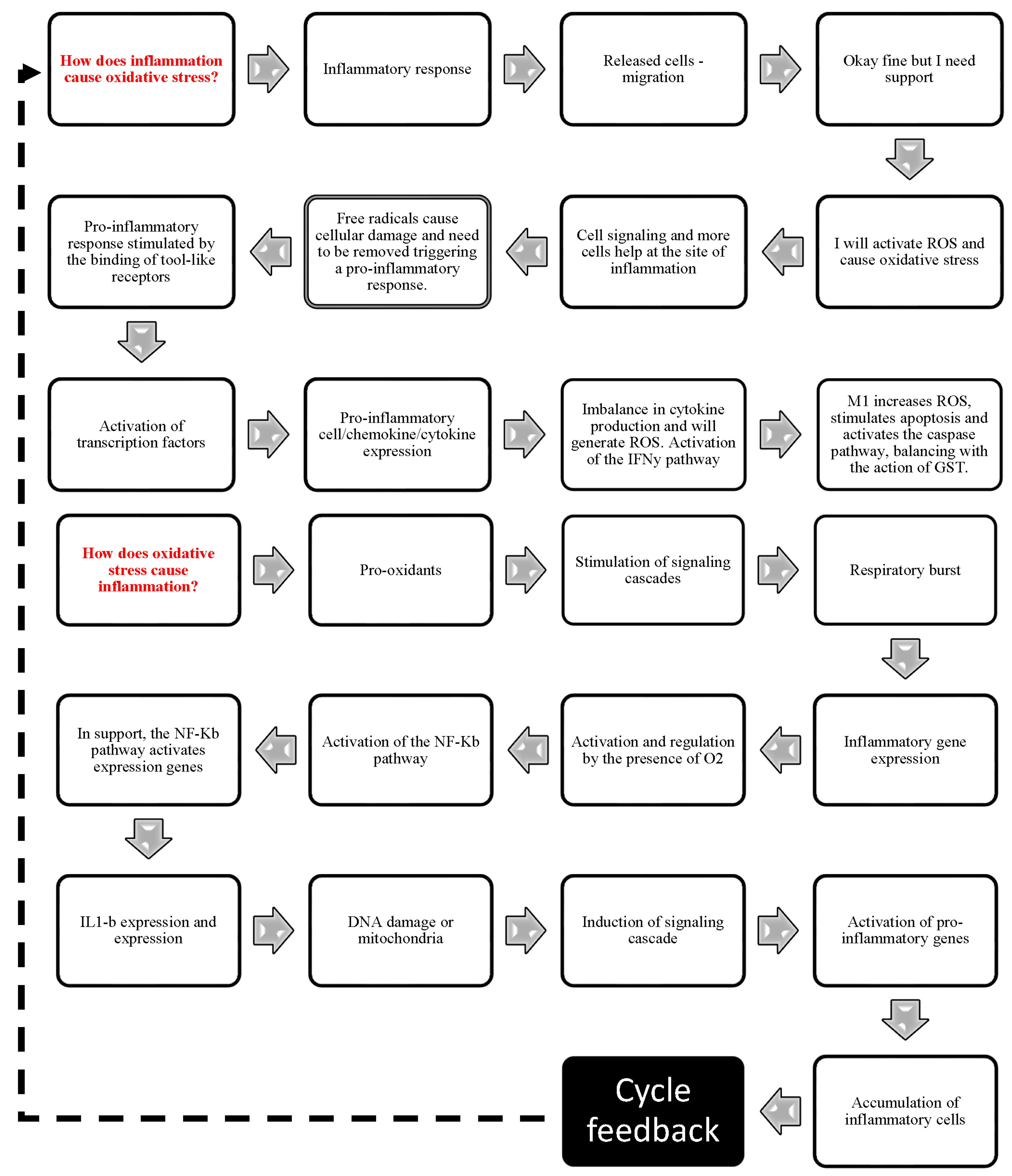
3.4.3. Relationship between Oxidative Stress and Inflammation
3.4.4. Classical Macrophages (M1)
3.4.5. Alternative Macrophages (M2)
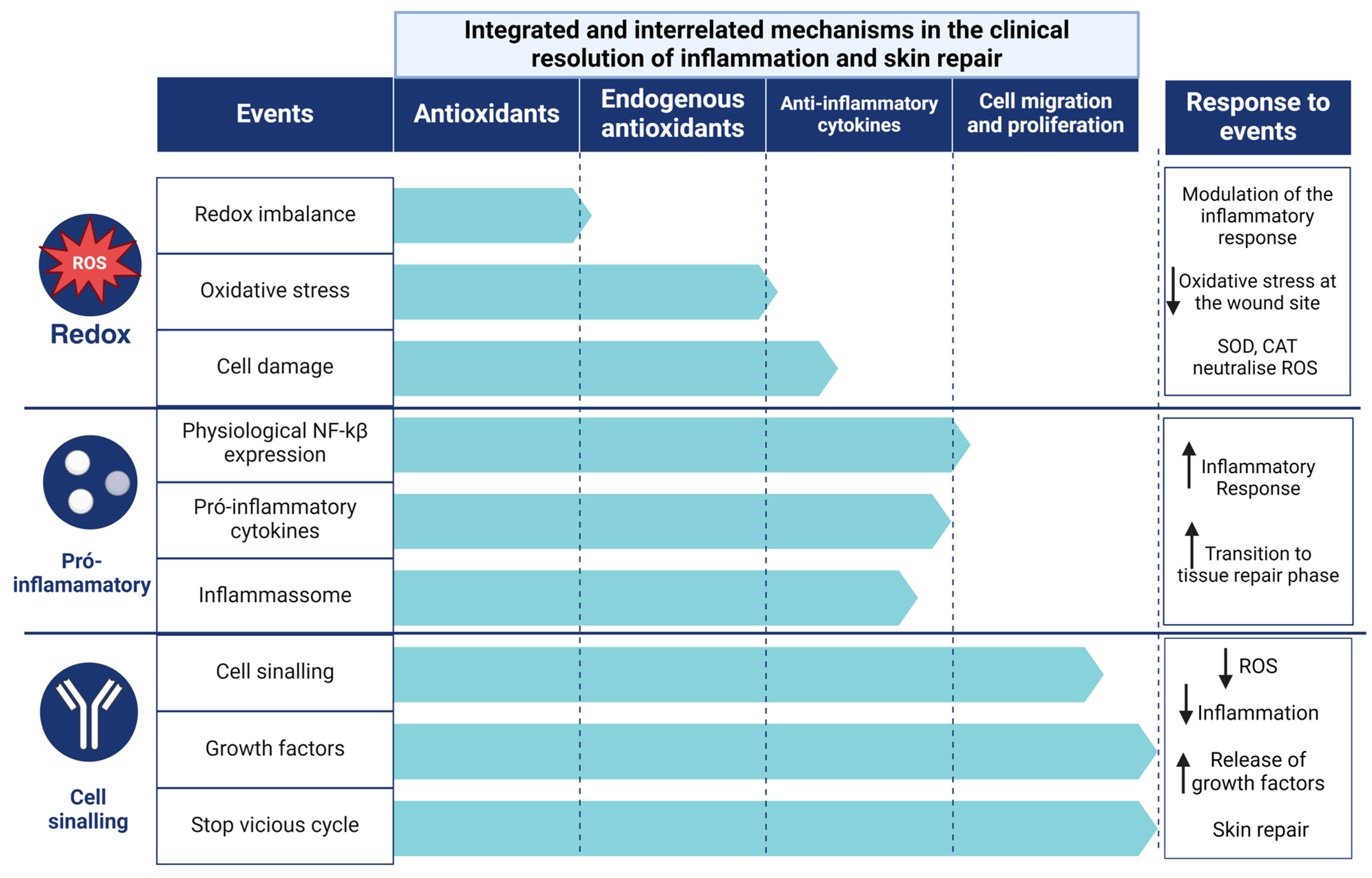
3.4.6. Clinical Perspectives
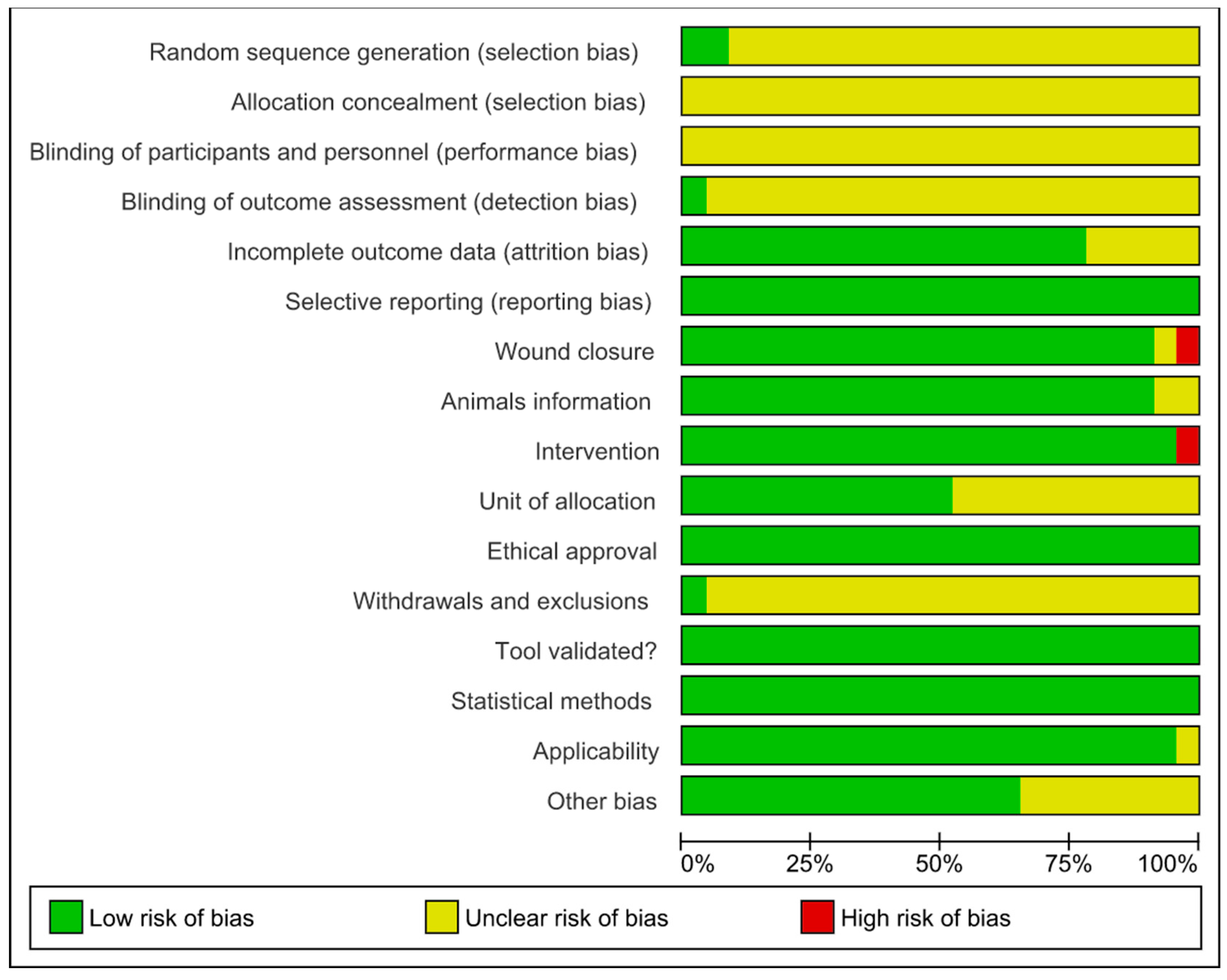
3.5. Risk of Bias and Methodological Quality Assessments
4. Discussion
4.1. General Characteristics of the Studies
4.2. Relationship between Inflammation and Oxidative Stress
4.3. Clinical Resolution of Inflammation and Skin Repair
4.4. Limitations
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lo, Z.J.; Lim, X.; Eng, D.; Car, J.; Hong, Q.; Yong, E.; Zhang, L.; Chandrasekar, S.; Tan, G.W.L.; Chan, Y.M.; et al. Clinical and Economic Burden of Wound Care in the Tropics: A 5-Year Institutional Population Health Review. Int. Wound J. 2020, 17, 790–803. [Google Scholar] [CrossRef]
- Altoé, L.S.; Alves, R.S.; Miranda, L.L.; Sarandy, M.M.; Bastos, D.S.S.; Gonçalves-Santos, E.; Novaes, R.D.; Gonçalves, R.V. Doxycycline Hyclate Modulates Antioxidant Defenses, Matrix Metalloproteinases, and COX-2 Activity Accelerating Skin Wound Healing by Secondary Intention in Rats. Oxidative Med. Cell. Longev. 2021, 2021, 4681041. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.S.; Alves, L.B.; Altoé, L.S.; Sarandy, M.M.; Freitas, M.B.; Silveira, N.J.F.; Novaes, R.D.; Gonçalves, R.V. Peptides from Animal Origin: A Systematic Review on Biological Sources and Effects on Skin Wounds. Oxidative Med. Cell. Longev. 2020, 2020, 4352761. [Google Scholar] [CrossRef]
- Laureano, A.; Rodrigues, A.M. Cicatrização De Feridas. Revista da SPDV-Educação Médica Contínua 2011, 69, 355. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Martin, P. Wound Healing—Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. New Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Stunova, A.; Vistejnova, L. Dermal Fibroblasts—A Heterogeneous Population with Regulatory Function in Wound Healing. Cytokine Growth Factor Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Meizlish, M.L.; Franklin, R.A.; Zhou, X.; Medzhitov, R. Tissue Homeostasis and Inflammation. Annu. Rev. Immunol. 2021, 39, 557–581. [Google Scholar] [CrossRef]
- Camps, J.; García-Heredia, A. Introduction: Oxidation and Inflammation, A Molecular Link Between Non-Communicable Diseases; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–4. [Google Scholar]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From Subclinical Condition to Pathological Biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Macrophage Polarity and Disease Control. Int. J. Mol. Sci. 2022, 23, 144. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M. The Many Faces of Macrophage Activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of Macrophage Polarization in Autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Covarrubias, A.; Byles, V.; Horng, T. ROS Sets the Stage for Macrophage Differentiation. Cell Res. 2013, 23, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E.C.; Walker, S.R.; Savery, K.; Frank, D.A.; Gaudet, S. Fold Change of Nuclear NF-ΚB Determines TNF-Induced Transcription in Single Cells. Mol. Cell 2014, 53, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-ΚB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of ProIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Ziblat, A.; Nuñez, S.Y.; Iraolagoitia, X.L.R.; Spallanzani, R.G.; Torres, N.I.; Sierra, J.M.; Secchiari, F.; Domaica, C.I.; Fuertes, M.B.; Zwirner, N.W. Interleukin (IL)-23 Stimulates IFN-γ Secretion by CD56bright Natural Killer Cells and Enhances IL-18-Driven Dendritic Cells Activation. Front. Immunol. 2018, 8, 1959. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, K.B.F.; Costa, N.M.B.; De Cássia Gonçalves Alfenas, R.; De Paula, S.O.; Minim, V.P.R.; Bressan, J. Estresse Oxidativo: Conceito, Implicações e Fatores Modulatórios. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Nery, R.A.; Kahlow, B.S.; Skare, T.L.; Tabushi, F.I.; do Amaral e Castro, A. Uric Acid and Tissue Repair. Arq. Bras. Cir. Dig. 2015, 28, 290–292. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of Wound Dressings Containing Silver on Skin and Immune Cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef]
- Cruvinel, W.M.; Mesquita Júnior, D.; Araújo, J.A.P.; Catelan, T.T.T.; de Souza, A.W.S.; da Silva, N.P.; Andrade, L.E.C. Sistema Imunitário: Parte I. Fundamentos Da Imunidade Inata Com Ênfase Nos Mecanismos Moleculares e Celulares Da Resposta Inflamatória. Rev. Bras. Reumatol. 2010, 50, 434–447. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (Sod), Catalase (Cat), Glutathione Peroxidase (Gpx), Glutathione-s-Transferase (Gst), and Nitric Oxide Synthase (Nos) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Da Mattosinhos, P.S. Potencial Anti-Inflamatório, Antioxidante e Regenerativo de Metabólitos Secundários de Brassicaceae Na Pele: Uma Revisão Sistemática; Universidade Federal de Viçosa: Viçosa, Brazil, 2022. [Google Scholar]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Mattosinhos, P.S.; Sarandy, M.M.; Novaes, R.D.; Esposito, D.; Gonçalves, R.V. Anti-Inflammatory, Antioxidant, and Skin Regenerative Potential of Secondary Metabolites from Plants of the Brassicaceae Family: A Systematic Review of In Vitro and In Vivo Preclinical Evidence (Biological Activities Brassicaceae Skin Diseases). Antioxidants 2022, 11, 1346. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.V.; Costa, A.M.A.; Grzeskowiak, L. Oxidative Stress and Tissue Repair: Mechanism, Biomarkers, and Therapeutics. Oxid. Med. Cell Longev. 2021, 2021, 6204096. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Grisham, M.B. Methods to Detect Nitric Oxide and Its Metabolites in Biological Samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Zaccardi, F.; Di Stasio, E.; Romitelli, F.; Santini, S.A.; Zuppi, C.; Ghirlanda, G. Oxidative Stress, Nitric Oxide, and Diabetes. Rev. Diabet. Stud. 2010, 7, 15–25. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 89, 11. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. Chin. J. Evid.-Based Med. 2014, 14, 1281–1285. [Google Scholar] [CrossRef]
- Back, P.I.; Balestrin, L.A.; Fachel, F.N.S.; Nemitz, M.C.; Falkembach, M.; Soares, G.; da Marques, M.S.; Silveira, T.; Dal Prá, M.; Horn, A.P.; et al. Hydrogels Containing Soybean Isoflavone Aglycones-Rich Fraction-Loaded Nanoemulsions for Wound Healing Treatment—In Vitro and in Vivo Studies. Colloids Surf. B Biointerfaces 2020, 196, 111301. [Google Scholar] [CrossRef]
- Dhall, S.; Alamat, R.; Castro, A.; Sarker, A.H.; Mao, J.H.; Chan, A.; Hang, B.; Martins-Green, M. Tobacco Toxins Deposited on Surfaces (Third Hand Smoke) Impair Wound Healing. Clin. Sci. 2016, 130, 1269–1284. [Google Scholar] [CrossRef]
- Dwivedi, D.; Dwivedi, M.; Malviya, S.; Singh, V. Evaluation of Wound Healing, Anti-Microbial and Antioxidant Potential of Pongamia Pinnata in Wistar Rats. J. Tradit. Complement. Med. 2017, 7, 79–85. [Google Scholar] [CrossRef]
- Ganeshkumar, M.; Ponrasu, T.; Krithika, R.; Iyappan, K.; Gayathri, V.S.; Suguna, L. Topical Application of Acalypha Indica Accelerates Rat Cutaneous Wound Healing by Up-Regulating the Expression of Type I and III Collagen. J. Ethnopharmacol. 2012, 142, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, M.; Gautam, M.K.; Ghildiyal, S.; Nath, G.; Goel, R.K. Mallotus Philippinensis Muell. Arg Fruit Glandular Hairs Extract Promotes Wound Healing on Different Wound Model in Rats. BMC Complement Altern. Med. 2015, 15, 123. [Google Scholar] [CrossRef]
- Gautam, M.K.; Purohit, V.; Agarwal, M.; Singh, A.; Goel, R.K. In Vivo Healing Potential of Aegle Marmelos in Excision, Incision, and Dead Space Wound Models. Sci. World J. 2014, 2014, 740107. [Google Scholar] [CrossRef] [PubMed]
- Jridi, M.; Sellimi, S.; Lassoued, K.B.; Beltaief, S.; Souissi, N.; Mora, L.; Toldra, F.; Elfeki, A.; Nasri, M.; Nasri, R. Wound Healing Activity of Cuttlefish Gelatin Gels and Films Enriched by Henna (Lawsonia Inermis) Extract. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 71–79. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Alam, J.; Patil, M.V.K.; Sinha, A.; Bodhankar, S.L. Wound Healing Potential of Naringin Ointment Formulation via Regulating the Expression of Inflammatory, Apoptotic and Growth Mediators in Experimental Rats. Pharm. Biol. 2016, 54, 419–432. [Google Scholar] [CrossRef]
- Leu, J.G.; Chen, S.A.; Chen, H.M.; Wu, W.M.; Hung, C.F.; Yao, Y.D.; Tu, C.S.; Liang, Y.J. The Effects of Gold Nanoparticles in Wound Healing with Antioxidant Epigallocatechin Gallate and α-Lipoic Acid. Nanomedicine 2012, 8, 767–775. [Google Scholar] [CrossRef]
- Lim, Y.; Phung, A.D.; Corbacho, A.M.; Aung, H.H.; Maioli, E.; Reznick, A.Z.; Cross, C.E.; Davis, P.A.; Valacchi, G. Modulation of Cutaneous Wound Healing by Ozone: Differences between Young and Aged Mice. Toxicol. Lett. 2006, 160, 127–134. [Google Scholar] [CrossRef]
- Murthy, S.; Gautam, M.K.; Goel, S.; Purohit, V.; Sharma, H.; Goel, R.K. Evaluation of in Vivo Wound Healing Activity of Bacopa Monniera on Different Wound Model in Rats. Biomed. Res. Int. 2013, 2013, 972028. [Google Scholar] [CrossRef]
- Nafiu, A.B.; Rahman, M.T. Anti-Inflammatory and Antioxidant Properties of Unripe Papaya Extract in an Excision Wound Model. Pharm. Biol. 2015, 53, 662–671. [Google Scholar] [CrossRef]
- Park, N.Y.; Valacchi, G.; Lim, Y. Effect of Dietary Conjugated Linoleic Acid Supplementation on Early Inflammatory Responses during Cutaneous Wound Healing. Mediat. Inflamm. 2010, 2010, 342328. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, S.M.; Jung, I.K.; Lim, Y.; Kim, J.H. Effects of Genistein on Early-Stage Cutaneous Wound Healing. Biochem. Biophys. Res. Commun. 2011, 410, 514–519. [Google Scholar] [CrossRef]
- Patel, M.; Nakaji-Hirabayashi, T.; Matsumura, K. Effect of Dual-Drug-Releasing Micelle–Hydrogel Composite on Wound Healing in Vivo in Full-Thickness Excision Wound Rat Model. J. Biomed. Mater. Res. A 2019, 107, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Sarandy, M.M.; Miranda, L.L.; Altoé, L.S.; Novaes, R.D.; Zanuncio, V.V.; Leite, J.P.V.; Gonçalves, R.V. Strychnos Pseudoquina Modulates the Morphological Reorganization of the Scar Tissue of Second Intention Cutaneous Wounds in Rats. PLoS ONE 2018, 13, e0195786. [Google Scholar] [CrossRef]
- Schanuel, F.S.; Romana-Souza, B.; Monte-Alto-Costa, A. Short-Term Administration of a High-Fat Diet Impairs Wound Repair in Mice. Lipids 2020, 55, 23–33. [Google Scholar] [CrossRef]
- Singh, S.V.B.; Park, H.; Khang, G.; Lee, D. Hydrogen Peroxide-Responsive Engineered Polyoxalate Nanoparticles for Enhanced Wound Healing. Macromol. Res. 2018, 26, 40–47. [Google Scholar] [CrossRef]
- Sungkar, A.; Widyatmoko, D.; Yarso, K.Y.; Wasita, B. The Effect of Duration of Wound Skin Tissue on Mda, Tnf-α, Il-6, Caspase 3, Vegf Levels, and Granulation Tissue Thickness in the White Rat (Rattus Novergicus). Bali Med. J. 2020, 9, 918–923. [Google Scholar] [CrossRef]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Attenuation of Dermal Wounds via Downregulating Oxidative Stress and Inflammatory Markers by Protocatechuic Acid Rich N-Butanol Fraction of Trianthema Portulacastrum Linn. in Wistar Albino Rats. Biomed. Pharmacother. 2017, 96, 86–97. [Google Scholar] [CrossRef]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Antioxidant and Anti-Inflammatory Properties of Prosopis Cineraria Based Phenolic Rich Ointment in Wound Healing. Biomed. Pharmacother. 2018, 108, 1572–1583. [Google Scholar] [CrossRef]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Ameliorative Effect of Biofabricated ZnO Nanoparticles of: Trianthema Portulacastrum Linn. on Dermal Wounds via Removal of Oxidative Stress and Inflammation. RSC Adv. 2018, 8, 21621–21635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gould, L.J. Hyperbaric Oxygen Reduces Matrix Metalloproteinases in Ischemic Wounds through a Redox-Dependent Mechanism. J. Investig. Dermatol. 2014, 134, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Arkan, M.C.; Bollrath, J.; Hsu, L.-C.; Goode, J.; Miething, C.; Göktuna, S.I.; Neuenhahn, M.; Fierer, J.; Paxian, S.; et al. NF-ΚB Is a Negative Regulator of IL-1β Secretion as Revealed by Genetic and Pharmacological Inhibition of IKKβ. Cell 2007, 130, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Criollo, A.; Senovilla, L.; Authier, H.; Maiuri, M.C.; Morselli, E.; Vitale, I.; Kepp, O.; Tasdemir, E.; Galluzzi, L.; Shen, S.; et al. The IKK Complex Contributes to the Induction of Autophagy. EMBO J. 2010, 29, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.K.; Lee, S.-J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy Proteins Regulate Innate Immune Responses by Inhibiting the Release of Mitochondrial DNA Mediated by the NALP3 Inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.-G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the Autophagy Protein Atg16L1 Enhances Endotoxin-Induced IL-1β Production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A Role for Mitochondria in NLRP3 Inflammasome Activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-ΚB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Balbino, C.A.; Pereira, L.M.; Curi, R. Mechanisms Involved in Wound Healing: A Revision. Braz. J. Pharm. Sci. 2005, 41, 27–51. [Google Scholar]
- Shi, Y.; Zhang, C.; Li, X. Traditional Medicine in India. J. Tradit. Chin. Med. Sci. 2021, 8, S51–S55. [Google Scholar] [CrossRef]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Indian Traditional Ayurvedic System of Medicine and Nutritional Supplementation. Evid.-Based Complement. Altern. Med. 2013, 2013, 376327. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.B.; Barroso, W.A.; Da Silva, N.N.; Silva, S.D.N.; Borges, A.C.R.; Abreu, I.C.; Borges, M.O.D.R. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int. J. Inflam. 2017, 2017, 3406215. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The Role of Oxidative Stress during Inflammatory Processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Pegoretti, V.; Swanson, K.A.; Bethea, J.R.; Probert, L.; Eisel, U.L.M.; Fischer, R. Inflammation and Oxidative Stress in Multiple Sclerosis: Consequences for Therapy Development. Oxid. Med. Cell Longev. 2020, 2020, 7191080. [Google Scholar] [CrossRef]
- Ramos-González, E.J.; Bitzer-Quintero, O.K.; Ortiz, G.; Hernández-Cruz, J.J.; Ramírez-Jirano, L.J. Relationship between Inflammation and Oxidative Stress and Its Effect on Multiple Sclerosis. Neurología 2021, 39, 292–301. [Google Scholar] [CrossRef]
- Bottino, D.A.; Lopes, F.G.; de Oliveira, F.J.; de Mecenas, A.S.; Clapauch, R.; Bouskela, E. Relationship between Biomarkers of Inflammation, Oxidative Stress and Endothelial/Microcirculatory Function in Successful Aging versus Healthy Youth: A Transversal Study. BMC Geriatr. 2015, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128032701. [Google Scholar]
- Alfaro, S.; Acuña, V.; Ceriani, R.; Cavieres, M.F.; Weinstein-Oppenheimer, C.R.; Campos-Estrada, C. Involvement of Inflammation and Its Resolution in Disease and Therapeutics. Int. J. Mol. Sci. 2022, 23, 10719. [Google Scholar] [CrossRef]
- Boaru, S.G.; Borkham-Kamphorst, E.; Tihaa, L.; Haas, U.; Weiskirchen, R. Expression Analysis of Inflammasomes in Experimental Models of Inflammatory and Fibrotic Liver Disease. J. Inflamm. 2012, 9, 49. [Google Scholar] [CrossRef]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns. J. Burn. Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.A.; Taggart, D.J.; Xu, G.; Fowler, J.D.; Wu, H.; Suo, Z. The Inhibitor of ΚB Kinase β (IKKβ) Phosphorylates IκBα Twice in a Single Binding Event through a Sequential Mechanism. J. Biol. Chem. 2023, 299, 102796. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Ghosh, P.; Bodhankar, S.L. Naringin, a Flavanone Glycoside, Promotes Angiogenesis and Inhibits Endothelial Apoptosis through Modulation of Inflammatory and Growth Factor Expression in Diabetic Foot Ulcer in Rats. Chem. Biol. Interact. 2014, 219, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Finnson, K.W.; Arany, P.R.; Philip, A. Transforming Growth Factor Beta Signaling in Cutaneous Wound Healing: Lessons Learned from Animal Studies. Adv. Wound Care 2013, 2, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Foreman, D.M.; Ferguson, M.W.J. Neutralisation of TGF-Β1 and TGF-Β2 or Exogenous Addition of TGF-Β3 to Cutaneous Rat Wounds Reduces Scarring. J. Cell Sci. 1995, 108, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Bian, Z.-M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-Inflammatory Cytokines Increase Reactive Oxygen Species through Mitochondria and NADPH Oxidase in Cultured RPE Cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef]
- Spencer, N.G.; Schilling, T.; Miralles, F.; Eder, C. Mechanisms Underlying Interferon-γ-Induced Priming of Microglial Reactive Oxygen Species Production. PLoS ONE 2016, 11, e0162497. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Adamiak, M.; Abdelbaset-Ismail, A.; Moore, J.B.; Zhao, J.; Abdel-Latif, A.; Wysoczynski, M.; Ratajczak, M.Z. Inducible Nitric Oxide Synthase (INOS) Is a Novel Negative Regulator of Hematopoietic Stem/Progenitor Cell Trafficking. Stem. Cell Rev. Rep. 2017, 13, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The Role of Nitric Oxide in Inflammation and Oxidative Stress. Immunopathol. Persa 2019, 5, e08. [Google Scholar] [CrossRef]
- Ahmed, I.; Ismail, N. M1 and M2 Macrophages Polarization via MTORC1 Influences Innate Immunity and Outcome of Ehrlichia Infection. J. Cell Immunol. 2020, 2, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.; Alharthi, H.; Alghamdi, A.; Sabico, S.; Al-Daghri, N.M. Role of NLRP3 Inflammasome Activation in Obesity-Mediated Metabolic Disorders. Int. J. Environ. Res. Public Health 2021, 18, 511. [Google Scholar] [CrossRef]
- Nosenko, M.A.; Ambaryan, S.G.; Drutskaya, M.S. Proinflammatory Cytokines and Skin Wound Healing in Mice. Mol. Biol. 2019, 53, 653–664. [Google Scholar] [CrossRef]
- Mauro, C.; Leow, S.C.; Anso, E.; Rocha, S.; Thotakura, A.K.; Tornatore, L.; Moretti, M.; De Smaele, E.; Beg, A.A.; Tergaonkar, V.; et al. NF-ΚB Controls Energy Homeostasis and Metabolic Adaptation by Upregulating Mitochondrial Respiration. Nat. Cell Biol. 2011, 18, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chisholm, A.D.C. Elegans Epidermal Wounding Induces a Mitochondrial ROS Burst That Promotes Wound Repair. Dev. Cell 2014, 31, 48–60. [Google Scholar] [CrossRef]
- Formentini, L.; Santacatterina, F.; Núñez de Arenas, C.; Stamatakis, K.; López-Martínez, D.; Logan, A.; Fresno, M.; Smits, R.; Murphy, M.P.; Cuezva, J.M. Mitochondrial ROS Production Protects the Intestine from Inflammation through Functional M2 Macrophage Polarization. Cell Rep. 2017, 19, 1202–1213. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Di Francesco, B.; Alesse, E.; Franzoso, G.; Zazzeroni, F. NF-ΚB and Mitochondria Cross Paths in Cancer: Mitochondrial Metabolism and Beyond. Semin. Cell Dev. Biol. 2019, 98, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Muliyil, S.; Narasimha, M. Mitochondrial ROS Regulates Cytoskeletal and Mitochondrial Remodeling to Tune Cell and Tissue Dynamics in a Model for Wound Healing. Dev. Cell 2014, 28, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 Inflammasome Activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Jin, C.; Flavell, R.A. Molecular Mechanism of NLRP3 Inflammasome Activation. J. Clin. Immunol. 2010, 30, 628–631. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Auf Dem Keller, U.; Kümin, A.; Braun, S.; Werner, S. Reactive Oxygen Species and Their Detoxification in Healing Skin Wounds. J. Investig. Dermatol. Symp. Proc. 2006, 11, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.; Schroder, K. NLRP3 Inflammasome Activation: The Convergence of Multiple Signalling Pathways on ROS Production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.-F.; Wang, Z.-C.; Lou, D.; Fang, Q.-Q.; Hu, Y.-Y.; Zhao, W.-Y.; Zhang, L.-Y.; Wu, L.-H.; Tan, W.-Q. Current Potential Therapeutic Strategies Targeting the TGF-β/Smad Signaling Pathway to Attenuate Keloid and Hypertrophic Scar Formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Zheng, D.; Ho, C.; Wen, D.; Sun, J.; Huang, L.; Liu, Y.; Li, Q.; Zhang, Y. HDAC5-mediated Smad7 silencing through MEF2A is critical for fibroblast activation and hypertrophic scar formation. Int. J. Biol. Sci. 2022, 18, 5724–5739. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Vabeiryureilai, M.; Lalrinzuali, K.; Jagetia, G.C. NF-ΚB and COX-2 Repression with Topical Application of Hesperidin and Naringin Hydrogels Augments Repair and Regeneration of Deep Dermal Wounds. Burns 2022, 48, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Trentin, D.S.; Silva, D.B.; Amaral, M.W.; Zimmer, K.R.; Silva, M.V.; Lopes, N.P.; Giordani, R.B.; Macedo, A.J. Tannins Possessing Bacteriostatic Effect Impair Pseudomonas Aeruginosa Adhesion and Biofilm Formation. PLoS ONE 2013, 8, e66257. [Google Scholar] [CrossRef] [PubMed]
- Trentin, D.D.S.; Giordani, R.B.; Zimmer, K.R.; Da Silva, A.G.; Da Silva, M.V.; Correia, M.T.D.S.; Baumvol, I.J.R.; MacEdo, A.J. Potential of Medicinal Plants from the Brazilian Semi-Arid Region (Caatinga) against Staphylococcus Epidermidis Planktonic and Biofilm Lifestyles. J. Ethnopharmacol. 2011, 137, 327–335. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, F.B.; Sarandy, M.M.; Novaes, R.D.; Valacchi, G.; Gonçalves, R.V. OxInflammatory Responses in the Wound Healing Process: A Systematic Review. Antioxidants 2024, 13, 823. https://doi.org/10.3390/antiox13070823
Lopes FB, Sarandy MM, Novaes RD, Valacchi G, Gonçalves RV. OxInflammatory Responses in the Wound Healing Process: A Systematic Review. Antioxidants. 2024; 13(7):823. https://doi.org/10.3390/antiox13070823
Chicago/Turabian StyleLopes, Fernanda Barbosa, Mariáurea Matias Sarandy, Rômulo Dias Novaes, Giuseppe Valacchi, and Reggiani Vilela Gonçalves. 2024. "OxInflammatory Responses in the Wound Healing Process: A Systematic Review" Antioxidants 13, no. 7: 823. https://doi.org/10.3390/antiox13070823





