Apple Blossom Agricultural Residues as a Sustainable Source of Bioactive Peptides through Microbial Fermentation Bioprocessing
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Microorganisms and Culture Conditions
2.3. Fermentation of Apple Flower
2.4. Carbohydrate, Organic Acid, and Ethanol Quantification
2.5. Total Protein Quantification
2.6. Low-Molecular-Weight (LMW) Peptide Profiling and Quantification
2.7. Identification of Low-Molecular-Weight Peptides by UHPLC/HRMS2
2.8. Amino Acid Profiling
2.9. Total Free Phenolic Compound Quantification
2.10. Identification and Quantification of Free Phenolic Compounds
2.11. In Vitro Antifungal Activity
2.12. In Vitro Antioxidant Assays
2.13. Statistical Analysis
3. Results
3.1. Apple Flower Fermentation
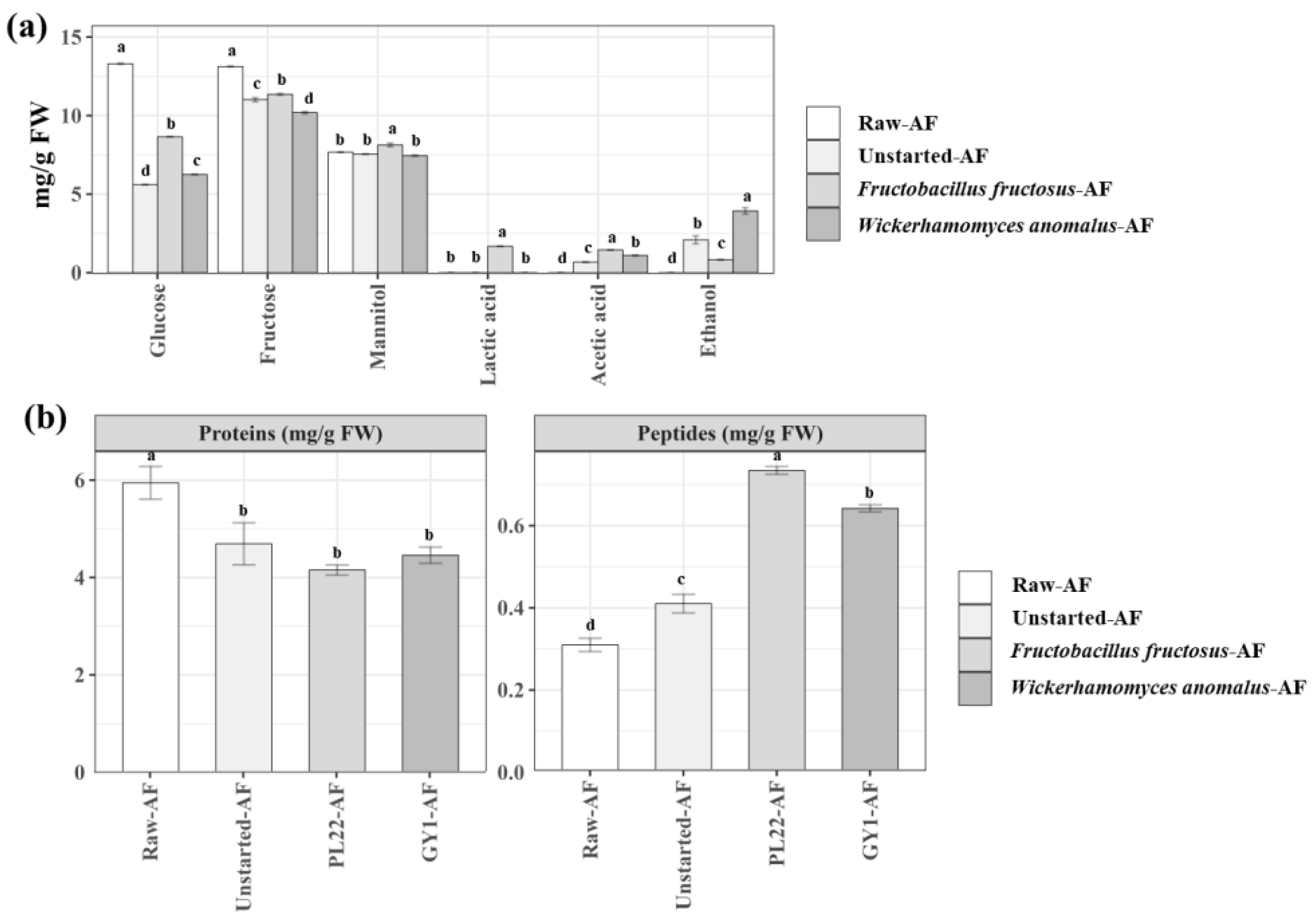
3.2. Analysis of Carbohydrate, Organic Acid, and Ethanol Concentrations
3.3. Total Protein and Peptide Profiles
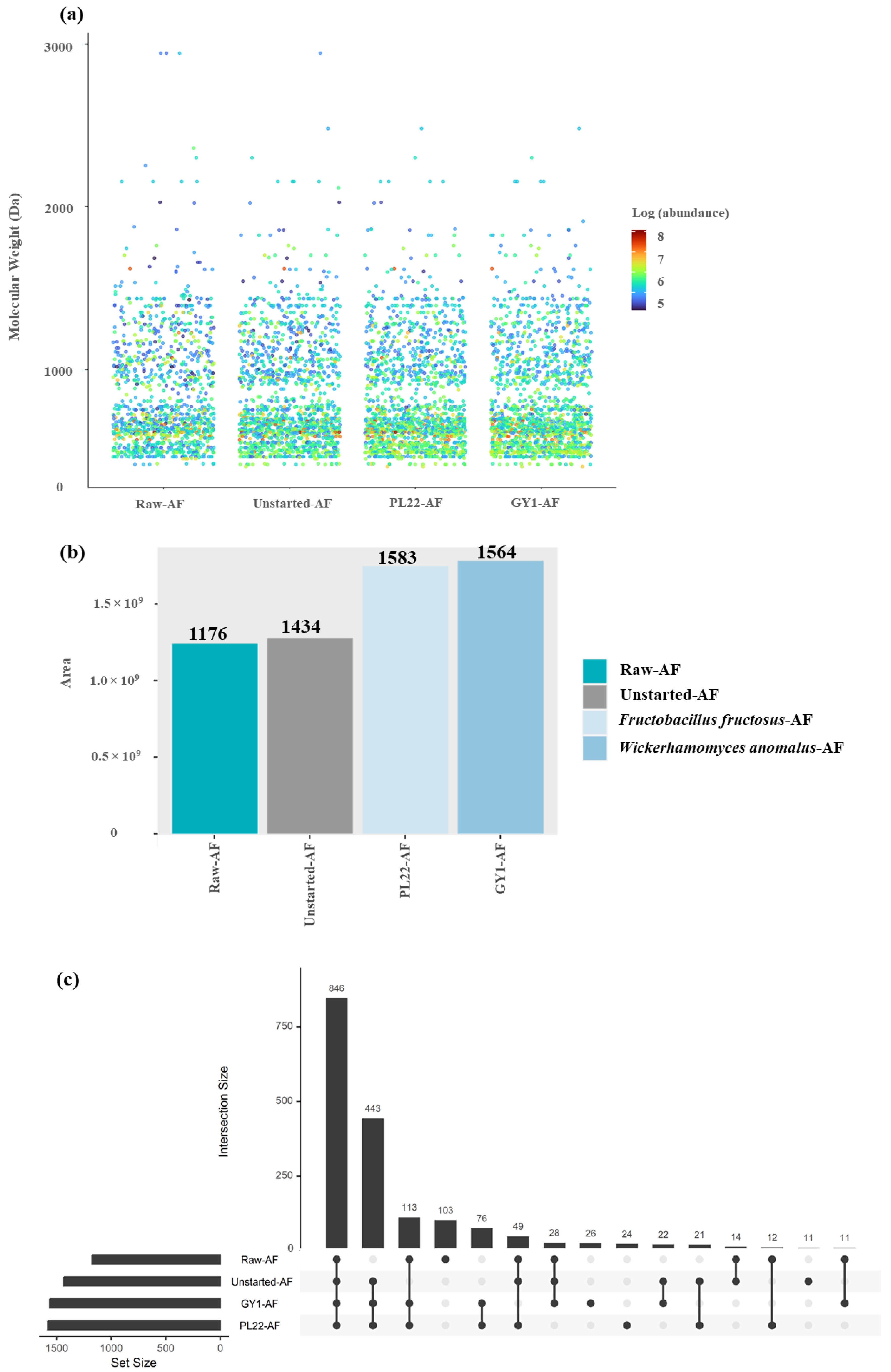
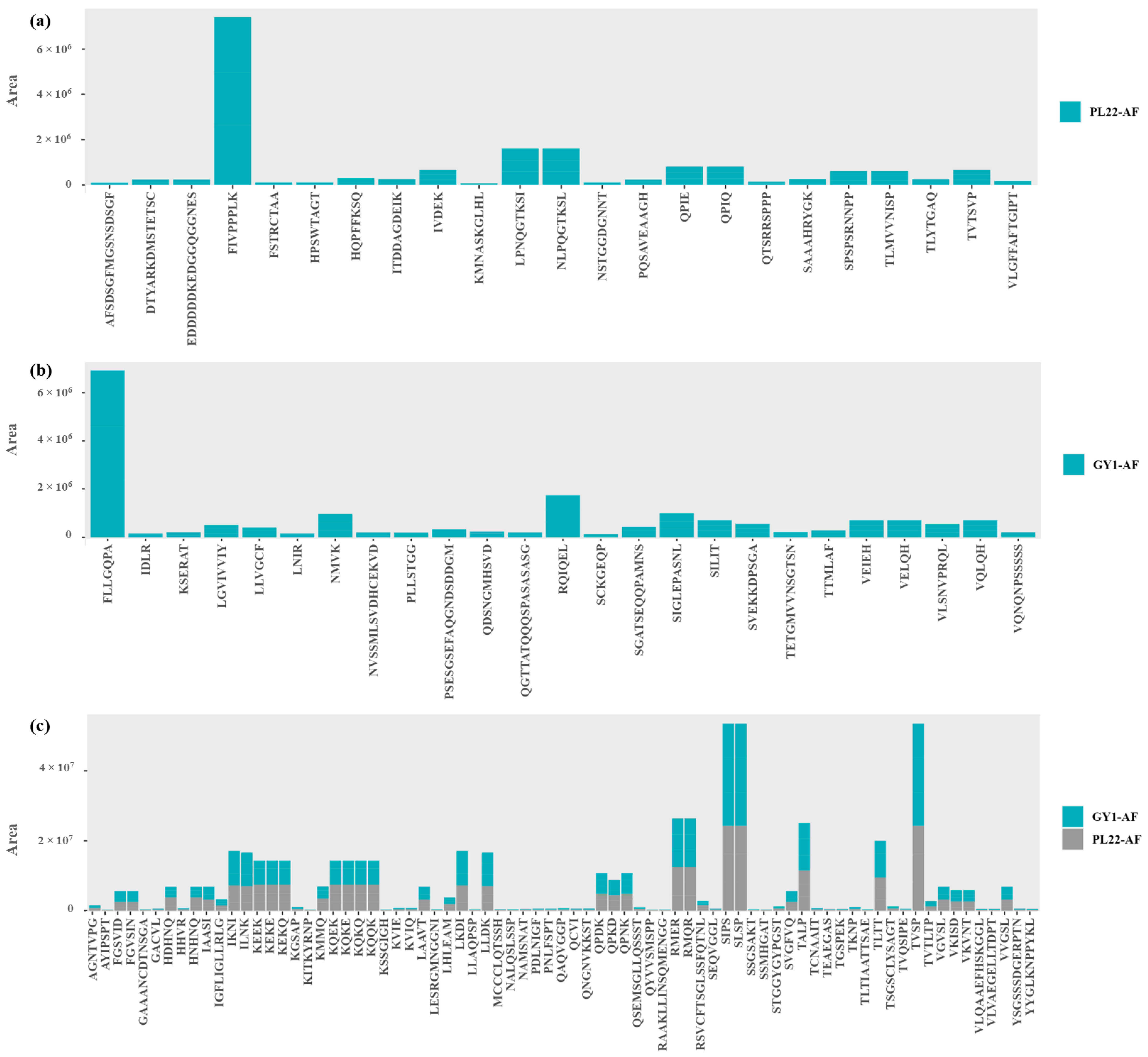
3.4. Peptidomic Analysis
3.5. Amino Acid Profile
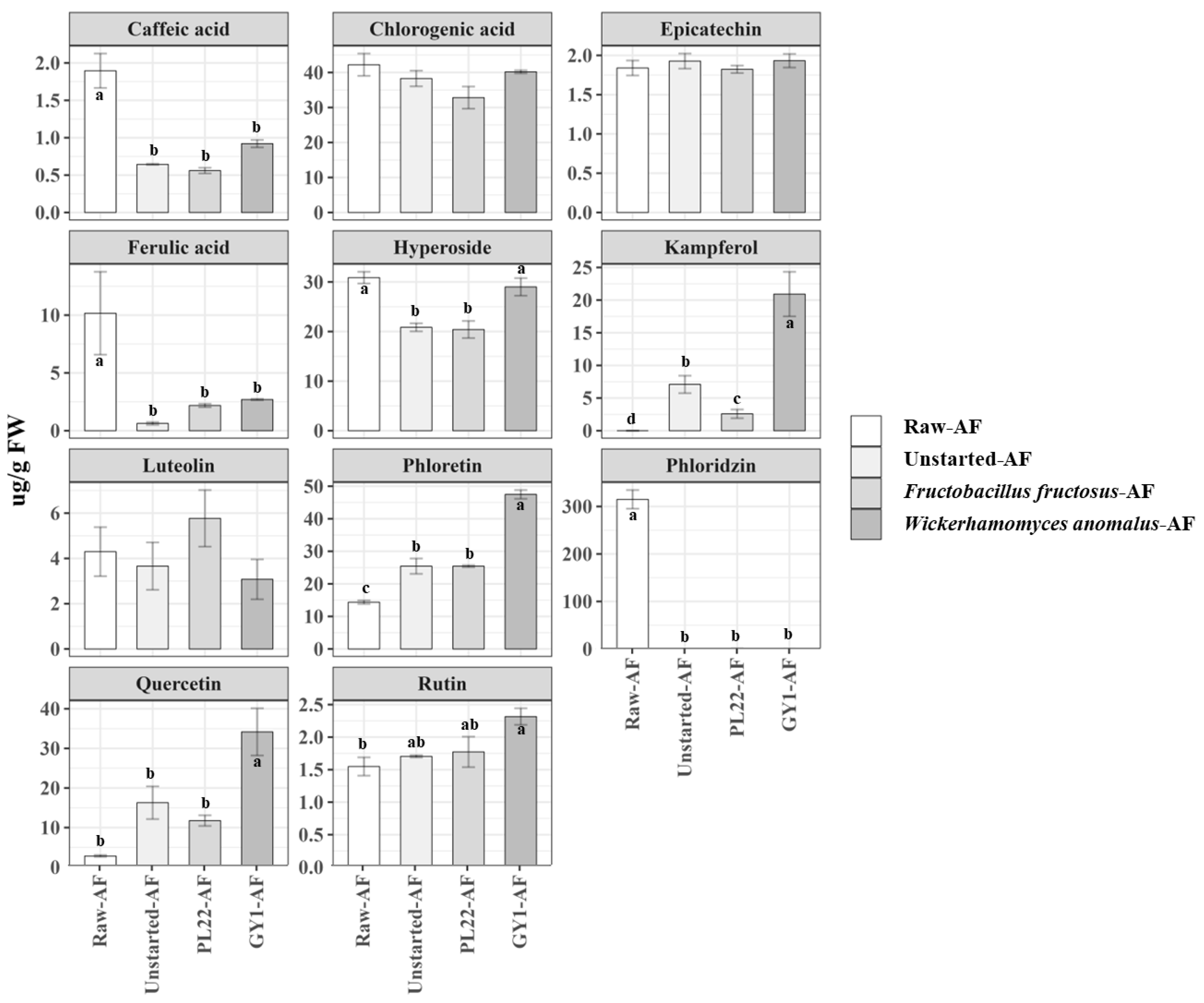
3.6. Total Free Phenolic Content
3.7. Phenolic Compound Profile
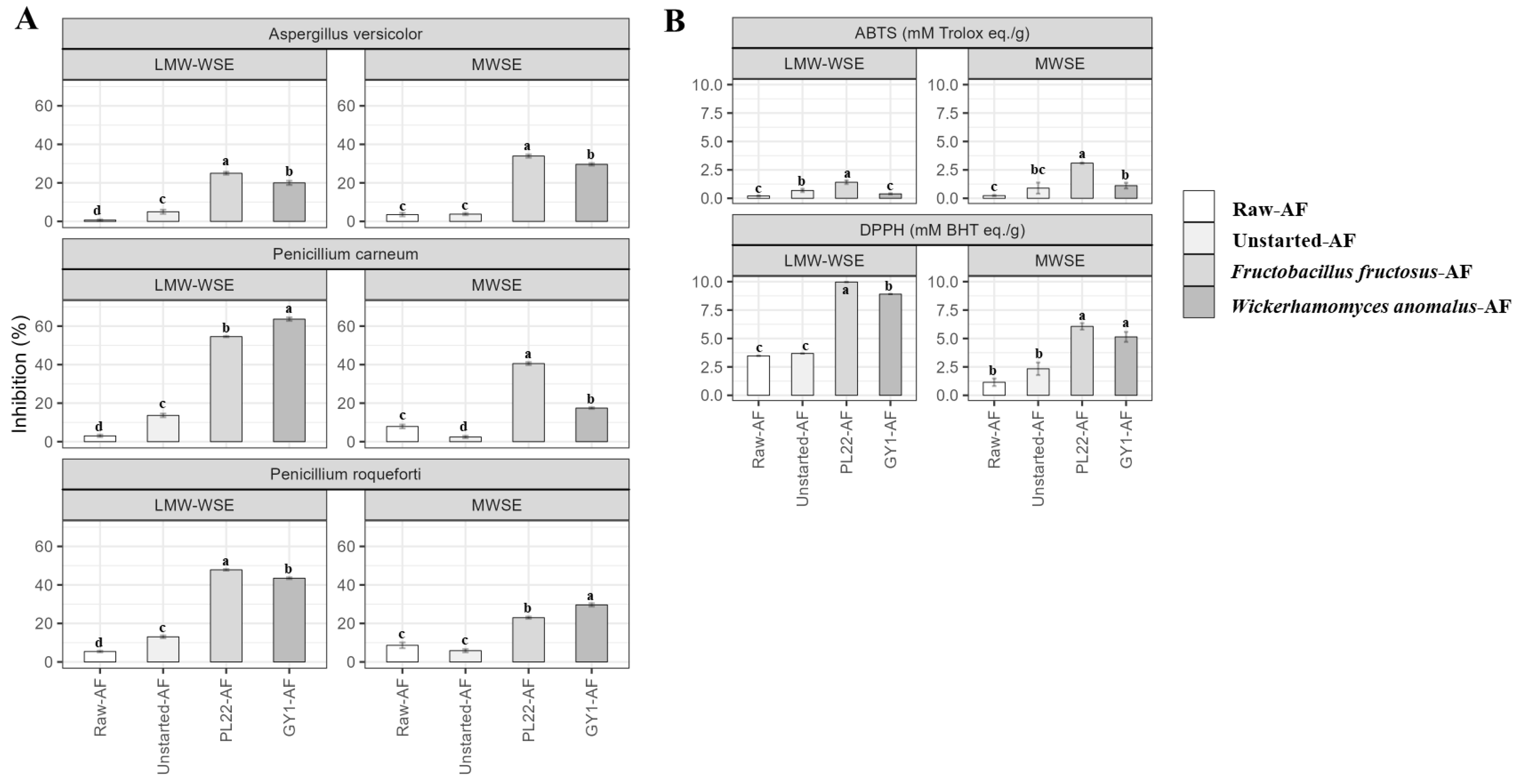
3.8. Antifungal Activity
3.9. Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aleklett, K.; Hart, M.; Shade, A. The microbial ecology of flowers: An emerging frontier in phyllosphere research. Botany 2014, 92, 253–266. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ricci, D.; Giomaro, G. Flowers volatile profile of a rare red apple tree from Marche region (Italy). J. Oleo Sci. 2014, 63, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Hehnen, D.; Hanrahan, I.; Lewis, K.; McFerson, J.; Blanke, M. Mechanical flower thinning improves fruit quality of apples and promotes consistent bearing. Sci. Hortic. 2012, 134, 241–244. [Google Scholar] [CrossRef]
- Duan, Y.; Mehariya, S.; Kumar, A.; Singh, E.; Yang, J.; Kumar, S.; Li, H.; Awasthi, M.K. Apple orchard waste recycling and valorization of valuable product-A review. Bioengineered 2021, 12, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, J.J.; Zhang, X.; Zhang, N.S.; Guo, Y.P.; Ren, X.L.; Mei, L.X. Analysis of nutrients, bioactive compounds, and antioxidants in apple flowers at two stages of flowering. Acta Hortic. 2019, 1261, 109–114. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. Novel solid-state fermentation of bee-collected pollen emulating the natural fermentation process of bee bread. Food Microbiol. 2019, 82, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Dey, G. Studies on Value-added Fermentation of Madhucalatifolia Flower and Its potential as a Nutrabeverage. Int. J. Biotechnol. Bioeng. Res. 2013, 4, 215–226. [Google Scholar]
- Sedláčková, V.H.; Grygorieva, O.; Fatrcová-Šramková, K.; Vergun, O.; Vinogradova, Y.; Ivanišová, E.; Brindza, J. The morphological and antioxidant characteristics of inflorescences within wild-growing genotypes of elderberry (Sambucus nigra L.). Potravinarstvo 2018, 12, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Kalemba-Drozdz, M.; Kwiecien, I.; Szewczyk, A.; Cierniak, A.; Grzywacz-Kisielewska, A. Fermented Vinegars from Apple Peels, Raspberries, Rosehips, Lavender, Mint, and Rose Petals: The Composition, Antioxidant Power, and Genoprotective Abilities in Comparison to Acetic Macerates, Decoctions, and Tinctures. Antioxidants 2020, 9, 1121. [Google Scholar] [CrossRef]
- Rahman, Z.; Aeri, V. Enhancement of lutein content in Calendula officinalis Linn. By solid-state fermentation with lactobacillus species. J. Food Sci. Technol. 2022, 59, 4794–4800. [Google Scholar] [CrossRef]
- Mendonça, G.R.; Pinto, R.A.; Praxedes, É.A.; Abreu, V.K.G.; Dutra, R.P.; Pereira, A.F.; Lemos, T.D.O.; Reis, A.S.D.; Pereira, A.L.F. Kombucha based on unconventional parts of the Hibiscus sabdariffa L.: Microbiological, physico-chemical, antioxidant activity, cytotoxicity and sensorial characteristics. Int. J. Gastron. Food Sci. 2023, 34, 100804. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Chang, L. Effects of lactic acid bacteria fermentation on the phytochemicals content, taste and aroma of blended edible rose and shiitake beverage. Food Chem. 2023, 405, 134722. [Google Scholar] [CrossRef] [PubMed]
- Tlais, A.Z.A.; Kanwal, S.; Filannino, P.; Albiac, M.A.; Gobbetti, M.; Di Cagno, R. Effect of sequential or ternary starters-assisted fermentation on the phenolic and glucosinolate profiles of sauerkraut in comparison with spontaneous fermentation. Food Res. Int. 2022, 156, 111116. [Google Scholar] [CrossRef] [PubMed]
- Albiac, M.A.; Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. How fructophilic lactic acid bacteria may reduce the FODMAPs content in wheat-derived baked goods: A proof of concept. Microb. Cell. Fact. 2020, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Addante, R.; Pontonio, E.; Gobbetti, M. Metabolism of Fructophilic Lactic Acid Bacteria Isolated from the Apis mellifera L. Bee Gut: Phenolic Acids as External Electron Acceptors. Appl. Environ. Microbiol. 2016, 82, 6899–6911. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Jadhav, A.; Patil, U. Functional characterization and in vitro screening of Fructobacillus fructosus MCC 3996 isolated from Butea monosperma flower for probiotic potential. Lett. Appl. Microbiol. 2020, 70, 331–339. [Google Scholar] [CrossRef]
- De Simone, N.; Rocchetti, M.T.; la Gatta, B.; Spano, G.; Drider, D.; Capozzi, V.; Russo, P.; Fiocco, D.; Properties, A. Functional Characterisation and Application of Fructobacillus fructosus and Lactiplantibacillus plantarum Isolated from Artisanal Honey. Probiotics Antimicrob. Proteins 2023, 15, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Simsek, D.; Kiymaci, M.E.; Tok, K.C.; Gumustas, M.; Altanlar, N. Investigation of the probiotic and metabolic potential of Fructobacillus tropaeoli and Apilactobacillus kunkeei from apiaries. Arch. Microbiol. 2022, 204, 432. [Google Scholar] [CrossRef]
- Verón, H.E.; Di Risio, H.D.; Isla, M.I.; Torres, S. Isolation and selection of potential probiotic lactic acid bacteria from Opuntia ficus-indica fruits that grow in Northwest Argentina. LWT 2017, 84, 231–240. [Google Scholar] [CrossRef]
- Romeo, F.V.; Granuzzo, G.; Foti, P.; Ballistreri, G.; Caggia, C.; Rapisarda, P. Microbial Application to Improve Olive Mill Wastewater Phenolic Extracts. Molecules 2021, 26, 1944. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, W.; Duan, M.; She, X.; Zhu, S.; Zhou, X.; Song, J.; Zhu, D. Effects of exogenous strain fermentation on protein structure and allergenicity of Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.). Food Biosci. 2023, 53, 102541. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.; Manzanares, P. Challenges of the Non-Conventional Yeast Wickerhamomyces anomalus in Winemaking. Fermentation 2018, 4, 68. [Google Scholar] [CrossRef]
- Lanhuang, B.; Yang, Q.; Godana, E.A.; Zhang, H. Efficacy of the Yeast Wickerhamomyces anomalus in Biocontrol of Gray Mold Decay of Tomatoes and Study of the Mechanisms Involved. Foods 2022, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Cassone, A.; Rizzello, C.G.; Nionelli, L.; Cardinali, G.; Gobbetti, M. Antifungal activity of Wickerhamomyces anomalus and Lactobacillus plantarum during sourdough fermentation: Identification of novel compounds and long-term effect during storage of wheat bread. Appl. Environ. Microbiol. 2011, 77, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Gambacorta, G.; Tlais, A.Z.A.; Cantatore, V.; Gobbetti, M. Volatilome and Bioaccessible Phenolics Profiles in Lab-Scale Fermented Bee Pollen. Foods 2021, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, E.; Lattanzi, A.; Dousset, X.; Minervini, F.; De Angelis, M.; Lacaze, G.; Onno, B.; Gobbetti, M. Lactic acid bacterium and yeast microbiotas of sixteen French traditional sourdoughs. Int. J. Food Microbiol. 2015, 215, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, E.A.; Shanab, S.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Shamsia, S.; Helal, A.; Conte, A. Angiotensin-converting enzyme inhibitory peptides from goats’ milk released by in vitro gastro-intestinal digestion. Int. Dairy J. 2017, 71, 6–16. [Google Scholar] [CrossRef]
- Church, W.R.; Walker, L.E.; Houghten, R.A.; Reisfeld, R.A. Anti-HLA antibodies of predetermined specificity: A chemically synthesized peptide induces antibodies specific for HLA-A, B heavy chain. Proc. Natl. Acad. Sci. USA 1983, 80, 255–258. [Google Scholar] [CrossRef]
- Pontonio, E.; Verni, M.; Dingeo, C.; Diaz-de-Cerio, E.; Pinto, D.; Rizzello, C.G. Impact of Enzymatic and Microbial Bioprocessing on Antioxidant Properties of Hemp (Cannabis sativa L.). Antioxidants 2020, 9, 1258. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Da Ros, A.; Filannino, P.; Vincentini, O.; Gobbetti, M.; Di Cagno, R. Biotechnological re-cycling of apple by-products: A reservoir model to produce a dietary supplement fortified with biogenic phenolic compounds. Food Chem. 2021, 336, 127616. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Cassone, A.; Coda, R.; Gobbetti, M. Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chem. 2011, 127, 952–959. [Google Scholar] [CrossRef]
- Yu, L.; Perret, J.; Harris, M.; Wilson, J.; Haley, S. Antioxidant properties of bran extracts from “Akron” wheat grown at different locations. J. Agric. Food Chem. 2003, 51, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Taghizadeh, M.S.; Niazi, A.; Moghadam, A.; Afsharifar, A.R. Novel bioactive peptides of Achillea eriophora show anticancer and antioxidant activities. Bioorg. Chem. 2021, 110, 104777. [Google Scholar] [CrossRef]
- Calderón-Jurado, M.; Cruz-Álvarez, O.; Juárez-López, P.; Hernández-Rodríguez, O.A.; Orozco-Meléndez, L.R.; Morales-Maldonado, E.R.; Ojeda-Barrios, D.L.; Alia-Tejacal, I. Bioactive compounds, minerals and antioxidants of edible flowers of peach and apple. Int. J. Food Prop. 2023, 26, 1855–1866. [Google Scholar] [CrossRef]
- Sooresh, M.M.; Willing, B.P.; Bourrie, B.C.T. Opportunities and Challenges of Understanding Community Assembly in Spontaneous Food Fermentation. Foods 2023, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, I.; Di Cagno, R.; Buchin, S.; De Angelis, M.; Gobbetti, M. Use of autochthonous mesophilic lactic acid bacteria as starter cultures for making Pecorino Crotonese cheese: Effect on compositional, microbiological and biochemical attributes. Food Res. Int. 2019, 116, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Tlais, A.Z.A.; Cantatore, V.; Gobbetti, M. Fructose-rich niches traced the evolution of lactic acid bacteria toward fructophilic species. Crit. Rev. Microbiol. 2019, 45, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Hou, X.; Tian, Q.; Hui, M. Gene mining and flavour metabolism analyses of Wickerhamomyces anomalus Y-1 isolated from a Chinese liquor fermentation starter. Front. Microbiol. 2022, 13, 891387. [Google Scholar] [CrossRef] [PubMed]
- Rodarte, M.P.; Dias, D.R.; Vilela, D.M.; Schwan, R.F. Proteolytic activities of bacteria, yeasts and filamentous fungi isolated from coffee fruit (Coffea arabica L.). Acta. Sci. Agron. 2011, 33, 457–464. [Google Scholar]
- Välikangas, T.; Suomi, T.; Elo, L.L. A comprehensive evaluation of popular proteomics software workflows for label-free proteome quantification and imputation. Brief. Bioinform. 2017, 19, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Capriotti, A.L.; Capuano, F.; Cavaliere, C.; Montone, A.M.I.; Montone, C.M.; Piovesana, S.; Chiozzi, R.Z.; Lagana, A. Identification and Antimicrobial Activity of Medium-Sized and Short Peptides from Yellowfin Tuna (Thunnus albacares) Simulated Gastrointestinal Digestion. Foods 2020, 9, 1185. [Google Scholar] [CrossRef]
- Tonini, S.; Tlais, A.Z.A.; Galli, B.D.; Helal, A.; Tagliazucchi, D.; Filannino, P.; Zannini, E.; Gobbetti, M.; Di Cagno, R. Lentils protein isolate as a fermenting substrate for the production of bioactive peptides by lactic acid bacteria and neglected yeast species. Microb. Biotechnol. 2024, 17, e14387. [Google Scholar] [CrossRef]
- Tang, X.; He, Z.; Dai, Y.; Xiong, Y.L.; Xie, M.; Chen, J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J. Agric. Food Chem. 2010, 58, 587–593. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Sosalagere, C.; Kehinde, B.A.; Sharma, P. Isolation and functionalities of bioactive peptides from fruits and vegetables: A reviews. Food Chem. 2022, 366, 130494. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Singh, J.; Bhat, Z.F.; Jaglan, S. Multifunctional apple seed protein hydrolysates: Impact of enzymolysis on the biochemical, techno-functional and in vitro α-glucosidase, pancreatic lipase and angiotensin-converting enzyme inhibition activities. Int. J. Biol. Macromol. 2024, 257, 128553. [Google Scholar] [CrossRef] [PubMed]
- Prasongsuk, S.; Ployngam, S.; Wacharasindhu, S.; Lotrakul, P.; Punnapayak, H. Effects of sugar and amino acid supplementation on Aureobasidium pullulans NRRL 58536 antifungal activity against four Aspergillus species. Appl. Microbiol. Biotechnol. 2013, 97, 7821–7830. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Millán, C.; Ortega, J.M.; Mauricio, J.C. Concentration of amino acids in wine after the end of fermentation by Saccharomyces cerevisiae strains. J. Sci. Food Agric. 2003, 83, 830–835. [Google Scholar] [CrossRef]
- Fernandez, M.; Zuniga, M. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 2006, 32, 155–183. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M.; Eun, J.B.; Simal-Gandara, J. Influence of enzymatic hydrolysis and molecular weight fractionation on the antioxidant and lipase/α-amylase inhibitory activities in vitro of watermelon seed protein hydrolysates. Molecules 2022, 27, 7897. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.R.; Prabhakaran, N.; Ragunath, K.S.; Srinivasan, R.; Ponni, K.K.; Balaji, G.; Latha, K. Unearthing the genes of plant-beneficial marine yeast-Wickerhamomyces anomalus strain MSD1. bioRxiv 2020. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.; Rodriguez, L.G.R.; Zorzoli, A.; Dorfmueller, H.C.; Raya, R.R.; Mozzi, F. Genomic diversity in Fructobacillus spp. isolated from fructose-rich niches. PLoS ONE 2023, 18, e0281839. [Google Scholar] [CrossRef] [PubMed]
- Satora, P.; Tarko, T.; Sroka, P.; Blaszczyk, U. The influence of Wickerhamomyces anomalus killer yeast on the fermentation and chemical composition of apple wines. FEMS Yeast Res. 2014, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Behzad, S.; Sureda, A.; Barreca, D.; Nabavi, S.F.; Rastrelli, L.; Nabavi, S.M. Health effects of phloretin: From chemistry to medicine. Phytochem. Rev. 2017, 16, 527–533. [Google Scholar] [CrossRef]
- Wang, L.; Yue, L.; Chi, Z.; Wang, X. Marine killer yeasts active against a yeast strain pathogenic to crab Portunus trituberculatus. Dis. Aquat. Organ. 2008, 80, 211–218. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Del Rio, D.; Lazzi, C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019, 276, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Zhao, G.; Ng, K.R.; Mark, R.; Chen, W.N. Metabolic Engineering of Saccharomyces cerevisiae for De Novo Production of Kaempferol. J. Agric. Food Chem. 2019, 67, 5596–5606. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Bastola, T.; Tripathi, J.; Tripathi, M.; Rokaya, R.K.; Dhakal, B.; Rabin, D.C.; Bhandari, R.; Poudel, A. Estimation of Total Quercetin and Rutin Content in Malus domestica of Nepalese Origin by HPLC Method and Determination of Their Antioxidative Activity. J. Food Qual. 2020, 2020, 8853426. [Google Scholar] [CrossRef]
- Boudaoud, S.; Aouf, C.; Devillers, H.; Sicard, D.; Segond, D. Sourdough yeast-bacteria interactions can change ferulic acid metabolism during fermentation. Food Microbiol. 2021, 98, 103790. [Google Scholar] [CrossRef]
- Berraquero-Garcia, C.; Perez-Galvez, R.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M.; Garcia-Moreno, P.J. Encapsulation of Bioactive Peptides by Spray-Drying and Electrospraying. Foods 2023, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple by-Products: Antioxidant Properties and Food Applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Leggieri, M.C.; Pietri, A.; Battilani, P.; Growth, M.F. Mycotoxin Production and Release in Grana Cheese. Microorganisms 2020, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Mechmeche, M.; Kachouri, F.; Ksontini, H.; Hamdi, M. Production of bioactive peptides from tomato seed isolate by Lactobacillus plantarum fermentation and enhancement of antioxidant activity. Food Biotechnol. 2017, 31, 94–113. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Rizzello, C.G. Bioprocessing of brewers’ spent grain enhances its antioxidant activity: Characterization of phenolic compounds and bioactive peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
| Samples | Raw-AF | Unstarted-AF | PL22-AF | GY1-AF |
|---|---|---|---|---|
| Asp | 50.3 ± 0.9 b | 84.1 ± 2.4 a | 46.0 ± 0.4 b | 88.1 ± 0.3 a |
| Thr | 32.0 ± 0.5 c | 40.8 ± 1.2 b | 45.6 ± 0.8 a | 42.5 ± 0.3 ab |
| Ser | 81.8 ± 1.1 a | 0.0 ± 0.0 c | 37.9 ± 0.8 b | 0.0 ± 0.0 c |
| Asn | 1293.9 ± 2.2 a | 998.9 ± 13.6 c | 1168.6 ± 15.2 b | 963.2 ± 2.9 c |
| Glu | 6.8 ± 0.7 bc | 16.9 ± 3.0 b | 0.0 ± 0.0 c | 58.4 ± 1.9 a |
| Gln | 1167.27 ± 22.25 a | 868.62 ± 26.81 c | 1011.62 ± 2.85 b | 805.7 ± 9.5 c |
| Ala | 220.9 ± 12.4 b | 270.0 ± 12.1 ab | 281.7 ± 4.4 a | 231.1 ± 1.8 ab |
| Val | 18.9 ± 1.1 b | 34.9 ± 0.8 a | 40.4 ± 0.8 a | 9.0 ± 1.6 c |
| Met | 74.0 ± 1.7 b | 88.4 ± 2.1 a | 90.5 ± 0.9 a | 60.1 ± 1.0 c |
| Ile | 10.6 ± 1.3 b | 16.9 ± 0.5 b | 24.1 ± 1.7 a | 16.7 ± 0.2 b |
| Leu | 0.0 ± 0.0 b | 5.0 ± 1.2 b | 21.1 ± 2.2 a | 3.67 ± 1.5 b |
| Phe | 285.5 ± 2.0 a | 211.1 ± 8.8 b | 284.9 ± 0.8 a | 184.2 ± 1.8 c |
| GABA | 184.4 ± 4.1 b | 786.4 ± 16.6 a | 722.6 ± 36.5 a | 819.1 ± 3.9 a |
| His | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 4.7 ± 0.78 a | 0.0 ± 0.0 b |
| Arg | 140.8 ± 1.6 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| Pro | 103.1 ± 5.3 ab | 112.2 ± 4.5 ab | 122.3 ± 1.4 a | 98.8 ± 2.8 b |
| TFAAs | 3670.2 ± 75.3 ab | 3534.2 ± 123.9 b | 3902.0 ± 7.2 a | 3380.48 ± 24.8 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonini, S.; Tlais, A.Z.A.; Filannino, P.; Di Cagno, R.; Gobbetti, M. Apple Blossom Agricultural Residues as a Sustainable Source of Bioactive Peptides through Microbial Fermentation Bioprocessing. Antioxidants 2024, 13, 837. https://doi.org/10.3390/antiox13070837
Tonini S, Tlais AZA, Filannino P, Di Cagno R, Gobbetti M. Apple Blossom Agricultural Residues as a Sustainable Source of Bioactive Peptides through Microbial Fermentation Bioprocessing. Antioxidants. 2024; 13(7):837. https://doi.org/10.3390/antiox13070837
Chicago/Turabian StyleTonini, Stefano, Ali Zein Alabiden Tlais, Pasquale Filannino, Raffaella Di Cagno, and Marco Gobbetti. 2024. "Apple Blossom Agricultural Residues as a Sustainable Source of Bioactive Peptides through Microbial Fermentation Bioprocessing" Antioxidants 13, no. 7: 837. https://doi.org/10.3390/antiox13070837
APA StyleTonini, S., Tlais, A. Z. A., Filannino, P., Di Cagno, R., & Gobbetti, M. (2024). Apple Blossom Agricultural Residues as a Sustainable Source of Bioactive Peptides through Microbial Fermentation Bioprocessing. Antioxidants, 13(7), 837. https://doi.org/10.3390/antiox13070837








