Influences of Stocking Density on Antioxidant Status, Nutrients Composition, and Lipid Metabolism in the Muscles of Cyprinus carpio under Rice–Fish Co-Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental System, Design, and Sampling
2.2. Assessment of Oxidative Stress Indicators
2.3. Analyzing Amino Acids in Muscle
2.4. Determination of Fatty Acids in Muscle
2.5. Untargeted Metabolomic Profiling in Muscle
2.6. Transcriptome Sequencing and Analysis
2.7. Quantitative Real-Time PCR Test
2.8. Statistical Analysis
3. Results
3.1. Alterations in Oxidative Stress Indicators
3.2. Alterations in Amino Acids Composition
3.3. Alterations in Fatty Acids Composition
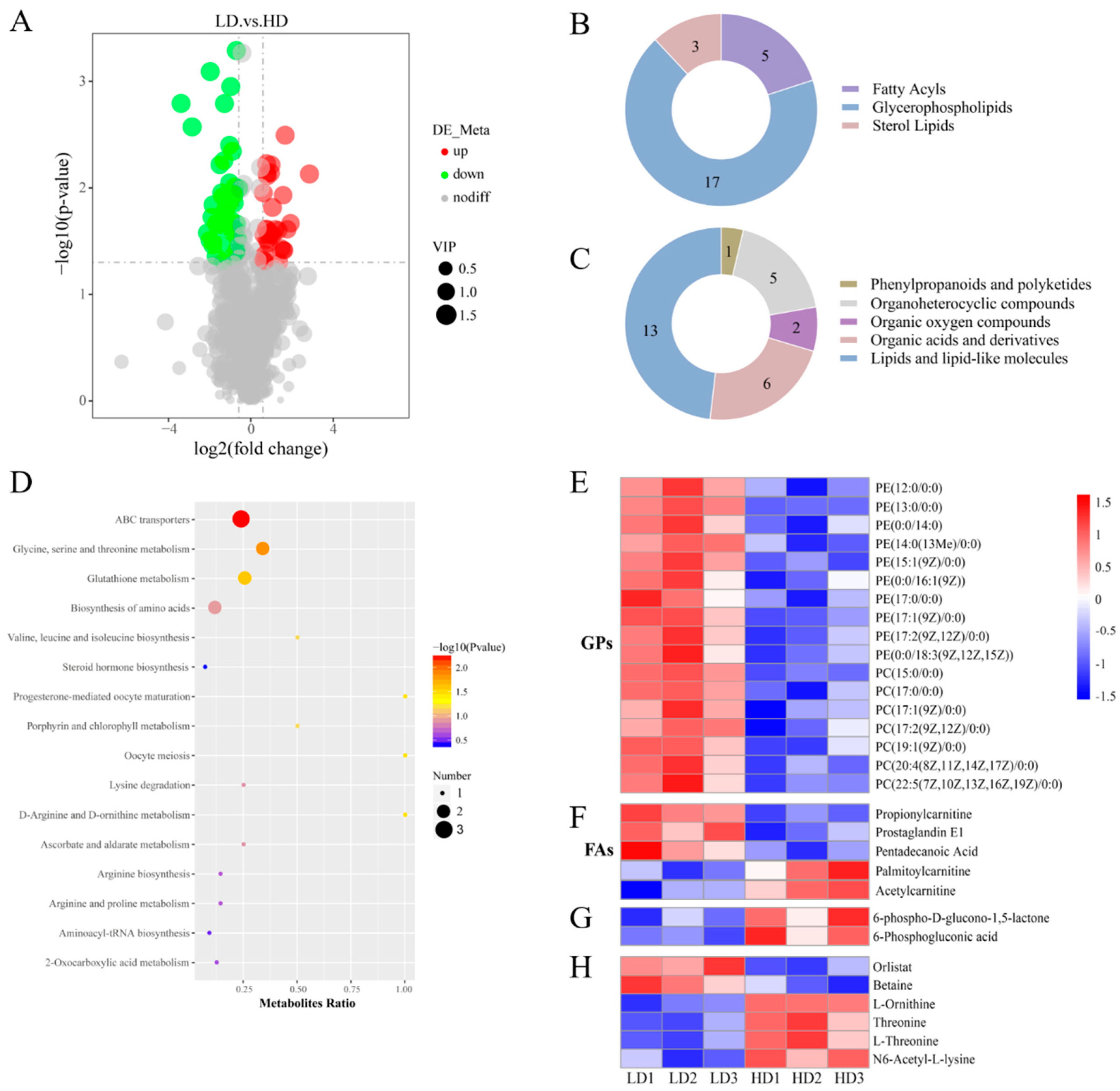
3.4. Metabolites Profile in Muscle
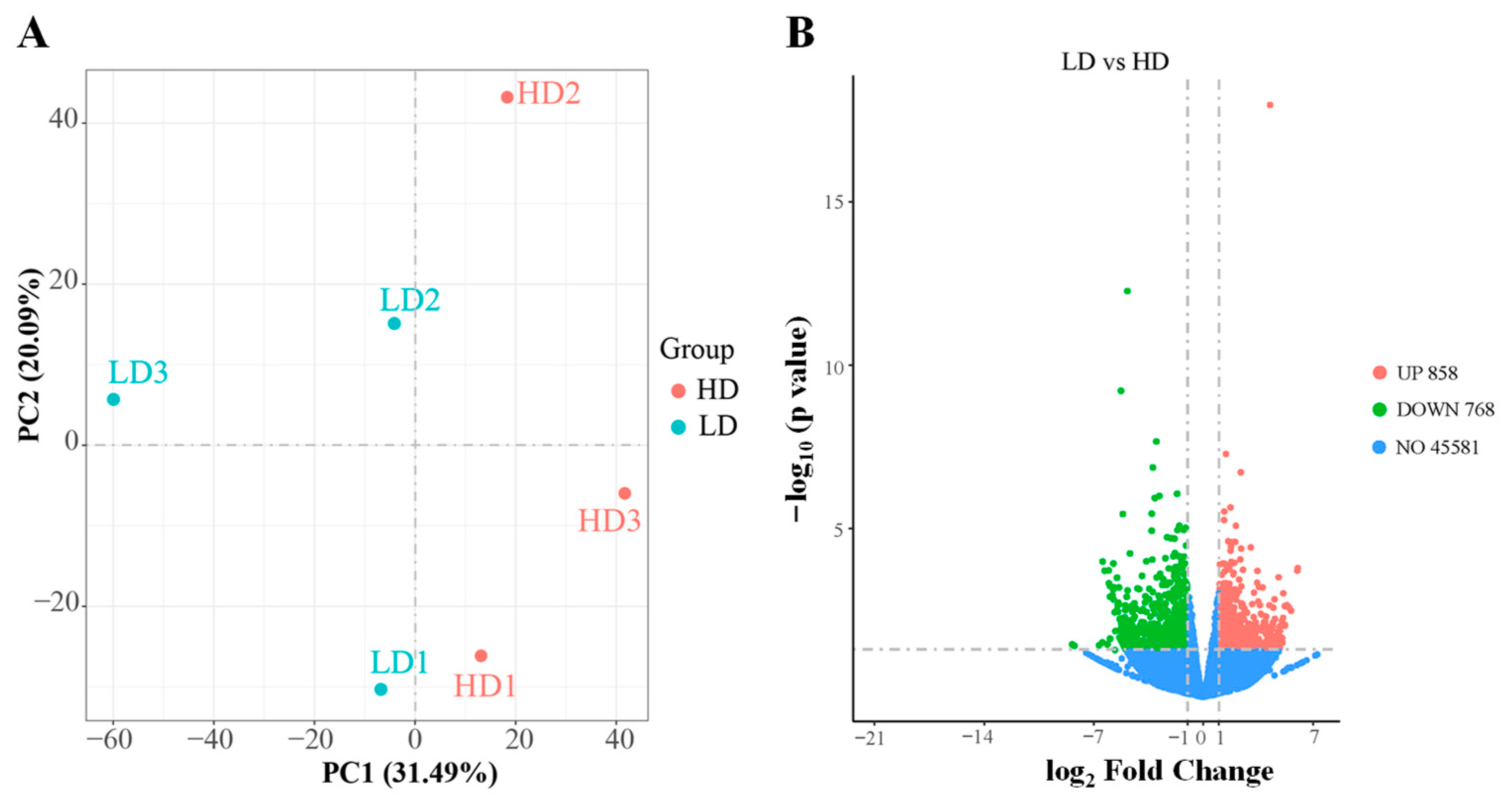
3.5. Transcriptome Profile in Muscle
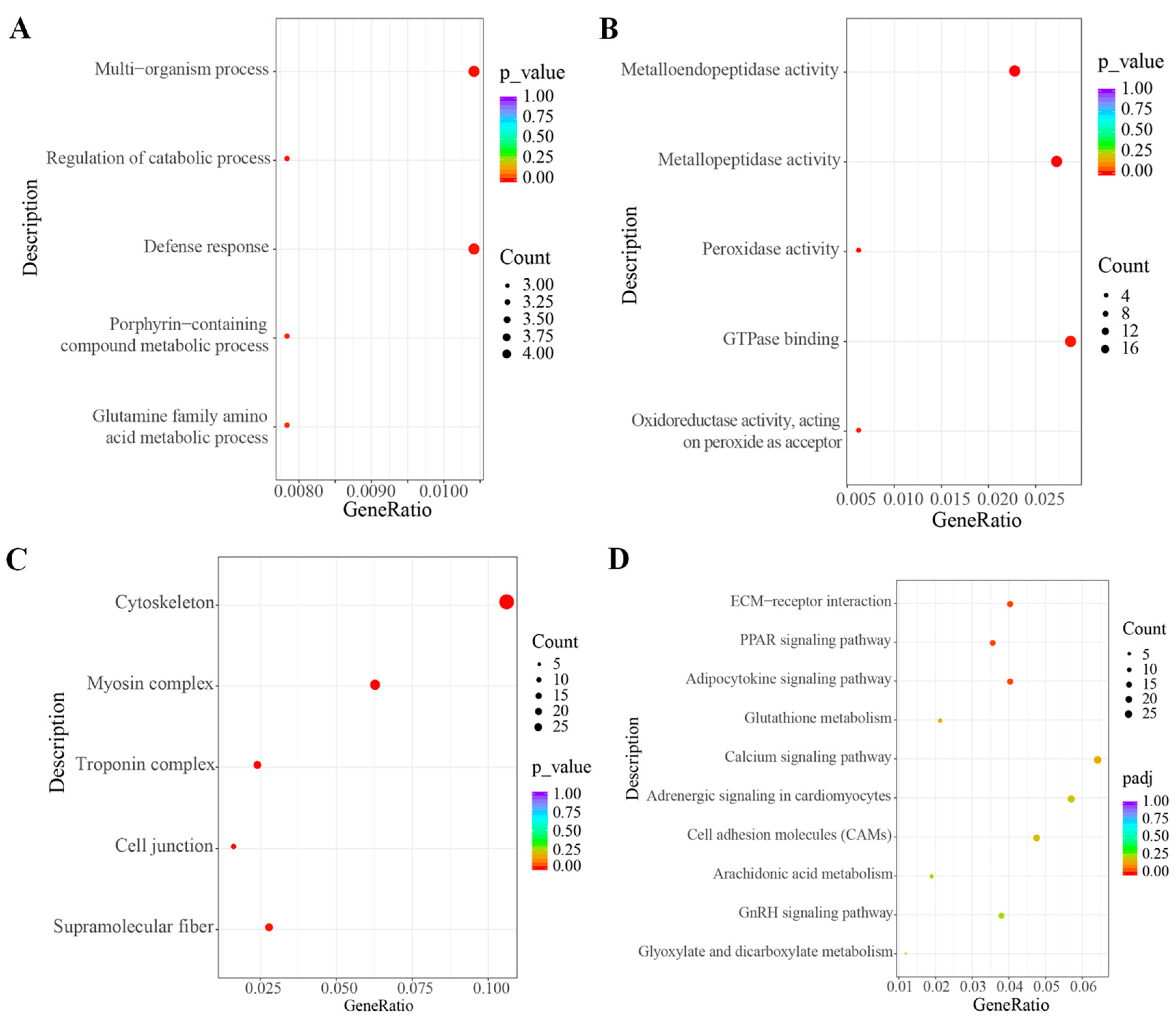
3.6. Enrichment Analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
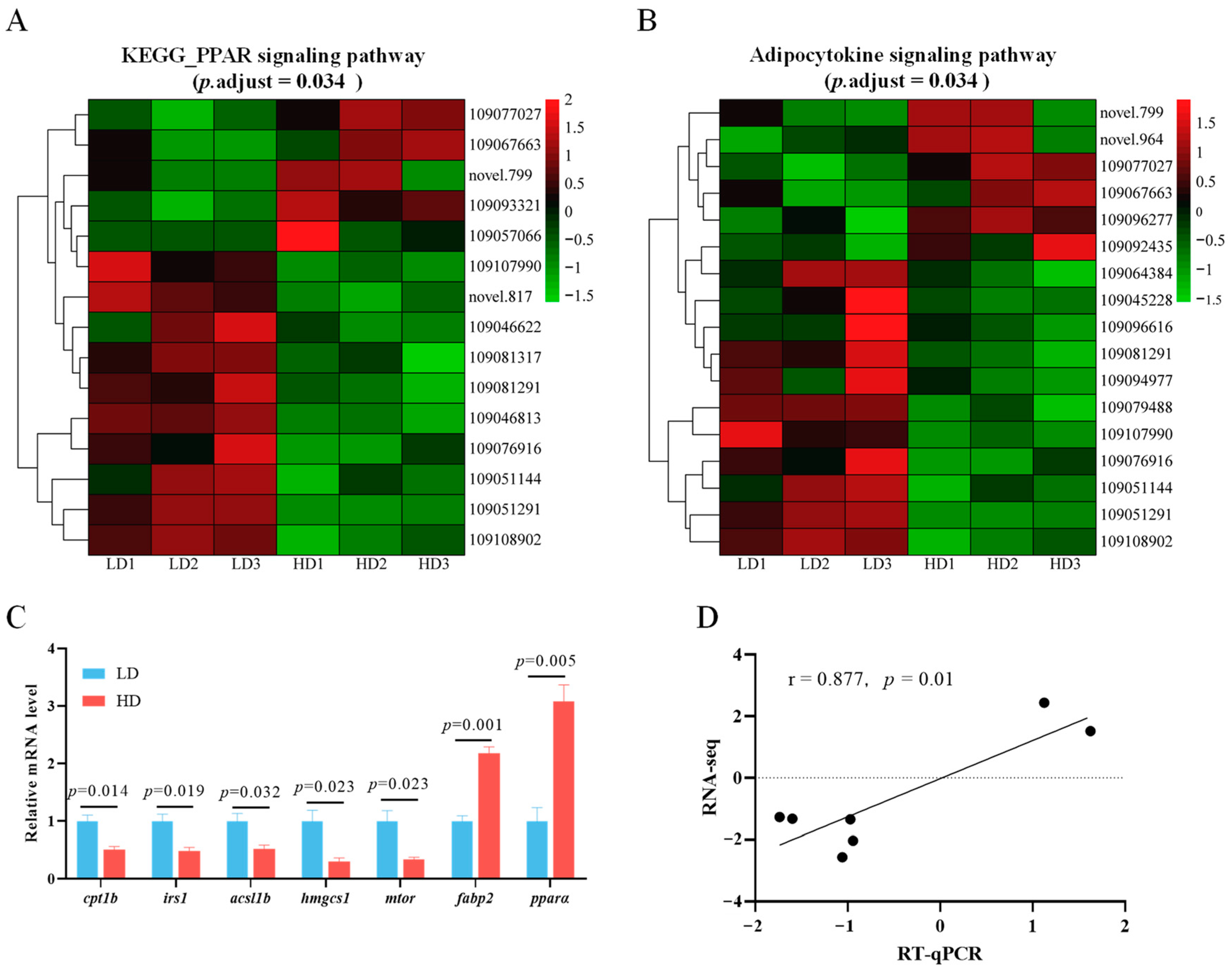
3.7. Alterations of Key Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacDonald, G.M. Climate Change and water in Southwestern North America special feature: Water, climate change, and sustainability in the southwest. Proc. Natl. Acad. Sci. USA 2010, 107, 21256–21262. [Google Scholar] [CrossRef] [PubMed]
- Kc, K.B.; Dias, G.M.; Veeramani, A.; Swanton, C.J.; Fraser, D.; Steinke, D.; Lee, E.; Wittman, H.; Farber, J.M.; Dunfield, K.; et al. When too much isn’t enough: Does current food production meet global nutritional needs? PLoS ONE 2018, 13, 0205683. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Campanati, C.; Willer, D.; Schubert, J.; Aldridge, D.C. Sustainable Intensification of Aquaculture through Nutrient Recycling and Circular Economies: More Fish, Less Waste, Blue Growth. Rev. Fish. Sci. Aquac. 2022, 30, 143–169. [Google Scholar] [CrossRef]
- Ahmed, N.; Garnett, S.T. Integrated rice-fish farming in Bangladesh: Meeting the challenges of food security. Food Secur. 2011, 3, 81–92. [Google Scholar] [CrossRef]
- Frei, M.; Becker, K. Integrated rice-fish culture: Coupled production saves resources. Nat. Resour. Forum 2005, 29, 135–143. [Google Scholar] [CrossRef]
- Feng, J.F.; Li, F.B.; Zhou, X.Y.; Xu, C.C.; Fang, F.P. Nutrient removal ability and economical benefit of a rice-fish co-culture system in aquaculture pond. Ecol. Eng. 2016, 94, 315–319. [Google Scholar] [CrossRef]
- Ge, L.; Sun, Y.; Li, Y.J.; Wang, L.Y.; Guo, G.Q.; Song, L.L.; Wang, C.; Wu, G.G.; Zang, X.Y.; Cai, X.M.; et al. Ecosystem sustainability of rice and aquatic animal co-culture systems and a synthesis of its underlying mechanisms. Sci. Total Environ. 2023, 880, 163314. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Liu, J.; Geng, Y.C.; Wang, H.Y.; Pan, J.T.; Zhang, D.; Rehim, A.; Aon, M.; Liu, H.B. Co-culture of rice and aquatic animals: An integrated system to achieve production and environmental sustainability. J. Clean. Prod. 2020, 249, 119310. [Google Scholar] [CrossRef]
- Yu, X.J.; Hao, X.J.; Dang, Z.Q.; Yang, L.K. Report on the development of integrated rice-fish farming industry in China (2023). China Fish 2023, 08, 19–26. [Google Scholar]
- Jia, R.; Wang, L.; Hou, Y.R.; Feng, W.R.; Li, B.; Zhu, J. Effects of stocking density on the growth performance, physiological parameters, redox status and lipid metabolism of Micropterus salmoides in integrated rice-fish farming systems. Antioxidants 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Hossain, M.A.; Haque, M.A.; Mondol, M.M.R.; Harun-Ur-Rashid, M.; Das, S.K. Determination of suitable stocking density for good aquaculture practice-based carp fattening in ponds under drought-prone areas of Bangladesh. Aquaculture 2022, 547, 737485. [Google Scholar] [CrossRef]
- Manley, C.B.; Rakocinski, C.F.; Lee, P.G.; Blaylock, R.B. Stocking density effects on aggressive and cannibalistic behaviors in larval hatchery-reared spotted seatrout, Cynoscion nebulosus. Aquaculture 2014, 420, 89–94. [Google Scholar] [CrossRef]
- Ellis, T.; North, B.; Scott, A.P.; Bromage, N.R.; Porter, M.; Gadd, D. The relationships between stocking density and welfare in farmed rainbow trout. J. Fish Biol. 2002, 61, 493–531. [Google Scholar] [CrossRef]
- Vera, L.M.; Al-Khamees, S.; Hervé, M. Stocking Density Affects Circadian Rhythms of Locomotor Activity in African Catfish, Clarias gariepinus. Chronobiol. Int. 2011, 28, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Ogut, H.; Reno, P. Prevalence of furunculosis in Chinook salmon depends on density of the host exposed by cohabitation. N. Am. J. Aquac. 2004, 66, 191–197. [Google Scholar] [CrossRef]
- Jia, R.; Liu, B.L.; Han, C.; Huang, B.; Lei, J.L. Influence of stocking density on growth performance, antioxidant status, and physiological response of Juvenile Turbot, Scophthalmus maximu, reared in land-based recirculating aquaculture system. J. World Aquac. Soc. 2016, 47, 587–599. [Google Scholar] [CrossRef]
- Refaey, M.M.; Li, D.P.; Tian, X.; Zhang, Z.M.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus. Aquaculture 2018, 492, 73–81. [Google Scholar] [CrossRef]
- Oke, V.; Goosen, N.J. The effect of stocking density on profitability of African catfish (Clarias gariepinus) culture in extensive pond systems. Aquaculture 2019, 507, 385–392. [Google Scholar] [CrossRef]
- Sadhu, N.; Sharma, S.R.K.; Joseph, S.; Dube, P.; Philipose, K.K. Chronic stress due to high stocking density in open sea cage farming induces variation in biochemical and immunological functions in Asian seabass (Lates calcarifer, Bloch). Fish Physiol. Biochem. 2014, 40, 1105–1113. [Google Scholar] [CrossRef]
- He, Y.; Yu, H.Y.; Zhao, H.G.; Zhu, H.; Zhang, Q.J.; Wang, A.Q.; Shen, Y.B.; Xu, X.Y.; Li, J.L. Transcriptomic analysis to elucidate the effects of high stocking density on grass carp (Ctenopharyngodon idella). BMC Genom. 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Du, F.K.; Li, Y.; Tang, Y.K.; Su, S.Y.; Yu, J.H.; Yu, F.; Li, J.L.; Li, H.X.; Wang, M.Y.; Xu, P. Response of the gut microbiome of Megalobrama amblycephala to crowding stress. Aquaculture 2019, 500, 586–596. [Google Scholar] [CrossRef]
- Rahman, M.M. Role of common carp (Cyprinus carpio) in aquaculture production systems. Front. Life Sci. 2015, 8, 399–410. [Google Scholar] [CrossRef]
- Rahman, M.M. Effects of co-cultured common carp on nutrients and food web dynamics in rohu aquaculture ponds. Aquac. Environ. Interact. 2015, 6, 223–232. [Google Scholar] [CrossRef]
- Wang, D.; Wu, F. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2023. [Google Scholar]
- Hartvich, P.; Flajshans, M.; Nydl, V.; Vondra, T.; Pavlícek, T. Growth testing of two breeds of common carps Cyprinus carpio L. (Hungarian mirror and Trebon scaly carp), in ponds with low and high stocking density. Aquac. Res. 2003, 34, 1015–1021. [Google Scholar] [CrossRef]
- Ruane, N.M.; Carballo, E.C.; Komen, J. Increased stocking density influences the acute physiological stress response of common carp Cyprinus carpio (L.). Aquac. Res. 2002, 33, 777–784. [Google Scholar] [CrossRef]
- SC/T 1135.1-2017; Technical Specification for Integrated Farming of Rice and Aquaculture Animal. Part 1: General Principle. Ministry of Agriculture and Rural Affairs: Beijing, China, 2018.
- Szollosi, R.; Varga, I.S. Total antioxidant power in some species of labiatae (Adaptation of FRAP method). Acta Biol. Szeged. 2002, 46, 125–127. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. A simple and efficient strategy to enhance the antioxidant activities of amino-substituted glutathione peroxidase mimics. Chem. A Eur. J. 2008, 14, 8640–8651. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. Assay of superoxide dismutase activity in a plate assay using WST-1. Free Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Jarvis, K.L.; Hyland, K.J. Protein measurement using bicinchoninic acid-elimination of interfering substances. Anal. Biochem. 1989, 180, 136–139. [Google Scholar] [CrossRef] [PubMed]
- GB5009.168-2016; Determination of Fatty Acids in Food. National Health and Family Planning Commission: Beijing, China, 2017.
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Guo, L.; Liu, B.S.; Guo, H.Y.; Zhu, K.C.; Zhang, N.; Jiang, S.G.; Zhang, D.C. Effects of stocking density on the growth performance, serum biochemistry, muscle composition and HSP70 gene expression of juvenile golden pompano Trachinotus ovatus (Linnaeus, 1758). Aquaculture 2020, 518, 734841. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Dong, Y.; Jia, R.; Hou, Y.; Diao, W.; Li, B.; Zhu, J. Effects of stocking density on the growth performance, mitophagy, endocytosis and metabolism of Cherax quadricarinatus in integrated rice-crayfish farming systems. Front. Physiol. 2022, 13, 1040712. [Google Scholar] [CrossRef] [PubMed]
- Onxayvieng, K.; Piria, M.; Fuka, M.M.; Gavrilovic, A.; Liang, X.; Liu, L.; Tang, R.; Li, L.; Li, D.P. High stocking density alters growth performance, blood biochemical profiles, and hepatic antioxidative capacity in gibel carp (Carassius gibelio). Fish Physiol. Biochem. 2021, 47, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.; Afonso, A.; Perez-Jimenez, A.; Oliva-Teles, A.; de las Heras, V.; Mancera, J.M.; Serradeiro, R.; Costas, B. Evaluation of different stocking densities in a Senegalese sole (Solea senegalensis) farm: Implications for growth, humoral immune parameters and oxidative status. Aquaculture 2015, 438, 6–11. [Google Scholar] [CrossRef]
- Mathew, S.; Kumar, K.A.; Anandan, R.; Nair, P.G.V.; Devadasan, K. Changes in tissue defence system in white spot syndrome virus (WSSV) infected Penaeus monodon. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.; Arudi, R.L.; Sutherland, M.W. A study of the reactivity of HO2/O2- with unsaturated fatty acids. J. Biol. Chem. 1983, 258, 4759–4761. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Dong, Y.; Hou, Y.R.; Feng, W.R.; Li, B.; Zhu, J. Transcriptome analysis reveals the effect of stocking density on energy metabolism in the gills of Cherax quadricarinatus under rice-crayfish co-culture. Int. J. Mol. Sci. 2023, 24, 11345. [Google Scholar] [CrossRef]
- Ding, C.H.; Jia, R.; Hou, Y.R.; Li, B.; Zhu, J. Effects of stocking density on the antioxidant capacity, muscle nutrient composition and metabolism function of Micropterus salmoides in integrated rice-bass farming systems. J. Fish. Sci. China 2023, 30, 1000–1014. [Google Scholar] [CrossRef]
- Taylor, E.N.; Beckmann, M.; Villarreal-Ramos, B.; Vordermeier, H.M.; Hewinson, G.; Rooke, D.; Mur, L.A.J.; Koets, A.P. Metabolomic changes in naturally MAP-infected Holstein-Friesian heifers indicate immunologically related biochemical reprogramming. Metabolites 2021, 11, 727. [Google Scholar] [CrossRef]
- Xie, S.W.; He, J.Y.; Masagounder, K.; Liu, Y.J.; Tian, L.X.; Tan, B.P.; Niu, J. Dietary lysine levels modulate the lipid metabolism, mitochondrial biogenesis and immune response of grass carp, Ctenopharyngodon idellus. Anim. Feed Sci. Technol. 2022, 291, 115375. [Google Scholar] [CrossRef]
- Rahman, M.M.; Li, X.Q.; Sharifuzzaman, S.M.; He, M.; Poolsawat, L.; Yang, H.; Leng, X.J. Dietary threonine requirement of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2021, 543, 736884. [Google Scholar] [CrossRef]
- Alvarez-Curto, E.; Milligan, G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem. Pharmacol. 2016, 114, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.M.; Ballantyne, J.S.; Leatherland, J.F. High stocking density alters the energy-metabolism of brook charr, Salvelinus-fontinalis. Aquaculture 1990, 88, 371–381. [Google Scholar] [CrossRef]
- Chatterjee, M.T.; Khalawan, S.A.; Curran, B.P.G. Cellular lipid composition influences stress activation of the yeast general stress response element (STRE). Microbiology 2000, 146, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Bai, Y.C.; Xu, S.G.; Yang, X.F.; Cheng, B. Intestinal metabolomics of juvenile lenok (Brachymystax lenok) in response to heat stress. Fish Physiol. Biochem. 2022, 48, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Novoa, B.; Pereiro, P.; López-Muñoz, A.; Varela, M.; Forn-Cuní, G.; Anchelin, M.; Dios, S.; Romero, A.; Martinez-López, A.; Medina-Gali, R.M.; et al. Rag1 immunodeficiency-induced early aging and senescence in zebrafish are dependent on chronic inflammation and oxidative stress. Aging Cell 2019, 18, 13020. [Google Scholar] [CrossRef] [PubMed]
- Vanella, A.; Russo, A.; Acquaviva, R.; Campisi, A.; Di Giacomo, C.; Sorrenti, V.; Barcellona, M.L. L-Propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol. Toxicol. 2000, 16, 99–104. [Google Scholar] [CrossRef]
- Venegas-Calerón, M.; Sayanova, O.; Napier, J.A. An alternative to fish oils: Metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog. Lipid Res. 2010, 49, 108–119. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of omega-6 polyunsaturated fatty acids (PUFAs) versus deficiency of omega-3 PUFAs in modern-day diets: The disturbing factor for their “balanced antagonistic metabolic functions” in the human body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Soufan, O.; Xia, J.G.; Tang, R.; Li, L.; Li, D.P. Transcriptome and physiological analysis reveal alterations in muscle metabolisms and immune responses of grass carp (Ctenopharyngodon idellus) cultured at different stocking densities. Aquaculture 2019, 503, 186–197. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, H.; Alhamoud, Y.; Chen, Y.; Zhuang, J.; Liu, T.; Cai, L.; Shen, W.; Wu, X.; Zheng, W. Integrated metabolomic and gene expression analyses to study the effects of glycerol monolaurate on flesh quality in large yellow croaker (Larimichthys crocea). Food Chem. 2022, 367, 130749. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.; Jia, R.; Hou, Y.; Gong, J.; Zhang, L.; Li, B.; Zhu, J. Effects of Different Stocking Densities on the Growth, Antioxidant Status, and Intestinal Bacterial Communities of Carp in the Rice–Fish Co-Culture System. Fishes 2024, 9, 244. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, K.S.; Xing, G.S.; Li, L.P.; Ma, B.C.; Hu, Z.J.; Duan, L.F.; Liu, X.G. Phospholipid remodeling is critical for stem cell pluripotency by facilitating mesenchymal-to-epithelial transition. Sci. Adv. 2019, 5, 7525. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.Q.; Wang, R.J.; Zhang, D.Y.; Guan, Z.Y.; Ding, T.T.; Zhang, J.N.; Zhao, X.J. Quercetin modulates the liver metabolic profile in a chronic unpredictable mild stress rat model based on metabolomics technology. Food Funct. 2023, 14, 1726–1739. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Guo, Y.; Balasubramanian, B.; Zhao, Z.H.; Liu, W.C. Heat stress alters serum lipid metabolism of Chinese indigenous broiler chickens-a lipidomics study. Environ. Sci. Pollut. Res. 2021, 28, 10707–10717. [Google Scholar] [CrossRef] [PubMed]
- Hafez, I.M.; Cullis, P.R. Roles of lipid polymorphism in intracellular delivery. Adv. Drug Deliv. Rev. 2001, 47, 139–148. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Liu, C.; Rao, W.X.; Chen, P.; Lei, K.K.; Mai, K.S.; Zhang, W.B. Dietary phospholipids improve growth performance and change the lipid composition and volatile flavor compound profiles in the muscle of abalone Haliotis discus hannai by affecting the glycerophospholipid metabolism. Aquacult. Rep. 2023, 30, 101567. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metab. Clin. Exp. 2021, 114, 154338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Rubenstrunk, A.; Noel, B.; Rigou, G.; Delataille, P.; Millatt, L.J.; Baron, M.; Lucas, A.; Tailleux, A.; Hum, D.W.; et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2013, 58, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.X.; Jia, R.; Hou, Y.R.; Dong, Y.; Li, B.; Zhu, J. Effects of stocking density on the growth performance, physiological parameters, antioxidant status and lipid metabolism of Pelteobagrus fulvidraco in the integrated rice-fish farming system. Animals 2023, 13, 1721. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Y.; Wen, H.S.; Li, Y.; Li, J.F.; He, F.; Ni, M. Effects of stocking density on lipid deposition and expression of lipid-related genes in Amur sturgeon (Acipenser schrenckii). Fish Physiol. Biochem. 2017, 43, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Carbone, F.; La Rocca, C.; Formisano, L.; Matarese, G. Role of adipokines signaling in the modulation of T cells function. Front. Immunol. 2013, 4, 332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, Z.Y.; Wang, J.Y.; Cao, Q.S.; Yang, H.; Zhang, Y.Y. Transcriptomic analysis of lipid metabolism in zebrafish offspring of parental long-term exposure to bisphenol A. Environ. Sci. Pollut. Res. 2023, 30, 51654–51664. [Google Scholar] [CrossRef]
- Chen, J.Q.; Cai, B.S.; Tian, C.X.; Jiang, D.N.; Shi, H.J.; Huang, Y.; Zhu, C.H.; Li, G.L.; Deng, S.P. RNA sequencing (RNA-Seq) analysis reveals liver lipid metabolism divergent adaptive response to low- and high-salinity stress in Spotted Scat (Scatophagus argus). Animals 2023, 13, 1503. [Google Scholar] [CrossRef]


| Gene | Primer Sequence (5′-3′) |
|---|---|
| β-actin | F: ATCCGTAAAGACCTGTATGCCA |
| R: GGGGAGCAATGATCTTGATCTTCA | |
| carnitine palmitoyltransferase 1B (cpt1b) | F: GAAGAAACGTGCCATACGCTT |
| R: AGGATGGCACTCAACACCG | |
| insulin receptor substrate 1 (irs1) | F: GAGTGAGGAGTTCAGGCCAC |
| R: AACGAATGGCTGTGTTTGCC | |
| acyl-CoA synthetase long chain family member 1b (acsl1b) | F: CAGCAGTGTGGCATCGACAT |
| R: GGGGCAGGTAAGAGATGTGTGT | |
| 3-hydroxy-3-methylglutaryl-CoA synthase 1 (hmgcs1) | F: AAGAAGGTTTCGCCCGATGT |
| R: CAGGAAGTGATGAGTCCGGC | |
| mechanistic target of rapamycin kinase (mtor) | F: TGGGTGTTTCTTTTCCCTCGT |
| R: TGGGTTGTTTCTCCCAGGTC | |
| fatty acid binding protein 2 (fabp2) | F: TCAGCACTTTCCGCACACT |
| R: TTTCCGTTGTCCTTGCGTGT | |
| peroxisome proliferator-activated receptor alpha (pparα) | F: GGGCGGATACCCCAATCTGA |
| R: GCGTGCTTTGGCTTTGTTCA |
| Amino Acids (g/100 g) | Groups | ||
|---|---|---|---|
| LD | HD | p-Value | |
| Threonine | 0.67 ± 0.03 | 0.64 ± 0.02 | p = 0.386 |
| Valine | 0.74 ± 0.03 | 0.70 ± 0.02 | p = 0.268 |
| Methionine | 0.39 ± 0.02 | 0.39 ± 0.01 | p = 0.936 |
| Isoleucine | 0.67 ± 0.03 | 0.63 ± 0.02 | p = 0.293 |
| Leucine | 1.23 ± 0.06 | 1.17 ± 0.04 | p = 0.378 |
| Phenylalanine | 0.68 ± 0.03 | 0.64 ± 0.02 | p = 0.340 |
| Lysine | 1.52 ± 0.07 | 1.44 ± 0.05 | p = 0.384 |
| EAA | 5.90 ± 0.26 | 5.60 ± 0.19 | p = 0.372 |
| Histidine | 0.58 ± 0.03 | 0.53 ± 0.02 | p = 0.235 |
| Arginine | 0.89 ± 0.04 | 0.84 ± 0.03 | p = 0.297 |
| Aspartic acid | 1.49 ± 0.08 | 1.40 ± 0.05 | p = 0.373 |
| Serine | 0.51 ± 0.02 | 0.48 ± 0.02 | p = 0.189 |
| Glutamic acid | 1.92 ± 0.10 | 1.80 ± 0.08 | p = 0.355 |
| Glycine | 0.82 ± 0.04 | 0.70 ± 0.02 | p = 0.028 |
| Alanine | 0.96 ± 0.04 | 0.89 ± 0.03 | p = 0.210 |
| Cystine | 0.15 ± 0.02 | 0.15 ± 0.01 | p = 0.878 |
| Tyrosine | 0.47 ± 0.02 | 0.47 ± 0.02 | p = 0.943 |
| Proline | 0.52 ± 0.02 | 0.46 ± 0.01 | p = 0.064 |
| NEAA | 6.85 ± 0.32 | 6.36 ± 0.22 | p = 0.247 |
| TAA | 14.22 ± 0.65 | 13.33 ± 0.46 | p = 0.292 |
| Fatty Acids (mg/100 g) | Groups | ||
|---|---|---|---|
| LD | HD | p-Value | |
| C14:0 | 4.3 ± 0.29 | 5.5 ± 0.28 | p = 0.015 |
| C16:0 | 101.9 ± 3.09 | 131.5 ± 5.89 | p = 0.004 |
| C18:0 | 37.0 ± 1.19 | 48.2 ± 1.78 | p < 0.001 |
| SFA | 143.2 ± 4.13 | 185.2 ± 7.59 | p = 0.001 |
| C16:1 | 14.3 ± 0.95 | 19.9 ± 1.91 | p = 0.032 |
| C18:1n9c | 111.0 ± 5.69 | 158.7 ± 12.09 | p = 0.007 |
| C20:1 | 6.6 ± 0.36 | 10.6 ± 1.17 | p = 0.012 |
| C22:1n9 | 4.0 ± 0.17 | 5.0 ± 0.66 | p = 0.249 |
| MUFA | 135.1 ± 6.38 | 194.1 ± 14.43 | p = 0.006 |
| C20:2 | 4.2 ± 0.21 | 4.9 ± 0.19 | p = 0.025 |
| C18:3n3 | 7.4 ± 0.44 | 8.7 ± 0.51 | p = 0.095 |
| C20:5n3 | 10.0 ± 0.31 | 12.7 ± 0.67 | p = 0.007 |
| C22:6n3 | 61.5 ± 2.62 | 75.1 ± 5.52 | p = 0.057 |
| C18:2n6c | 74.4 ± 4.82 | 100.9 ± 5.29 | p = 0.006 |
| C20:3n6 | 6.3 ± 0.43 | 8.4 ± 0.63 | p = 0.024 |
| C20:4n6 | 25.5 ± 1.42 | 25.9 ± 1.89 | p = 0.850 |
| PUFA | 189.2 ± 7.25 | 236.6 ± 7.52 | p = 0.002 |
| n-3 PUFA | 78.9 ± 2.86 | 96.4 ± 5.83 | p = 0.027 |
| n-6 PUFA | 106.2 ± 5.57 | 135.2 ± 5.39 | p = 0.006 |
| n-3/n-6 | 0.75 ± 0.04 | 0.72 ± 0.06 | p = 0.659 |
| Samples | Raw Reads | Clean Reads | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|
| LD-1 | 44196060 | 43603302 | 97.43 | 93.01 | 48.92 |
| LD-2 | 40773658 | 40071582 | 97.63 | 93.44 | 49.19 |
| LD-3 | 41394172 | 40804442 | 97.42 | 93.05 | 48.66 |
| HD-1 | 43968464 | 43151978 | 97.45 | 93.13 | 49.29 |
| HD-2 | 41525792 | 40895330 | 97.12 | 92.41 | 49.44 |
| HD-3 | 46374674 | 45760408 | 97.36 | 92.91 | 49.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, Y.; Li, B.; Hou, Y.; Zhang, L.; Jia, R.; Zhu, J. Influences of Stocking Density on Antioxidant Status, Nutrients Composition, and Lipid Metabolism in the Muscles of Cyprinus carpio under Rice–Fish Co-Culture. Antioxidants 2024, 13, 849. https://doi.org/10.3390/antiox13070849
Rong Y, Li B, Hou Y, Zhang L, Jia R, Zhu J. Influences of Stocking Density on Antioxidant Status, Nutrients Composition, and Lipid Metabolism in the Muscles of Cyprinus carpio under Rice–Fish Co-Culture. Antioxidants. 2024; 13(7):849. https://doi.org/10.3390/antiox13070849
Chicago/Turabian StyleRong, Yongrong, Bing Li, Yiran Hou, Liqiang Zhang, Rui Jia, and Jian Zhu. 2024. "Influences of Stocking Density on Antioxidant Status, Nutrients Composition, and Lipid Metabolism in the Muscles of Cyprinus carpio under Rice–Fish Co-Culture" Antioxidants 13, no. 7: 849. https://doi.org/10.3390/antiox13070849







