Abstract
The widespread use of carbamate pesticides has raised significant environmental and health concerns, particularly regarding water contamination and the disruption of defense systems in organisms. Despite these concerns, research on the differential impacts of pesticides on male and female organisms remains limited. This study focused on methomyl, investigating sex-specific differences in liver antioxidant defenses and inflammatory response indices in male and female zebrafish after 56 days of exposure to environmentally relevant concentrations (0, 0.05, 0.10, and 0.20 mg/L). Our findings indicate that methomyl exposure significantly increased ROS content in zebrafish livers, inducing oxidative stress and activating enzymatic antioxidant defenses such as SOD, CAT, and GSH-Px activities. Sub-chronic exposure altered the expression of apoptosis-related genes (Bax/Bcl2a and Caspases3a), resulting in liver cell apoptosis in a concentration-dependent manner, with the 0.20 mg/L concentration causing the most severe damage. Additionally, methomyl exposure at environmentally relevant concentrations triggered persistent inflammatory responses in liver tissues, evidenced by increased transcription levels of inflammatory factor genes and the activation of toll-like receptors, heightening susceptibility to exogenous allergens. It is noteworthy that oxidative damage indicators (AST, ROS, MDA) and inflammatory gene expressions (IL-1β, TNF-α) were significantly higher in female livers compared to male livers at 0.10–0.20 mg/L methomyl exposure. Consequently, our study underscores the potential adverse effects of environmental methomyl exposure on aquatic organisms and highlights the need for heightened consideration of the risks posed by environmental endocrine disruptors to female health and safety.
1. Introduction
According to the Food and Agriculture Organization of the United Nations, global commodity production of primary crops is projected to reach 9.5 billion tons in 2021, representing an approximate 54% increase from the production levels in 2000 [1]. The use of pesticides, such as insecticides and herbicides, has played a significant role in achieving this outcome. However, it is estimated that only 10% of the pesticides applied actually affect the target crops, with the remaining 90% persisting in the environment and entering the ecological cycle, posing a potential threat to non-target ecosystems and human health [2].
Methomyl (S-methyl-N-[(methylcarbamoxyl) oxy] thioacetimidate, CAS 16752-77-5, C5H10N2O2S) is an oxime carbamate insecticide used to control a wide range of insect classes. It is highly hepatotoxic, cytotoxic, and neurotoxic, primarily by accumulating in organisms and inhibiting acetylcholinesterase (AChE) activity, leading to nerve and tissue failure. Despite regulations and restrictions in some countries, methomyl remains a problem due to its high water solubility (57.9 g/L at 25 °C), long half-life (surface water, 6 days; groundwater, 25 weeks), and weak to moderate soil adsorption [3,4]. Methomyl readily contaminates surface and groundwater, endangering ecological equilibrium and human health, particularly in areas of extensive use [5]. Of concern to the community is that exposure to pesticides like methomyl induces adverse outcomes such as endocrine disorders, cancer, diabetes, obesity, and cardiovascular diseases [6,7,8], for which the common pathogenic mechanisms include increased oxidative stress and persistent inflammation [9,10,11,12]. However, the influence of sex differences in organisms on the toxicological hazards of methomyl exposure remains largely unexplored.
Zebrafish (Danio rerio) is a recognized model organism [13] for toxicology studies due to its sensitivity to exogenous compounds, genetic similarity to humans, and disease characteristics, and zebrafish is widely used to explore the toxicity mechanisms of environmental pollutants [14,15]. The liver, a key organ for xenobiotic biotransformation, contains enzymes like alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transpeptidase (γ-GT), which are classical markers of liver damage from environmental toxicants [16,17,18]. Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) are the first line of defense against oxidative stress, and their significant increase in methomyl-exposed fish suggests a heightened primary defense [19,20]. In addition, inflammation is critical in defending and supporting the body following injury, trauma, and infection [21]. Recognition of various external stimuli by the innate immune system leads to the activation of multiple intracellular signaling pathways, including the NF-κB signaling pathway. NF-κB, a transcription factor involved in the sustained inflammatory response, regulates the expression of various pro-inflammatory genes and promotes the production of inflammatory factors (IL-1β, TNF-α, IL-6), serving as a key mediator of the inflammatory response [22]. Studies have documented that hepatic transcription factor expression and enzyme activities are regulated by sex [23,24], which may affect biological sensitivity to toxic contaminants, including methomyl. Recognizing these differences is, therefore, critical for accurately assessing toxicant risk and understanding the broader effects of toxicant exposure.
Therefore, we planned to expose zebrafish to environmental concentrations of methomyl over an extended period, analyzing the effects of exposure concentration and sex-specific differences on antioxidant enzyme activities, apoptosis, and the transcription levels of inflammation-related genes. The aim was to understand the sex-specific toxicological effects of methomyl exposure in zebrafish, provide critical insights for personalized medicine approaches, aid in the development of sex-specific guidelines for methomyl exposure limits, and establish a foundation for comprehensive and inclusive safety regulations.
2. Materials and Methods
2.1. Ethics Statement
The experimental procedures adhered to the guidelines set forth by the Ethics Committee at the Freshwater Fisheries Research Centre of the Chinese Academy of Fishery Sciences (FFRC, Wuxi, China) under approval number 2011AA1004020012.
2.2. Fish and Chemicals
Wild-type AB zebrafish lines were sourced from Shanghai Fauci Biotechnology Co. Ltd. (Shanghai, China) and acclimated to new environment in 100 × 50 × 60 cm glass tanks for one week.
Methomyl (CAS 16752-77-5), with a purity of 97% (w/w), served as the chemical agent in our study. All reagents, which were sourced from Sigma-Aldrich (St. Louis, MO, USA) and Sangon Biotech (Shanghai, China), were of analytical reagent grade.
2.3. Experimental Design
A total of 800 juvenile zebrafish (body length 22.9 ± 1.66 mm, weight 0.20 ± 0.06 g) were randomly assigned to four concentration gradients of methomyl (0, 0.05, 0.10, and 0.20 mg/L), with four replicates for each treatment concentration (50 zebrafish/100 L methomyl/replicate). The selection of these concentration gradients was based on the residue level (0–0.097 mg/L) of methomyl in environmental water sources [25,26,27] and the safe concentration (0.212 mg/L) from the acute toxicity experiment of Meng [28].
To ensure the consistency of exposure concentrations, the methomyl solution was replaced with an equivalent volume of fresh solution every three days. Water samples were collected at 0 h (initial concentration) and 48 h post-exposure, then extracted and concentrated using solid-phase extraction (SPE). A Waters HLB extraction column was used for this purpose. The column was activated with 5 mL of methanol and 5 mL of pure water. The water samples were added to the column, which was subsequently eluted twice, first with 2 mL of methanol and then with 3 mL of methanol. The eluent was collected, shaken well, and diluted with methanol. The solution was then filtered using a 0.22 µm organic phase filter membrane and transferred into a 1.5 mL Waters sample vial. The actual concentration of methomyl was quantified using high-performance liquid chromatography–triple quadrupole mass spectrometry (HPLC-MS, Xevo TQ-S, Water, Germany), following the method described by Meng [19]. During the acclimatization period and sub-chronic exposure periods, zebrafish were fed twice daily (8:00 and 17:00) with Artemia salina eggs (crude protein ≥ 52%, crude fat ≥ 11%). The photoperiod was 14 h light/10 h dark, the water temperature was maintained at 26 ± 1 °C, the pH was maintained at 7.4 ± 0.2, and the dissolved oxygen levels were kept within the range of 6.5–7.0 mg/L.
2.4. Sample Collection
After 56 d of exposure, the fish were starved for 24 h and subsequently anesthetized using 100 mg/L MS-222 (Argent Chemical Laboratories, Redmond, WA, USA). From each replicate tank, six adult male and six adult female zebrafish were randomly selected and dissected to collect liver samples using a stereoscopic microscope (XTZ-AT, Shanghai, China). The liver tissues were rapidly frozen in liquid nitrogen and stored at −80 °C for subsequent enzyme activity measurements. Additionally, six adult male and six adult female zebrafish were randomly selected from each replicate tank for RNA extraction to assess gene expression changes, and the tissues were snap-frozen in liquid nitrogen and stored at −80 °C. Furthermore, liver tissues from six females and six males were collected from each concentration group and fixed in 4% paraformaldehyde for apoptosis staining to assess apoptotic activity.
2.5. Antioxidant Enzyme Activity Assay
The livers and pre-cooled phosphate-buffered saline (PBS) were mixed at a ratio of weight:volume = 1:19 before homogenization using a multi-sample tissue grinder (Tissuelyser-32L, Jingxin, Shanghai, China), and then centrifuged for 15 min at 3000× g and 4 °C (four liver tissues mixed into one sample, n = 6). The activities of ALT, AST, γ-GT, SOD, CAT, GSH-Px, and the contents of malondialdehyde (MDA) and reactive oxygen species (ROS) were examined in a multifunctional microplate reader (SpectraMax Mini, Molecular Devices, Silicon Valley, CA, USA) according to the instructions of Elisa kits. Additionally, the total protein (TP) content was determined using a biochemical kit according to the Bradford method. All kits were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China).
2.6. Apoptosis Analysis
Fixed liver tissues were dehydrated, embedded, and sectioned into slices approximately 3 μm thick following the outlined protocol. Hepatic cell apoptosis was detected using a TUNEL (terminal dUTP nick-end labeling) kit (Yanjin Biotechnology Co., Ltd. Shanghai, China). The paraffin sections were immersed in xylene I (20 min), xylene II (20 min), and ethanol (100, 100, 85, 75%; 5 min at each concentration), and then washed with water for 2 min as described by Song et al. [29]. The tissues were covered with DNase-free proteinase K (20 μg/mL), incubated at 37 °C for 25 min, and washed with PBS three times for 5 min each time. Tissue samples were placed in a wet box, covered with TUNEL reaction solution, and incubated at 37 °C for 1 h. After incubating with 3,3-diaminobenzidine (DAB) for 5 min, sections were restained with hematoxylin for 12 s. The sections were dewaxed in xylene solution (Sinopharm, Shanghai, China) and then stained using TUNEL kits (Yanjin, Shanghai, China). The stained sections were observed and photographed under an ortho-fluorescent microscope (Eclipse C1, Nikon, Japan) equipped with an imaging system (DS-U3, Nikon, Japan). Images were analyzed using ImageJ software (v 1.54, National Institutes of Health, Silver Spring, Bethesda, MA, USA), with observations made in at least six different microscope fields per group.
2.7. Quantitative Real-Time PCR (qRT-PCR)
Gene expression analysis was conducted following the Minimum Information Guidelines for Publication of Quantitative Real-Time PCR Experiments (MIQE). Four liver samples were randomly selected as one sample, for a total of six samples for each concentration group. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA), with the RNA concentration and quality assessed via a micro-spectrophotometer (Merinton SMA 4000, Ann Arbor, MI, USA) and 1% agarose gel, respectively. Approximately 500 ng of RNA (Abs 260/280 nm~2.0) was reverse-transcribed into cDNA using a HiScript III RT Super Mix kit (Vazyme, Nanjing, China). RT-qPCR analyses were performed on a CFX96™ Real-time PCR System (Bio-Rad, Hercules, CA, USA) using an AceQ® qPCR SYBR® Green Master Mix kit (Vazyme). Each reaction was performed in triplicate. β-actin mRNA served as a reference gene, with relative gene transcript levels calculated using the 2−ΔΔCq method [29]. All primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China) and are detailed in Table 1.

Table 1.
Sequences of primers used for RT-qPCR.
2.8. Statistical Analysis
The number of apoptotic cells was analyzed using the Shapiro–Wilk test and Levene’s test, revealing that the data conformed to a normal distribution, but not to the principle of homogeneity of variance. Consequently, Welch’s test and Games–Howell multiple comparisons were used to determine the significance of these data.
Other data from the exposed groups were presented as a ratio of the levels in the control group, referring to the study by Meng [30]. These data conformed to both normal distribution and homogeneity of variance, so we employed factorial (two-way) analysis of variance (ANOVA) with Bonferroni’s post hoc multiple comparisons correction to determine statistical significance. Significant interactions between the exposure concentration (C) and sex (S) warranted simple effect analysis to inspect treatment differences. A p-value < 0.05 was deemed statistically significant. Results are presented as mean ± standard deviation (SD) in bar charts.
Additionally, the significance of all raw data between the control and exposed groups was also analyzed using one-way analysis of variance (ANOVA) with Bonferroni’s post hoc multiple comparisons correction. These results are also expressed as mean ± standard deviation (SD) in a table, and statistical analyses were conducted using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Actual Methomyl Concentrations
The initial methomyl concentrations in the groups designated as 0, 0.05, 0.10, and 0.20 mg/L were measured as 0, 0.057, 0.114, and 0.232 mg/L, respectively. After 48 h of exposure, these concentrations were recorded as 0, 0.049, 0.098, and 0.208 mg/L, respectively. This precise quantification validates the experimental conditions and ensures the scientific rigor and reliability of the subsequent findings, thus allowing the discussion and interpretation of the results to be based on the nominal concentrations with confidence.
3.2. Apoptosis of Zebrafish Livers
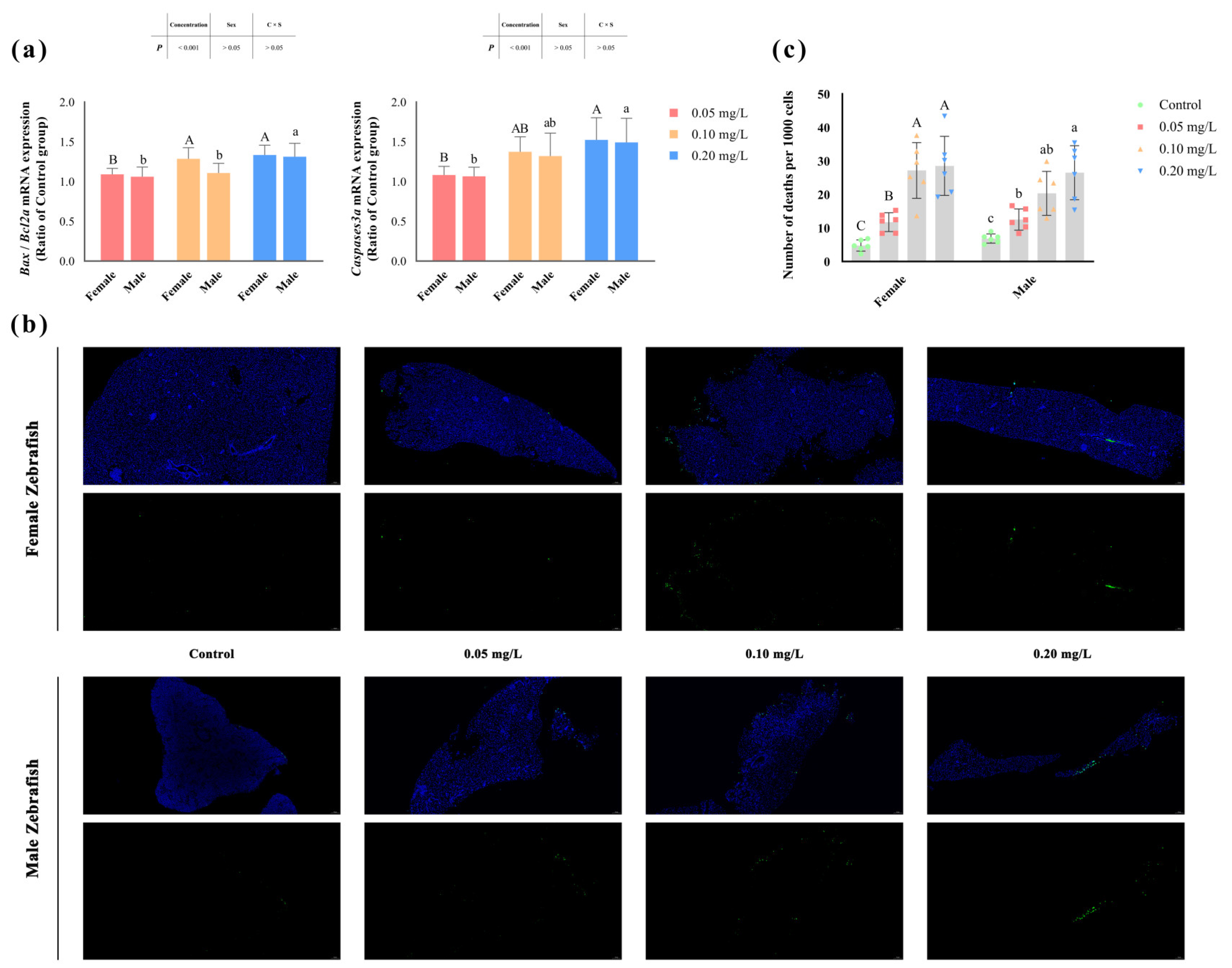
The degree of apoptosis in zebrafish liver significantly increased (p < 0.05) after methomyl exposure (Figure 1b), and the number of apoptotic cells rose significantly as the concentration increased, reaching its highest value at 0.20 mg/L (Figure 1c). Similarly, the transcription levels of the Bax/Bcl2a and Caspases3a genes also showed a concentration-dependent increase (p < 0.05) (Table 2). However, no significant differences were observed between male and female zebrafish (p > 0.05) (Figure 1a).
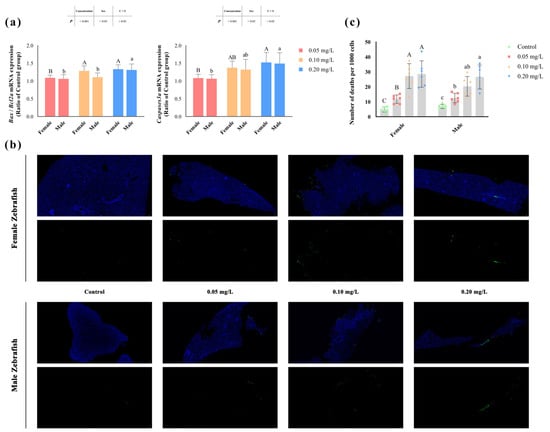
Figure 1.
Apoptosis in the livers of male and female zebrafish after sub-chronic exposure to methomyl. (a) Changes in transcription levels of zebrafish hepatic Bax/Bcl2a and Caspases3a genes compared to the control group (expressed as ratios relative to the levels in the control group). (b) Representative images of TUNEL staining of liver sections from the control group and methomyl-exposed groups under a fluorescence microscope. The green dots represent dead cells, and the blue dots indicate live cells, scale bar: 50 μm. (c) Number of apoptotic cells observed per 1000 cells. Different letters indicate significant differences between concentration groups, p < 0.05.

Table 2.
Changes in hepatic gene transcription levels in male and female zebrafish after 56 days of methomyl exposure.
3.3. Effects of Methomyl Exposure on Hepatic Antioxidant Defense System of Female and Male Zebrafish
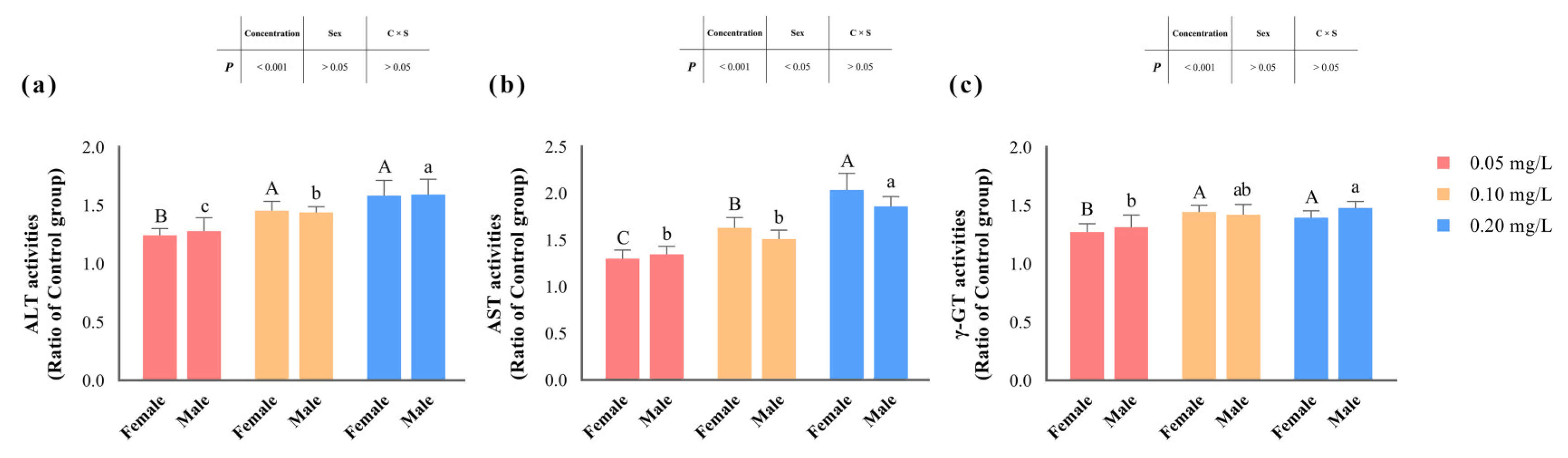
The activities of ALT, AST, and γ-GT significantly increased (p < 0.05) with rising concentrations of methomyl exposure compared to the control group, reaching their peak levels in the 0.20 mg/L-exposed group (Table 3, and Figure 2). However, there were no significant differences in the changes in ALT and γ-GT activities between male and female zebrafish (p > 0.05). Notably, only the changes in AST activity were significantly influenced (p < 0.05) by the sex of the zebrafish. Furthermore, none of the changes in enzyme activities exhibited an interaction effect between concentration and sex (p > 0.05).

Table 3.
Changes in liver function indices in male and female zebrafish after 56 days of methomyl exposure.
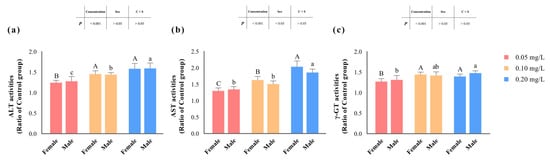
Figure 2.
Changes in hepatic ALT (a), AST (b), and γ-GT (c) activities in zebrafish after sub-chronic exposure to methomyl compared to the control group (expressed as ratios relative to the levels in the control group). Different letters indicate significant differences between concentration groups, p < 0.05.
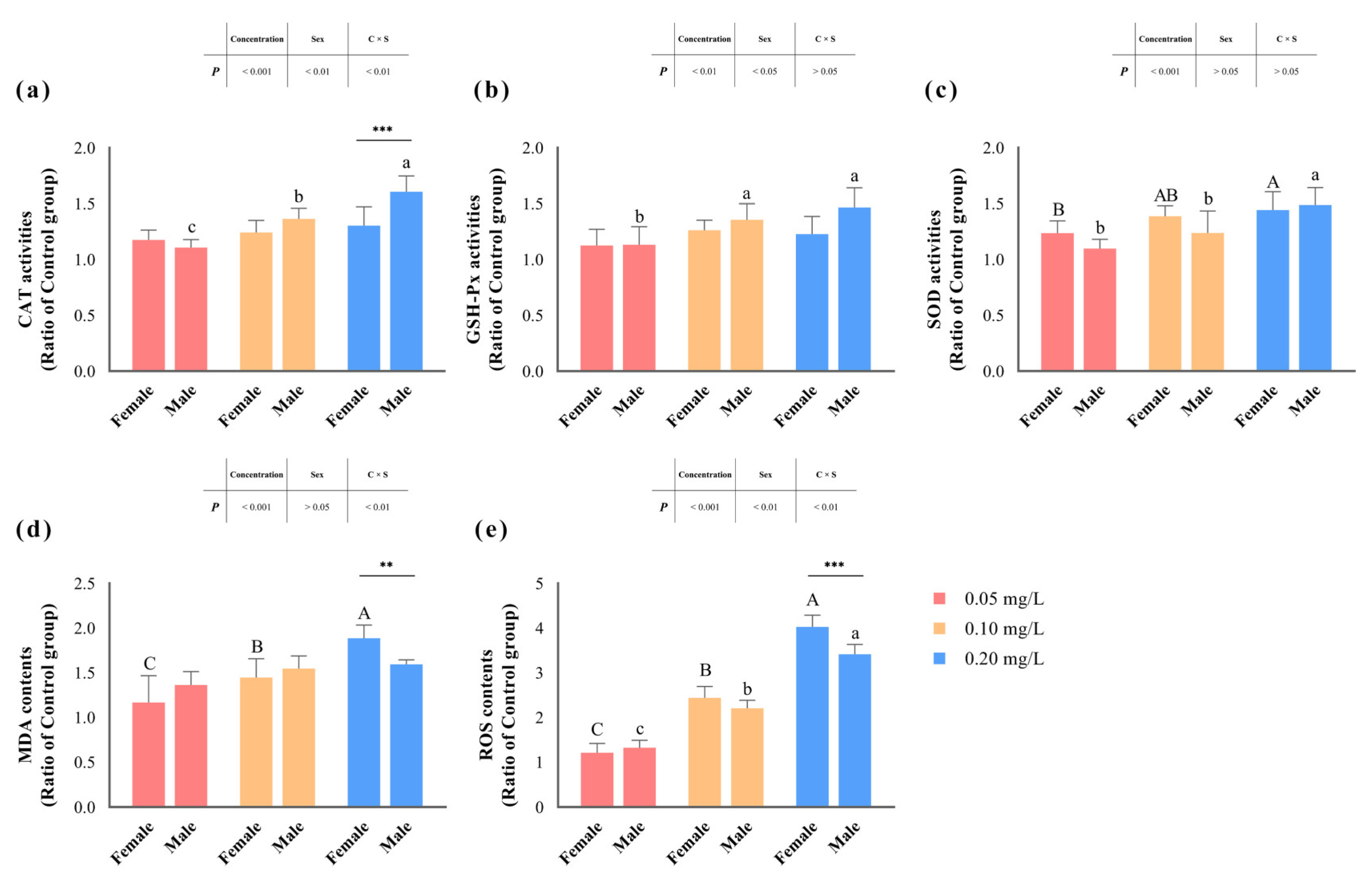
In our assessment of key enzyme activities within the antioxidant defense system, we observed significant effects (p < 0.05) of methomyl exposure concentration on the activities of CAT, SOD, and GSH-Px (Table 4 and Figure 3). Among them, the activities of CAT and GSH-Px displayed significant differentiation based on male/female sex (p < 0.05). Notably, CAT activity exhibited an interaction effect between concentration and sex (p < 0.05), with the maximum observed in the livers of male zebrafish exposed to 0.20 mg/L methomyl.

Table 4.
Changes in hepatic oxidative stress levels in male and female zebrafish after 56 days of methomyl exposure.
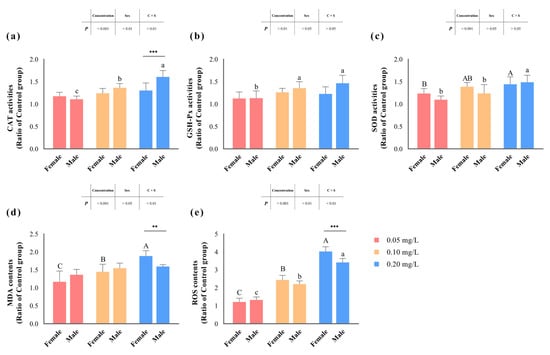
Figure 3.
Changes in zebrafish hepatic CAT (a), GSH-Px (b), SOD (c), MDA (d), and ROS (e) levels after sub-chronic exposure to methomyl compared to the control group (expressed as ratios relative to the levels in the control group). Different letters indicate significant differences between concentration groups, p < 0.05. Asterisks (*) denote significant concentration–sex interaction effects for this concentration group. **: p < 0.01, ***: p < 0.001.
Similarly, hepatic MDA and ROS contents (expressed as a ratio of the control group) significantly increased (p < 0.05) with increasing methomyl exposure concentrations, showing a concentration–effect relationship. Interestingly, changes in MDA content did not show significant correlations with sex differences (p > 0.05), but were significantly influenced by the interaction effects of concentration and sex (p < 0.05). In addition, changes in ROS content were significantly impacted (p < 0.05), not only by exposure concentration and sex differences, but also by the interaction effect. Both MDA and ROS content peaked in the livers of female fish at the 0.20 mg/L exposure concentration.
3.4. Effects of Methomyl Exposure on Transcription Levels of Genes Associated with Hepatic Inflammation of Female and Male Zebrafish
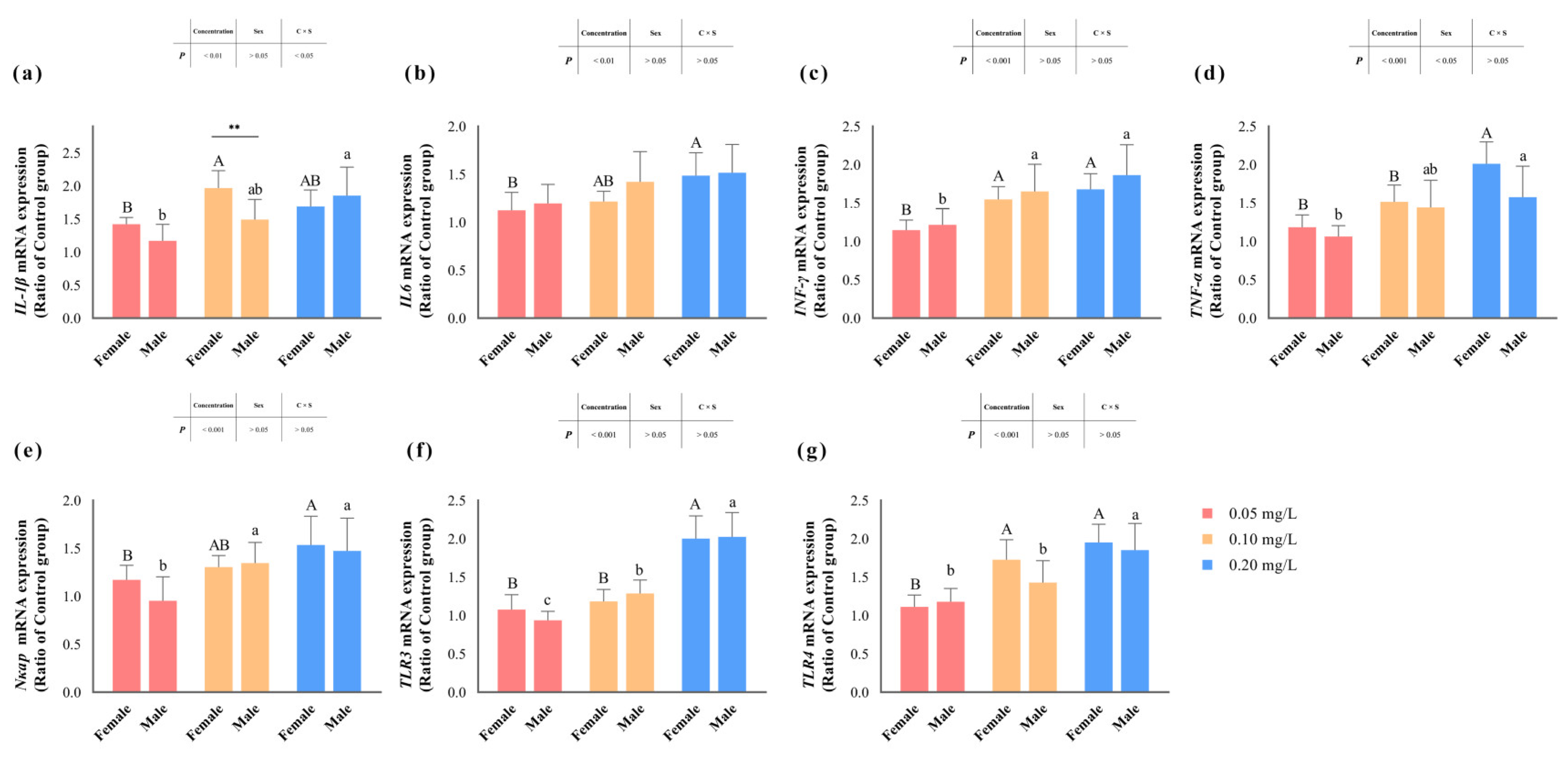
Figure 4 illustrates the transcription levels of zebrafish liver inflammation-related genes after methomyl exposure. Genes encoding pro-inflammatory factors, such as IL6, INF-γ and Nκap, as well as toll-like receptors (TLRs) like TLR3 and TLR4, displayed significant sensitivity to methomyl concentration (p < 0.05) (Table 2), showing an increasing trend with rising methomyl concentrations and reaching maximum levels in the group exposed to 0.20 mg/L methomyl. However, no discernible sex differences or interaction effects were observed in these genes (p > 0.05). The transcription levels of the TNF-α gene significantly increased (p < 0.05) compared to the control group, indicating a concentration–effect relationship. Interestingly, this alteration was significantly associated with sex, with females showing significantly higher levels compared to males (p < 0.05).
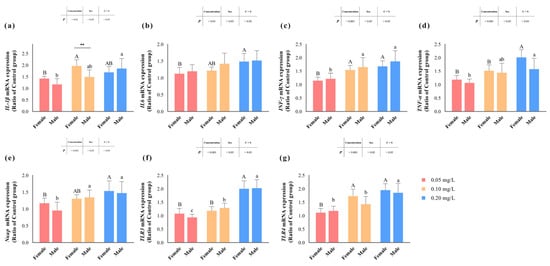
Figure 4.
Changes in transcript levels of IL-1β (a), IL6 (b), INF-γ (c), TNF-α (d), Nκap (e), TLR3 (f), and TLR4 (g) genes in zebrafish livers after sub-chronic exposure to methomyl compared to the control group (expressed as ratios relative to the levels in the control group). Different letters indicate significant differences between concentration groups, p < 0.05. Asterisks (*) denote significant concentration–sex interaction effects for this concentration group. **: p < 0.01.
Additionally, the transcription levels of the IL1β gene demonstrated a distinctive pattern: while not significantly influenced by sex differences (p > 0.05), they were notably affected by methomyl concentration and the interaction effect of concentration and sex (p < 0.05). Specifically, females displayed significantly higher transcription levels compared to males at the exposure concentration of 0.10 mg/L. Overall, the transcription levels of the IL1β gene exhibited a significant and consistent increase in the livers of male zebrafish after methomyl exposure, contrasting with the increasing-then-decreasing trend observed in the livers of female zebrafish.
4. Discussion
Carbamate insecticides, such as methomyl, have been extensively utilized in agricultural pest control, resulting in their frequent detection in the environment. Previous studies have elucidated various detrimental effects of methomyl on organisms, including impacts on embryonic development [30], oxidative stress [31], apoptosis [32], energy metabolism [33], sex hormone secretion [34], immune response [35], and motor behavior [36]. However, little attention has been paid to whether there is a differential effect of methomyl on male and female fish. Our study seeks to address this knowledge gap by comparing the differences in hepatic antioxidant function, apoptosis, and inflammatory responses between male and female zebrafish after sub-chronic exposure to environmentally relevant concentrations of methomyl.
The liver serves as the primary organ for detoxification of xenobiotics and elimination of toxic substances in fish, but pollutants possess the potential to inflict structural damage upon it. SOD, CAT, and GSH-Px enzymes play crucial roles in the antioxidant defense system, and their activities are modulated by environmental pollution, thereby triggering the production of reactive oxygen species (ROS) and subsequent oxidative stress. In this experiment, we observed a substantial increase in ROS and MDA contents (utilized to evaluate cellular ROS oxidation) with escalating concentrations of methomyl exposure, peaking at the 0.20 mg/L exposure concentration. We also noted a pronounced concentration-by-sex interaction effect, with MDA and ROS alterations in the livers of females at the 0.20 mg/L concentration surpassing those in male fish.
Furthermore, our study revealed an upward trend in CAT, SOD, and GSH-PX activities in the liver tissues of both female and male zebrafish after methomyl exposure, with significant alterations correlating with concentration. Simultaneously, CAT and GSH-Px activities were significantly influenced by the sex of zebrafish, with male zebrafish exhibiting higher activity levels than females at a concentration of 0.20 mg/L methomyl. Numerous studies on the toxic effects of pesticides such as diazinon [37], cypermethrin [38], atrazine [39,40], methomyl [41,42], quinalphos [43], lambda-cyhalothrin [44], carbosulfan [45], organochlorine pesticides (OCPs) [46], 2,4-dichlorophenoxyacetic acid, and azinphosmethyl [47] have documented similar results of induced oxidative stress in fish, including zebrafish, Oreochromis Niloticus, Oncorhynchus mykiss, and Cyprinus Carpio. Interestingly, differences in pesticide species, exposure concentrations, exposure durations, and exposure subjects may account for the varying outcomes in terms of changes in enzyme activities. For example, studies on diazinon [37] and cypermethrin [38] exposure have been shown to disrupt antioxidant defenses and reduce the activity of CAT, SOD, and GSH-Px enzymes in tissues, and exposure to methomyl, quinalphos [43], lambda-cyhalothrin [44], and atrazine [39] has been reported to induce the activation of antioxidant enzyme defenses, elevating their activities. These results ultimately suggest that exposure to pollutants elevates ROS levels, inducing oxidative stress in the organism and causing toxic damage. Therefore, based on this experiment, we concluded that chronic exposure to environmental concentrations of methomyl increased the ROS levels and MDA content in zebrafish livers, induced stress and oxidative damage, and activated the antioxidant defense system. Combined with the observation of lower ROS and MDA contents in the livers of male zebrafish compared to female zebrafish, we hypothesized that exposure to 0.20 mg/L methomyl posed a greater risk of oxidative toxicity injury to the livers of female zebrafish.
ALT and AST are widely employed as conventional markers to evaluate liver function and injury, with their release indicating hepatocyte necrosis or membrane damage [48]. Additionally, γ-GT plays a pivotal role in maintaining glutathione homeostasis and oxidative stress balance by cleaving intact glutathione and transferring glutamyl to various molecular acceptors in ROS-exposed cells [49,50]. Our study revealed that hepatic ALT, AST, and γ-GT activities significantly increased after methomyl exposure, displaying a concentration–effect relationship. This finding is consistent with results from studies on the toxicity of pesticides such as methomyl [51], chlorpyrifos [52], glyphosate, and methidathion [53]. It is interesting to us that, when male and female zebrafish were separately exposed to methomyl, the increase in ALT activity was significantly higher in females than in males at a concentration of 0.20 mg/L methomyl. This suggests that methomyl exposure caused more pronounced liver damage in female fish. Moreover, methomyl induced apoptosis in zebrafish liver cells in a concentration-dependent manner, as evidenced by TUNEL staining and the transcriptional levels of Bax/Bcl2a and Caspases3a, which are important regulators in the apoptotic pathway [54].
ROS is widely acknowledged as a key mediator in necroptosis and can act in concert with the NF-κB pathway to regulate programmed cell death [55] and inflammatory responses [56] in animals. While the inflammatory response is vital for triggering the immune system’s defensive reaction against various danger signals [57], prolonged or chronic inflammation can lead to the release of proinflammatory cytokines (IL-1β, IL6, INF-γ, TNF-α), disrupting inflammatory processes’ delicate balance and resulting in severe symptoms and complications [58,59]. Additionally, TLRs play a crucial role in inflammatory and antiviral responses by sensing pathogen-associated molecular patterns (PAMPs) [60], with TLR3 and TLR4 being particularly sensitive to allergens and environmental pollutants [61,62], thereby activating the NF-κB pathway [63,64]. In our study, the transcription levels of the IL-1β, IL6, INF-γ, and TNF-α genes were significantly affected by methomyl concentration, as is consistent with exposure results for other pesticides such as Chlorpyrifos [10], Abamectin [12], oxyfluorfen [65], and organochlorine pesticides [66]. Similarly, the transcription levels of the TLR3, TLR4, and Nkap genes increased significantly after exposure, indicating that methomyl exposure induced an inflammatory response in zebrafish, likely related to increased susceptibility to allergens [67]. The data showed a clear concentration–effect relationship, with higher exposure concentrations correlating with greater toxicity hazards. Notably, TNF-α transcription levels were significantly correlated with sex differences, and IL-1β was significantly affected by the interaction effect of concentration and sex, especially in the 0.10 mg/L exposure group. The alterations in the transcription levels of these genes were significantly higher in females than in males, suggesting that female zebrafish exposed to environmental concentrations of methomyl may have a higher risk of chronic inflammation compared to male zebrafish.
5. Conclusions
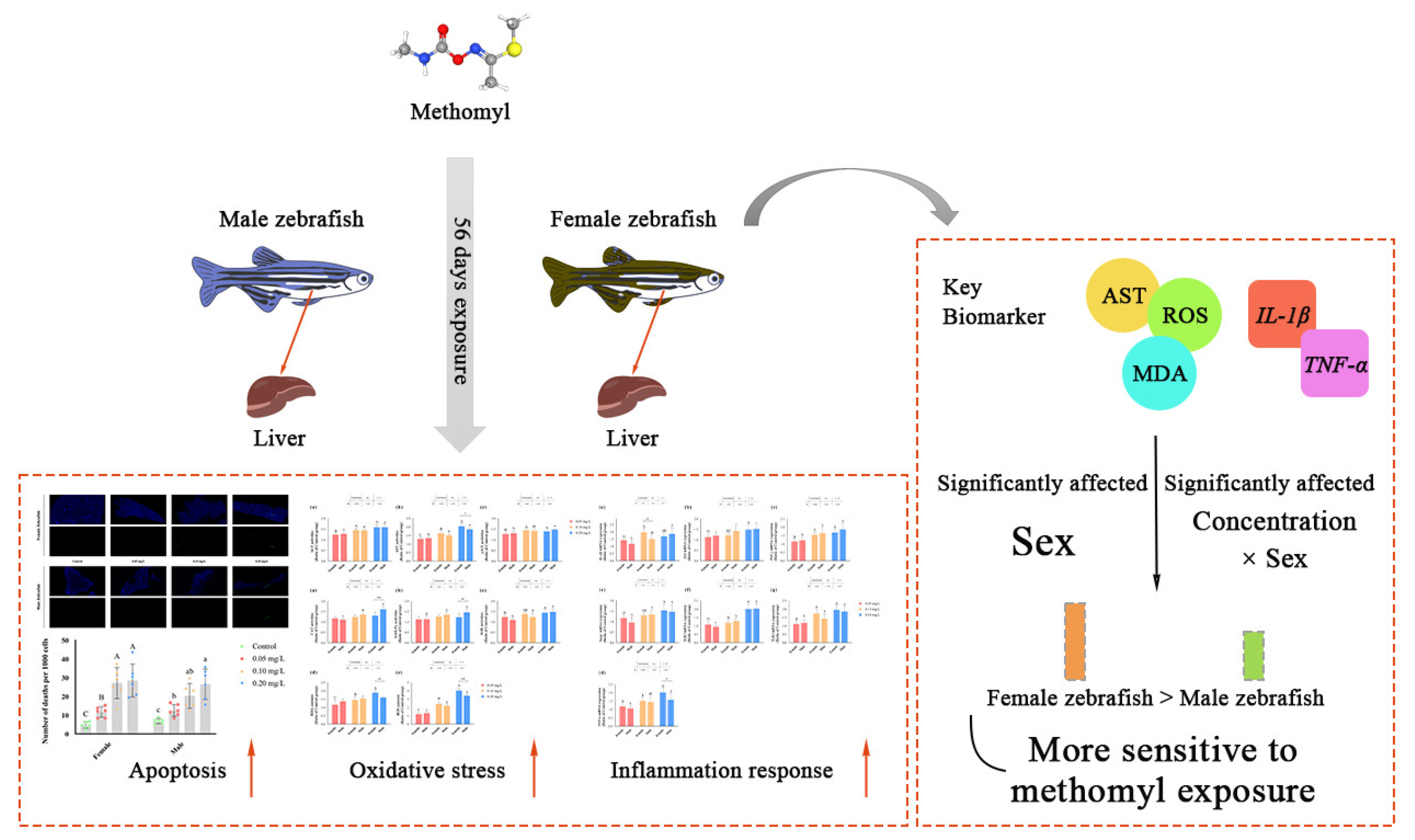
The liver plays a pivotal role in the metabolism and detoxification of xenobiotics. Our study demonstrated that sub-chronic exposure to methomyl at concentrations of 0.10–0.20 mg/L for 56 days resulted in a significant increase in ROS levels in the zebrafish livers, accompanied by the activation of the antioxidant defense system. Methomyl exposure also upregulated the expression of apoptotic genes Bax/Bcl2a and Caspases3a and increased the number of apoptotic cells. This exposure-induced oxidative stress was accompanied by chronic low-grade inflammation, with genes encoding inflammatory factors being highly expressed at 0.10–0.20 mg/L exposure concentrations. This heightened inflammatory response may increase the susceptibility of zebrafish to pathogenic bacteria. In conclusion, the results demonstrate that methomyl exposure induced oxidative stress and inflammatory responses in zebrafish, and female zebrafish were more susceptible to the toxic effects of methomyl than male zebrafish (Figure 5). Furthermore, it is noteworthy that even concentrations of 0.10 to 0.20 mg/L, which are lower than the theoretical safe concentration derived from acute toxicity tests, were capable of inducing changes in liver transcription factors and enzyme activities after 56 days of sub-chronic exposure. Consequently, pesticide risk assessments should not solely rely on acute toxicity data, but must also consider the potential toxic effects arising from prolonged exposure.
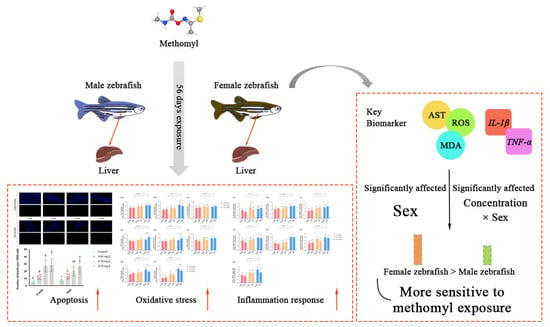
Figure 5.
Summary of sub-chronic methomyl exposure in zebrafish livers: sex-susceptible biomarker responses. Key indicators such as AST, ROS, MDA, IL-1β, and TNF-α exhibited heightened sensitivity in females, suggesting that these markers can serve as effective biomarkers for assessing sex differences in response to methomyl exposure. Different letters indicate significant differences between concentration groups, p < 0.05, asterisks (*) denote significant concentration–sex interaction effects for this concentration group. **: p < 0.01, ***: p < 0.001.
The findings of our study underscore the imperative to develop sex-specific guidelines regarding exposure limits for pesticides such as methomyl and highlight the critical importance of integrating the toxic effects of long-term exposure into the pesticide risk assessment framework to ensure robust and protective measures for public health, addressing both acute and chronic toxicological impacts.
Author Contributions
Conceptualization: S.M., X.M., B.X. and J.L.; methodology: M.L. and X.C.; formal analysis and investigation: M.L.; writing—original draft preparation: M.L.; writing—review and editing: S.M., L.F. and H.X.; funding acquisition: S.M. and J.L.; resources: L.Q., D.L. and J.X.; supervision: C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Central Public-interest Scientific Institution Basal Research Fund, CAFS: Project of Innovation Team on Fishery Eco-Environment Monitoring and Remediation of Yangtze River Basin (NO. 2024TD18).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee at the Freshwater Fisheries Research Centre of the Chinese Academy of Fishery Sciences (FFRC, Wuxi, China) (protocol code: 2011AA1004020012 and date of approval: 2023).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
Acetylcholinesterase, AChE; Alanine aminotransferase, ALT; Aspartate aminotransferase, AST; Gamma-glutamyl transpeptidase, γ-GT; Superoxide dismutase, SOD; Catalase, CAT; Glutathione peroxidase, GSH-Px; Malondialdehyde, MDA; Reactive oxygen species, ROS; Total protein, TP; Interleukin 1 beta, IL-1β; Interleukin 6, IL6; Tumor necrosis factor alpha, TNF-α; Nuclear factor-kappaB activating protein, Nκap; Interferon 1, IFN-γ; Toll-like receptor 3, TLR3; Toll-like receptor 4, TLR4; B-cell lymphoma2 associated X, Bax; B-cell lymphoma2 apoptosis regulator a, Bcl2a; Caspase3, apoptosis-related cysteine peptidase a, Caspases3a; Beta-actin, β-actin.
References
- FAO. Agricultural Production Statistics 2000–2021; FAOSTAT Anal. Brief Ser. No 60; FAO: Rome, Italy, 2022. [Google Scholar]
- Selvam, A.D.G.; Thatheyus, A.J.; Vidhya, R. Biodegradation of the Synthetic Pyrethroid, Fenvalerate by Bacillus cereus Mtcc 1305. World J. Environ. Eng. 2013, 1, 21–26. [Google Scholar] [CrossRef]
- Yang, G.-P.; Zhao, Y.-H.; Lu, X.-L.; Gao, X.-C. Adsorption of Methomyl on Marine Sediments. Colloids Surf. Physicochem. Eng. Asp. 2005, 264, 179–186. [Google Scholar] [CrossRef]
- Zheng, Y.; Fateh, B.; Xu, G. Effects of Methomyl on the Intestinal Microbiome and Hepatic Transcriptome of Tilapia, and the Modifying Effects of Mint Co-Culture. Aquat. Toxicol. 2023, 263, 106675. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, T.J.; Stone, A.T. Reduction of the Carbamate Pesticides Oxamyl and Methomyl by Dissolved FeII and CuI. Environ. Sci. Technol. 2001, 35, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Legler, J.; Hamers, T.; van der Sluijs, M.V.; Schoeters, G.; van der Ven, L.; Eggesbo, M.; Koppe, J.; Feinberg, M.; Trnovec, T. The OBELIX Project: Early Life Exposure to Endocrine Disruptors and Obesity. Am. J. Clin. Nutr. 2011, 94, S1933–S1938. [Google Scholar] [CrossRef] [PubMed]
- Cestonaro, L.V.; Garcia, S.C.; Nascimento, S.; Gauer, B.; Sauer, E.; Göethel, G.; Peruzzi, C.; Nardi, J.; Fão, N.; Piton, Y.; et al. Biochemical, Hematological and Immunological Parameters and Relationship with Occupational Exposure to Pesticides and Metals. Environ. Sci. Pollut. Res. 2020, 27, 29291–29302. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Arias, M.A.; Medina-Díaz, I.M.; Bernal-Hernández, Y.Y.; Agraz-Cibrián, J.M.; González-Arias, C.A.; Barrón-Vivanco, B.S.; Herrera-Moreno, J.F.; Verdín-Betancourt, F.A.; Zambrano-Zaragoza, J.F.; Rojas-García, A.E. Hematological Indices as Indicators of Inflammation Induced by Exposure to Pesticides. Environ. Sci. Pollut. Res. 2023, 30, 19466–19476. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.Z.; Hafida, M.; Merzouk, S.A.; Loukidi, B.; Taouli, K.; Narce, M. Hemostatic, Inflammatory, and Oxidative Markers in Pesticide User Farmers. Biomarkers 2016, 21, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, Y.; Bi, Y.; Wu, Q.; Xu, S. Baicalin Suppressed Necroptosis and Inflammation against Chlorpyrifos Toxicity; Involving in ER Stress and Oxidative Stress in Carp Gills. Fish Shellfish Immunol. 2023, 139, 108883. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, P. Lupeol Application Ameliorates Inflammation, Oxidative Stress Mediated Toxicicity and Apoptosis in Pesticides Model. Biol. Bull. 2023, 50, 244–249. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, P.; Liu, F.; Zhang, W.; Yang, H.; Li, X.; Dong, J. Abamectin Causes Toxicity to the Carp Respiratory System by Triggering Oxidative Stress, Inflammation, and Apoptosis and Inhibiting Autophagy. Environ. Sci. Pollut. Res. 2023, 30, 55200–55213. [Google Scholar] [CrossRef] [PubMed]
- Hallauer, J.; Geng, X.; Yang, H.-C.; Shen, J.; Tsai, K.-J.; Liu, Z. The Effect of Chronic Arsenic Exposure in Zebrafish. Zebrafish 2016, 13, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Mou, Z.; Li, R.; Hossen, M.A.; Dai, J.; Qin, W.; Lee, K. Characterization and Preliminary Safety Evaluation of Nano-SiO2 Isolated from Instant Coffee. Ecotoxicol. Environ. Saf. 2021, 224, 112694. [Google Scholar] [CrossRef] [PubMed]
- Glaberman, S.; Padilla, S.; Barron, M.G. Evaluating the Zebrafish Embryo Toxicity Test for Pesticide Hazard Screening. Environ. Toxicol. Chem. 2017, 36, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Lo, D.; Wang, Y.-T.; Wu, M.-C. Hepatoprotective Effect of Silymarin on Di(2-Ethylhexyl)Phthalate (DEHP) Induced Injury in Liver FL83B Cells. Environ. Toxicol. Pharmacol. 2014, 38, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, H.; Dambrós, B.F.; Rodrigues, K.; Cesconetto, P.A.; Zamoner, A.; Sousa de Moura, K.R.; Gomes Castro, A.J.; Van Der Kraak, G.; Mena Barreto Silva, F.R. Acute Exposure to Bis(2-Ethylhexyl)Phthalate Disrupts Calcium Homeostasis, Energy Metabolism and Induces Oxidative Stress in the Testis of Danio rerio. Biochimie 2020, 175, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rieg, C.E.H.; Cattani, D.; Naspolini, N.F.; Cenci, V.H.; de Liz Oliveira Cavalli, V.L.; Jacques, A.V.; Nascimento, M.V.P.D.S.; Dalmarco, E.M.; De Moraes, A.C.R.; Santos-Silva, M.C.; et al. Perinatal Exposure to a Glyphosate Pesticide Formulation Induces Offspring Liver Damage. Toxicol. Appl. Pharmacol. 2022, 454, 116245. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Chen, X.; Song, C.; Fan, L.; Qiu, L.; Zheng, Y.; Chen, J.; Xu, P. Effect of Chronic Exposure to Pesticide Methomyl on Antioxidant Defense System in Testis of Tilapia (Oreochromis niloticus) and Its Recovery Pattern. Appl. Sci. 2021, 11, 3332. [Google Scholar] [CrossRef]
- Milan, F.S.; Maleki, B.R.S.; Moosavy, M.-H.; Mousavi, S.; Sheikhzadeh, N.; Khatibi, S.A. Ameliorating Effects of Dietary Haematococcus pluvialis on Arsenic-Induced Oxidative Stress in Rainbow Trout (Oncorhynchus mykiss) Fillet. Ecotoxicol. Environ. Saf. 2021, 207, 111559. [Google Scholar] [CrossRef] [PubMed]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, D.A.; Casero, D.; Zhang, Z.; Wang, D.; Kim, J.; Wu, X.; Vergnes, L.; Mirza, A.H.; Leon-Mimila, P.; Williams, K.J.; et al. Transcriptional Regulation of N6-Methyladenosine Orchestrates Sex-Dimorphic Metabolic Traits. Nat. Metab. 2021, 3, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, Y.; Wang, J.; Xie, T.; Zhang, J.; Jia, Y. Gender differences in the hematology, hepatic antioxidant capacity, and gill histology of turbot (Scophthalmus maximus) under hypoxic stress. J. Fish. Sci. China 2023, 30, 878–890. [Google Scholar]
- Saeid, M.H.E.; Turki, A.M.A.; Wable, M.I.A.; Nasser, G.A. Evaluation of Pesticide Residues in Saudi Arabia Ground Water. Res. J. Environ. Sci. 2010, 5, 171–178. [Google Scholar] [CrossRef]
- Van Scoy, A.R.; Yue, M.; Deng, X.; Tjeerdema, R.S. Environmental Fate and Toxicology of Methomyl. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2013; Volume 222, pp. 93–109. ISBN 978-1-4614-4716-0. [Google Scholar]
- Leandro, C.; Souza, V.; Dores, E.F.G.C.; Ribeiro, M.L. Determination of Pesticides Multiresidues in Shallow Groundwater in a Cotton-Growing Region of Mato Grosso, Brazil. J. Braz. Chem. Soc. 2008, 19, 1111–1117. [Google Scholar] [CrossRef]
- Meng, S.L.; Chen, F.; Chen, X.; Li, M.X.; Qiu, L.P.; Li, D.D.; Song, C.; Fan, L.M.; Chen, J.Z.; Xu, P. Acute Toxicity Effects of Methomyl on Organisms in Typical Aquatic Food Chain of Green Algae-Daphnia magna-Fish. Chin. Agric. Sci. Bull. 2023, 39, 121–126. [Google Scholar]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.L.; Li, M.X.; Lu, Y.; Chen, X.; Wang, W.P.; Song, C.; Fan, L.M.; Qiu, L.P.; Li, D.D.; Xu, H.M.; et al. Effect of Environmental Level of Methomyl on Hatching, Morphology, Immunity and Development Related Genes Expression in Zebrafish (Danio rerio) Embryo. Ecotoxicol. Environ. Saf. 2023, 268, 115684. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.-L.; Qu, J.-H.; Fan, L.-M.; Qiu, L.-P.; Chen, J.-Z.; Xu, P. Responses of Glutathione-Related Antioxidant Defense System in Serum of Nile Tilapia (Oreochromis niloticus) Exposed to Sublethal Concentration of Methomyl and Recovery Pattern. Environ. Toxicol. 2015, 30, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.L.; Liu, T.; Chen, X.; Qiu, L.P.; Hu, G.D.; Song, C.; Fan, L.; Zheng, Y.; Chen, J.Z.; Xu, P. Effect of Chronic Exposure to Methomyl on Tissue Damage and Apoptosis in Testis of Tilapia (Oreochromis niloticus) and Recovery Pattern. Bull. Environ. Contam. Toxicol. 2019, 102, 371–376. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ni, Y.; Jin, Y.; Fu, Z. Pesticides-Induced Energy Metabolic Disorders. Sci. Total Environ. 2020, 729, 139033. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.-L.; Qiu, L.-P.; Hu, G.-D.; Fan, L.-M.; Song, C.; Zheng, Y.; Wu, W.; Qu, J.-H.; Li, D.-D.; Chen, J.-Z.; et al. Effect of Methomyl on Sex Steroid Hormone and Vitellogenin Levels in Serum of Male Tilapia (Oreochromis niloticus) and Recovery Pattern. Environ. Toxicol. 2017, 32, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman Mohamed, A.; Abdel Rahman, A.N.; Salem, G.A.; Deib, M.M.E.; Nassan, M.A.; Rhouma, N.R.; Khater, S.I. The Antioxidant Role of a Taurine-Enriched Diet in Combating the Immunotoxic and Inflammatory Effects of Pyrethroids and/or Carbamates in Oreochromis niloticus. Animals 2021, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, C.A.; Pereira, T.C.B.; Teodoro, L.D.S.; Altenhofen, S.; Rübensam, G.; Bonan, C.D.; Bogo, M.R. Acute Toxicity of Methomyl Commercial Formulation Induces Morphological and Behavioral Changes in Larval Zebrafish (Danio rerio). Neurotoxicol. Teratol. 2022, 89, 107058. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, W.; Jiang, Y.; Chu, W. Diazinon Exposure Produces Histological Damage, Oxidative Stress, Immune Disorders and Gut Microbiota Dysbiosis in Crucian Carp (Carassius auratus gibelio). Environ. Pollut. 2021, 269, 116129. [Google Scholar] [CrossRef] [PubMed]
- Alak, G.; Yeltekin, A.Ç.; Özgeriş, F.B.; Parlak, V.; Uçar, A.; Sait Keleş, M.; Atamanalp, M. Therapeutic Effect of N- Acetyl Cysteine as an Antioxidant on Rainbow Trout’s Brain in Cypermethrin Toxicity. Chemosphere 2019, 221, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Paulino, M.G.; Souza, N.E.S.; Fernandes, M.N. Subchronic Exposure to Atrazine Induces Biochemical and Histopathological Changes in the Gills of a Neotropical Freshwater Fish, Prochilodus lineatus. Ecotoxicol. Environ. Saf. 2012, 80, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-S.; Shao, B.; Song, Y.; Xie, H.; Wang, J.; Wang, J.-H.; Liu, W.; Hou, X.-X. DNA Damage and Effects on Antioxidative Enzymes in Zebra Fish (Danio rerio) Induced by Atrazine. Toxicol. Mech. Methods 2011, 21, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.L.; Hu, G.D.; Qiu, L.P.; Song, C.; Fan, L.M.; Chen, J.Z.; Xu, P. Effects of Chronic Exposure of Methomyl on the Antioxidant System in Kidney of Nile Tilapia (Oreochromis niloticus) and Recovery Pattern. J. Toxicol. Environ. Health A 2013, 76, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.L.; Chen, J.Z.; Xu, P.; Qu, J.H.; Fan, L.M.; Song, C.; Qiu, L.P. Hepatic Antioxidant Enzymes SOD and CAT of Nile Tilapia (Oreochromis niloticus) in Response to Pesticide Methomyl and Recovery Pattern. Bull. Environ. Contam. Toxicol. 2014, 92, 388–392. [Google Scholar] [CrossRef]
- Hemalatha, D.; Amala, A.; Rangasamy, B.; Nataraj, B.; Ramesh, M. Sublethal Toxicity of Quinalphos on Oxidative Stress and Antioxidant Responses in a Freshwater Fish Cyprinus Carpio. Environ. Toxicol. 2016, 31, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, A.; Niu, F.; Zhang, Y. Antioxidant Vitamin E Protects Embryos of Xenopus Tropicalis against Lambda-Cyhalothrin Induced Embryotoxicity. Environ. Sci. Pollut. Res. 2019, 26, 21629–21640. [Google Scholar] [CrossRef] [PubMed]
- Capkin, E.; Altinok, I. Effects of Chronic Carbosulfan Exposure on Liver Antioxidant Enzyme Activities in Rainbow Trout. Environ. Toxicol. Pharmacol. 2013, 36, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Karaca, M.; Varışlı, L.; Korkmaz, K.; Özaydın, O.; Perçin, F.; Orhan, H. Organochlorine Pesticides and Antioxidant Enzymes Are Inversely Correlated with Liver Enzyme Gene Expression in Cyprinus carpio. Toxicol. Lett. 2014, 230, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Ozcan Oruc, E.; Sevgiler, Y.; Uner, N. Tissue-Specific Oxidative Stress Responses in Fish Exposed to 2,4-D and Azinphosmethyl. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 137, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, X.; Qian, H.; Li, X.; Su, J.; Zhang, G.; Li, X. Associations of Arsenic Exposure with Liver Injury in US Adults: NHANES 2003–2018. Environ. Sci. Pollut. Res. 2023, 30, 48260–48269. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, M.H. Gamma-Glutamyl Transpeptidase: Redox Regulation and Drug Resistance. In Advances in Cancer Research; Townsend, D.M., Tew, K.D., Eds.; Redox and Cancer Part A; Academic Press: Cambridge, MA, USA, 2014; Volume 122, pp. 103–141. [Google Scholar]
- Hegazi, M.M.; Attia, Z.I.; Ashour, O.A. Oxidative Stress and Antioxidant Enzymes in Liver and White Muscle of Nile Tilapia Juveniles in Chronic Ammonia Exposure. Aquat. Toxicol. 2010, 99, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Gaber, O.A.; Asran, A.E.A.; Elfayoumi, H.M.K.; El-Shahawy, G.; Khider, F.K.; Abdel-Tawab, H.; Mahmoud, K.A. Influence of Methomyl (Copter 90%) on Certain Biochemical Activities and Histological Structures of Land Snails Monacha cartusiana. Saudi J. Biol. Sci. 2022, 29, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Akhlaghi, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Rakhshaninejad, M. Combined Effects of Exposure to Sub-Lethal Concentration of the Insecticide Chlorpyrifos and the Herbicide Glyphosate on the Biochemical Changes in the Freshwater Crayfish Pontastacus leptodactylus. Ecotoxicology 2020, 29, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Güngördü, A.; Uçkun, M.; Yoloğlu, E. Integrated Assessment of Biochemical Markers in Premetamorphic Tadpoles of Three Amphibian Species Exposed to Glyphosate- and Methidathion-Based Pesticides in Single and Combination Forms. Chemosphere 2016, 144, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Jia, K.; Huang, L.; Liao, X.; Guo, X.; Lu, H. Hepatotoxicity of Tricyclazole in Zebrafish (Danio rerio). Chemosphere 2019, 232, 171–179. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, X.; Liu, Q.; Li, X. Tea Polyphenols Alleviates Acetochlor-Induced Apoptosis and Necroptosis via ROS/MAPK/NF-κB Signaling in Ctenopharyngodon idellus Kidney Cells. Aquat. Toxicol. 2022, 246, 106153. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, Q.; Chen, D.; Liu, Y. Atrazine Exposure Induces Necroptosis through the P450/ROS Pathway and Causes Inflammation in the Gill of Common Carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2022, 131, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-Sensing Receptors in Sterile Inflammation and Inflammatory Diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Arida, A.; Protogerou, A.D.; Kitas, G.D.; Sfikakis, P.P. Systemic Inflammatory Response and Atherosclerosis: The Paradigm of Chronic Inflammatory Rheumatic Diseases. Int. J. Mol. Sci. 2018, 19, 1890. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR Signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-S.; Fang, D.-A.; Xu, D.-P. Toll-like Receptors (TLRs) Respond to Tributyltin Chloride (TBT-Cl) Exposure in the River Pufferfish (Takifugu obscurus): Evidences for Its Toxic Injury Function. Fish Shellfish Immunol. 2020, 99, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Zettel, K.; Korff, S.; Zamora, R.; Morelli, A.E.; Darwiche, S.; Loughran, P.A.; Elson, G.; Shang, L.; Salgado-Pires, S.; Scott, M.J.; et al. Toll-Like Receptor 4 on Both Myeloid Cells and Dendritic Cells Is Required for Systemic Inflammation and Organ Damage after Hemorrhagic Shock with Tissue Trauma in Mice. Front. Immunol. 2017, 8, 1672. [Google Scholar] [CrossRef] [PubMed]
- Bugge, M.; Bergstrom, B.; Eide, O.K.; Solli, H.; Kjønstad, I.F.; Stenvik, J.; Espevik, T.; Nilsen, N.J. Surface Toll-like Receptor 3 Expression in Metastatic Intestinal Epithelial Cells Induces Inflammatory Cytokine Production and Promotes Invasiveness. J. Biol. Chem. 2017, 292, 15408–15425. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Xiong, H.; Yuan, W.; Huang, L.; Xu, J.; Lu, C.; Hu, Y.; Huang, K.; Luo, Q.; Ma, J.; et al. Diflovidazin Damages the Hematopoietic Stem Cells to Zebrafish Embryos via the TLR4/ NF-κB/ P53 Pathway. Fish Shellfish Immunol. 2023, 135, 108672. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, J.; Jia, K.; Zheng, Z.; Chen, X.; Bai, Z.; Yang, Y.; Chen, B.; Yuan, W.; Chen, W.; et al. Oxyfluorfen Induces Hepatotoxicity through Lipo-Sugar Accumulation and Inflammation in Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 230, 113140. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.K.; Sharma, T.; Banerjee, B.D. Organochlorine Pesticides Induce Inflammation, ROS Production, and DNA Damage in Human Epithelial Ovary Cells: An in Vitro Study. Chemosphere 2020, 246, 125691. [Google Scholar] [CrossRef]
- Dupuy, C.; Cabon, J.; Louboutin, L.; Le Floch, S.; Morin, T.; Danion, M. Cellular, Humoral and Molecular Responses in Rainbow Trout (Oncorhynchus mykiss) Exposed to a Herbicide and Subsequently Infected with Infectious Hematopoietic Necrosis Virus. Aquat. Toxicol. 2019, 215, 105282. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).