Ocular Inflammation and Oxidative Stress as a Result of Chronic Intermittent Hypoxia: A Rat Model of Sleep Apnea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CIH Protocol
2.3. Sample Collection and Preparation
2.4. Immunohistochemistry
2.5. Protein Analysis
2.6. Thiobarbituric Acid Reactive Substance (TBARS) Assay
2.7. Milliplex Inflammation Panel
2.8. Statistics
3. Results
3.1. Chronic Intermittent Hypoxia Increases Hypoxia-Inducible Factor-1α Levels
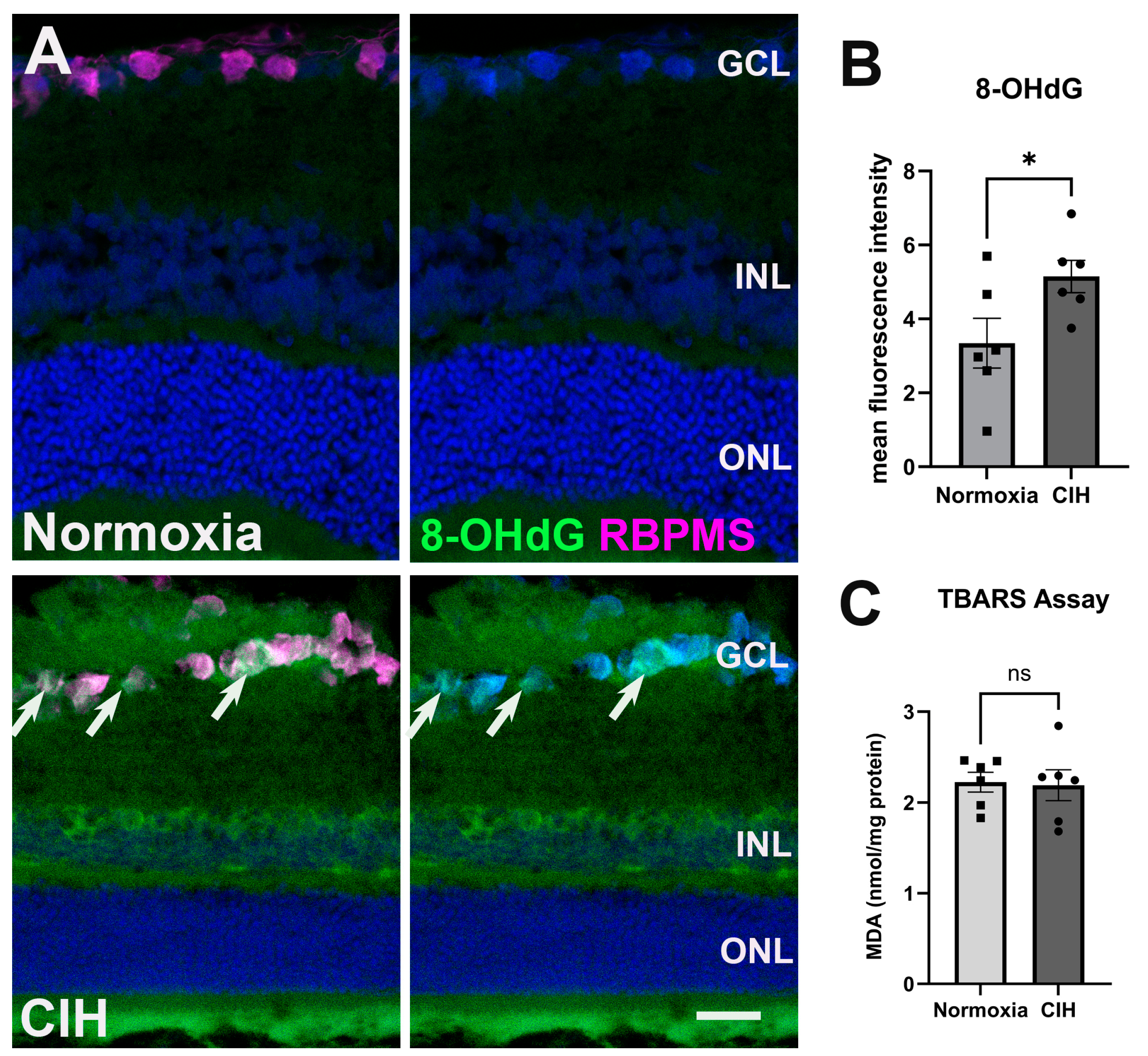
3.2. Chronic Intermittent Hypoxia Increases Oxidative Stress
3.3. Chronic Intermittent Hypoxia Induces Inflammation
3.4. Chronic Intermittent Hypoxia Induces Microglia Activation
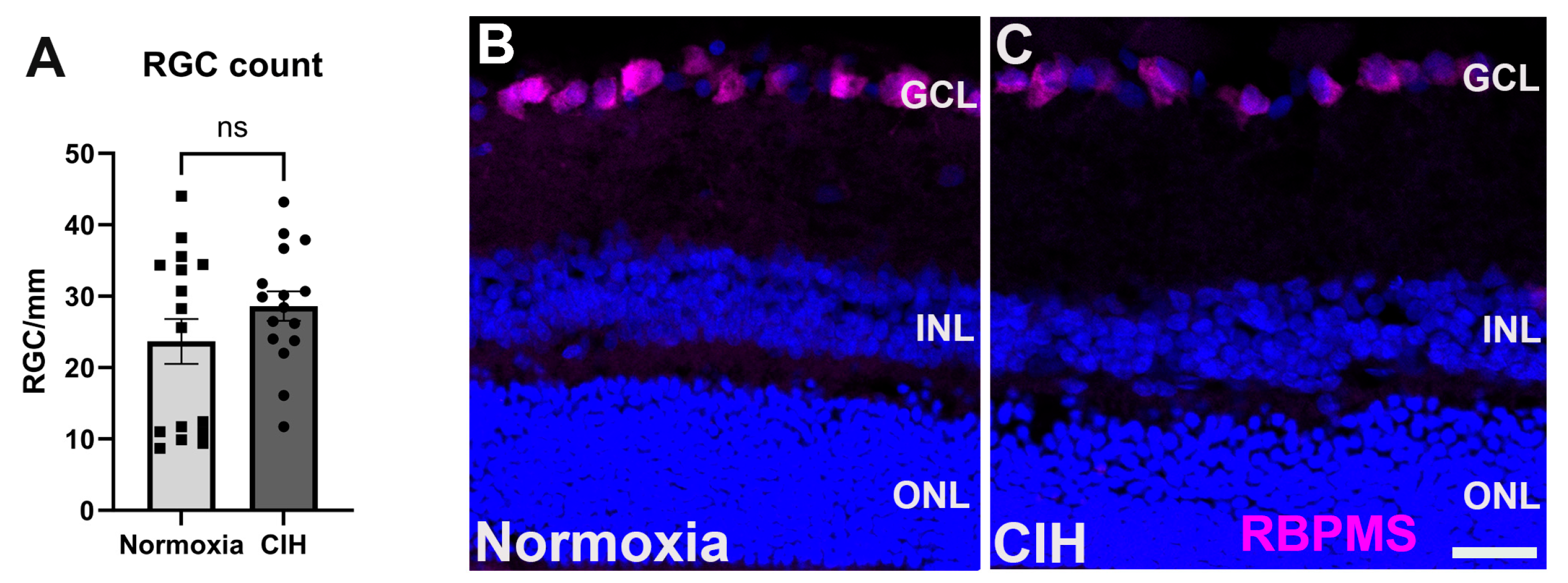
3.5. Effect of Chronic Intermittent Hypoxia on RGC Count
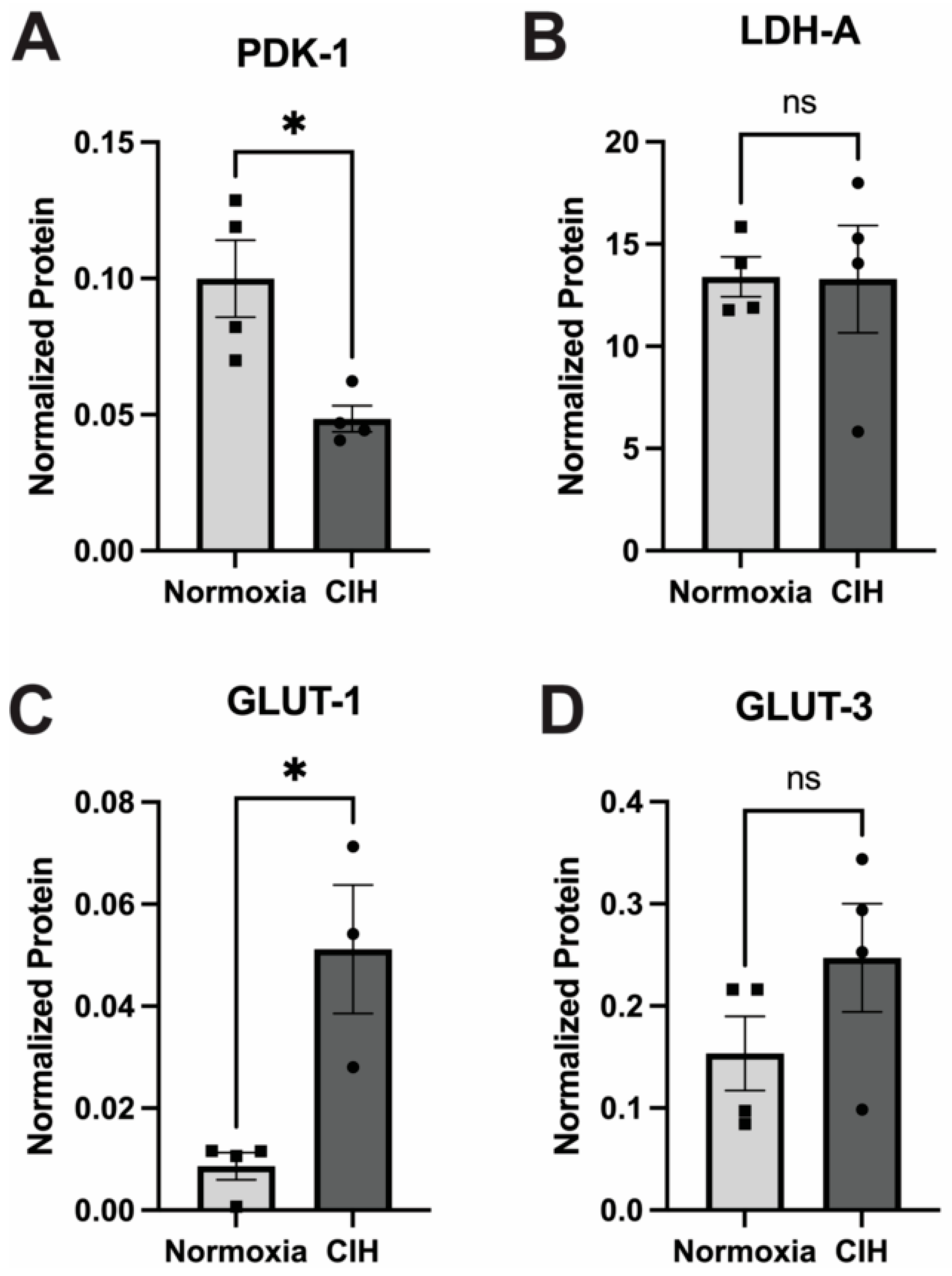
3.6. Effect of Chronic Intermittent Hypoxia on Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Sawaguchi, S.; Araie, M. Differentiating Diagnosed and Undiagnosed Primary Angle-Closure Glaucoma and Open-Angle Glaucoma: A Population-Based Study. Ophthalmol. Glaucoma 2022, 5, 160–169. [Google Scholar] [CrossRef]
- Sheybani, A.; Scott, R.; Samuelson, T.W.; Kahook, M.Y.; Bettis, D.I.; Ahmed, I.I.K.; Stephens, J.D.; Kent, D.; Ferguson, T.J.; Herndon, L.W. Open-Angle Glaucoma: Burden of Illness, Current Therapies, and the Management of Nocturnal IOP Variation. Ophthalmol. Ther. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Tribble, J.R.; Hui, F.; Quintero, H.; El Hajji, S.; Bell, K.; Di Polo, A.; Williams, P.A. Neuroprotection in glaucoma: Mechanisms beyond intraocular pressure lowering. Mol. Asp. Med. 2023, 92, 101193. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Calkins, D.J. The Neurovascular Unit in Glaucomatous Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Konieczka, K.; Liu, X.; Chen, M.; Yao, K.; Wang, K.; Flammer, J. Role of ocular blood flow in normal tension glaucoma. Adv. Ophthalmol. Pract. Res. 2022, 2, 100036. [Google Scholar] [CrossRef]
- Kuroda, F.; Iwase, T.; Yamamoto, K.; Ra, E.; Terasaki, H. Correlation between blood flow on optic nerve head and structural and functional changes in eyes with glaucoma. Sci. Rep. 2020, 10, 729. [Google Scholar] [CrossRef]
- Adzigbli, L.; Sokolov, E.P.; Wimmers, K.; Sokolova, I.M.; Ponsuksili, S. Effects of hypoxia and reoxygenation on mitochondrial functions and transcriptional profiles of isolated brain and muscle porcine cells. Sci. Rep. 2022, 12, 19881. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Chuang, L.-H.; Yu, C.-C.; Lo, Y.-L.; Chen, H.S.L.; Huang, P.-W.; Yeung, L.; Lai, C.-C. Prospective evaluation of the comorbidity of obstructive sleep apnea in patients with glaucoma. J. Clin. Sleep Med. 2022, 18, 47–56. [Google Scholar] [CrossRef]
- Cheong, A.J.Y.; Wang, S.K.X.; Woon, C.Y.; Yap, K.H.; Ng, K.J.Y.; Xu, F.W.X.; Alkan, U.; Ng, A.C.W.; See, A.; Loh, S.R.H.; et al. Obstructive sleep apnoea and glaucoma: A systematic review and meta-analysis. Eye 2023, 37, 3065–3083. [Google Scholar] [CrossRef]
- Lee, T.-E.; Kim, J.S.; Yeom, S.W.; Lee, M.G.; Lee, J.H.; Lee, H.-J. Long-term effects of obstructive sleep apnea and its treatment on open-angle glaucoma: A big-data cohort study. J. Clin. Sleep Med. 2023, 19, 339–346. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Villalaín, I.; Asencio, M.; García, J.; García-Rio, F. Sleep apnea and eye diseases: Evidence of association and potential pathogenic mechanisms. J. Clin. Sleep Med. 2022, 18, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-Y.; Su, W.-W.; Liu, C.-H.; Chen, H.S.-L.; Wu, S.-C.; Chang, S.H.L.; Chen, K.-J.; Wu, W.-C.; Chen, N.-H.; Li, H.-Y.; et al. Correlation between structural progression in glaucoma and obstructive sleep apnea. Eye 2019, 33, 1459–1465. [Google Scholar] [CrossRef]
- Zhou, M.; Li, D.-L.; Kai, J.-Y.; Zhang, X.-F.; Pan, C.-W. Sleep duration and the risk of major eye disorders: A systematic review and meta-analysis. Eye 2023, 37, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Carnero, E.; Bragard, J.; Urrestarazu, E.; Rivas, E.; Polo, V.; Larrosa, J.M.; Antón, V.; Peláez, A.; Moreno-Montañés, J. Continuous intraocular pressure monitoring in patients with obstructive sleep apnea syndrome using a contact lens sensor. PLoS ONE 2020, 15, e0229856. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Pengo, M.F.; Parati, G. Obstructive sleep apnea syndrome and autonomic dysfunction. Auton Neurosci 2019, 221, 102563. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Oh, S.; Baek, S.U.; Ahn, S.J.; Park, K.H.; Jeoung, J.W. Ocular Perfusion Pressure and the Risk of Open-Angle Glaucoma: Systematic Review and Meta-analysis. Sci. Rep. 2020, 10, 10056. [Google Scholar] [CrossRef] [PubMed]
- Christou, E.E.; Kostikas, K.; Asproudis, C.; Zafeiropoulos, P.; Stefaniotou, M.; Asproudis, I. Retinal microcirculation characteristics in obstructive sleep apnea/hypopnea syndrome evaluated by OCT-angiography: A literature review. Int. Ophthalmol. 2022, 42, 3977–3991. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-H.; Tang, Y.; Niu, X.; Sun, H.-Y. The role of nitric oxide (NO) levels in patients with obstructive sleep apnea-hypopnea syndrome: A meta-analysis. Sleep Breath. 2021, 25, 9–16. [Google Scholar] [CrossRef]
- Harańczyk, M.; Konieczyńska, M.; Płazak, W. Endothelial dysfunction in obstructive sleep apnea patients. Sleep Breath. 2022, 26, 231–242. [Google Scholar] [CrossRef]
- Fang, Y.-Y.; Luo, M.; Yue, S.; Han, Y.; Zhang, H.-J.; Zhou, Y.-H.; Liu, K.; Liu, H.-G. 7,8-Dihydroxyflavone protects retinal ganglion cells against chronic intermittent hypoxia-induced oxidative stress damage via activation of the BDNF/TrkB signaling pathway. Sleep Breath. 2022, 26, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Nsiah, N.Y.; Morgan, A.B.; Donkor, N.; Inman, D.M. Long-term HIF-1α stabilization reduces respiration, promotes mitophagy, and results in retinal cell death. Sci. Rep. 2023, 13, 20541. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Santos, V.; Cruz, A.-J.N.; Shih, A.Y. Does Perinatal Intermittent Hypoxia Affect Cerebrovascular Network Development? Dev. Neurosci. 2023, 46, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Reiser, G. How the brain fights fatty acids’ toxicity. Neurochem. Int. 2021, 148, 105050. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.R.; Zhang, L.; Lin, Y.N.; Sun, X.W.; Ding, Y.J.; Li, N.; Li, H.P.; Li, S.Q.; Zhou, J.P.; Li, Q.Y. Chronic intermittent hypoxia-induced mitochondrial dysfunction mediates endothelial injury via the TXNIP/NLRP3/IL-1β signaling pathway. Free Radic. Biol. Med. 2021, 165, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Murenu, E.; Gerhardt, M.-J.; Biel, M.; Michalakis, S. More than meets the eye: The role of microglia in healthy and diseased retina. Front. Immunol. 2022, 13, 1006897. [Google Scholar] [CrossRef]
- Guo, L.; Choi, S.; Bikkannavar, P.; Cordeiro, M.F. Microglia: Key Players in Retinal Ageing and Neurodegeneration. Front. Cell. Neurosci. 2022, 16, 804782. [Google Scholar] [CrossRef]
- Jassim, A.H.; Inman, D.M. Evidence of Hypoxic Glial Cells in a Model of Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1–15. [Google Scholar] [CrossRef]
- Jassim, A.H.; Nsiah, N.Y.; Inman, D.M. Ocular Hypertension Results in Hypoxia within Glia and Neurons throughout the Visual Projection. Antioxidants 2022, 11, 888. [Google Scholar] [CrossRef]
- Prochownik, E.V.; Wang, H. The Metabolic Fates of Pyruvate in Normal and Neoplastic Cells. Cells 2021, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Patko, E.; Szabo, E.; Vaczy, A.; Molitor, D.; Tari, E.; Li, L.; Csutak, A.; Toth, G.; Reglodi, D.; Atlasz, T. Protective Effects of Pituitary Adenylate-Cyclase-Activating Polypeptide on Retinal Vasculature and Molecular Responses in a Rat Model of Moderate Glaucoma. Int. J. Mol. Sci. 2023, 24, 13256. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Moszyńska, A.; Króliczewski, J.; Cabaj, A.; Bartoszewska, S.; Charzyńska, A.; Gebert, M.; Dąbrowski, M.; Collawn, J.F.; Bartoszewski, R. The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell. Mol. Biol. Lett. 2022, 27, 109. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ruan, W.; Bobrow, B.; Carmeliet, P.; Eltzschig, H.K. Targeting hypoxia-inducible factors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2024, 23, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Lu, C.; An, L.; Gao, Q.; Xie, W.; Miao, F.; Chen, X.; Pan, Y.; Wang, Q. SIRT1 relieves Necrotizing Enterocolitis through inactivation of Hypoxia-inducible factor (HIF)-1a. Cell Cycle 2020, 19, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Lee, E.; You, Y.-H.; Ahn, Y.-B.; Song, K.-H.; Kim, J.-W.; Ko, S.-H. Role of sirtuin-1 (SIRT1) in hypoxic injury in pancreatic β-cells. J. Drug Target. 2021, 29, 88–98. [Google Scholar] [CrossRef]
- Jassim, A.H.; Fan, Y.; Pappenhagen, N.; Nsiah, N.Y.; Inman, D.M. Oxidative Stress and Hypoxia Modify Mitochondrial Homeostasis During Glaucoma. Antioxid. Redox Signal. 2021, 35, 1341–1357. [Google Scholar] [CrossRef]
- Mamun, A.A.; Hayashi, H.; Yamamura, A.; Nayeem, M.J.; Sato, M. Hypoxia induces the translocation of glucose transporter 1 to the plasma membrane in vascular endothelial cells. J. Physiol. Sci. 2020, 70, 44. [Google Scholar] [CrossRef]
- Peng, W.; Tan, C.; Mo, L.; Jiang, J.; Zhou, W.; Du, J.; Zhou, X.; Liu, X.; Chen, L. Glucose transporter 3 in neuronal glucose metabolism: Health and diseases. Metabolism 2021, 123, 154869. [Google Scholar] [CrossRef]
- Mesentier-Louro, L.A.; Shariati, M.A.; Dalal, R.; Camargo, A.; Kumar, V.; Shamskhou, E.A.; de Jesus Perez, V.; Liao, Y.J. Systemic hypoxia led to little retinal neuronal loss and dramatic optic nerve glial response. Exp. Eye Res. 2020, 193, 107957. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-q.; Jiang, L.-y.; Fu, C.-p.; Wu, X.; Liu, Z.-l.; Xie, L.; Wu, X.-d.; Hao, S.-y.; Li, S.-q. Heterozygous SOD2 deletion deteriorated chronic intermittent hypoxia-induced lung inflammation and vascular remodeling through mtROS-NLRP3 signaling pathway. Acta Pharmacol. Sin. 2020, 41, 1197–1207. [Google Scholar] [CrossRef]
- Kuang, G.; Halimitabrizi, M.; Edziah, A.-A.; Salowe, R.; O’Brien, J.M. The potential for mitochondrial therapeutics in the treatment of primary open-angle glaucoma: A review. Front. Physiol. 2023, 14, 1184060. [Google Scholar] [CrossRef] [PubMed]
- Hurley, D.J.; Normile, C.; Irnaten, M.; O’Brien, C. The Intertwined Roles of Oxidative Stress and Endoplasmic Reticulum Stress in Glaucoma. Antioxidants 2022, 11, 886. [Google Scholar] [CrossRef]
- Shi, R.; Wu, Y.; Chen, H.; Zhang, Z.; Bao, S.; Qu, J.; Zhou, M. The causal effect of oxidative stress on the risk of glaucoma. Heliyon 2024, 10, e24852. [Google Scholar] [CrossRef]
- Fan Gaskin, J.C.; Shah, M.H.; Chan, E.C. Oxidative Stress and the Role of NADPH Oxidase in Glaucoma. Antioxidants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Peng, B.; Huang, G.; Diao, C.; Qin, Y.; Hong, Y.; Lin, J.; Lin, Y.; Jiang, L.; Tang, N.; et al. Inhibition of NOX4 with GLX351322 alleviates acute ocular hypertension-induced retinal inflammation and injury by suppressing ROS mediated redox-sensitive factors activation. Biomed. Pharmacother. 2023, 165, 115052. [Google Scholar] [CrossRef]
- Kondkar, A.A.; Azad, T.A.; Sultan, T.; Osman, E.A.; Almobarak, F.A.; Al-Obeidan, S.A. Elevated Plasma Level of 8-Hydroxy-2′-deoxyguanosine Is Associated with Primary Open-Angle Glaucoma. J. Ophthalmol. 2020, 2020, 6571413. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Jin, J.; Zhao, C.; Zhao, B.; Liu, Y. NLRP3-GSDMD-dependent IL-1β Secretion from Microglia Mediates Learning and Memory Impairment in a Chronic Intermittent Hypoxia-induced Mouse Model. Neuroscience 2024, 539, 51–65. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, Y.; Ai, L.; Huang, L.; Liu, Z.; He, C.; Bai, Q.; Li, Y. Chronic intermittent hypoxia-induced oxidative stress activates TRB3 and phosphorylated JNK to mediate insulin resistance and cell apoptosis in the pancreas. Clin. Exp. Pharmacol. Physiol. 2024, 51, e13843. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.-J. Hypoxia and aging. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ana, R.d.; Gliszczyńska, A.; Sanchez-Lopez, E.; Garcia, M.L.; Krambeck, K.; Kovacevic, A.; Souto, E.B. Precision Medicines for Retinal Lipid Metabolism-Related Pathologies. J. Pers. Med. 2023, 13, 635. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shao, M.; Li, Y.; Li, X.; Wan, Y.; Sun, X.; Cao, W. Relationship between Oxidative Stress Biomarkers and Visual Field Progression in Patients with Primary Angle Closure Glaucoma. Oxidative Med. Cell. Longev. 2020, 2020, 2701539. [Google Scholar] [CrossRef] [PubMed]
- Himori, N.; Inoue Yanagimachi, M.; Omodaka, K.; Shiga, Y.; Tsuda, S.; Kunikata, H.; Nakazawa, T. The Effect of Dietary Antioxidant Supplementation in Patients with Glaucoma. Clin. Ophthalmol. 2021, 15, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Lopez-Bernal, M.D.; Cobo-Martinez, A.; Zanon-Moreno, V.; Pinazo-Duran, M.D.; del-Rio-Vellosillo, M. Glaucoma and Antioxidants: Review and Update. Antioxidants 2020, 9, 1031. [Google Scholar] [CrossRef]
- Li, J.; Savransky, V.; Nanayakkara, A.; Smith, P.L.; O’Donnell, C.P.; Polotsky, V.Y. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J. Appl. Physiol. 2007, 102, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhao, Y.; Mo, H.; Yang, H.; Yue, F.; Hu, K. Intermittent hypoxia increases ROS/HIF-1α ‘related oxidative stress and inflammation and worsens bleomycin-induced pulmonary fibrosis in adult male C57BL/6J mice. Int. Immunopharmacol. 2021, 100, 108165. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, H.; Niu, Y.; Yang, X.; Li, Z.; Wang, K.; Bi, H.; Pang, X. Chronic intermittent hypoxia induces gut microbial dysbiosis and infers metabolic dysfunction in mice. Sleep Med. 2022, 91, 84–92. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Hsu, P.-Y.; Su, M.-C.; Chin, C.-H.; Liou, C.-W.; Wang, T.-Y.; Lin, Y.-Y.; Lee, C.P.; Lin, M.-C.; Hsiao, C.-C. miR-21-5p Under-Expression in Patients with Obstructive Sleep Apnea Modulates Intermittent Hypoxia with Re-Oxygenation-Induced-Cell Apoptosis and Cytotoxicity by Targeting Pro-Inflammatory TNF-α-TLR4 Signaling. Int. J. Mol. Sci. 2020, 21, 999. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.L.; Martins, F.O.; Olea, E.; Prieto-Lloret, J.; Braga, P.C.; Sacramento, J.F.; Sequeira, C.O.; Negrinho, A.P.; Pereira, S.A.; Alves, M.G.; et al. Chronic Intermittent Hypoxia-Induced Dysmetabolism Is Associated with Hepatic Oxidative Stress, Mitochondrial Dysfunction and Inflammation. Antioxidants 2023, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhao, G.-L.; Cheng, S.; Wang, Z.; Yang, X.-L. Activation of retinal glial cells contributes to the degeneration of ganglion cells in experimental glaucoma. Prog. Retin. Eye Res. 2023, 93, 101169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, R.; Luo, X.; Wang, F.; Sun, X. The Interaction Between Microglia and Macroglia in Glaucoma. Front. Neurosci. 2021, 15, 610788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, W.; Zhang, M.; Tian, X.; Li, Y.; Lü, Y. Imbalance of Microglial TLR4/TREM2 in LPS-Treated APP/PS1 Transgenic Mice: A Potential Link Between Alzheimer’s Disease and Systemic Inflammation. Neurochem. Res. 2019, 44, 1138–1151. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cho, K.S.; Vu, T.H.K.; Shen, C.H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat. Commun. 2018, 9, 3209. [Google Scholar] [CrossRef] [PubMed]
- DeMaio, A.; Mehrotra, S.; Sambamurti, K.; Husain, S. The role of the adaptive immune system and T cell dysfunction in neurodegenerative diseases. J. Neuroinflamm. 2022, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Shestopalov, V.I.; Spurlock, M.; Gramlich, O.W.; Kuehn, M.H. Immune Responses in the Glaucomatous Retina: Regulation and Dynamics. Cells 2021, 10, 1973. [Google Scholar] [CrossRef]
- Zhu, J.; Kang, J.; Li, X.; Wang, M.; Shang, M.; Luo, Y.; Xiong, M.; Hu, K. Chronic intermittent hypoxia vs chronic continuous hypoxia: Effects on vascular endothelial function and myocardial contractility. Clin. Hemorheol. Microcirc. 2020, 74, 417–427. [Google Scholar] [CrossRef]
- Olfa, H.; Quentin, B.; Jean-Louis, P.; Claire, A.; Elise, B.; Gilles, F.; Charles, K.; Anne, B.-M. Intermittent hypoxia-related alterations in vascular structure and function: A systematic review and meta-analysis of rodent data. Eur. Respir. J. 2022, 59, 2100866. [Google Scholar] [CrossRef]
- Basavarajappa, D.; Galindo-Romero, C.; Gupta, V.; Agudo-Barriuso, M.; Gupta, V.B.; Graham, S.L.; Chitranshi, N. Signalling pathways and cell death mechanisms in glaucoma: Insights into the molecular pathophysiology. Mol. Asp. Med. 2023, 94, 101216. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, X. Retinal Ganglion Cell Death in Glaucoma: Advances and Caveats. Curr. Eye Res. 2023, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]




| Antigen | Species | Manufacturer | Catalog Number | Dilution |
|---|---|---|---|---|
| Hif-1α | Rabbit | Novus | NB100-134 | 1:500 |
| Iba-1 | Rabbit | Wako/Sigma | 019-19741 | 1:250 |
| RBPMS | Rabbit | Genetex | GTX 118619 | 1:250 |
| Mouse | Novus | OTI3B7 | 1:250 | |
| TNF-α | Mouse | Abcam | Ab1793 | 1:50 |
| 8-OHdG | Mouse | QED Bioscience | 12501 | 1:100 |
| IL-6 | Rabbit | Invitrogen | PA5-120041 | 1:100 |
| Antigen | Species | Manufacturer | Catalog Number | Dilution |
|---|---|---|---|---|
| Hif-1α | Rabbit | Novus | NB100-134 | 1:25 |
| PDK1 | Rabbit | Cell Signaling | C47H1 | 1:100 |
| LDH-A | Rabbit | Novus | NBP1-48336 | 1:100 |
| SIRT-1 | Rabbit | Novus | MBP2-27205 | 1:100 |
| GLUT 1 | Rabbit | Novus | NB110-39113 | 1:50 |
| GLUT 3 | Mouse | R&D Systems | MAB1415 | 1:25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donkor, N.; Gardner, J.J.; Bradshaw, J.L.; Cunningham, R.L.; Inman, D.M. Ocular Inflammation and Oxidative Stress as a Result of Chronic Intermittent Hypoxia: A Rat Model of Sleep Apnea. Antioxidants 2024, 13, 878. https://doi.org/10.3390/antiox13070878
Donkor N, Gardner JJ, Bradshaw JL, Cunningham RL, Inman DM. Ocular Inflammation and Oxidative Stress as a Result of Chronic Intermittent Hypoxia: A Rat Model of Sleep Apnea. Antioxidants. 2024; 13(7):878. https://doi.org/10.3390/antiox13070878
Chicago/Turabian StyleDonkor, Nina, Jennifer J. Gardner, Jessica L. Bradshaw, Rebecca L. Cunningham, and Denise M. Inman. 2024. "Ocular Inflammation and Oxidative Stress as a Result of Chronic Intermittent Hypoxia: A Rat Model of Sleep Apnea" Antioxidants 13, no. 7: 878. https://doi.org/10.3390/antiox13070878
APA StyleDonkor, N., Gardner, J. J., Bradshaw, J. L., Cunningham, R. L., & Inman, D. M. (2024). Ocular Inflammation and Oxidative Stress as a Result of Chronic Intermittent Hypoxia: A Rat Model of Sleep Apnea. Antioxidants, 13(7), 878. https://doi.org/10.3390/antiox13070878






