Natural Product Auraptene Targets SLC7A11 for Degradation and Induces Hepatocellular Carcinoma Ferroptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Reagents and Antibodies
2.3. Cell Viability Assay
2.4. Colony Formation Assay
2.5. Cell Death Analysis
2.6. ROS and Lipid ROS Levels Detection
2.7. Western Blot
2.8. Measurement of Glutathione (GSH)
2.9. Statistical Analysis
3. Results
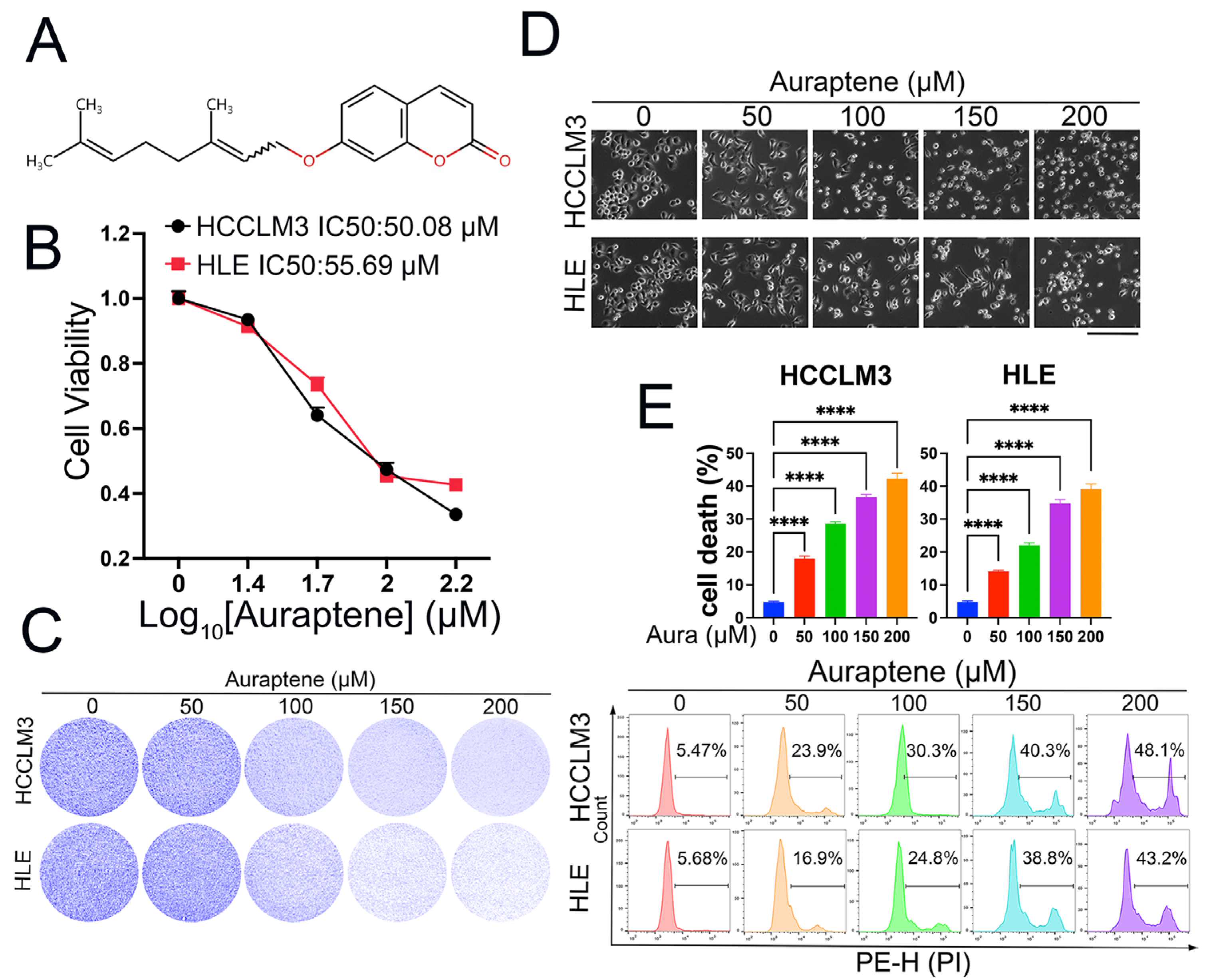
3.1. Auraptene Exerts Anti-Tumor Effects in HCC Cells
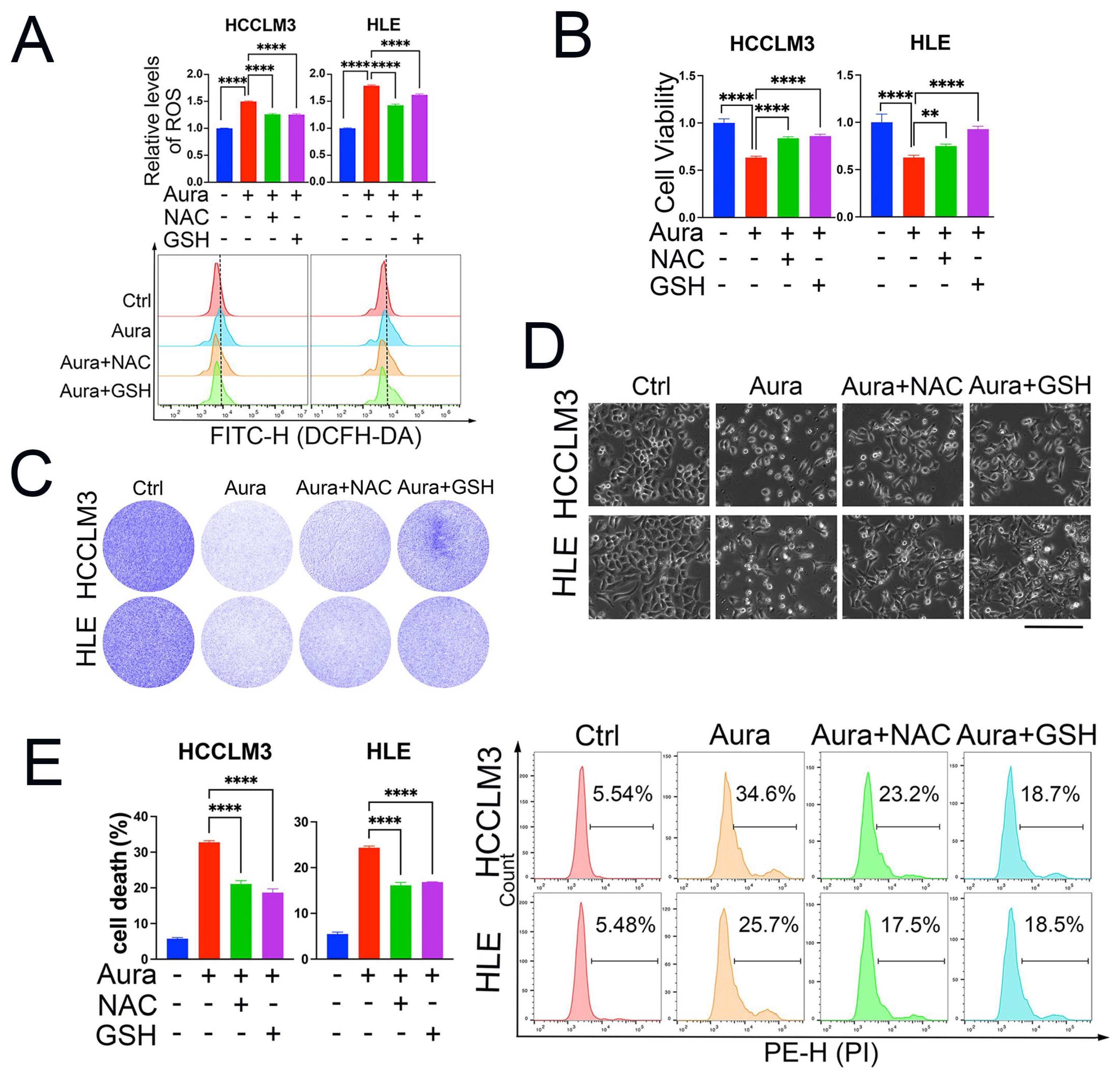
3.2. ROS Induction Is Responsible for Auraptene-Induced Cell Growth Inhibition and Cell Death
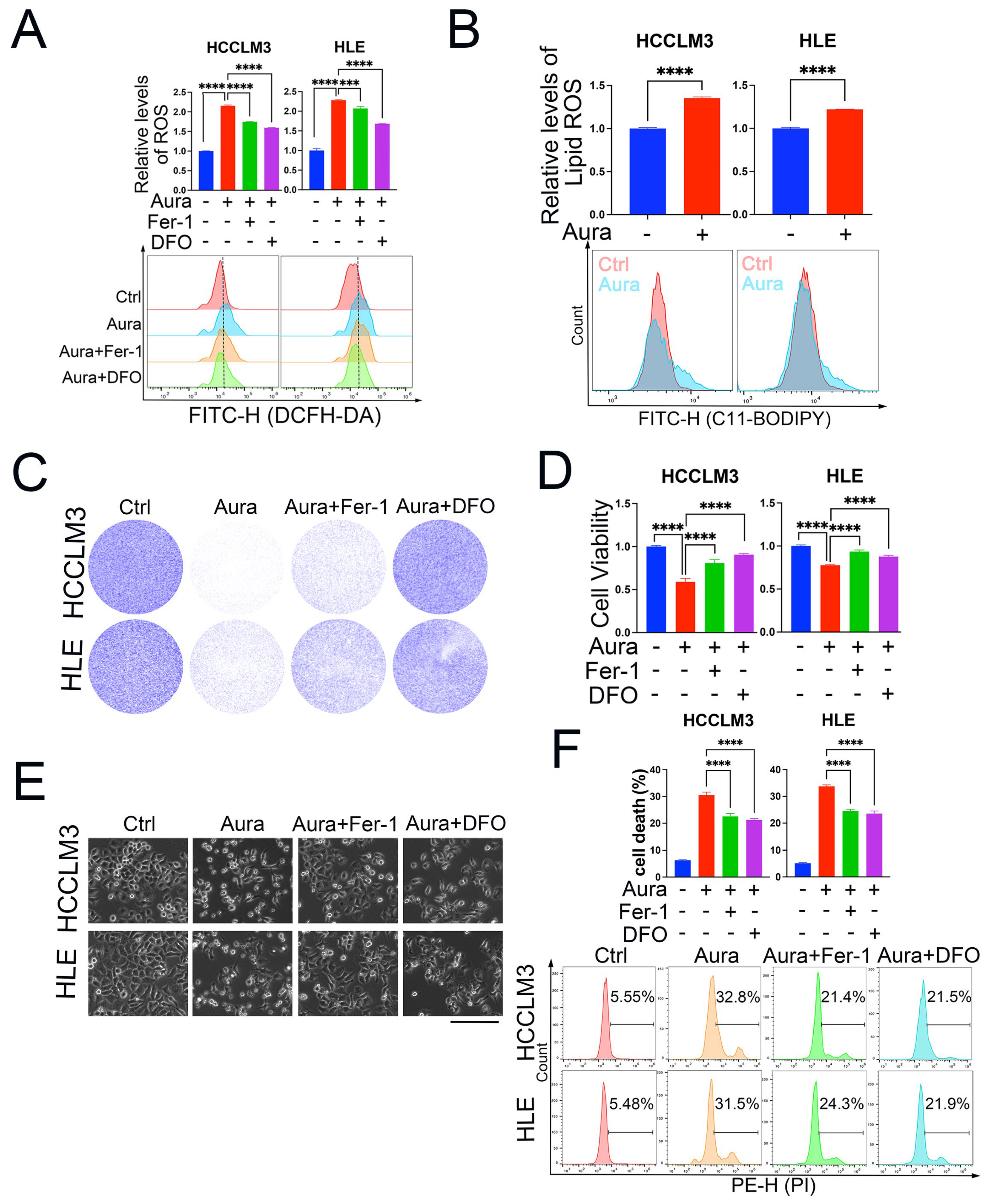
3.3. Auraptene Induces HCC Cell Ferroptosis
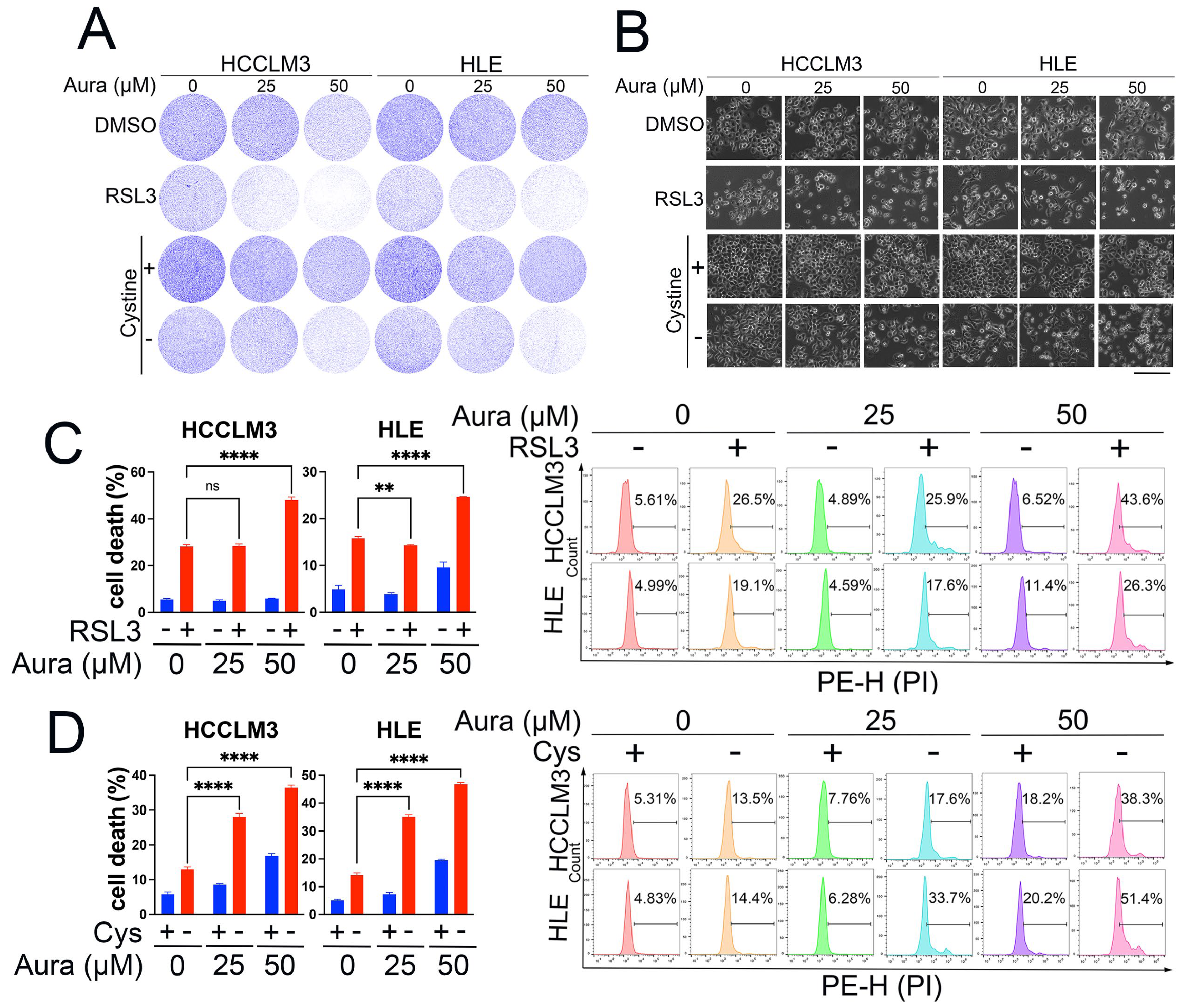
3.4. A Low Dose of Auraptene Sensitizes HCC Cells to Ferroptosis
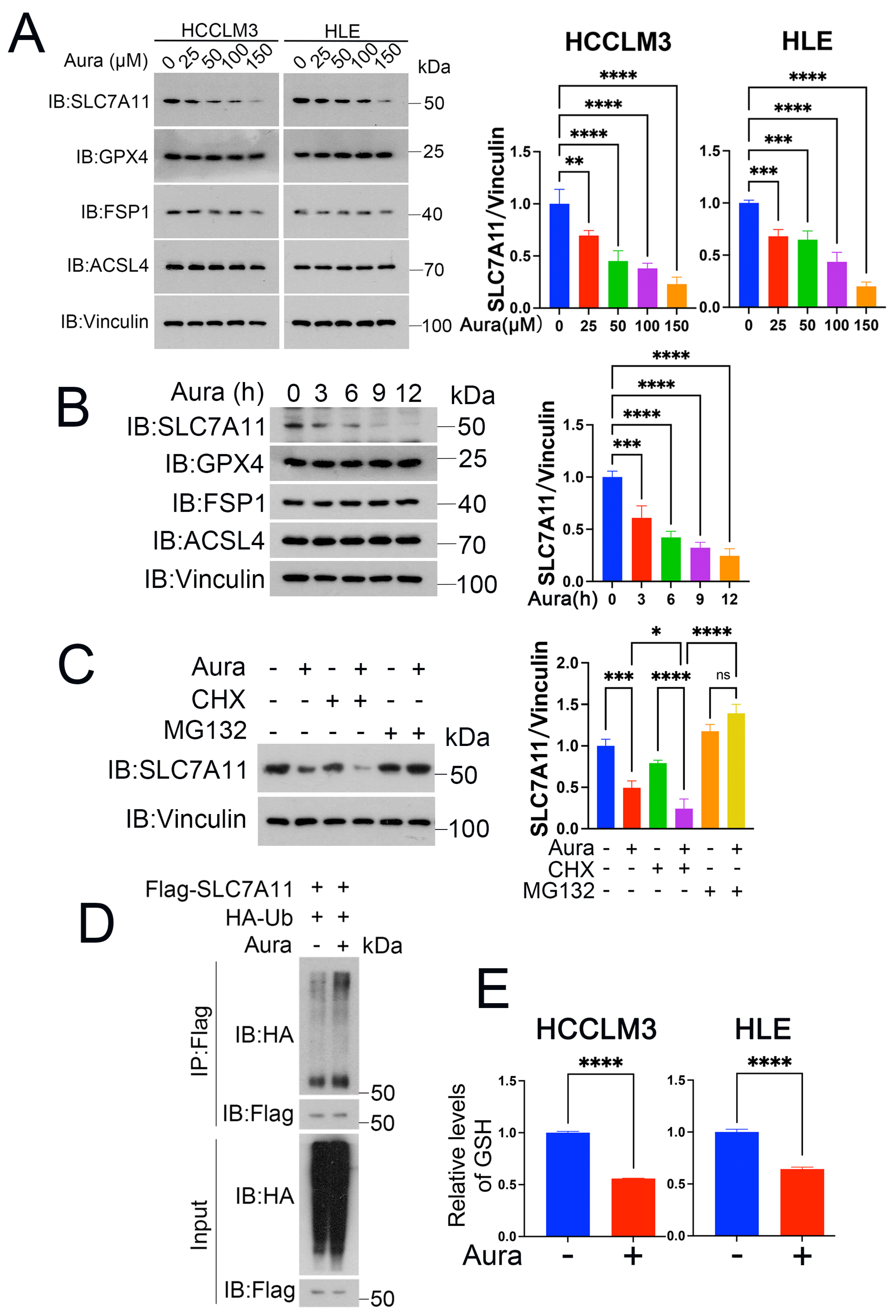
3.5. Auraptene Degrades SLC7A11
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagaraju, G.P.; Dariya, B.; Kasa, P.; Peela, S.; El-Rayes, B.F. Epigenetics in hepatocellular carcinoma. Semin. Cancer Biol. 2022, 86 Pt 3, 622–632. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Zhang, W.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S.; Rong, D.; Reiter, F.P.; et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct. Target. Ther. 2020, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Kalathur, R.K.R.; Coto-Llerena, M.; Ercan, C.; Buechel, D.; Shuang, S.; Piscuoglio, S.; Dill, M.T.; Camargo, F.D.; Christofori, G.; et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol. Med. 2021, 13, e14351. [Google Scholar] [CrossRef] [PubMed]
- Ladd, A.D.; Duarte, S.; Sahin, I.; Zarrinpar, A. Mechanisms of drug resistance in HCC. Hepatology 2024, 79, 926–940. [Google Scholar] [CrossRef]
- Xia, S.; Pan, Y.; Liang, Y.; Xu, J.; Cai, X. The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine 2020, 51, 102610. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Wu, Y.; Wang, M.; Lu, Y.; Fang, Z.; Wang, H.; Li, Y. Increased ATF2 expression predicts poor prognosis and inhibits sorafenib-induced ferroptosis in gastric cancer. Redox Biol. 2023, 59, 102564. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Dai, H.Q.; Huang, X.W.; Feng, J.; Deng, J.H.; Wang, Z.X.; Yang, X.M.; Liu, Y.J.; Wu, Y.; Chen, P.H.; et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol. Sin. 2021, 42, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef]
- Bian, X.; Jiang, H.; Meng, Y.; Li, Y.P.; Fang, J.; Lu, Z. Regulation of gene expression by glycolytic and gluconeogenic enzymes. Trends Cell Biol. 2022, 32, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Soula, M.; Weber, R.A.; Zilka, O.; Alwaseem, H.; La, K.; Yen, F.; Molina, H.; Garcia-Bermudez, J.; Pratt, D.A.; Birsoy, K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020, 16, 1351–1360. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Zandkarimi, F.; Liu, Y.; Duan, S.; Li, Z.; Kon, N.; Zhang, Z.; Jiang, X.; Stockwell, B.R.; et al. Regulation of VKORC1L1 is critical for p53-mediated tumor suppression through vitamin K metabolism. Cell Metab. 2023, 35, 1474–1490.e8. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Yang, S.; Li, L.; Dai, Q.; Borcherding, N.; Wagner, B.A.; Buettner, G.R.; Spitz, D.R.; Leslie, K.K.; Zhang, J. Metadherin enhances vulnerability of cancer cells to ferroptosis. Cell Death Dis. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wu, X.; Xu, F.; Ma, H.; Wu, M.; Xia, Y. Targeting ferroptosis pathway to combat therapy resistance and metastasis of cancer. Front. Pharmacol. 2022, 13, 909821. [Google Scholar] [CrossRef] [PubMed]
- Kazunori, O.; Akemi, K.; Toshio, Y.; Hirohisa, N.; Michihiko, N.; Yoshinori, I.; Masamichi, Y. Evaluation of Auraptene Content in Citrus Fruits and Their Products. J. Agric. Food Chem. 2000, 48, 1763–1769. [Google Scholar]
- Yoichi, S.; Shogo, K.; Yusuke, M.; Yasufumi, K.; Masafumi, F.; Kana, S.; Satoshi, S.; Toshihide, H.H.; Yuto, K.; Maki, K.; et al. Auraptene, a citrus peel-derived natural product, prevents myocardial infarction-induced heart failure by activating PPARα in rats. Phytomedicine 2022, 107, 154457. [Google Scholar]
- Akasaka, Y.; Hasei, S.; Ohata, Y.; Kanna, M.; Nakatsu, Y.; Sakoda, H.; Fujishiro, M.; Kushiyama, A.; Ono, H.; Matsubara, A.; et al. Auraptene Enhances AMP-Activated Protein Kinase Phosphorylation and Thereby Inhibits the Proliferation, Migration and Expression of Androgen Receptors and Prostate-Specific Antigens in Prostate Cancer Cells. Int. J. Mol. Sci. 2023, 24, 16011. [Google Scholar] [CrossRef]
- Ghorbani, M.; Soukhtanloo, M.; Farrokhi, A.S.; Hassanian, S.M.; Ghorbani, F.; Afshari, A.R.; Taherian, M.; Sadeghian, M.H. Auraptene-induced cytotoxic effects in acute myeloid leukemia cell lines. Med. Oncol. 2023, 40, 231. [Google Scholar] [CrossRef]
- Hosseini, F.; Ahmadi, A.; Hassanzade, H.; Gharedaghi, S.; Rassouli, F.B.; Jamialahmadi, K. Inhibition of melanoma cell migration and invasion by natural coumarin auraptene through regulating EMT markers and reducing MMP-2 and MMP-9 activity. Eur. J. Pharmacol. 2024, 971, 176517. [Google Scholar] [CrossRef] [PubMed]
- Izadi, A.; Soukhtanloo, M.; Mirzavi, F.; Jalili-Nik, M.; Sadeghi, A. Alpha-Lipoic Acid, Auraptene, and Particularly Their Combination Prevent the Metastasis of U87 Human Glioblastoma Cells. Evid. Based Complement. Altern. Med. 2023, 2023, 8618575. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Soukhtanloo, M.; Mostafavi-Pour, Z. Anti-tumor effects of Auraptene through induction of apoptosis and oxidative stress in a mouse model of colorectal cancer. Tissue Cell 2023, 81, 102004. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, Z.; Ye, S.; Liu, B.; Liu, Y.; Chen, J.; Xue, Q. Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis-related genes through cDNA microarray. J. Cancer Res. Clin. Oncol. 2003, 129, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Dor, I.; Namba, M.; Sato, J. Establishment and some biological characteristics of human hepatoma cell lines. Gann = Gan 1975, 66, 385. [Google Scholar]
- Bian, X.L.; Chen, H.Z.; Yang, P.B.; Li, Y.P.; Zhang, F.N.; Zhang, J.Y.; Wang, W.J.; Zhao, W.X.; Zhang, S.; Chen, Q.T.; et al. Nur77 suppresses hepatocellular carcinoma via switching glucose metabolism toward gluconeogenesis through attenuating phosphoenolpyruvate carboxykinase sumoylation. Nat. Commun. 2017, 8, 14420. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Kathryn, M.L.; Michael, R.L.; Rachid, S.; Eleina, M.Z.; Caroline, E.G.; Darpan, N.P.; Andras, J.B.; Alexandra, M.C.; Wan Seok, Y.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar]
- Hassannia, B.; Vandenabeele, P.; Berghe, T.V. Targeting ferroptosis to iron out cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Bekric, D.; Ocker, M.; Mayr, C.; Stintzing, S.; Ritter, M.; Kiesslich, T.; Neureiter, D. Ferroptosis in hepatocellular carcinoma: Mechanisms, drug targets and approaches to clinical translation. Cancers 2022, 14, 1826. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, W.; Guan, J.; Liu, H.; Dan, Y.; Zhu, L.; Song, Y.; Zhou, Y.; Zhao, X.; Zhang, Y.; et al. SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 2023, 30, 137–151. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, J.; Huang, S.; Chen, X.; Chang, A.C.Y.; Wang, C.; Zhang, J.; Zhang, H. Hydrogen sulfide protects cardiomyocytes from doxorubicin-induced ferroptosis through the SLC7A11/GSH/GPx4 pathway by Keap1 S-sulfhydration and Nrf2 activation. Redox Biol. 2024, 70, 103066. [Google Scholar] [CrossRef] [PubMed]
- Yizhong, Y.; Hongqi, T.; Qinglei, H.; Lavanya, K.; Guang, L.; Amber, H.; Xiaoguang, L.; Chao, M.; Shiqi, W.; Zhuang, L.; et al. SLC7A11 expression level dictates differential responses to oxidative stress in cancer cells. Nat. Commun. 2023, 14, 3673. [Google Scholar]
- Deguang, L.; Yan, F.; Fereshteh, Z.; Hua, W.; Zeda, Z.; Jinnie, K.; Yanyan, C.; Wei, G.; Brent, R.S.; Xuejun, J. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 2023, 186, 2748–2764.e22. [Google Scholar]
- Guang, L.; Zhuang, L.; Boyi, G. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, Y.; Chen, L.; Gao, C.; Dai, B.; Yu, W.; Yang, H.; Pi, J.; Bian, X. Natural Product Auraptene Targets SLC7A11 for Degradation and Induces Hepatocellular Carcinoma Ferroptosis. Antioxidants 2024, 13, 1015. https://doi.org/10.3390/antiox13081015
Li D, Li Y, Chen L, Gao C, Dai B, Yu W, Yang H, Pi J, Bian X. Natural Product Auraptene Targets SLC7A11 for Degradation and Induces Hepatocellular Carcinoma Ferroptosis. Antioxidants. 2024; 13(8):1015. https://doi.org/10.3390/antiox13081015
Chicago/Turabian StyleLi, Donglin, Yingping Li, Liangjie Chen, Chengchang Gao, Bolei Dai, Wenjia Yu, Haoying Yang, Junxiang Pi, and Xueli Bian. 2024. "Natural Product Auraptene Targets SLC7A11 for Degradation and Induces Hepatocellular Carcinoma Ferroptosis" Antioxidants 13, no. 8: 1015. https://doi.org/10.3390/antiox13081015
APA StyleLi, D., Li, Y., Chen, L., Gao, C., Dai, B., Yu, W., Yang, H., Pi, J., & Bian, X. (2024). Natural Product Auraptene Targets SLC7A11 for Degradation and Induces Hepatocellular Carcinoma Ferroptosis. Antioxidants, 13(8), 1015. https://doi.org/10.3390/antiox13081015





