Ribonuclease Inhibitor 1 (RNH1) Regulates Sperm tsRNA Generation for Paternal Inheritance through Interacting with Angiogenin in the Caput Epididymis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Western Blot Analysis
2.4. Immunofluorescence (IF) Analysis
2.5. Co-Immunoprecipitation
2.6. Plasmids and Transfection
2.7. Cell Treatment
2.8. The Construction of the Inflammation Model
2.9. RNA Extraction, Reverse Transcription, and Quantitative RT-PCR Analysis
2.10. Small RNA qPCR
2.11. Extraction and Identification of Exosomes
2.12. Nuclear and Cytoplasmic Proteins Extraction
2.13. Statistical Analysis
3. Results
3.1. RNH1 Is Highly Expressed in Mouse Caput Epididymis
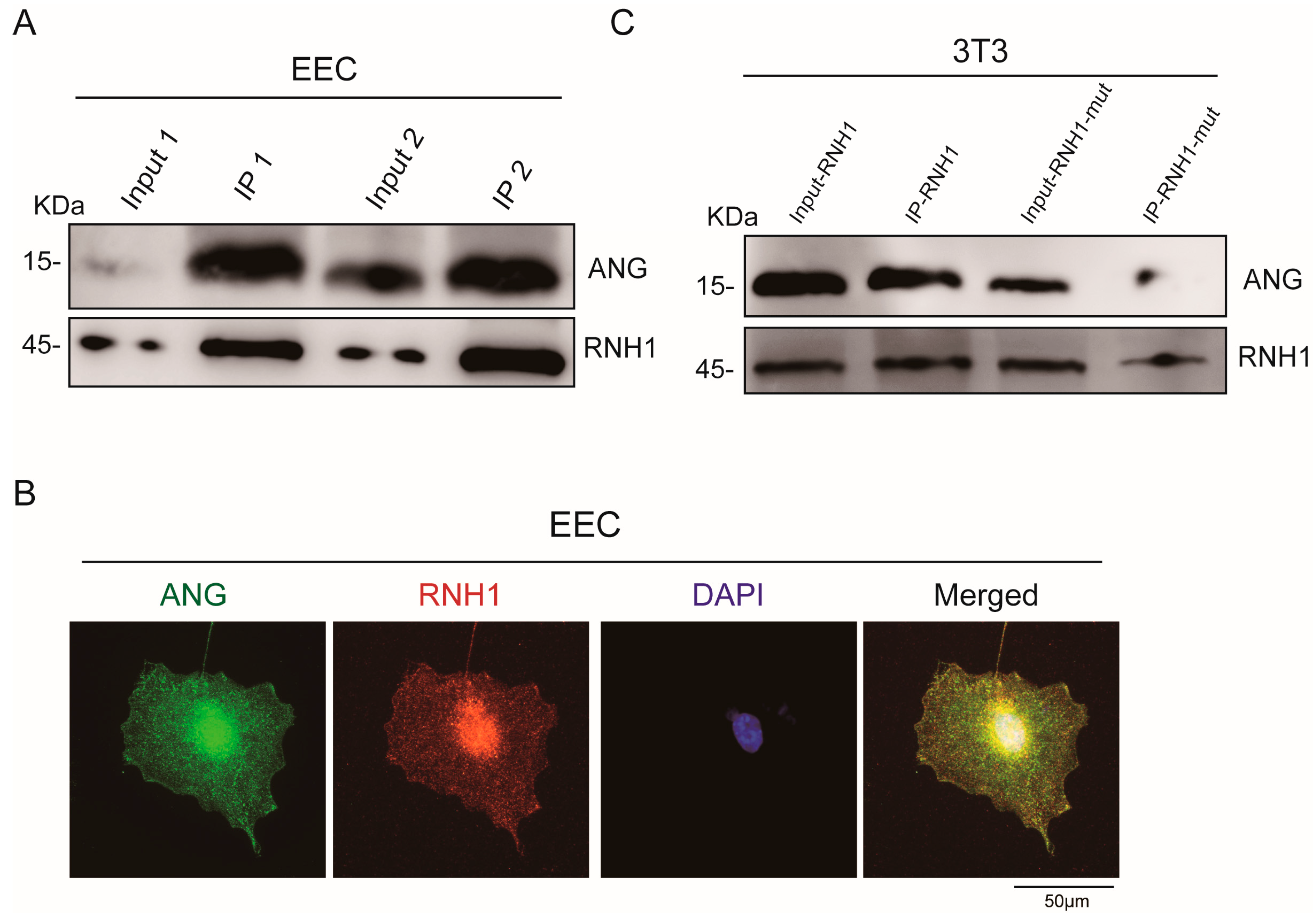
3.2. Interaction between RNH1 and ANG in EEC
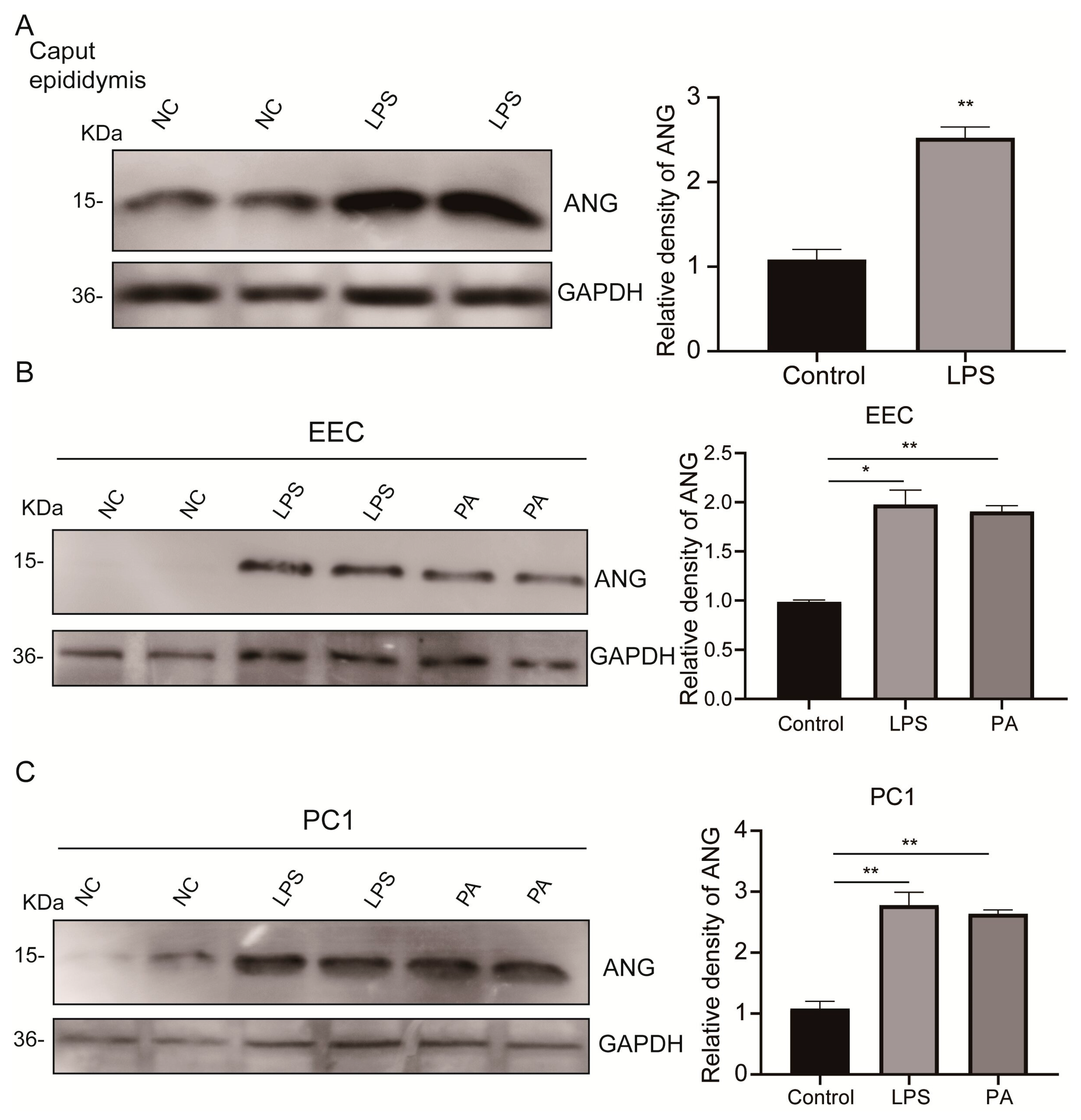
3.3. Upregulated Epididymal Cytoplasmic ANG Levels under Environmental Stress In Vivo and In Vitro
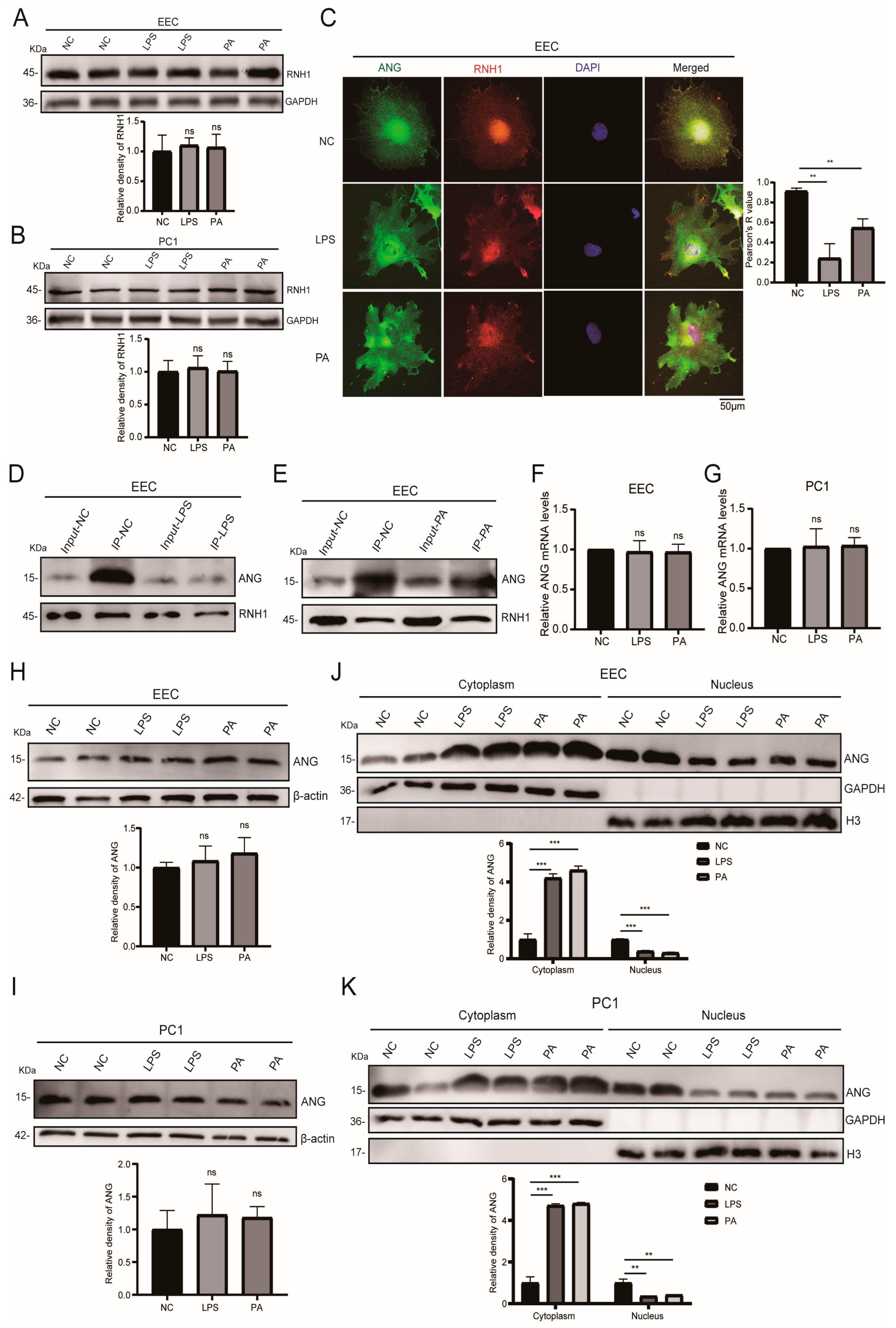
3.4. Inflammation and Oxidative Stress Weakened the Interaction of RNH1 and ANG in EEC
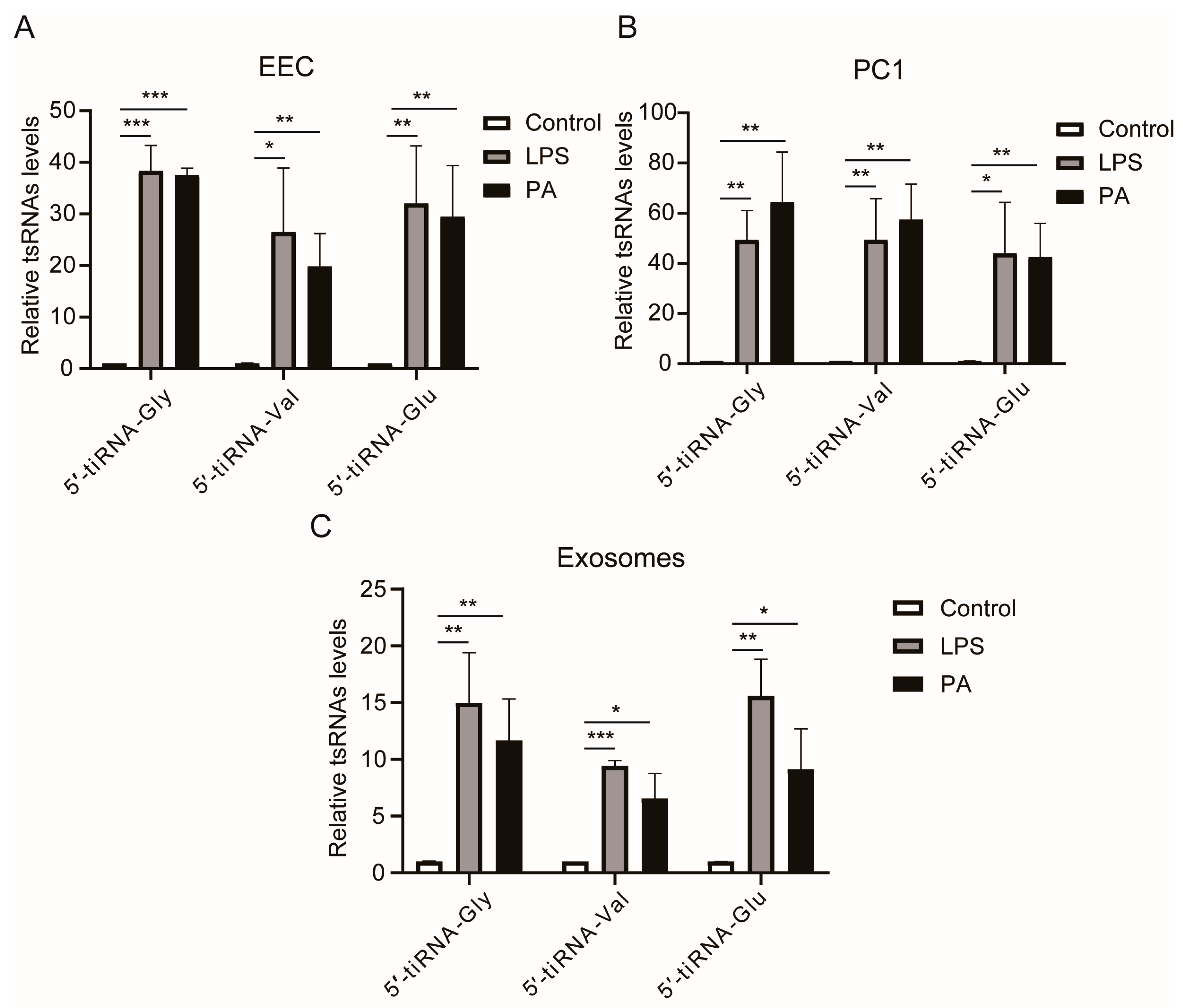
3.5. LPS or PA Treatment Increases Levels of tsRNAs in Epididymal Epithelial Cells
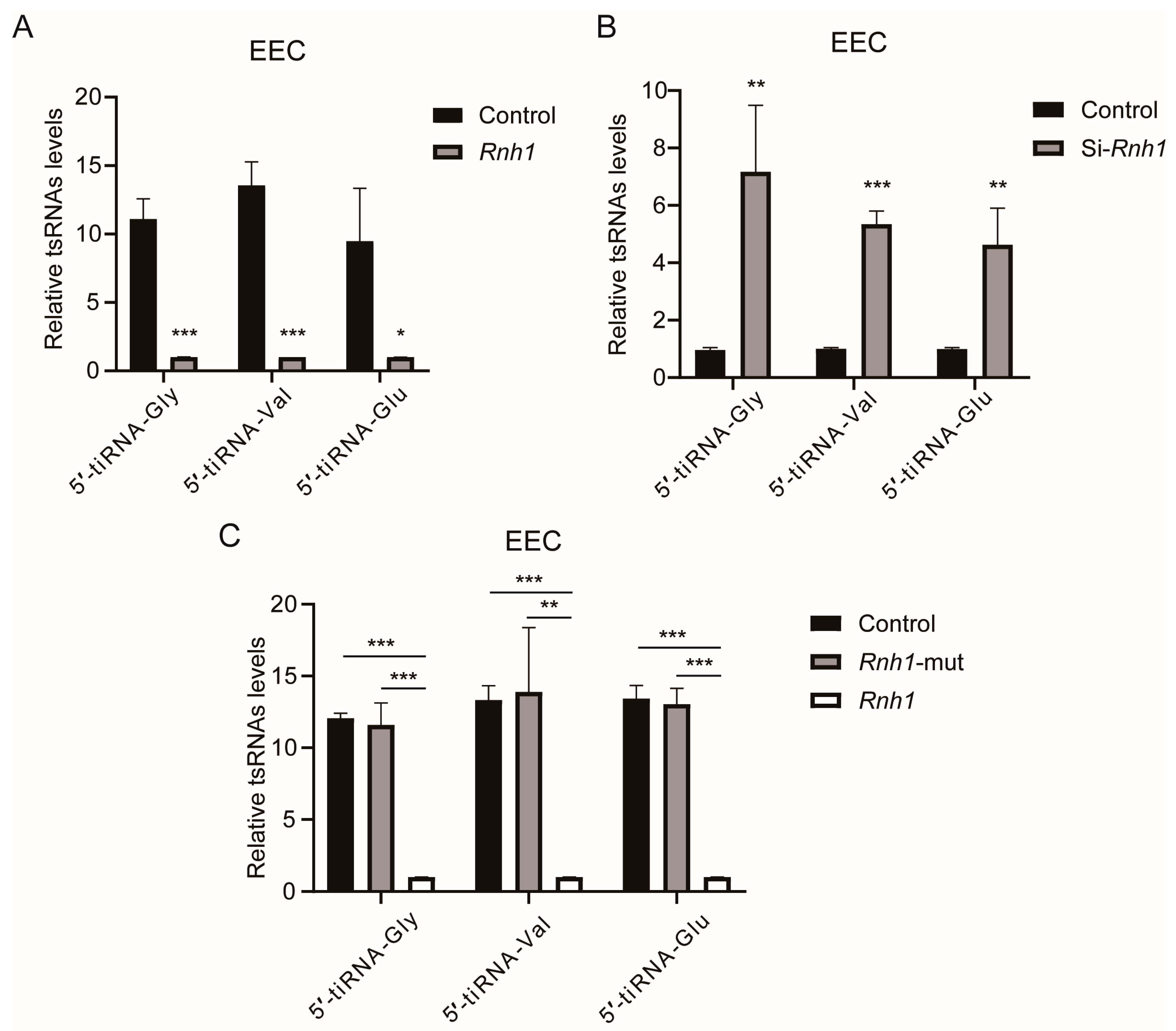
3.6. RNH1 Participates in the Process of tsRNA Generation Produced by ANG in EEC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.; Schatten, H.; Sun, Q.Y. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum. Reprod. Update 2015, 21, 194–208. [Google Scholar] [CrossRef]
- Non, A.L.; Binder, A.M.; Kubzansky, L.D.; Michels, K.B. Genome-wide DNA methylation in neonates exposed to maternal depression, anxiety, or SSRI medication during pregnancy. Epigenetics 2014, 9, 964–972. [Google Scholar] [CrossRef] [PubMed]
- CARE Study Group. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: A large prospective observational study. BMJ 2008, 337, a2332. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Quan, Y.; Wang, Z.; Liu, Y.; Ding, Z. Maternal obesity alters methylation level of cytosine in CpG island for epigenetic inheritance in fetal umbilical cord blood. Hum. Genom. 2022, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, J.; Hernandez, R.; Li, X.; Konchadi, P.; Miyake, Y.; Chen, Q.; Zhou, T.; Zhou, C. Paternal phthalate exposure-elicited offspring metabolic disorders are associated with altered sperm small RNAs in mice. Environ. Int. 2023, 172, 107769. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Maekawa, T.; Ly, N.H.; Fujita, S.I.; Muratani, M.; Ando, M.; Katou, Y.; Araki, H.; Miura, F.; Shirahige, K.; et al. ATF7-Dependent Epigenetic Changes Are Required for the Intergenerational Effect of a Paternal Low-Protein Diet. Mol. Cell 2020, 78, 445–458.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Xin, Y.; Sun, X.; Zhang, Y.; Chen, Y.; Liu, S.; He, B. Small Noncoding RNAs Contribute to Sperm Oxidative Stress-Induced Programming of Behavioral and Metabolic Phenotypes in Offspring. Oxid. Med. Cell. Longev. 2022, 2022, 6877283. [Google Scholar] [CrossRef] [PubMed]
- Conine, C.C.; Sun, F.; Song, L.; Rivera-Pérez, J.A.; Rando, O.J. Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice. Dev. Cell 2018, 46, 470–480.e3. [Google Scholar] [CrossRef]
- Nätt, D.; Öst, A. Male reproductive health and intergenerational metabolic responses from a small RNA perspective. J. Intern. Med. 2020, 288, 305–320. [Google Scholar] [CrossRef]
- Trigg, N.A.; Eamens, A.L.; Nixon, B. The contribution of epididymosomes to the sperm small RNA profile. Reproduction 2019, 157, R209–R223. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.N.; McLaughlin, E.A.; Stanger, S.J.; Anderson, A.L.; Hutcheon, K.; Church, K.; Mihalas, B.P.; Tyagi, S.; Holt, J.E.; Eamens, A.L.; et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 2016, 6, 31794. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm acquire epididymis-derived proteins through epididymosomes. Hum. Reprod. 2022, 37, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Stanger, S.J.; Anderson, A.L.; Bernstein, I.R.; De Iuliis, G.N.; McCluskey, A.; McLaughlin, E.A.; Dun, M.D.; Nixon, B. Mechanisms of tethering and cargo transfer during epididymosome-sperm interactions. BMC Biol. 2019, 17, 35. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, L.; Sun, X.; Zhang, Z.; Liu, J.; Xin, Y.; Yu, J.; Jia, Y.; Sheng, J.; Hu, G.F.; et al. Angiogenin mediates paternal inflammation-induced metabolic disorders in offspring through sperm tsRNAs. Nat. Commun. 2021, 12, 6673. [Google Scholar] [CrossRef]
- Dickson, K.A.; Haigis, M.C.; Raines, R.T. Ribonuclease inhibitor: Structure and function. Prog. Nucleic Acid Res. Mol. Biol. 2005, 80, 349–374. [Google Scholar]
- Dickson, K.A.; Kang, D.K.; Kwon, Y.S.; Kim, J.C.; Leland, P.A.; Kim, B.M.; Chang, S.I.; Raines, R.T. Ribonuclease inhibitor regulates neovascularization by human angiogenin. Biochemistry 2009, 48, 3804–3806. [Google Scholar] [CrossRef]
- Yamasaki, S.; Ivanov, P.; Hu, G.F.; Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 2009, 185, 35–42. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, S.J.; Choi, E.Y.; Kim, S.; Kwak, H.J.; Yoo, B.C.; Yoo, H.; Lee, S.H.; Kim, D.; Park, J.B.; et al. PTEN modulates miR-21 processing via RNA-regulatory protein RNH1. PLoS ONE 2011, 6, e28308. [Google Scholar] [CrossRef]
- Lin, X.; Dinglin, X.; Cao, S.; Zheng, S.; Wu, C.; Chen, W.; Li, Q.; Hu, Q.; Zheng, F.; Wu, Z.; et al. Enhancer-Driven lncRNA BDNF-AS Induces Endocrine Resistance and Malignant Progression of Breast Cancer through the RNH1/TRIM21/mTOR Cascade. Cell Rep. 2020, 31, 107753. [Google Scholar] [CrossRef]
- Sangeeta, K.; Yenugu, S. Characterization of isolated rat caput epididymal primary epithelial cells: A molecular biology approach. Theriogenology 2019, 135, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, J.; Fu, L.; Fu, R.; Tang, N.; Quan, Y.; Xin, Z.; Ding, Z.; Liu, Y. Epididymal RNase T2 contributes to astheno-teratozoospermia and intergenerational metabolic disorder through epididymosome-sperm interaction. BMC Med. 2023, 21, 453. [Google Scholar] [CrossRef]
- Hayashi, N.; Yamasaki, A.; Ueda, S.; Okazaki, S.; Ohno, Y.; Tanaka, T.; Endo, Y.; Tomioka, Y.; Masuko, K.; Masuko, T.; et al. Oncogenic transformation of NIH/3T3 cells by the overexpression of L-type amino acid transporter 1, a promising anti-cancer target. Oncotarget 2021, 12, 1256–1270. [Google Scholar] [CrossRef]
- Selcer, K.; Balasubramonian, B.; Miller, D.; Kerr, J.; DiFrancesco, M.; Ojha, S.; Urbano, R. Steroid sulfatase in the mouse NIH-3T3 fibroblast cell line: Characterization, and downregulation by glucocorticoids. Steroids 2021, 174, 108890. [Google Scholar] [CrossRef] [PubMed]
- Seenundun, S.; Robaire, B. Time-dependent rescue of gene expression by androgens in the mouse proximal caput epididymidis-1 cell line after androgen withdrawal. Endocrinology 2007, 148, 173–188. [Google Scholar] [CrossRef]
- Hamzeh, M.; Robaire, B. Androgens activate mitogen-activated protein kinase via epidermal growth factor receptor/insulin-like growth factor 1 receptor in the mouse PC-1 cell line. J. Endocrinol. 2011, 209, 55–64. [Google Scholar] [CrossRef]
- Jing, J.; Peng, Y.; Fan, W.; Han, S.; Peng, Q.; Xue, C.; Qin, X.; Liu, Y.; Ding, Z. Obesity-induced oxidative stress and mitochondrial dysfunction negatively affect sperm quality. FEBS Open Bio 2023, 13, 763–778. [Google Scholar] [CrossRef]
- Diaz-Baena, M.; Galvez-Valdivieso, G.; Delgado-Garcia, E.; Pineda, M.; Piedras, P. Nuclease and ribonuclease activities in response to salt stress: Identification of PvRNS3, a T2/S-like ribonuclease induced in common bean radicles by salt stress. Plant Physiol. Biochem. 2020, 147, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, M.; Wu, J.; Ma, Y.; Li, T.; Chen, Q.; Zhang, X.; Xiang, L. Stress-induced RNASET2 overexpression mediates melanocyte apoptosis via the TRAF2 pathway in vitro. Cell Death Dis. 2014, 5, e1022. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Fu, R.; Ma, Z.; Hu, Y.; Liu, Y.; Ding, Z. Endoplasmic reticulum stress increases exosome biogenesis and packaging relevant to sperm maturation in response to oxidative stress in obese mice. Reprod. Biol. Endocrinol. 2022, 20, 161. [Google Scholar] [CrossRef]
- Monti, D.M.; Montesano Gesualdi, N.; Matousek, J.; Esposito, F.; D’Alessio, G. The cytosolic ribonuclease inhibitor contributes to intracellular redox homeostasis. FEBS Lett. 2007, 581, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.C.; Shapiro, R.; Acharya, K.R. Molecular recognition of human angiogenin by placental ribonuclease inhibitor--an X-ray crystallographic study at 2.0 A resolution. Embo J. 1997, 16, 5162–5177. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Kuscu, C.; Malik, A.; Shibata, E.; Dutta, A. Angiogenin generates specific stress-induced tRNA halves and is not involved in tRF-3-mediated gene silencing. J. Biol. Chem. 2019, 294, 16930–16941. [Google Scholar] [CrossRef]
- Sarangdhar, M.A.; Allam, R. Angiogenin (ANG)-Ribonuclease Inhibitor (RNH1) System in Protein Synthesis and Disease. Int. J. Mol. Sci. 2021, 22, 1287. [Google Scholar] [CrossRef] [PubMed]
- Bombaci, G.; Sarangdhar, M.A.; Andina, N.; Tardivel, A.; Yu, E.C.; Mackie, G.M.; Pugh, M.; Ozan, V.B.; Banz, Y.; Spinetti, T.; et al. LRR-protein RNH1 dampens the inflammasome activation and is associated with COVID-19 severity. Life Sci. Alliance 2022, 5, e202101226. [Google Scholar] [CrossRef]
- Jing, J.; Wang, Y.; Quan, Y.; Wang, Z.; Liu, Y.; Ding, Z. Maternal obesity alters C19MC microRNAs expression profile in fetal umbilical cord blood. Nutr. Metab. 2020, 17, 52. [Google Scholar] [CrossRef]
- Sarker, G.; Sun, W.; Rosenkranz, D.; Pelczar, P.; Opitz, L.; Efthymiou, V.; Wolfrum, C.; Peleg-Raibstein, D. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc. Natl. Acad. Sci. USA 2019, 116, 10547–10556. [Google Scholar] [CrossRef]
- Szyf, M. The epigenetics of perinatal stress. Dialogues Clin. Neurosci. 2019, 21, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xia, L.; Zhu, D.; Zeng, H.; Wei, B.; Lu, L.; Li, W.; Shi, Y.; Liu, J.; Zhang, Y.; et al. Paternal High-Fat Diet Altered Sperm 5’tsRNA-Gly-GCC Is Associated With Enhanced Gluconeogenesis in the Offspring. Front. Mol. Biosci. 2022, 9, 857875. [Google Scholar] [CrossRef]
- Rando, O.J.; Simmons, R.A. I’m eating for two: Parental dietary effects on offspring metabolism. Cell 2015, 161, 93–105. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, J.; Zhang, Y.; Wang, X.; Wang, M.; Su, P. Differential Expression Profiles and Potential Intergenerational Functions of tRNA-Derived Small RNAs in Mice After Cadmium Exposure. Front. Cell Dev. Biol. 2021, 9, 791784. [Google Scholar] [CrossRef]
- Telonis, A.G.; Loher, P.; Kirino, Y.; Rigoutsos, I. Nuclear and mitochondrial tRNA-lookalikes in the human genome. Front. Genet. 2014, 5, 344. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Anaya, J.; Mudunuri, S.B.; Dutta, A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Haussecker, D.; Huang, Y.; Lau, A.; Parameswaran, P.; Fire, A.Z.; Kay, M.A. Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA 2010, 16, 673–695. [Google Scholar] [CrossRef]
- Di Fazio, A.; Schlackow, M.; Pong, S.K.; Alagia, A.; Gullerova, M. Dicer dependent tRNA derived small RNAs promote nascent RNA silencing. Nucleic Acids Res. 2022, 50, 1734–1752. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.; Mu, Y.; Mu, J.; Chen, Y.; Ye, X.; Zhou, Q.; Xu, M.; Deng, Q.; Hu, Y.; Tang, Z.; et al. Dicer1 Promotes Colon Cancer Cell Invasion and Migration Through Modulation of tRF-20-MEJB5Y13 Expression Under Hypoxia. Front. Genet. 2021, 12, 638244. [Google Scholar] [CrossRef]
- Thompson, D.M.; Parker, R. Stressing out over tRNA cleavage. Cell 2009, 138, 215–219. [Google Scholar] [CrossRef]
- Ivanov, P.; Emara, M.M.; Villen, J.; Gygi, S.P.; Anderson, P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 2011, 43, 613–623. [Google Scholar] [CrossRef]
- Tao, E.W.; Wang, H.L.; Cheng, W.Y.; Liu, Q.Q.; Chen, Y.X.; Gao, Q.Y. A specific tRNA half, 5’tiRNA-His-GTG, responds to hypoxia via the HIF1α/ANG axis and promotes colorectal cancer progression by regulating LATS2. J. Exp. Clin. Cancer Res. 2021, 40, 67. [Google Scholar] [CrossRef]
- Luhtala, N.; Parker, R. Structure-function analysis of Rny1 in tRNA cleavage and growth inhibition. PLoS ONE 2012, 7, e41111. [Google Scholar] [CrossRef]
- Thompson, D.M.; Parker, R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J. Cell Biol. 2009, 185, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Megel, C.; Hummel, G.; Lalande, S.; Ubrig, E.; Cognat, V.; Morelle, G.; Salinas-Giegé, T.; Duchêne, A.M.; Maréchal-Drouard, L. Plant RNases T2, but not Dicer-like proteins, are major players of tRNA-derived fragments biogenesis. Nucleic Acids Res. 2019, 47, 941–952. [Google Scholar] [CrossRef]
- Crisóstomo, L.; Bourgery, M.; Rato, L.; Raposo, J.F.; Batterham, R.L.; Kotaja, N.; Alves, M.G. Testicular “Inherited Metabolic Memory” of Ancestral High-Fat Diet Is Associated with Sperm sncRNA Content. Biomedicines 2022, 10, 909. [Google Scholar] [CrossRef]
- Guo, Y.; Bai, D.; Liu, W.; Liu, Y.; Zhang, Y.; Kou, X.; Chen, J.; Wang, H.; Teng, X.; Zuo, J.; et al. Altered sperm tsRNAs in aged male contribute to anxiety-like behavior in offspring. Aging Cell 2021, 20, e13466. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Xue, Y.; Li, X.; Zhang, Z.; Zhou, W.; Marcolongo, P.; Benedetti, A.; Mao, S.; Han, L.; Ding, G.; et al. Inter- and Transgenerational Effects of Paternal Exposure to Inorganic Arsenic. Adv. Sci. 2021, 8, 2002715. [Google Scholar] [CrossRef]
- Moroianu, J.; Riordan, J.F. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc. Natl. Acad. Sci. USA 1994, 91, 1677–1681. [Google Scholar] [CrossRef]
- Hoang, T.T.; Raines, R.T. Molecular basis for the autonomous promotion of cell proliferation by angiogenin. Nucleic Acids Res. 2017, 45, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Sun, Y.; Kishimoto, K.; Olson, K.A.; Liu, S.; Hirukawa, S.; Hu, G.F. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res. 2005, 65, 1352–1360. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Tan, D.; Zhang, X.; Yan, M.; Zhang, Y.; Franklin, R.; Shahbazi, M.; Mackinlay, K.; Liu, S.; et al. PANDORA-seq expands the repertoire of regulatory small RNAs by overcoming RNA modifications. Nat. Cell Biol. 2021, 23, 424–436. [Google Scholar] [CrossRef]
- Kobe, B.; Deisenhofer, J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature 1995, 374, 183–186. [Google Scholar] [CrossRef]
- Ng, A.C.; Eisenberg, J.M.; Heath, R.J.; Huett, A.; Robinson, C.M.; Nau, G.J.; Xavier, R.J. Human leucine-rich repeat proteins: A genome-wide bioinformatic categorization and functional analysis in innate immunity. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4631–4638. [Google Scholar] [CrossRef]
- Balatti, V.; Nigita, G.; Veneziano, D.; Drusco, A.; Stein, G.S.; Messier, T.L.; Farina, N.H.; Lian, J.B.; Tomasello, L.; Liu, C.G.; et al. tsRNA signatures in cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 8071–8076. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, Q.; Zheng, Y.; Liu, Z.; Meng, X.; Zeng, W.; Lu, H. Human sperm tsRNA as potential biomarker and therapy target for male fertility. Reproduction 2021, 161, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; He, F.; Luo, J.; Dou, S.; Wang, Y.; Guo, A.; Lu, J. Drosophila tsRNAs preferentially suppress general translation machinery via antisense pairing and participate in cellular starvation response. Nucleic Acids Res. 2018, 46, 5250–5268. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Sun, Q.; Liu, X.; Wang, P.; Wu, R.; Ma, Z. tRF-Leu-CAG promotes cell proliferation and cell cycle in non-small cell lung cancer. Chem. Biol. Drug Des. 2017, 90, 730–738. [Google Scholar] [CrossRef]
- Gebetsberger, J.; Wyss, L.; Mleczko, A.M.; Reuther, J.; Polacek, N. A tRNA-derived fragment competes with mRNA for ribosome binding and regulates translation during stress. RNA Biol. 2017, 14, 1364–1373. [Google Scholar] [CrossRef]
- Anderson, P.; Ivanov, P. tRNA fragments in human health and disease. FEBS Lett. 2014, 588, 4297–4304. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yao, L.; Yu, X.; Ruan, Y.; Li, Z.; Guo, J. Action mechanisms and research methods of tRNA-derived small RNAs. Signal Transduct. Target. Ther. 2020, 5, 109. [Google Scholar] [CrossRef]
- Takahashi, Y.; Morales Valencia, M.; Yu, Y.; Ouchi, Y.; Takahashi, K.; Shokhirev, M.N.; Lande, K.; Williams, A.E.; Fresia, C.; Kurita, M.; et al. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice. Cell 2023, 186, 715–731.e19. [Google Scholar] [CrossRef]
- Kaneshiro, K.R.; Egelhofer, T.A.; Rechtsteiner, A.; Cockrum, C.; Strome, S. Sperm-inherited H3K27me3 epialleles are transmitted transgenerationally in cis. Proc. Natl. Acad. Sci. USA 2022, 119, e2209471119. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.L.; Meng, X.; Wang, C.; Dai, W.; Luo, Z.; Yin, Z.; Ju, Z.; Fu, X.; Yang, J.; Ye, Q.; et al. Histone H3K4me3 modification is a transgenerational epigenetic signal for lipid metabolism in Caenorhabditis elegans. Nat. Commun. 2022, 13, 768. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Tang, N.; Zhang, R.; Deng, H.; Chen, K.; Liu, Y.; Ding, Z. Ribonuclease Inhibitor 1 (RNH1) Regulates Sperm tsRNA Generation for Paternal Inheritance through Interacting with Angiogenin in the Caput Epididymis. Antioxidants 2024, 13, 1020. https://doi.org/10.3390/antiox13081020
Ma Z, Tang N, Zhang R, Deng H, Chen K, Liu Y, Ding Z. Ribonuclease Inhibitor 1 (RNH1) Regulates Sperm tsRNA Generation for Paternal Inheritance through Interacting with Angiogenin in the Caput Epididymis. Antioxidants. 2024; 13(8):1020. https://doi.org/10.3390/antiox13081020
Chicago/Turabian StyleMa, Zhuoyao, Ningyuan Tang, Ruiyan Zhang, Hanyu Deng, Kexin Chen, Yue Liu, and Zhide Ding. 2024. "Ribonuclease Inhibitor 1 (RNH1) Regulates Sperm tsRNA Generation for Paternal Inheritance through Interacting with Angiogenin in the Caput Epididymis" Antioxidants 13, no. 8: 1020. https://doi.org/10.3390/antiox13081020




