Evaluation of In Vitro-Derived Hop Plantlets, cv. Columbus and Magnum, as Potential Source of Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Plantlet Materials
2.3. Sample Extraction
2.4. Determination of Total Phenolic Content and Antioxidant Activity of Extracts
2.5. UPLC-ESI-QqQ-MS/MS Analysis
2.6. Statistical Analysis
3. Results
3.1. Total (Poly)Phenol Content and Antioxidant Activity
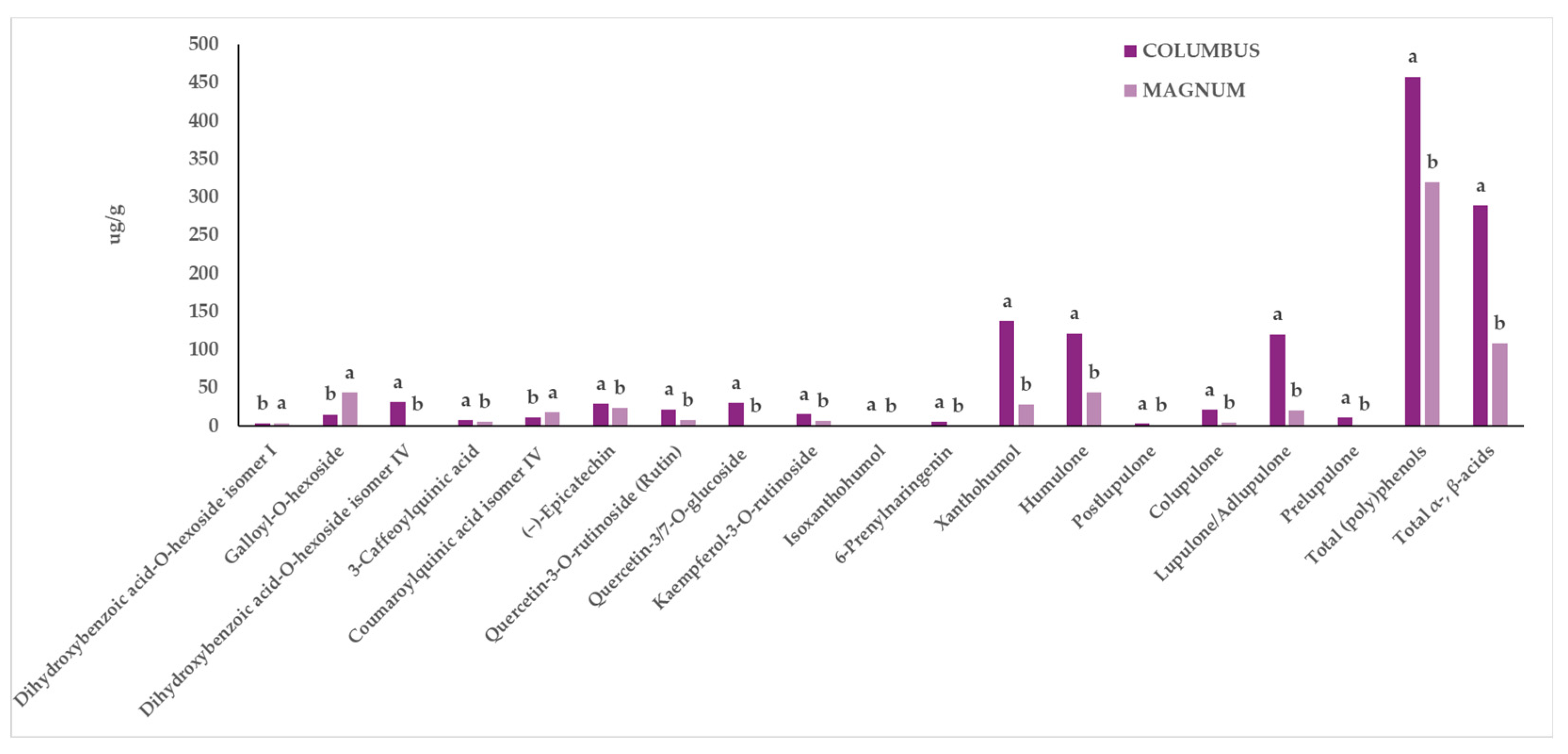
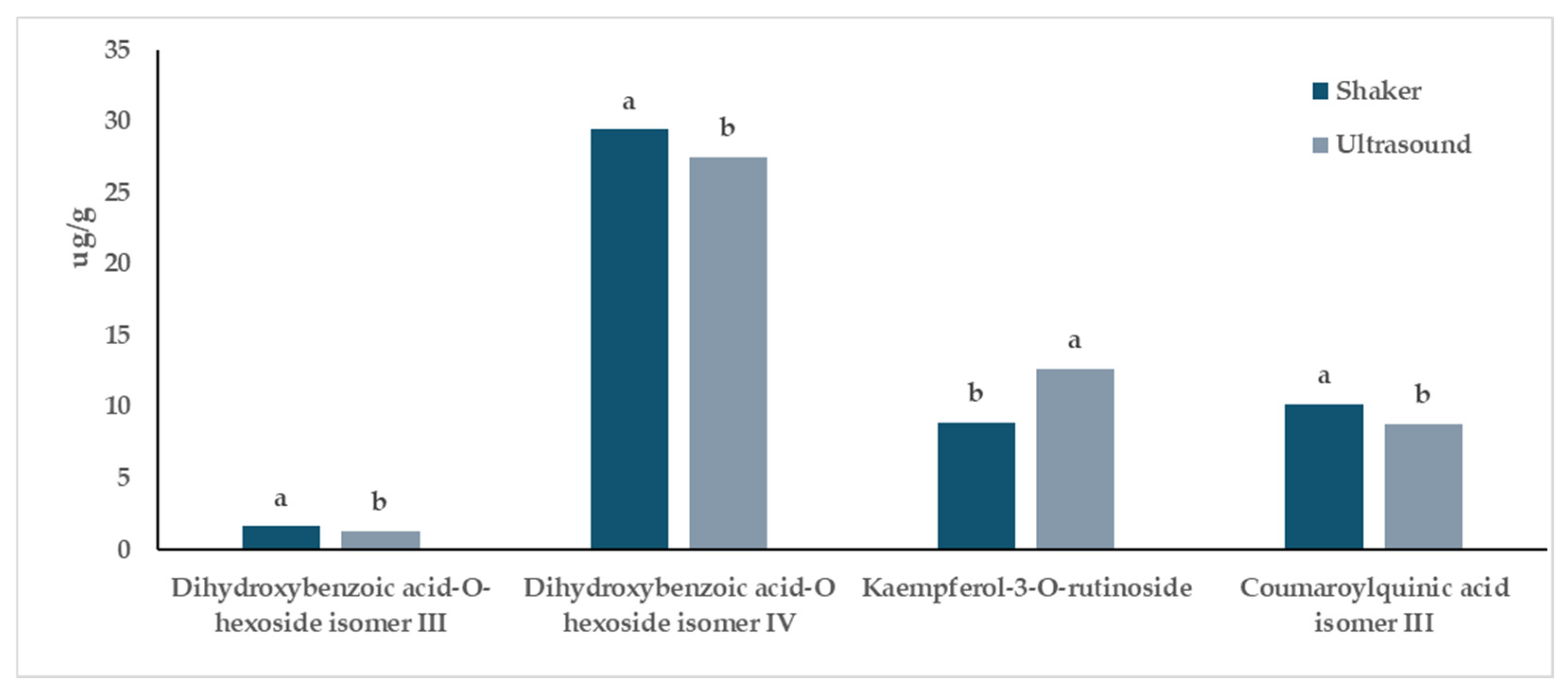
3.2. Characterization of Extracts from In Vitro-Derived Plantlets of Hop Genotypes through UPLC-ESI-QqQ-MS/MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Smole Možina, S.; Košir, I.J.; Kač, M.; Demšar, L.; et al. A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crops Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Carbone, K.; Amoriello, T.; Pagano, M.; Sperandio, G.; Assirelli, A.; Tarangioli, S.; Monteleone, A. Prospettive interessanti per il luppolo italiano. Inf. Agrar. 2017, 20, 49–51. [Google Scholar]
- Chiancone, B.; Guarrasi, V.; Leto, L.; Del Vecchio, L.; Calani, L.; Ganino, T.; Galaverni, M.; Cirlini, M. Vitro-derived hop (Humulus lupulus L.) leaves and roots as source of bioactive compounds: Antioxidant activity and polyphenolic profile. Plant Cell Tiss. Org. 2023, 153, 295–306. [Google Scholar] [CrossRef]
- Green, P.B. Self-organization and the formation of patterns in plants. In Dynamics of Cell and Tissue Motion. Mathematics and Biosciences in Interaction; Alt, W., Deutsch, A., Dunn, G.A., Eds.; Birkhäuser: Basel, Switzerland, 1997; pp. 243–249. [Google Scholar]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.S.; Lorenzo, J.M. Humulus lupulus L. as a natural source of functional biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus L., a very popular beer ingredient and medicinal plant: Overview of its phytochemistry, its bioactivity, and its biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Nezi, P.; Cicaloni, V.; Tinti, L.; Salvini, L.; Iannone, M.; Vitalini, S.; Garzoli, S. Metabolomic and proteomic profile of dried hop inflorescences (Humulus lupulus L. cv. Chinook and cv. Cascade) by SPME-GC-MS and UPLC-MS-MS. Separations 2022, 9, 204. [Google Scholar] [CrossRef]
- Nionelli, L.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Use of hop extract as antifungal ingredient for bread making and selection of autochthonous resistant starters for sourdough fermentation. Int. J. Food Microbiol. 2018, 266, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef]
- Roehrer, S.; Stork, V.; Ludwig, C.; Minceva, M.; Behr, J. Analyzing bioactive effects of the minor hop compound xanthohumol C on human breast cancer cells using quantitative proteomics. PLoS ONE 2019, 14, e0213469. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Neumann, H.F.; Frank, J.; Venturelli, S.; Egert, S. Bioavailability and cardiometabolic effects of xanthohumol: Evidence from animal and human studies. Mol. Nutr. Food Res. 2021, 66, 2100831. [Google Scholar] [CrossRef] [PubMed]
- Salter, S.; Brownie, S. Treating primary insomnia—The efficacy of valerian and hops. Aust. Fam. Physician 2010, 39, 433–437. [Google Scholar] [PubMed]
- Scarpa, G.M.; Prota, V.; Schianchi, N.; Manunta, F. In vitro cultures for the production of secondary metabolites. In Secondary Metabolites—Trends and Reviews; Vijayakumar, R., Raja, S.S.S., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Čeh, B.; Kac, M.; Košir, I.J.; Abram, V. Relationships between xanthohumol and polyphenol content in hop leaves and hop cones with regard to water supply and cultivar. Int. J. Mol. Sci. 2007, 8, 989–1000. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the potential of microwaves and ultrasounds in the green extraction of bioactive compounds from Humulus lupulus for the food and pharmaceutical industry. Ind. Crops Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Edible seaweeds and spirulina extracts for food application: In vitro and in situ evaluation of antimicrobial activity towards foodborne pathogenic bacteria. Foods 2020, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Muzykiewicz, A.; Nowak, A.; Zielonka-Brzezicka, J.; Florkowska, K.; Duchnik, W.; Klimowicz, A. Comparison of antioxidant activity of extracts of hop leaves harvested in different years. Herba Pol. 2019, 65, 1–9. [Google Scholar] [CrossRef]
- Keskin, Ş.; Şirin, Y.; Çakir, H.E.; Keskin, M. An investigation of Humulus lupulus L.: Phenolic composition, antioxidant capacity and inhibition properties of clinically important enzymes. S. Afr. J. Bot. 2019, 120, 170–174. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zhang, X.; Zeng, X. Effect of ultrasound on different quality parameters of apple juice. Ultrason. Sonochem. 2013, 20, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Paciulli, M.; Grimaldi, M.; Rinaldi, M.; Cavazza, A.; Flamminii, F.; Di Mattia, C.; Gennari, M.; Chiavaro, E. Microencapsulated olive leaf extract enhances physicochemical stability of biscuits. Future Foods 2023, 7, 100209. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Barteková, M.; Adameová, A.; Görbe, A.; Ferenczyová, K.; Pecháňová, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and synthetic antioxidants targeting cardiac oxidative stress and redox signaling in cardiometabolic diseases. Free Radic. Biol. Med. 2021, 169, 446–477. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.M.; Nauser, T. Fast antioxidant reaction of polyphenols and their metabolites. Antioxidants 2021, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I.; Hid, E.J.; Galleano, M. (Poly)phenols and the regulation of NADPH oxidases. Redox Biol. 2023, 67, 102927. [Google Scholar] [CrossRef]
- Mu, K.; Kitts, D.D. Intestinal polyphenol antioxidant activity involves redox signaling mechanisms facilitated by aquaporin activity. Redox Biol. 2023, 68, 102948. [Google Scholar] [CrossRef]
- da Rosa Almeida, A.; Maciel, M.V.D.O.B.; Cardoso Gasparini Gandolpho, B.; Machado, M.H.; Teixeira, G.L.; Bertoldi, F.C.; Barreto, P.L.M. Brazilian grown cascade hop (Humulus lupulus L.): LC-ESI-MS-MS and GC-MS analysis of chemical composition and antioxidant activity of extracts and essential oils. J. Am. Soc. Brew. Chem. 2021, 79, 156–166. [Google Scholar] [CrossRef]
- Paguet, A.S.; Siah, A.; Lefèvre, G.; Vandenberghe, M.; Lutun, D.; Degardin, N.; Samaillie, J.; Mathiron, D.; Dermont, C.; Michels, F.; et al. Phytochemical characterisation and aromatic potential for brewing of wild hops (Humulus lupulus L.) from Northern France: Towards a lead for local hop varieties. Food Chem. 2024, 433, 137302. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.J.; Vieira, J.S.; Gonçalves, L.M.; Pacheco, J.G.; Guido, L.F.; Barros, A.A. Isolation of phenolic compounds from hop extracts using polyvinylpolypyrrolidone: Characterization by high-performance liquid chromatography–diode array detection–electrospray tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3258–3268. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, A.; Rodolfi, M.; Ganino, T.; Marieschi, M.; Dall’Asta, C.; Bruni, R. Italian hop germplasm: Characterization of wild Humulus lupulus L. genotypes from Northern Italy by means of phytochemical, morphological traits and multivariate data analysis. Ind. Crops Prod. 2015, 70, 16–27. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 77–106. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Metaj, I.; Hajdini, D.; Gliha, K.; Košir, I.J.; Ocvirk, M.; Kolar, M.; Cerar, J. Extraction of polyphenols from slovenian hop (Humulus lupulus L.) Aurora variety using deep eutectic solvents: Choice of the extraction method vs. structure of the solvent. Plants 2023, 12, 2890. [Google Scholar] [CrossRef] [PubMed]

| Genotype | Extraction Method | TPC | DPPH• | ABTS+ | FRAP | ||||
|---|---|---|---|---|---|---|---|---|---|
| mg GAE/g | ±SD | mg TEAC/mL | ±SD | mg TEAC/ mL | ±SD | mg TEAC/ mL | ± SD | ||
| Columbus | Ultrasound | 6.10 | ±0.10 | 42.98 | ±1.04 | 82.59 | ±3.18 | 68.40 | ±1.44 |
| Shaker | 5.75 | ±0.29 | 41.18 | ±2.63 | 84.06 | ±4.68 | 66.17 | ±0.72 | |
| Magnum | Ultrasound | 5.53 | ±0.14 | 38.78 | ±1.03 | 82.42 | ±1.94 | 67.31 | ±0.88 |
| Shaker | 5.71 | ±0.18 | 39.89 | ±2.13 | 78.11 | ±2.81 | 65.83 | ±0.53 | |
| Statistical analysis of factors | |||||||||
| p | p | p | p | ||||||
| GENOTYPE (G) | 0.018 | 0.025 | 0.135 | 0.220 | |||||
| EXTRACTION METHOD (EM) | 0.431 | 0.754 | 0.472 | 0.006 | |||||
| G × EM | 0.033 | 0.197 | 0.156 | 0.508 | |||||
| Family | Compound | RT (min) | Parent Ion [M-H]− (m/z) | Product Ions | Standard Used for Quantification | |||
|---|---|---|---|---|---|---|---|---|
| Quantifier (m/z) | Qualifier(s) (m/z) | |||||||
| Hydroxybenzoic acids | 3,4,5-Trihydroxybenzoic acid (Gallic acid) | 2.36 | 169 | 125 | 97 | <LOQ | ||
| Dihydroxybenzoic acid- O-hexoside isomer I | 2.60 | 315 | 153 | 109 | 152 | 108 | 3,4-Dihydroxybenzoic acid | |
| Dihydroxybenzoic acid- O-hexoside isomer II | 2.65 | 315 | 152 | 153 | 109 | 108 | 3,4-Dihydroxybenzoic acid | |
| Dihydroxybenzoic acid- O-hexoside isomer III | 2.83 | 315 | 153 | 109 | 152 | 108 | 3,4-Dihydroxybenzoic acid | |
| Galloyl-O-hexoside | 2.87 | 331 | 169 | 125 | 3,4,5-Trihydroxybenzoic acid | |||
| Dihydroxybenzoic acid- O-hexoside isomer IV | 3.17 | 315 | 153 | 109 | 152 | 108 | 3,4-Dihydroxybenzoic acid | |
| 3,4-Dihydroxybenzoic acid (Protocatechuic acid) | 3.19 | 153 | 109 | 81 | <LOQ | |||
| Hydroxycinnamic acids | 3-Caffeoylquinic acid | 3.30 | 353 | 191 | 179 | 135 | 3-Caffeoylquinic acid | |
| Coumaroylquinic acid isomer I | 3.77 | 337 | 191 | 173 | 3-Caffeoylquinic acid | |||
| Coumaroylquinic acid isomer II | 3.82 | 337 | 191 | 173 | 3-Caffeoylquinic acid | |||
| 5-Caffeoylquinic acid | 3.84 | 353 | 191 | 179 | 135 | <LOQ | ||
| 4-Caffeoylquinic acid | 3.93 | 353 | 179 | 191 | 135 | <LOQ | ||
| Sinapic acid acyl-hexoside | 3.93 | 385 | 223 | 205 | 190 | Sinapic acid acyl- glucoside | ||
| Flavan-3-ols | (+)-Catechin | 3.97 | 289 | 245 | 109 | 203 | (+)-Catechin | |
| Hydroxycinnamic acids | Coumaroylquinic acid isomer III | 4.29 | 337 | 173 | 191 | 4-Caffeoylquinic acid | ||
| Sinapic acid acyl-glucoside | 4.29 | 385 | 205 | 223 | 190 | <LOQ | ||
| Coumaroylquinic acid isomer IV | 4.34 | 337 | 191 | 173 | 5-Caffeoylquinic acid | |||
| Flavan-3-ols | (−)-Epicatechin | 4.37 | 289 | 245 | 109 | 203 | (−)-Epicatechin | |
| Flavonols | Quercetin 3-O-rutinoside (Rutin) | 4.73 | 609 | 300 | 271 | 301 | 151 | Kaempferol 3-rutinoside |
| Quercetin 3/7-O-glucoside (Sum of Quercetin 3-O-glucoside and Quercetin-7-O-glucoside) | 4.86 | 463 | 300 | 271 | 255 | Quercetin 3/7-glucoside (Sum of Quercetin 3-glucoside and Quercetin-7-glucoside) | ||
| Kaempferol-3-O-rutinoside | 4.95 | 593 | 285 | 255 | 227 | Kaempferol 3-rutinoside | ||
| Prenylflavonoids | Isoxanthohumol | 6.88 | 353 | 119 | 233 | Isoxanthohumol | ||
| 8-Prenylnaringenin | 7.51 | 339 | 219 | 95 | 237 | <LOQ | ||
| Iso-α-acids | Isohumulone | 7.54 | 361 | 221 | 292 | 249 | Humulone | |
| Prenylflavonoids | 6-Prenylnaringenin | 8.04 | 339 | 219 | 119 | 133 | 6-Prenylnaringenin | |
| Xanthohumol | 8.29 | 353 | 119 | 233 | 175 | Xanthohumol | ||
| α-acids | Cohumulone | 9.42 | 347 | 278 | 235 | 223 | Humulone | |
| Humulone | 9.65 | 361 | 292 | 249 | 221 | Humulone | ||
| β-acids | Postlupulone | 9.91 | 385 | 273 | Humulone | |||
| Colupulone | 10.18 | 399 | 287 | 330 | Humulone | |||
| Lupulone/Adlupulone | 10.42 | 413 | 301 | 289 | Humulone | |||
| Prelupulone | 10.78 | 427 | 315 | 358 | Humulone | |||
| Columbus | Magnum | Factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | US | SK | US | SK | G | EM | G × EM | ||||
| µg/g | ±SD | µg/g | ±SD | µg/g | ±SD | µg/g | ±SD | p | p | p | |
| Dihydroxybenzoic acid-O-hexoside isomer I | 3.02 | ±0.06 | 2.93 | ±0.42 | 4.04 | ±0.03 | 3.76 | ±0.03 | 0.012 | 0.436 | 0.676 |
| Dihydroxybenzoic acid-O-hexoside isomer II | 40.79 | ±6.57 | 39.72 | ±0.13 | 27.59 | ±2.84 | 42.47 | ±2.74 | 0.244 | 0.146 | 0.106 |
| Dihydroxybenzoic acid-O-hexoside isomer III | 1.44 | ±0.04 | 1.61 | ±0.08 | 1.18 | ±0.13 | 1.61 | ±0.14 | 0.307 | 0.050 | 0.296 |
| Galloyl-O-hexoside | 14.26 | ±0.23 | 16.12 | ±0.72 | 47.78 | ±3.28 | 40.61 | ±0.65 | 0.000 | 0.197 | 0.058 |
| Dihydroxybenzoic acid-O-hexoside isomer IV | 29.29 | ±0.19 | 33.14 | ±1.19 | 21.05 | ±0.75 | 25.71 | ±1.56 | 0.002 | 0.016 | 0.722 |
| 3-Caffeoylquinic acid | 8.07 | ±1.28 | 7.25 | ±0.85 | 5.00 | ±0.28 | 5.60 | ±0.41 | 0.043 | 0.899 | 0.426 |
| Coumaroylquinic acid isomer I | 2.46 | ±0.35 | 2.69 | ±0.07 | 2.72 | ±0.08 | 2.71 | ±0.09 | 0.498 | 0.601 | 0.571 |
| Coumaroylquinic acid isomer II | 6.30 | ±0.93 | 6.85 | ±0.70 | 6.17 | ±0.26 | 8.85 | ±0.08 | 0.193 | 0.053 | 0.150 |
| Sinapic Acid Acyl-hexoside | 45.22 | ±1.12 | 47.02 | ±2.40 | 61.03 | ±0.83 | 41.58 | ±3.67 | 0.088 | 0.019 | 0.01 |
| (+)-Catechin | 39.24 | ±1.38 | 44.73 | ±6.19 | 82.64 | ±14.75 | 44.03 | ±14.99 | 0.124 | 0.206 | 0.115 |
| Coumaroylquinic acid isomer III | 8.55 | ±0.37 | 9.36 | ±0.41 | 9.10 | ±0.33 | 11.00 | ±0.56 | 0.062 | 0.034 | 0.271 |
| Coumaroylquinic acid isomer IV | 11.08 | ±1.03 | 10.57 | ±0.26 | 17.14 | ±0.20 | 17.82 | ±0.57 | 0.000 | 0.898 | 0.389 |
| (−)-Epicatechin | 33.47 | ±2.99 | 24.10 | ±0.29 | 13.09 | ±0.89 | 9.16 | ±1.80 | 0.001 | 0.021 | 0.206 |
| Quercetin-3-O-rutinoside (Rutin) | 22.12 | ±2.27 | 21.68 | ±3.01 | 10.36 | ±0.27 | 5.82 | ±0.53 | 0.002 | 0.262 | 0.342 |
| Quercetin-3/7-O-glucoside (Sum of Quercetin3-O-Glucoside and Quercetin-7-O-glucoside) | 31.36 | ±1.20 | 30.06 | ±5.94 | 0.00 | ±0.00 | 0.00 | ±0.00 | 0.000 | 0.776 | 0.776 |
| Kaempferol-3-O-rutinoside | 16.79 | ±1.31 | 13.70 | ±2.27 | 8.47 | ±0.57 | 4.06 | ±0.34 | 0.003 | 0.050 | 0.651 |
| Isoxanthohumol | 1.67 | ±0.01 | 1.61 | ±0.09 | 0.00 | ±0.00 | 0.00 | ±0.00 | 0.000 | 0.574 | 0.574 |
| Isohumulone | 0.56 | ±0.01 | 0.56 | ±0.00 | 0.55 | ±0.01 | 0.57 | ±0.00 | 0.719 | 0.388 | 0.138 |
| 6-Prenylnaringenin | 5.57 | ±0.19 | 5.17 | ±0.28 | 0.00 | ±0.00 | 0.00 | ±0.00 | 0.000 | 0.300 | 0.300 |
| Xanthohumol | 141.99 | ±7.36 | 133.63 | ±7.05 | 22.09 | ±1.43 | 34.28 | ±5.70 | 0.000 | 0.761 | 0.156 |
| Cohumulone | 12.50 | ±1.05 | 13.29 | ±1.07 | 7.70 | ±0.76 | 15.24 | ±4.02 | 0.548 | 0.129 | 0.196 |
| Humulone | 120.54 | ±15.25 | 121.97 | ±3.54 | 48.15 | ±7.32 | 92.95 | ±22.48 | 0.023 | 0.178 | 0.201 |
| Postlupulone | 2.99 | ±0.32 | 2.72 | ±0.18 | 0.47 | ±0.01 | 0.76 | ±0.16 | 0.000 | 0.971 | 0.237 |
| Colupulone | 22.39 | ±3.69 | 19.91 | ±0.55 | 2.99 | ±0.03 | 5.55 | ±1.37 | 0.001 | 0.985 | 0.273 |
| Lupulone/Adlupulone | 131.18 | ±20.23 | 108.85 | ±3.51 | 13.71 | ±0.11 | 26.45 | ±5.35 | 0.001 | 0.675 | 0.174 |
| Prelupulone | 11.98 | ±2.61 | 10.13 | ±0.09 | 1.07 | ±0.02 | 2.01 | ±0.49 | 0.002 | 0.750 | 0.352 |
| Total (poly)phenols | 463.24 | ±17.70 | 452.5 | ±21.1 | 340.01 | ±14.98 | 299.63 | ±12.52 | 0.000 | 0.089 | 0.282 |
| Total α- and β-acids | 301.57 | ±61.02 | 276.85 | ±12.38 | 74.08 | ±11.24 | 142.96 | ±47.90 | 0.000 | 0.475 | 0.171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leto, L.; Favari, C.; Agosti, A.; Del Vecchio, L.; Di Fazio, A.; Bresciani, L.; Mena, P.; Guarrasi, V.; Cirlini, M.; Chiancone, B. Evaluation of In Vitro-Derived Hop Plantlets, cv. Columbus and Magnum, as Potential Source of Bioactive Compounds. Antioxidants 2024, 13, 909. https://doi.org/10.3390/antiox13080909
Leto L, Favari C, Agosti A, Del Vecchio L, Di Fazio A, Bresciani L, Mena P, Guarrasi V, Cirlini M, Chiancone B. Evaluation of In Vitro-Derived Hop Plantlets, cv. Columbus and Magnum, as Potential Source of Bioactive Compounds. Antioxidants. 2024; 13(8):909. https://doi.org/10.3390/antiox13080909
Chicago/Turabian StyleLeto, Leandra, Claudia Favari, Anna Agosti, Lorenzo Del Vecchio, Andrea Di Fazio, Letizia Bresciani, Pedro Mena, Valeria Guarrasi, Martina Cirlini, and Benedetta Chiancone. 2024. "Evaluation of In Vitro-Derived Hop Plantlets, cv. Columbus and Magnum, as Potential Source of Bioactive Compounds" Antioxidants 13, no. 8: 909. https://doi.org/10.3390/antiox13080909








