Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine
Abstract
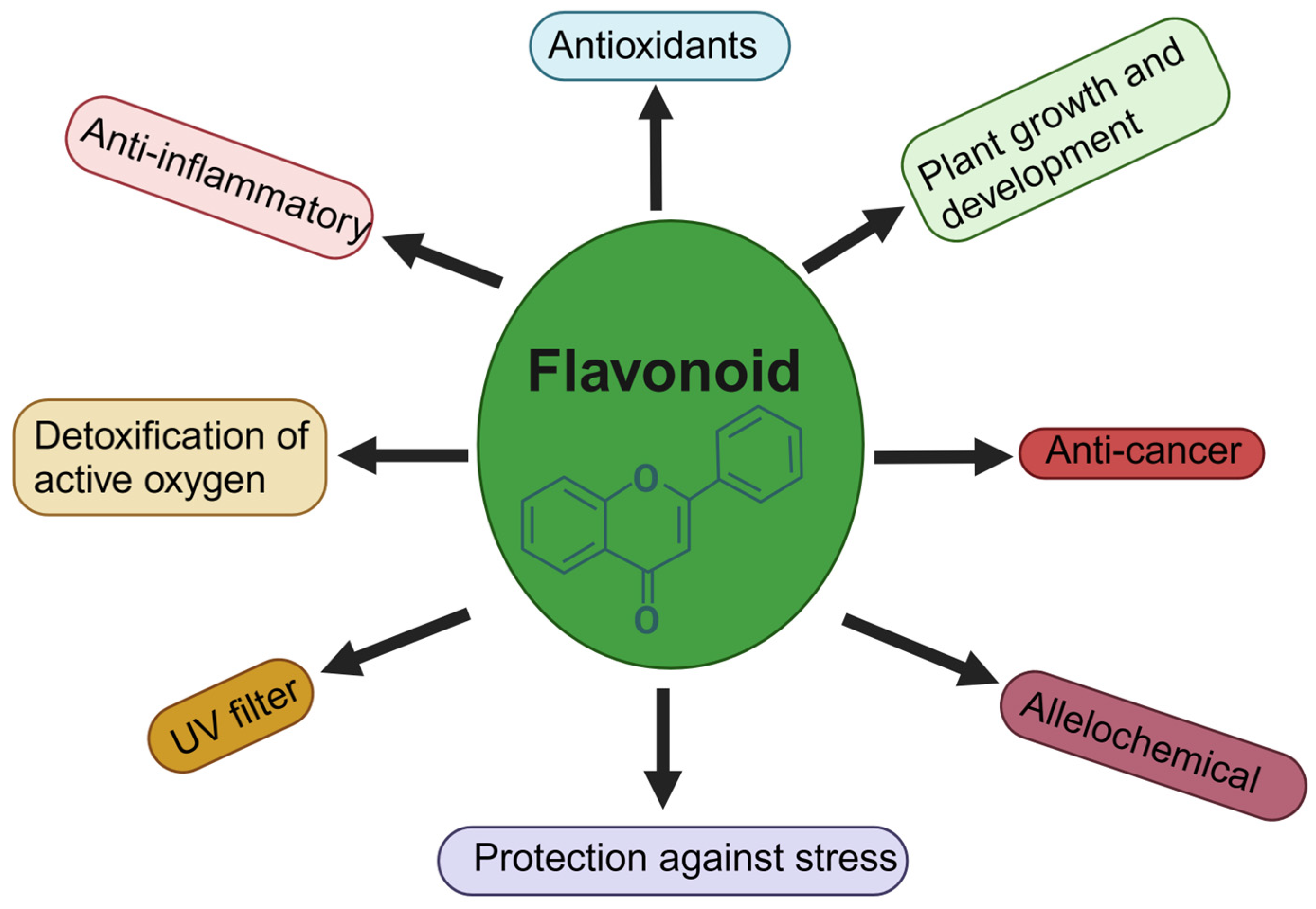
1. Introduction
2. South African Flora: A Rich Source of Flavonoids
3. Antioxidant Potential of Flavonoids
3.1. Anti-Inflammatory and Anti-Oxidative Flavonoids
3.2. Anti-Cancer Effects of Flavonoids
3.3. Anti-Hypertensive Effects of Flavonoids
3.4. Anti-Microbial Effects of Flavonoids
4. Flavonoid-Mediated Nanoparticles and Their Efficacy
5. Conclusions and Suggestions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Services D of H & H. Antioxidants. Department of Health & Human Services. Available online: http://www.betterhealth.vic.gov.au/health/healthyliving/antioxidants (accessed on 7 July 2024).
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or Negative Actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.F.; He, J.; Ahmed, A.; Yang, Y.; Li, M.; Wan, C. Chemical Components and Biological Activities of the Genus Phyllanthus: A Review of the Recent Literature. Molecules 2018, 23, 2567. [Google Scholar] [CrossRef]
- GBIF Backbone Taxonomy. Available online: https://www.gbif.org/dataset/d7dddbf4-2cf0-4f39-9b2a-bb099caae36c (accessed on 12 July 2024).
- Sepeur, S. Nanotechnology: Technical Basics and Applications; Vincentz Network GmbH & Co KG: Hanover, Germany, 2008; 172p. [Google Scholar]
- Kiran, K.R.; Swathy, P.S.; Paul, B.; Prasada, K.S.; Rao, M.R.; Joshi, M.B.; Rai, P.S.; Satyamoorthy, K.; Muthusamy, A. Untargeted metabolomics and DNA barcoding for discrimination of Phyllanthus species. J. Ethnopharmacol. 2021, 273, 113928. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Doll, R. An Overview of the epidemiological evidence linking diet and cancer. Proc. Nutr. Soc. 1990, 49, 119–131. [Google Scholar] [CrossRef]
- Kyle, J.; Duthie, G. Nutritional Relevance of Flavonoids in Disease Prevention. Nat. Prod. Commun. 2006, 1, 1049–1060. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.L.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef]
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated Dietary Flavonoid Intake and Major Food Sources of U.S. Adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Mukhtar, H. Cancer chemoprevention through dietary antioxidants: Progress and promise. Antioxid. Redox Signal. 2008, 10, 475–510. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Wendlocha, D.; Krzykawski, K.; Mielczarek-Palacz, A.; Kubina, R. Selected Flavonols in Breast and Gynecological Cancer: A Systematic Review. Nutrients 2023, 15, 2938. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Aguilera, C.M.; Gil, A. A Systematic Review of the Efficacy of Bioactive Compounds in Cardiovascular Disease: Phenolic Compounds. Nutrients 2015, 7, 5177–5216. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, M.; Bilotto, S.; Tedesco, I.; Laratta, B.; Russo, G.L. Dietary polyphenols in cancer prevention: The example of the flavonoid quercetin in leukemia. Ann. N. Y. Acad. Sci. 2012, 1259, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.; Dobson, V.; Duthie, S.; Kyle, J.; Collins, A. Absorption and DNA protective effects of flavonoid glycosides from an onion meal. Eur. J. Nutr. 2000, 39, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Gu, F.L.; Zhan, Q.; Palvannan, T.; Mohd Yusoff, A.R. Flavonoids mediated ‘Green’ nanomaterials: A novel nanomedicine system to treat various diseases—Current trends and future perspective. Mater. Lett. 2018, 210, 26–30. [Google Scholar] [CrossRef]
- Roy, P.; Parveen, S.; Ghosh, P.; Ghatak, K.; Dasgupta, S. Flavonoid loaded nanoparticles as an effective measure to combat oxidative stress in Ribonuclease A. Biochimie 2019, 162, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Stevens Barrón, J.C.; Chapa González, C.; Álvarez Parrilla, E.; De la Rosa, L.A. Nanoparticle-Mediated Delivery of Flavonoids: Impact on Proinflammatory Cytokine Production: A Systematic Review. Biomolecules 2023, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; de Beer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Marnewick, J.L.; Rautenbach, F.; Venter, I.; Neethling, H.; Blackhurst, D.M.; Wolmarans, P.; Macharia, M. Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J. Ethnopharmacol. 2011, 133, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Gelderblom, W.; Louw, A.; de Beer, D. South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides—A review. J. Ethnopharmacol. 2008, 119, 376–412. [Google Scholar] [CrossRef]
- Rejmánek, M. Cape plants: A conspectus of the Cape flora of South Africa. Divers. Distrib. 2001, 7, 303–304. [Google Scholar] [CrossRef]
- Van Heerden, F. Hoodia gordonii: A natural appetite suppressant. J. Ethnopharmacol. 2008, 119, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Vermaak, I.; Hamman, J.H.; Viljoen, A.M. Hoodia gordonii: An up-to-date review of a commercially important anti-obesity plant. Planta Medica 2011, 77, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Tulp, O.; Harbi, N.A.; Mihalov, J.; DerMarderosian, A. Effect of Hoodia plant on food intake and body weight in lean and obese LA/Ntul//-cp rats. FASEB J. 2001, 15, A404. [Google Scholar]
- Swart, C.; Rowswell, R.; Donaldson, J.; Barker, N. Population structure and survival of the critically endangered cycad Encephalartos latifrons in South Africa. S. Afr. J. Bot. 2019, 127, 80–90. [Google Scholar] [CrossRef]
- Timmer, A.; Günther, J.; Motschall, E.; Rücker, G.; Antes, G.; Kern, W.V. Pelargonium sidoides extract for treating acute respiratory tract infections. Cochrane Database Syst. Rev. 2013, CD006323. [Google Scholar] [CrossRef] [PubMed]
- Gericke, N.; Viljoen, A.M. Sceletium—A review update. J. Ethnopharmacol. 2008, 119, 653–663. [Google Scholar] [CrossRef] [PubMed]
- de Beer, S.; Campbell, J.; Leisegang, K.; Ma, X. A comparison between the anthropological and modern day uses of this indigenous herb. In Proceedings of the SA Medical Research Council 6th Annual Research Conference; 2012. Available online: https://repository.uwc.ac.za/handle/10566/6575?show=full (accessed on 24 July 2024).
- Gupta, R.K.; Patel, A.K.; Shah, N.; Choudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative stress and antioxidants in disease and cancer: A review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Szewczyk, K.; Bogucka-Kocka, A.; Vorobets, N.; Grzywa-Celińska, A.; Granica, S. Phenolic Composition of the Leaves of Pyrola rotundifolia L. and Their Antioxidant and Cytotoxic Activity. Molecules 2020, 25, 1749. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Afolayan, A.J. Antibacterial and antioxidant activities of hydroalcoholic stem bark extract of Schotia latifolia Jacq. Asian Pac. J. Trop. Med. 2011, 4, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Aderogba, M.A.; Okoh, E.K.; Idowu, T.O. Evaluation of the Antioxidant Activity of the Secondary Metabolites from Piliostigma reticulatum (DC.) Hochst. J. Biol. Sci. 2005, 5, 239–242. [Google Scholar]
- Kamara, B.I.; Brandt, E.V.; Ferreira, D.; Joubert, E. Polyphenols from Honeybush Tea (Cyclopia intermedia). J. Agric. Food Chem. 2003, 51, 3874–3879. [Google Scholar] [CrossRef]
- Aust, S.D.; Morehouse, L.A.; Thomas, C.E. Role of metals in oxygen radical reactions. J. Free. Radic. Biol. Med. 1985, 1, 3–25. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The Effects of Favonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M. Macrophage-mediated oxidation of extracellular low density lipoprotein requires an initial binding of the lipoprotein to its receptor. J. Lipid Res. 1994, 35, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amsha, R.; Croft, K.D.; Puddey, I.B.; Proudfoot, J.M.; Beilin, L.J. Phenolic content of various beverages determines the extent of inhibition of human serum and low-density lipoprotein oxidation in vitro: Identification and mechanism of action of some cinnamic acid derivatives from red wine. Clin. Sci. 1996, 91, 449–458. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis is an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Brown, N.J.; Vaughan, D.E.; Harrison, D.G.; Mehta, J.L. Established and Emerging Plasma Biomarkers in the Prediction of First Atherothrombotic Events. Circulation 2004, 109 (Suppl. 1), IV-6–IV-19. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef]
- Lapuente, M.; Estruch, R.; Shahbaz, M.; Casas, R. Relation of Fruits and Vegetables with Major Cardiometabolic Risk Factors, Markers of Oxidation, and Inflammation. Nutrients 2019, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Meng-Zhen, S.; Ju, L.; Lan-Chun, Z.; Cai-Feng, D.; Shu-Da, Y.; Hao-Fei, Y.; Wei-Yan, H. Potential therapeutic use of plant flavonoids in AD and PD. Heliyon 2022, 8, e11440. [Google Scholar] [CrossRef]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary Flavonoids for Immunoregulation and Cancer: Food Design for Targeting Disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef]
- Fortunato, L.R.; Alves, C.D.F.; Teixeira, M.M.; Rogerio, A.P. Quercetin: A flavonoid with the potential to treat asthma. Braz. J. Pharm. Sci. 2012, 48, 589–599. [Google Scholar] [CrossRef]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.-H.; Kang, E.S.; Kim, S.K. Antidiabetic Agents from Medicinal Plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Flavonoid-Rich Food Could Improve Your Gut Microbiome and Lower Your Blood Pressure. Available online: https://www.sciencefocus.com/news/flavonoid-food-gut-microbiome-blood-pressure (accessed on 8 July 2024).
- Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef]
- Hunter, T. Protein kinases and phosphatases: The Yin and Yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef]
- Tunon, M.J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S.; González-Gallego, J. Potential of flavonoids as anti-inflammatory agents: Modulation of pro-inflammatory gene expression and signal transduction pathways. Curr. Drug Metab. 2009, 10, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Elisha, I.L.; Dzoyem, J.-P.; McGaw, L.J.; Botha, F.S.; Eloff, J.N. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Complement. Altern. Med. 2016, 16, 307. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Candiracci, M.; Piatti, E.; Dominguez-Barragán, M.; García-Antrás, D.; Morgado, B.; Ruano, D.; Gutiérrez, J.F.; Parrado, J.; Castaño, A. Anti-inflammatory Activity of a Honey Flavonoid Extract on Lipopolysaccharide-Activated N13 Microglial Cells. J. Agric. Food Chem. 2012, 60, 12304–12311. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Bjørklund, G.; Lysiuk, R.; Vella, A.; Lenchyk, L.; Upyr, T. Targeting Cancer with Phytochemicals via Their Fine Tuning of the Cell Survival Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 3568. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Kundu, J.K.; Na, H.-K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Medica 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Gupta, S.C.; Hevia, D.; Patchva, S.; Park, B.; Koh, W.; Aggarwal, B.B. Upsides and downsides of reactive oxygen species for cancer: The roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal. 2012, 16, 1295–1322. [Google Scholar] [CrossRef]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Kumar, S.; Chashoo, G.; Saxena, A.K.; Pandey, A.K. Parthenium hysterophorus: A Probable Source of Anticancer, Antioxidant and Anti-HIV Agents. BioMed Res. Int. 2013, 2013, 810734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamson, D.W.; Brignall, M.S. Antioxidants and cancer, part 3: Quercetin. Altern. Med. Rev. J. Clin. Ther. 2000, 5, 196–208. [Google Scholar]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Popescu, I.D.; Zipeto, D.; Tzanakakis, G.; Nikitovic, D.; Fenga, C.; Stratakis, C.A.; Spandidos, D.A.; Tsatsakis, A.M. Inflammation and Metabolism in Cancer Cell—Mitochondria Key Player. Front. Oncol. 2019, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Hadi, S.M.; Asad, S.F.; Singh, S.; Ahmad, A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Marques, V.; Marinho, H.S.; Cyrne, L.; Antunes, F. Modulation of NF-kappaB-dependent gene expression by H2O2: A major role for a simple chemical process in a complex biological response. Antioxid. Redox Signal. 2009, 11, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Marnewick, J.; Joubert, E.; Joseph, S.; Swanevelder, S.; Swart, P.; Gelderblom, W. Inhibition of tumour promotion in mouse skin by extracts of rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), unique South African herbal teas. Cancer Lett. 2005, 224, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Magcwebeba, T.U.; Swart, P.; Swanevelder, S.; Joubert, E.; Gelderblom, W.C.A. In Vitro Chemopreventive Properties of Green Tea, Rooibos and Honeybush Extracts in Skin Cells. Molecules 2016, 21, 1622. [Google Scholar] [CrossRef]
- Kaushik, S.; Shyam, H.; Agarwal, S.; Sharma, R.; Nag, T.C.; Dwivedi, A.K.; Balapure, A.K. Genistein potentiates Centchroman induced antineoplasticity in breast cancer via PI3K/Akt deactivation and ROS dependent induction of apoptosis. Life Sci. 2019, 239, 117073. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhang, Q.Y.; Kang, X.M.; Wang, J.X.; Zhao, W.H. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol. 2010, 21, 263–268. [Google Scholar] [CrossRef]
- Pandey, P.; Sayyed, U.; Tiwari, R.K.; Siddiqui, M.H.; Pathak, N.; Bajpai, P. Hesperidin Induces ROS-Mediated Apoptosis along with Cell Cycle Arrest at G2/M Phase in Human Gall Bladder Carcinoma. Nutr. Cancer 2019, 71, 676–687. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Wang, J.; Li, J.; Liao, F.; Dong, W. Hesperetin induces apoptosis of esophageal cancer cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species. Tumor Biol. 2016, 37, 3451–3459. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Wu, D.; Wang, J.; Dong, W. Hesperetin induces the apoptosis of hepatocellular carcinoma cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, ATP and calcium. Med. Oncol. 2015, 32, 101. [Google Scholar] [CrossRef]
- Palit, S.; Kar, S.; Sharma, G.; Das, P.K. Hesperetin Induces Apoptosis in Breast Carcinoma by Triggering Accumulation of ROS and Activation of ASK1/JNK Pathway. J. Cell. Physiol. 2015, 230, 1729–1739. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin suppresses growth of human placental choriocarcinoma via reactive oxygen species-mediated P38 and JNK MAPK pathways. Phytomedicine 2018, 50, 238–246. [Google Scholar] [CrossRef]
- Ahamad, S.; Siddiqui, S.; Jafri, A.; Ahmad, S.; Afzal, M. Arshad induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS ONE 2014, 9, e110003. [Google Scholar] [CrossRef]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Naringenin-Induced Apoptotic Cell Death in Prostate Cancer Cells Is Mediated via the PI3K/AKT and MAPK Signaling Pathways. J. Cell. Biochem. 2017, 118, 1118–1131. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Cui, J.; Zhang, Y.; Wang, Z.L.; Chong, T.; Wang, Z.M. Isoflavones and Prostate Cancer: A Review of Some Critical Issues. Chin. Med. J. 2016, 129, 341–347. [Google Scholar] [CrossRef]
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef]
- Taparia, S.S.; Khanna, A. Procyanidin-rich extract of natural cocoa powder causes ROS-mediated caspase-3 dependent apoptosis and reduction of pro-MMP-2 in epithelial ovarian carcinoma cell lines. Biomed. Pharmacother. 2016, 83, 130–140. [Google Scholar] [CrossRef]
- Dev, S.S.; Farghadani, R.; Abidin, S.A.Z.; Othman, I.; Naidu, R. Flavonoids as receptor tyrosine kinase inhibitors in lung cancer. J. Funct. Foods 2023, 110, 105845. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, Y.; Jiang, Y.B.; Ping, L.B.; Cheng, H.; Zhang, J. EGFR promoter methylation detection in cervical cancer by a hybridization-fluorescence polarization assay. Diagn. Mol. Pathol. 2013, 22, 102–106. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Q.; Zhang, Y.; He, J.; Zheng, J. EBP50 interacts with EGFR and regulates EGFR signaling to affect the prognosis of cervical cancer patients. Int. J. Oncol. 2016, 49, 1737–1745. [Google Scholar] [CrossRef]
- Chen, X.; Xu, P.; Zhang, H.; Su, X.; Guo, L.; Zhou, X.; Wang, J.; Huang, P.; Zhang, Q.; Sun, R. EGFR and ERK activation resists flavonoid quercetin-induced anticancer activities in human cervical cancer cells in vitro. Oncol. Lett. 2021, 22, 754. [Google Scholar] [CrossRef]
- Martín, M.Á.; Serrano, A.B.G.; Ramos, S.; Pulido, M.I.; Bravo, L.; Goya, L. Cocoa flavonoids up-regulate antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J. Nutr. Biochem. 2010, 21, 196–205. [Google Scholar] [CrossRef]
- Rodríguez-Ramiro, I.; Martín, M.; Ramos, S.; Bravo, L.; Goya, L. Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on Caco2 cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef]
- Martín, M.A.; Goya, L.; Ramos, S. Preventive Effects of Cocoa and Cocoa Antioxidants in Colon Cancer. Diseases 2016, 4, 6. [Google Scholar] [CrossRef]
- Jeon, J.-S.; Kwon, S.; Ban, K.; Hong, Y.K.; Ahn, C.; Sung, J.-S.; Choi, I. Regulation of the Intracellular ROS Level Is Critical for the Antiproliferative Effect of Quercetin in the Hepatocellular Carcinoma Cell Line HepG2. Nutr. Cancer 2019, 71, 861–869. [Google Scholar] [CrossRef]
- Shang, H.; Lu, H.; Lee, C.; Chiang, H.; Chu, Y.; Chen, A.; Lin, Y.; Chung, J. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ. Toxicol. 2018, 33, 1168–1181. [Google Scholar] [CrossRef]
- Wu, P.; Meng, X.; Zheng, H.; Zeng, Q.; Chen, T.; Wang, W.; Zhang, X.; Su, J. Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer. Molecules 2018, 23, 2592. [Google Scholar] [CrossRef]
- Choi, J.-B.; Kim, J.-H.; Lee, H.; Pak, J.-N.; Shim, B.S.; Kim, S.-H. Reactive Oxygen Species and p53 Mediated Activation of p38 and Caspases is Critically Involved in Kaempferol Induced Apoptosis in Colorectal Cancer Cells. J. Agric. Food Chem. 2018, 66, 9960–9967. [Google Scholar] [CrossRef]
- Seydi, E.; Salimi, A.; Rasekh, H.R.; Mohsenifar, Z.; Pourahmad, J. Selective Cytotoxicity of Luteolin and Kaempferol on Cancerous Hepatocytes Obtained from Rat Model of Hepatocellular Carcinoma: Involvement of ROS-Mediated Mitochondrial Targeting. Nutr. Cancer 2018, 70, 594–604. [Google Scholar] [CrossRef]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef]
- Salmani, J.M.M.; Zhang, X.-P.; Jacob, J.A.; Chen, B.-A. Apigenin’s anticancer properties and molecular mechanisms of action: Recent advances and future prospectives. Chin. J. Nat. Med. 2017, 15, 321–329. [Google Scholar] [CrossRef]
- Lim, W.; Ryu, S.; Bazer, F.W.; Kim, S.; Song, G. Chrysin attenuates progression of ovarian cancer cells by regulating signaling cascades and mitochondrial dysfunction. J. Cell. Physiol. 2018, 233, 3129–3140. [Google Scholar] [CrossRef]
- Park, W.; Park, S.; Lim, W.; Song, G. Chrysin disrupts intracellular homeostasis through mitochondria-mediated cell death in human choriocarcinoma cells. Biochem. Biophys. Res. Commun. 2018, 503, 3155–3161. [Google Scholar] [CrossRef]
- Alipour, B.; Rashidkhani, B.; Edalati, S. Dietary flavonoid intake, total antioxidant capacity and lipid oxidative damage: A cross-sectional study of Iranian women. Nutrition 2016, 32, 566–572. [Google Scholar] [CrossRef]
- Sorrenti, V.; Vanella, L.; Acquaviva, R.; Cardile, V.; Giofrè, S.; DI Giacomo, C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015, 47, 1303–1310. [Google Scholar] [CrossRef]
- Cvorovic, J.; Tramer, F.; Granzotto, M.; Candussio, L.; Decorti, G.; Passamonti, S. Oxidative stress-based cytotoxicity of delphinidin and cyanidin in colon cancer cells. Arch. Biochem. Biophys. 2010, 501, 151–157. [Google Scholar] [CrossRef]
- Khan, F.; Niaz, K.; Maqbool, F.; Hassan, F.I.; Abdollahi, M.; Venkata, K.C.N.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef]
- Teekaraman, D.; Elayapillai, S.P.; Viswanathan, M.P.; Jagadeesan, A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Interact. 2019, 300, 91–100. [Google Scholar] [CrossRef]
- Li, H.; Chen, C. Quercetin Has Antimetastatic Effects on Gastric Cancer Cells via the Interruption of uPA/uPAR Function by Modulating NF-κb, PKC-δ, ERK1/2, and AMPKα. Integr. Cancer Ther. 2017, 17, 511–523. [Google Scholar] [CrossRef]
- Xiang, T.; Fang, Y.; Wang, S.X. Quercetin suppresses HeLa cells by blocking PI3K/Akt pathway. J. Huazhong Univ. Sci. Technol. 2014, 34, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ge, X.; Tian, X.; Zhang, Y.; Zhang, J.; Zhang, P. Soy isoflavone: The multipurpose phytochemical (Review). Biomed. Rep. 2013, 1, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.A.; Cotterchio, M.; Anderson, L.N.; Kreiger, N.; Kirsh, V.A.; Thompson, L.U. Use of isoflavone supplements is associated with reduced postmenopausal breast cancer risk. Int. J. Cancer 2013, 132, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Tan, J.-H.; Ou, T.-M.; Huang, Z.-S. Natural products and their derivatives as G-quadruplex binding ligands. Sci. China Chem. 2013, 56, 1351–1363. [Google Scholar] [CrossRef]
- Zubair, M.S.; Anam, S.; Maulana, S.; Arba, M. In Vitro and In Silico Studies of Quercetin and Daidzin as Selective Anticancer Agents. Indones. J. Chem. 2021, 21, 310–317. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- McArthur, K.; Kile, B.T. Apoptotic Caspases: Multiple or Mistaken Identities? Trends Cell Biol. 2018, 28, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.-E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.R. Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase, and modulate lipid homeostasis in adipose tissue and liver. Exp. Biol. Med. 2006, 231, 1287–1299. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, T.; Raj, J.U. Endothelial and Smooth Muscle Cell Interactions in the Pathobiology of Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2016, 54, 451–460. [Google Scholar] [CrossRef]
- Puzserova, A.; Bernatova, I. Blood pressure regulation in stress: Focus on nitric oxide-dependent mechanisms. Physiol. Res. 2016, 65, S309–S342. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Xu, H.-T.; Yu, J.-J.; Gao, J.-L.; Lei, J.; Yin, Q.-S.; Li, B.; Pang, M.-X.; Su, M.-X.; Mi, W.-J.; et al. Luteolin Ameliorates Hypertensive Vascular Remodeling through Inhibiting the Proliferation and Migration of Vascular Smooth Muscle Cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Thamcharoen, N.; Susantitaphong, P.; Wongrakpanich, S.; Chongsathidkiet, P.; Tantrachoti, P.; Pitukweerakul, S.; Avihingsanon, Y.; Praditpornsilpa, K.; Jaber, B.L.; Eiam-Ong, S. Effect of N- and T-type calcium channel blocker on proteinuria, blood pressure and kidney function in hypertensive patients: A meta-analysis. Hypertens. Res. 2015, 38, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Leeya, Y.; Mulvany, M.J.; Queiroz, E.F.; Marston, A.; Hostettmann, K.; Jansakul, C. Hypotensive activity of an n-butanol extract and their purified compounds from leaves of Phyllanthus acidus (L.) Skeels in rats. Eur. J. Pharmacol. 2010, 649, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.J.; Symons, J.D.; Jalili, T. Therapeutic potential of quercetin to decrease blood pressure: Review of efficacy and mechanisms. Adv. Nutr. 2012, 3, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Morón, R.; Sánchez, M.; Zarzuelo, A.; Galisteo, M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese zucker rats. Obesity 2008, 16, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, M.; Crown, O.; Akinmoladun, A.; Akindahunsi, A. Rutin and quercetin show greater efficacy than nifedipin in ameliorating hemodynamic, redox, and metabolite imbalances in sodium chloride-induced hypertensive rats. Hum. Exp. Toxicol. 2014, 33, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Omobowale, T.O.; Ola-Davies, O.E.; Asenuga, E.R.; Ajibade, T.O.; Adejumobi, O.A.; Arojojoye, O.A.; Afolabi, J.M.; Ogunpolu, B.S.; Falayi, O.O.; et al. Quercetin attenuates hypertension induced by sodium fluoride via reduction in oxidative stress and modulation of HSP 70/ERK/PPARγ signaling pathways. Biofactors 2018, 44, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Texier, O.; Favier, M.-L.; Agullo, G.; Demigné, C.; Régérat, F.; Rémésy, C. Quercetin metabolites in plasma of rats fed diets containing rutin or quercetin. J. Nutr. 1995, 125, 1911–1922. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects1. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Saponara, S.; Testai, L.; Iozzi, D.; Martinotti, E.; Martelli, A.; Chericoni, S.; Sgaragli, G.; Fusi, F.; Calderone, V. (+/−)-Naringenin as large conductance Ca(2+)-activated K+ (BKCa) channel opener in vascular smooth muscle cells. Br. J. Pharmacol. 2006, 149, 1013–1021. [Google Scholar] [CrossRef]
- Schnorr, O.; Brossette, T.; Momma, T.Y.; Kleinbongard, P.; Keen, C.L.; Schroeter, H.; Sies, H. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch. Biochem. Biophys. 2008, 476, 211–215. [Google Scholar] [CrossRef]
- Lu, X.L.; Liu, J.X.; Wu, Q.; Long, S.M.; Zheng, M.Y.; Yao, X.L.; Ren, H.; Wang, Y.G.; Su, W.W.; Cheung, R.T.F.; et al. Protective effects of puerarin against Aß40-induced vascular dysfunction in zebrafish and human endothelial cells. Eur. J. Pharmacol. 2014, 732, 76–85. [Google Scholar] [CrossRef]
- Mita, M.; Tanaka, H.; Yanagihara, H.; Nakagawa, J.I.; Hishinuma, S.; Sutherland, C.; Walsh, M.P.; Shoji, M. Membrane depolarization-induced RhoA/Rho-associated kinase activation and sustained contraction of rat caudal arterial smooth muscle involves genistein-sensitive tyrosine phosphorylation. J. Smooth Muscle Res. 2013, 49, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, T.; Zhou, S.; Sun, L.; Zhang, L.; Yu, G. Mg2+-dependent modulation of BKCa channels by genistein in rat arteriolar smooth muscle cells. J. Cell. Physiol. 2014, 229, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Omokhua-Uyi, A.G.; Abdalla, M.A.; Leonard, C.M.; Aro, A.; Uyi, O.O.; Van Staden, J.; McGaw, L.J. Flavonoids isolated from the South African weed Chromolaena odorata (Asteraceae) have pharmacological activity against uropathogens. BMC Complement. Med. Ther. 2020, 20, 233. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Day, A.J.; Mellon, F.; Barron, D.; Sarrazin, G.; Morgan, M.R.; Williamson, G. Human metabolism of dietary flavonoids: Identification of plasma metabolites of quercetin. Free Radic. Res. 2001, 35, 941–952. [Google Scholar] [CrossRef]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-O-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic Res. 2000, 33, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Sesink, A.L.A.; O’Leary, K.A.; Hollman, P.C.H. Quercetin glucuronides but not glucosides are present in human plasma after consumption of quercetin-3-glucoside or quercetin-4′-glucoside. J. Nutr. 2001, 131, 1938–1941. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Gee, J.M.; DuPont, M.S.; Johnson, I.T.; Williamson, G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: The role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003, 65, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Cañada, F.; Díaz, J.C.; A Kroon, P.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Walgren, R.A.; Lin, J.T.; Kinne, R.K.; Walle, T. Cellular uptake of dietary flavonoid quercetin 4’-beta-glucoside by sodium-dependent glucose transporter SGLT1. J. Pharmacol. Exp. Ther. 2000, 294, 837–843. [Google Scholar] [PubMed]
- Islam, M.A.; Punt, A.; Spenkelink, B.; Murk, A.J.; van Leeuwen, F.X.R.; Rietjens, I.M.C.M. Conversion of major soy isoflavone glucosides and aglycones in in vitro intestinal models. Mol. Nutr. Food Res. 2014, 58, 503–515. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, H.; Yu, J.; Zhu, L.; Yan, T.; Wu, P.; Lu, L.; Wang, Y.; Hu, M.; Liu, Z. SGLT-1 Transport and Deglycosylation inside Intestinal Cells Are Key Steps in the Absorption and Disposition of Calycosin-7-O-β-d-Glucoside in Rats. Drug Metab. Dispos. 2016, 44, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y.; Chang, W.; Zhou, M. The Effects and Mechanisms of Flavonoids on Cancer Prevention and Therapy: Focus on Gut Microbiota. Int. J. Biol. Sci. 2022, 18, 1451–1475. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, P.; Harish, S.; Ponnusamy, S.; Muthamizhchelvan, C. A novel strategy of nanosized herbal Plectranthus amboinicus, Phyllanthus niruri and Euphorbia hirta treated TiO2 nanoparticles for antibacterial and anticancer activities. Bioprocess Biosyst. Eng. 2021, 44, 1593–1616. [Google Scholar] [CrossRef] [PubMed]
- Dzerefos, C.M.; Witkowski, E. Density and potential utilisation of medicinal grassland plants from Abe Bailey Nature Reserve, South Africa. Biodivers. Conserv. 2001, 10, 1875–1896. [Google Scholar] [CrossRef]
- Makunga, N.P. Turning folklore into an ethnomedicinal catalogue Medicinal Plants of South Africa (2nd edition), B-E. van Wyk, B. van Oudtshoorn and N. Gericke: Book review. S. Afr. J. Sci. 2009, 105, 250. [Google Scholar]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Phenolic composition and antioxidant activities of 11 celery cultivars. J. Food Sci. 2010, 75, C9–C13. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Miar, S.; Majid Zadehzare, M.; Akbari, H. Evaluation of Effectiveness of Herbal Medication in Cancer Care: A Review Study. Iran. J. Cancer Prev. 2012, 5, 144–156. [Google Scholar] [PubMed]
- Ahmad, N.; Danish, S.; Sahai, N.; Dutta, R. Biosynthesis, Characterization of Gold Nanoparticles Using M. indica Leaf Extract and Their Anticancer Activity. Int. J. Nanobiotechnol. 2016, 2, 7–11. [Google Scholar]
- Dube, P.; Meyer, S.; Madiehe, A.; Meyer, M. Antibacterial activity of biogenic silver and gold nanoparticles synthesized from Salvia africana-luteaandSutherlandia frutescens. Nanotechnology 2020, 31, 505607. [Google Scholar] [CrossRef] [PubMed]
- Elbagory, A.M.; Meyer, M.; Cupido, C.N.; Hussein, A.A. Inhibition of Bacteria Associated with Wound Infection by Biocompatible Green Synthesized Gold Nanoparticles from South African Plant Extracts. Nanomaterials 2017, 7, 417. [Google Scholar] [CrossRef]
- Rajagopal, T.; Jemimah, I.A.A.; Ponmanickam, P.; Ayyanar, M. Synthesis of silver nanoparticles using Catharanthus roseus root extract and its larvicidal effects. J. Environ. Biol. 2015, 36, 1283–1289. [Google Scholar]
- Elbagory, A.M.; Hussein, A.A.; Meyer, M. The In Vitro Immunomodulatory Effects of Gold Nanoparticles Synthesized From Hypoxis hemerocallidea Aqueous Extract And Hypoxoside On Macrophage And Natural Killer Cells. Int. J. Nanomed. 2019, 14, 9007–9018. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood–Brain Barrier Transfer—Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; Tessaro, L.; Lima, A.K.O.; Velloso, I.P.S.; Conte-Junior, C.A. Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer. Nutrients 2023, 15, 3136. [Google Scholar] [CrossRef] [PubMed]
- Zahra, M.; Abrahamse, H.; George, B.P. Green nanotech paradigm for enhancing sesquiterpene lactone therapeutics in cancer. Biomed. Pharmacother. 2024, 173, 116426. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef]
- Reddy, N.J.; Vali, D.N.; Rani, M.; Rani, S.S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C 2014, 34, 115–122. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Vijaya, J.J.; Kaviyarasu, K.; Kombaiah, K.; Kennedy, L.J.; Ramalingam, R.J.; Munusamy, M.A.; Al-Lohedan, H.A. Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. J. Photochem. Photobiol. B Biol. 2017, 169, 178–185. [Google Scholar] [CrossRef]
- Khodadadi, B.; Bordbar, M.; Nasrollahzadeh, M. Green synthesis of Pd nanoparticles at Apricot kernel shell substrate using Salvia hydrangea extract: Catalytic activity for reduction of organic dyes. J. Colloid Interface Sci. 2017, 490, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Vennila, K.; Jayakumar, R.; Yusoff, A.R.M.; Hadibarata, T.; Palvannan, T. Phyto-synthesis of silver nanoparticles using Alternanthera tenella leaf extract: An effective inhibitor for the migration of human breast adenocarcinoma (MCF-7) cells. Bioprocess Biosyst. Eng. 2016, 39, 651–659. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Preethi, J.; Vijayan, R.; Yusoff, A.R.M.; Ameen, F.; Suresh, S.; Balagurunathan, R.; Palvannan, T. Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesised silver nanoparticles using Coriandrum sativum leaf extract. J. Photochem. Photobiol. B Biol. 2016, 163, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, B.S.; Kondath, S.; Anantanarayanan, R.; Rajaram, R. Kaempferol mediated synthesis of gold nanoparticles and their cytotoxic effects on MCF-7 cancer cell line. Process Biochem. 2015, 50, 1966–1976. [Google Scholar] [CrossRef]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surfaces B Biointerfaces 2009, 68, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, A.; Govindaraju, K.; Singaravelu, G.; Sadiq, A.M.; Kumar, V.G. Cardioprotective potential of biobased gold nanoparticles. Colloids Surfaces B Biointerfaces 2014, 117, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Nazeruddin, G.; Prasad, N.; Prasad, S.; Shaikh, Y.; Waghmare, S.; Adhyapak, P. Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind. Crop. Prod. 2014, 60, 212–216. [Google Scholar] [CrossRef]
- Luo, J.; Kong, J.L.; Dong, B.Y.; Huang, H.; Wang, K.; Wu, L.H.; Hou, C.C.; Liang, Y.; Li, B.; Chen, Y.Q. Baicalein attenuates the quorum sensing-controlled virulence factors of Pseudomonas aeruginosa and relieves the inflammatory response in P. aeruginosa-infected macrophages by downregulating the MAPK and NFκB signal-transduction pathways. Drug Des. Dev. Ther. 2016, 10, 183–203. [Google Scholar] [CrossRef]
- Rajkumari, J.; Busi, S.; Vasu, A.C.; Reddy, P. Facile green synthesis of baicalein fabricated gold nanoparticles and their antibiofilm activity against Pseudomonas aeruginosa PAO1. Microb. Pathog. 2017, 107, 261–269. [Google Scholar] [CrossRef]
- Muniyappan, N.; Nagarajan, N. Green synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activities. Process. Biochem. 2014, 49, 1054–1061. [Google Scholar] [CrossRef]
- Sysak, S.; Czarczynska-Goslinska, B.; Szyk, P.; Koczorowski, T.; Mlynarczyk, D.T.; Szczolko, W.; Lesyk, R.; Goslinski, T. Metal Nanoparticle-Flavonoid Connections: Synthesis, Physicochemical and Biological Properties, as Well as Potential Applications in Medicine. Nanomaterials 2023, 13, 1531. [Google Scholar] [CrossRef] [PubMed]
- Kollur, S.P.; Prasad, S.K.; Pradeep, S.; Veerapur, R.; Patil, S.S.; Amachawadi, R.G.; Lamraoui, G.; Al-Kheraif, A.A.; Elgorban, A.M.; Syed, A.; et al. Luteolin-Fabricated ZnO Nanostructures Showed PLK-1 Mediated Anti-Breast Cancer Activity. Biomolecules 2021, 11, 385. [Google Scholar] [CrossRef]
- Salaheldin, T.A.; Adhami, V.M.; Fujioka, K.; Mukhtar, H.; Mousa, S.A. Photochemoprevention of ultraviolet Beam Radiation-induced DNA damage in keratinocytes by topical delivery of nanoformulated Epigallocatechin-3-gallate. Nanomed. Nanotechnol. Biol. Med. 2022, 44, 102580. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Sun, D.-W.; Pu, H. Interfacing metal-polyphenolic networks upon photothermal gold nanorods for triplex-evolved biocompatible bactericidal activity. J. Hazard. Mater. 2022, 426, 127824. [Google Scholar] [CrossRef] [PubMed]
- Alhadrami, H.A.; Orfali, R.; Hamed, A.A.; Ghoneim, M.M.; Hassan, H.M.; Hassane, A.S.I.; Rateb, M.E.; Sayed, A.M.; Gamaleldin, N.M. Flavonoid-Coated Gold Nanoparticles as Efficient Antibiotics against Gram-Negative Bacteria—Evidence from In Silico-Supported In Vitro Studies. Antibiotics 2021, 10, 968. [Google Scholar] [CrossRef]
- Das, S.; Pramanik, T.; Jethwa, M.; Roy, P. Flavonoid-Decorated Nano-gold for Antimicrobial Therapy Against Gram-negative Bacteria Escherichia coli. Appl. Biochem. Biotechnol. 2021, 193, 1727–1743. [Google Scholar] [CrossRef]
- Sivakumar, S.; Tamizhazhagan, A.; Abdhul, K. Synthesis, characterization and anti-bacterial activity of silver nanoparticles from leaf extract of Phyllanthus urinaria L. Eur. J. Biomed. Pharm. Sci. 2017, 12, 544–553. [Google Scholar]



| Plant Species | Flavonoids | Traditional Uses | References |
|---|---|---|---|
| Rooibos (Aspalathus linearis) | Flavonols like quercetin and aspalathin | A traditional herbal infusion that is consumed for its refreshing taste and potential health benefits | [30,31] |
| Cyclopia intermedia (Honey bush) | Various flavonoids | Soothing coughs and easing respiratory issues like tuberculosis, pneumonia, and catarrh | [33] |
| Hoodia gordonii | Quercetin, kaempferol | Appetite-suppressing properties | [34,35] |
| Cycads (Various species) | Various flavonoids among diverse phytochemical constituents | Consumption as a starchy food source, raising conservation concerns | [37] |
| Pelargonium sidoides (Umckaloabo) | Quercetin derivatives | Treating respiratory infections | [38] |
| Sceletium tortuosum | Mesembrine alkaloids (distinctive flavonoid type) | Mood enhancement and stress reduction | [40] |
| Flavonoids | Cancer Type | Cell Line | Effect | Mechanism | References |
|---|---|---|---|---|---|
| Genistein | Breast cancer | MDA-MB-231 and MCF-7 | G2/M phase arrest, Apoptosis | ROS dependency | [92] |
| Daidzein | Breast cancer | MCF-7 | Apoptosis induction | ROS generation | [93] |
| Hesperetin | Gall bladder, esophageal, hepatocellular, and breast cancer | MCF-7 | Apoptosis trigger | Mitochondrial pathway, ROS production | [94,95,96,97] |
| Naringenin | Choriocarcinoma, epidermoid carcinoma, and prostate cancer | JAR, JEG 3 A431, PC3 and LNCaP | Anticancer effects | ROS generation, apoptotic pathways | [98,99,100] |
| Cocoa Catechins/Procyanidins | Ovarian cancer | OAW42 and OVCAR3 | Apoptosis | DNA damage, apoptotic changes | [103] |
| Cocoa Catechins/Procyanidins | Adenocarcinoma | Caco2 | Oxidative Stress Protection | Reduced ROS production | [109] |
| Cocoa Polyphenolic Extract | Liver cancer | HepG2 | ERK1/2 pathway activation | Increased antioxidant enzyme activity | [108] |
| Quercetin | Liver cancer | HepG2 | Chemo preventive properties | Reduced proliferation, decreased ROS levels | [111] |
| Quercetin | Gastric adenocarcinoma and breast cancer | AGS and MCF-7 | Apoptosis induction | Increased ROS production | [112] |
| Kaempferol | Bladder cancer | EJ | Growth inhibition | Apoptosis, S phase arrest, ROS modulation | [113] |
| Kaempferol | Colorectal cancer | HCT116, HCT15, and SW480 | Apoptosis activation | Caspase activation, ROS generation | [114] |
| Kaempferol | Hepatocellular carcinoma | HepG2 | Cytotoxic effects | Mitochondrial targeting, ROS mediation | [115] |
| Apigenin | Ovarian cancer | A2780, OVCAR-3, and SKOV-3 | Apoptosis promotion | ROS signaling alteration | [116] |
| Apigenin | Cervical cancer | HeLa, SiHa, CaSki, and C33A | Apoptosis activation | ROS generation, mitochondrial pathway | [117] |
| Chrysin | Choriocarcinoma, bladder cancer, and ovarian cancer | JAR, JEG3, ES2 and OV90 | Induction of death | Increased ROS, lipid peroxidation | [118,119] |
| Cyanidin | Prostate cancer | DU145 and LnCap | Induced cell death | ROS modulation | [121] |
| Cyanidin and Delphinidin | Colorectal cancer | LoVo and LoVo/ADR | Cytotoxic effects | ROS accumulation | [122] |
| Flavonoids | Nanomaterials | Size (nm) | Biomedical Applications | References |
|---|---|---|---|---|
| Quercetin | Ag-SeNPs | 30–35 nm | Exhibit antioxidant, antimicrobial, and anticancer activities. | [189] |
| Proanthocyanidin | AuNPs | 17–29 nm | Efficient cardio-protective potential with good biocompatibility | [192] |
| Luteolin | AgNPs | 13 nm | Antimicrobial activity against B. subtilus | [193] |
| Kaempferol | AuNPs | 16.5 nm | Anticancer activity against human breast cancer. | [190] |
| Apiin | AuNPs | 21 nm | Anticancer activity | [191] |
| Baicalein | AuNPs | 39 nm | Antibiofilm activity against P. aeruginosa | [195] |
| Flavonoids (Dalbergia spinosa) | AgNPs | 18 nm | Anti-inflammatory and antibacterial (E. coli, P. aeruginosa, S. aureus, and B. subtilis) activities | [196] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, M.; Abrahamse, H.; George, B.P. Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine. Antioxidants 2024, 13, 922. https://doi.org/10.3390/antiox13080922
Zahra M, Abrahamse H, George BP. Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine. Antioxidants. 2024; 13(8):922. https://doi.org/10.3390/antiox13080922
Chicago/Turabian StyleZahra, Mehak, Heidi Abrahamse, and Blassan P. George. 2024. "Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine" Antioxidants 13, no. 8: 922. https://doi.org/10.3390/antiox13080922
APA StyleZahra, M., Abrahamse, H., & George, B. P. (2024). Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine. Antioxidants, 13(8), 922. https://doi.org/10.3390/antiox13080922








